Abstract
The role of complement in the development of maladaptive immunity in experimental allergic asthma is unclear. In this study, we show that C3a receptor (C3aR)-deficient mice are protected from the development of Th2 immunity in a model of house dust mite-induced asthma. C5a receptor (C5aR)-targeting of C3aR-deficient mice during allergen sensitization not only reversed the protective effect but enhanced Th2 cytokine production, airway inflammation, and airway responsiveness, suggesting that the reduced allergic phenotype in C3aR-deficient mice results from protective C5aR signaling. In support of this view, C5aR expression in C3aR-deficient pulmonary dendritic cells (DCs) was increased when compared with wild-type DCs. Moreover, C5aR targeting regulated the frequency of pulmonary plasmacytoid DCs expressing costimulatory molecules B7-H1 and B7-DC. Ex vivo targeting of B7-H1 and B7-DC increased Th2 cytokine production from T cells of wild-type but not of C5aR-targeted mice, suggesting a protective role for C5a through regulation of B7 molecule expression on plasmacytoid DCs.
Allergic asthma is a chronic airway inflammatory disease arising as a result of inappropriate immune responses, mediated by Th2 cells to common environmental Ags in genetically susceptible individuals. The major pathophysiological characteristics of asthma include bronchoconstriction, airway hyperresponsiveness (AHR),3 and airway inflammation, which are causally linked to a maladaptive Th2 immune response (1).
The complement system has been shown to contribute to inflammatory responses during the effector phase, in particular by effects of the anaphylatoxins (ATs) C3a and C5a (2, 3). The ATs have long been recognized as potent proinflammatory mediators that promote leukocyte migration and activation, induce smooth muscle contraction, and increase vascular permeability. All of these functions are of particular relevance for the pathophysiology of allergic asthma (3). In addition to this expected proallergic function of complement in allergic asthma, a protective role has been described. C5-deficient mice are more susceptible to the development of AHR and airway inflammation than wild-type (WT) controls (4 – 6). This effect has recently been assigned to C5a receptor (C5aR)-mediated signaling during allergen sensitization. Pharmacological targeting of the C5aR results in a marked increase in Th2 immunity in mouse models of allergic asthma (2). These data suggest a dual role for C5a/C5aR signaling in asthma pathogenesis and pathology.
In contrast to C5a, the role for C3a is less clear. Although most available data suggest a proallergic role for C3a receptor (C3aR)-mediated signaling during the effector phase of asthma, its regulatory impact on the development of Th2 adaptive immunity is controversial. In OVA-induced asthma models, C3aR deficiency is associated with reduced bronchoconstriction in guinea pigs and decreased AHR in mice on the BALB/c background. However, these animals were not protected from airway eosinophilic inflammation, serum IgE secretion, or Th2 cytokine production (7, 8). In contrast, in a model of mixed Aspergillus fumigatus- and OVA-induced pulmonary allergy, C3aR-deficient mice (C3aRKOs) on the C57BL/6 background showed significant attenuation in AHR, airway eosinophilia, pulmonary Th2 cytokines, IgE titers, and mucus secretion, suggesting a critical role for C3aR signaling in the development of Th2 immunity in this model (9). The contentious data may result from species/strain differences, the nature of allergen, and/or the method used for immunization.
In the present study, we have used the well characterized model of house dust mite (HDM)-induced allergic asthma. The HDM Dermatophagoides pteronyssinus is an important source of indoor allergens. Approximately 10% of individuals with asthma suffer from HDM-mediated allergy (10). In contrast to models where systemic delivery of allergen in the context of potent adjuvants has to be used to promote an allergic phenotype, administration of crude extracts of HDM into the airways effectively elicits allergic sensitization and airway inflammation in BALB/c mice (2, 11). Here we compared HDM-mediated immune responses elicited in WT, C5aR-deficient mice (C5aRKOs), and C3aRKOs side-by-side. Our data reveal a protective role for C5a in the development of pulmonary allergy and maladaptive Th2 immunity by regulating the accumulation of tolerogenic dendritic cells (DCs) expressing costimulatory molecules B7-H1 and B7-DC in the lungs.
Materials and Methods
Mice
BALB/c (The Jackson Laboratory), C5aRKOs, and C3aRKOs on a BALB/c background were bred and maintained in the Cincinnati Children’s Hospital Medical Center specific pathogen-free facility and used at 8–12 wk of age. Animal care was provided in accordance with National Institute of Health guidelines. These studies were reviewed and approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee.
Induction of the allergic phenotype and C5aR blockade in vivo
BALB/c, C5aRKOs, and C3aRKOs were immunized with 100 μg/40 μl of HDM (crude extract; Greer Laboratories) or PBS (as control) intratracheally (i.t.) on days 0, 7, 14, and 21. C5aR was blocked in HDM-treated BALB/c and C3aRKOs by i.t. administration of 50 μg/40 μl of the neutralizing anti-C5aR mAb (clone 20/70; Hycult Biotechnology) on days –1, 6, 13, and 20. Animals treated with 50 μg/40 μl of Rat IgG2b mAb (Zymed Laboratories) served as isotype controls.
Allergen-induced airway hyperresponsiveness
AHR was determined as described (12). Briefly, mice were anesthetized 72 h after the last i.t. HDM exposure, intubated, and ventilated at a rate of 120 breaths per minute with a constant tidal volume of air (0.2 ml), and paralyzed with 25 mg/kg weight of decamethonium bromide. After establishment of a stable airway pressure, 25 μg/kg weight of acetylcholine was injected i.v. and dynamic airway pressure measured as airway pressure time index in cm H2O × sec−1 was followed for 5 min.
Collection of blood and bronchoalveolar lavage (BAL) measurements
Blood was drawn from abdominal aorta and centrifuged. Serum was stored at −80°C for IgE measurements. BAL samples were obtained by cannulating the trachea, injecting 1.0 ml of ice-cold PBS, and subsequently aspirating the BAL fluid. BAL cells were washed once in PBS and counted using a hemocytometer (Paul Marienfeld). Differential cell counts were obtained from BAL cells spun down onto slides and treated with Diff-Quick stain (Dade Behring). A total of 500 cells were morphologically differentiated by light microscopy.
Th2 cytokine production and isolation of pulmonary cells
Liberase/DNase I digests of the lungs were prepared to obtain single lung cell suspensions. Single cell suspensions (2.5 × 105) were restimulated ex vivo with 30 μg/ml HDM and incubated for 72 h in RPMI 1640 culture medium. IL-4, IL-5, IL-10, and IL-13 production in supernatants was determined using DuoSet ELISA kits (R&D Systems) following the manufacturer’s protocol. In some experiments, cell suspensions were labeled with CD11c microbeads and sorted by MACS (Miltenyi Biotec) to obtain CD11c+ lung DCs for RT-PCR analysis. Alternatively, cell suspensions were stained with allophycocyanin anti-CD11c and PE anti-CD4 Abs (eBioscience) and sorted by flow cytometry (FACSVantage SE) to obtain lung DCs and T cells for coculture assay.
Serum IgE ELISA
To determine total serum IgE levels, microtiter plates (Nunc) were coated overnight at 4°C with 50 μl of purified rat anti-mouse IgE mAb (BD Pharmingen) diluted in PBS at a concentration of 2 μg/ml. Unspecific binding was blocked with 10% FBS/PBS. An eight-point standard curve was obtained using 2-fold serial dilutions of purified mouse IgE (BD Pharmingen). Ten-fold dilutions of mouse sera were incubated in wells overnight at 4°C. Total IgE binding was detected using biotin-conjugated rat anti-mouse IgE mAb (BD Pharmingen), followed by avidin-peroxidase (Sigma-Aldrich), and one-step ABTS (Pierce) as substrates. To assess serum HDM-specific IgE levels, 50 μl of HDM diluted in 0.1 M Na2HPO4 (PH 8.2) at a concentration of 100 μg/ml were used to coat the plates. Two-fold dilutions of mouse sera were incubated in wells. HDM-specific IgE binding was detected in the same way as described above.
RT-PCR analysis
Quantification of C5aR and C3aR gene expression in CD11c+ lung DCs was performed as described (13). Briefly, lung DCs were prepared with TRIzol reagent (Invitrogen), and RNA was extracted and quantified by spectrophotometry. After DNase digestion using a Deoxyribonuclease I kit (Sigma-Aldrich), cDNA was obtained using Superscript II Reverse Transcriptase (Invitrogen) following the manufacturer’s protocol. Standards for real-time RT-PCR were obtained from a macrophage cell line (J774.A1) stimulated for 2 h with LPS (200 ng/ml) at 37°C. Gene expression levels were determined by real-time RT-PCR using iQ-SYBRgreen reaction mix(Bio-Rad) containing 5 μl cDNA and 500 nM primer. The following primers were used: GAPDH (f) 5′-TGC ACC ACC AAC TGC TTA-3′, (r) 5′-GGA TGC AGG GAT GAT GTT C-3′; C5aR (f) 5′-CAG GCG GTGTAG AGG AGA AG-3′, (r) 5′-GAA GGA AGG AAG GAG GAG AGG-3′; C3aR (f) 5′-TGA TCT GTT CAT TAT GGA CAA TC-3′, (r) 5′-TGA TCT GTT CAT TAT GGA CAA TC-3′.
Statistical analysis
Statistical analysis was performed using the SigmaStat version 3.5 statistical package (Systat Software). Statistical difference of data was assessed using an unpaired Student’s t test. Values of p < 0.05 were considered statistically significant.
Results
Opposing roles for C5aR and C3aR signaling in the development of maladaptive Th2 immunity in experimental allergic asthma
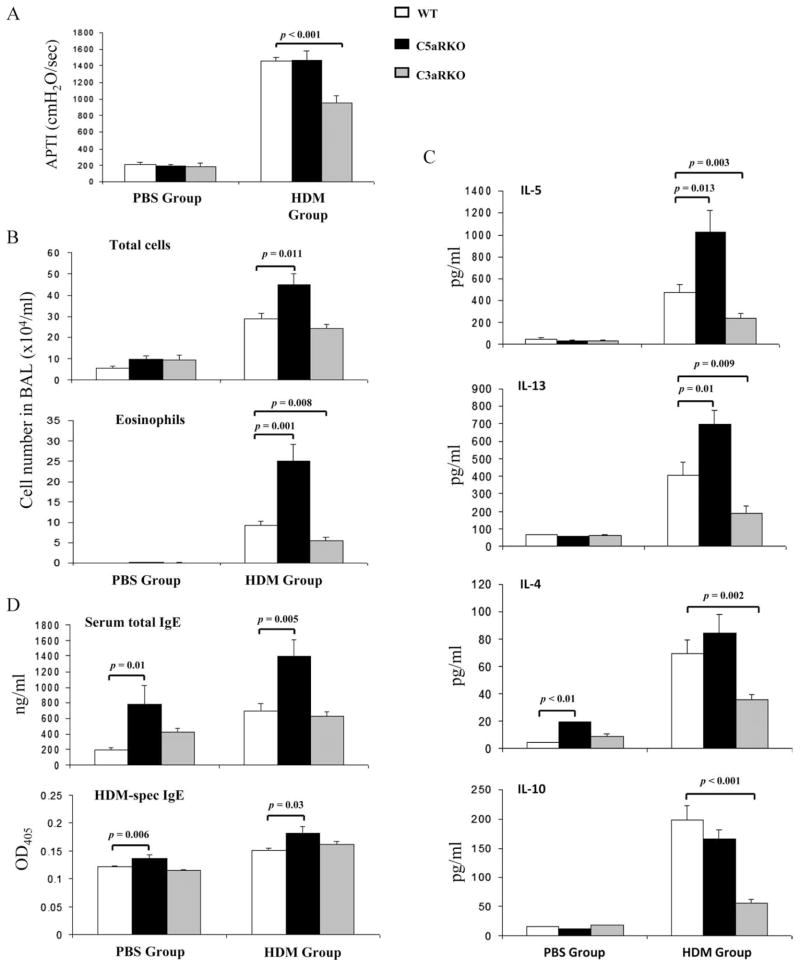
To examine the role for C5aR and C3aR signaling in the development of maladaptive Th2-mediated immune responses, we used a mouse model of HDM-induced experimental allergic asthma (Fig. 1). Intra-tracheal HDM administration significantly increased airway responsiveness, total cell numbers, and eosinophil numbers in BAL fluid as well as serum total IgE and allergen-specific IgE levels in WT BALB/c mice (Fig. 2, A, B, and D). Furthermore, pulmonary cells from HDM-treated WT mice produced significantly more IL-5, IL-13, IL-4, and IL-10 compared with PBS controls (Fig. 2C).
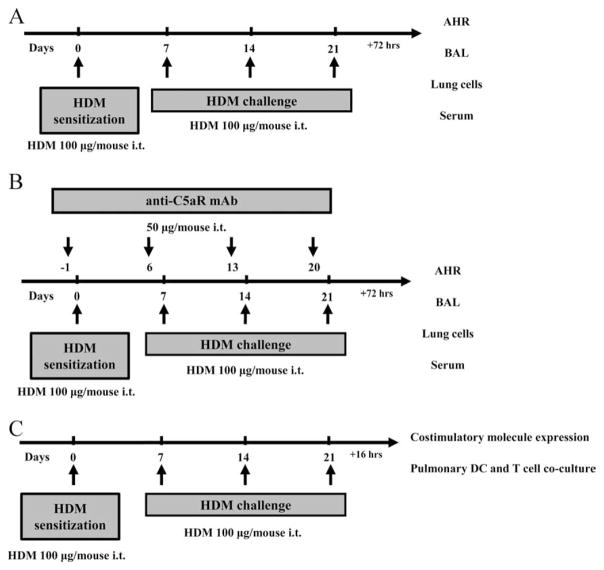
FIGURE 1.
Protocols underlying the mouse model of HDM-induced experimental allergic asthma. A, Mice were exposed to i.t. HDM at the indicated time points. Seventy-two hours after the final HDM exposure, airway responsiveness was determined. Subsequently BAL, lung cells, and blood samples were collected. B, To block C5aR signaling, mice were treated with the anti-C5aR mAb i.t. 24 h before each HDM exposure on days –1, 6, 13, and 20. C, Pulmonary DCs and T cells were isolated and cocultured 16 h after the final HDM exposure. The expression of costimulatory molecules on pulmonary DCs was also measured.
FIGURE 2.
The lack of C3aR or C5aR signaling has opposing effects on the development of the allergic phenotype in response to pulmonary HDM exposure. A, Airway responsiveness to i.v. acetylcholine. Airway responsiveness is expressed as the time-integrated change in airway pressure over baseline pressure. B, Total and eosinophil cell counts in BAL. C, Cytokine profile of pulmonary cells harvested from mice 72 h after the final in vivo HDM exposure. Supernatants were collected after 72 h in vitro cell culture. D, Serum concentrations of total IgE and HDM-specific IgE. Values shown are the mean ± SEM. n = 10 –25 per group. APTI, airway pressure time index.
Importantly, C5aRKOs suffered from substantially increased total cell numbers, which were mainly due to a 2.5-fold increase in airway eosinophils as compared with WT mice (Fig. 2B). This increase in inflammatory cells was associated with increased total and HDM-specific serum IgE levels as well as increased production of IL-5 and IL-13 from pulmonary cells restimulated with HDM ex vivo for 3 days after the final allergen challenge (Fig. 2, B–D). AHR and production of IL-4 and IL-10 in HDM-treated C5aRKOs were similar to WT mice (Fig. 2, A and C). Of note, we observed significantly increased levels of IL-4 and serum IgEs in PBS-treated C5aRKOs as compared with WT controls (Fig. 2, C and D), suggesting a protective regulatory role for C5aR signaling on the development of Th2 adaptive immunity under steady state conditions. In contrast, C3aRKOs showed significantly decreased allergen-induced AHR, airway eosinophilia, and production of Th2 cytokines as compared with WT mice (Fig. 2, A–C). However, total and HDM-specific IgE production in C3aRKOs was similar to that in WT controls (Fig. 2D). These data suggest a positive regulatory role for C3aR signaling on the development of Th2 immunity, airway inflammation, and AHR. An alternative explanation is that the decreased allergic phenotype in the absence of C3aR results from protective C5aR signaling during allergen sensitization.
Ablation of C5aR signaling reverts reduced Th2 immune responses in C3aRKOs upon pulmonary HDM exposure
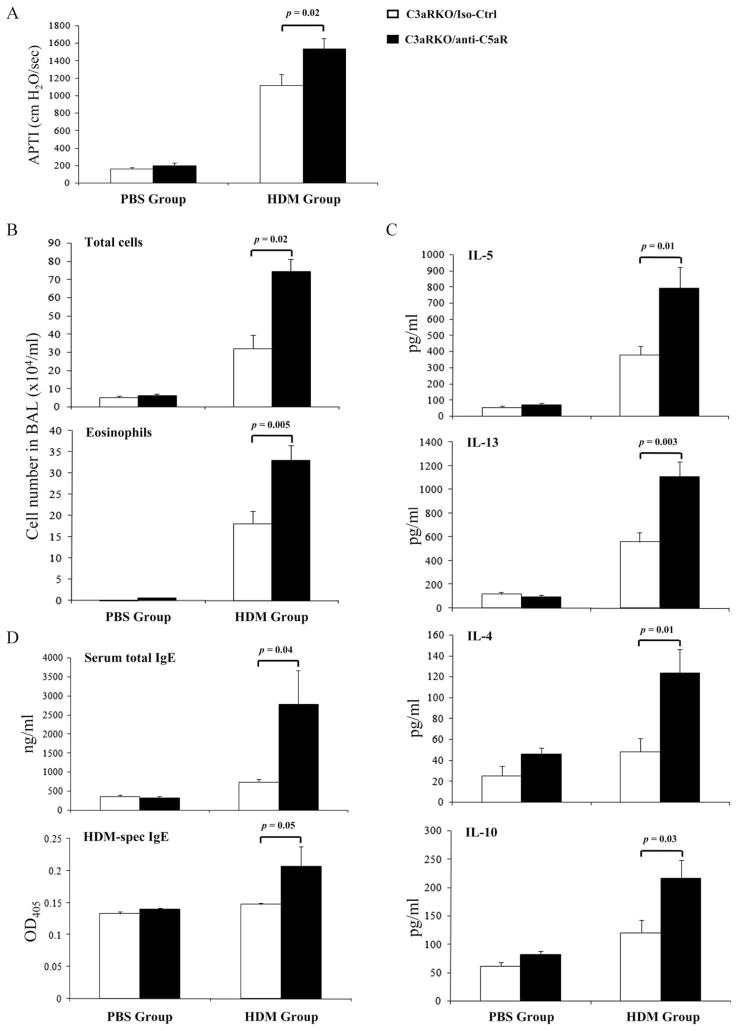
To evaluate this hypothesis, we blocked C5aR-mediated signaling in C3aRKOs by pharmacological targeting of C5aR before each HDM treatment, using a neutralizing anti-C5aR mAb (14) (Fig. 1). The anti-C5aR mAb has been demonstrated to neutralize C5aR-specific signaling in several in vivo models of immune complex disease (15) and allergic asthma (2, 16). Blocking C5aR significantly increased AHR in C3aRKOs as compared with isotype Ab-treated controls (Iso-Ctrl) (Fig. 3A). Similarly, C5aR blockade dramatically enhanced the development of airway eosinophilia and increased the production of total and HDM-specific serum IgE and of Th2 cytokines as compared with the Iso-Ctrl group (Fig. 3, B–D). These data suggest that the decreased Th2 immunity in the absence of C3aR signaling results rather from a shift toward protective C5aR signaling than from the absence of C3aR signaling.
FIGURE 3.
C5aR targeting in C3aRKOs reverts the impaired Th2 immune responses. A, Airway responsiveness to i.v. acetylcholine. B, Total and eosinophil cell counts in BAL. C, Cytokine profile of pulmonary cells harvested from mice 72 h after the final in vivo HDM exposure. D, Serum concentrations of total IgE and HDM-specific IgE. Values shown are the mean ± SEM; n = 5–15 per group.
Reciprocal modulation of C5aR and C3aR expression in pulmonary DCs as a potential mechanism to regulate the development of Th2 immune responses
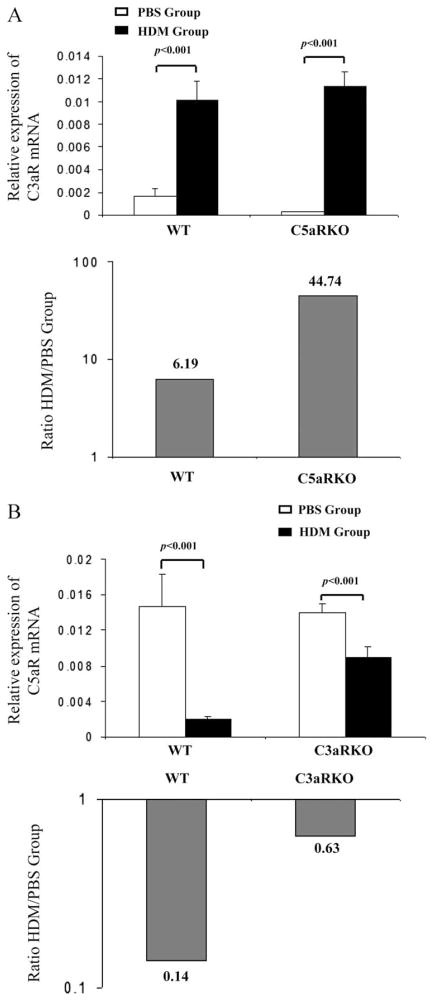
In a previous study we found that different pulmonary DC subsets express C5aRs (2). DCs are crucial immune regulators that not only mediate and maintain inhalation tolerance but also trigger the development of maladaptive Th2 immunity in asthmatics (17). To investigate the potential mechanisms underlying the opposing effects of C5aR- and C3aR-mediated signaling observed in the HDM model of allergic asthma, we examined the expression of C5aR and C3aR mRNA in pulmonary CD11c+ DCs isolated from mice following the final airway HDM exposure by real-time RT-PCR. C3aR expression in pulmonary DCs increased during the course of HDM exposure in WT and in C5aRKO mice (Fig. 4A, upper panel). Pulmonary HDM exposure increased C3aR expression 45-fold in C5aR-deficient pulmonary DCs but only 6-fold in WT DCs (Fig. 4A, lower panel). In contrast, C5aR expression in pulmonary DCs decreased upon HDM exposure in WT and in C3aRKO mice (Fig. 4B, upper panel). More importantly, HDM exposure resulted in a 7-fold reduction in C5aR expression in WT DCs whereas in C3aR-deficient DCs the decrease in C5aR expression was minor (Fig. 4B, lower panel). These data indicate cross-regulation between C5aR and C3aR in pulmonary DCs, which is consistent with the idea that the reduced allergic phenotype in C3aRKOs is mainly driven by C5aR signaling in pulmonary DCs.
FIGURE 4.
Reciprocal modulation of C3aR and C5aR expression in pulmonary dendritic cells after HDM exposure. A, Relative expression of C3aR mRNA in pulmonary CD11c+ dendritic cells from BALB/c WT and C5aRKOs (upper panel). Fold increase of C3aR mRNA expression in HDM-treated group relative to PBS-treated group (lower panel). B, Relative expression of C5aR mRNA in pulmonary CD11c+ dendritic cells from BALB/c WT and C3aRKOs (upper panel). Fold decrease of C5aR mRNA expression in HDM-treated group relative to PBS-treated group (lower panel). Values shown are the mean ± SEM. n = 10 –25 per group.
C5a regulates B7-H1 and B7-DC expression on plasmacytoid DCs (pDCs) but not on myeloid DCs (mDCs)
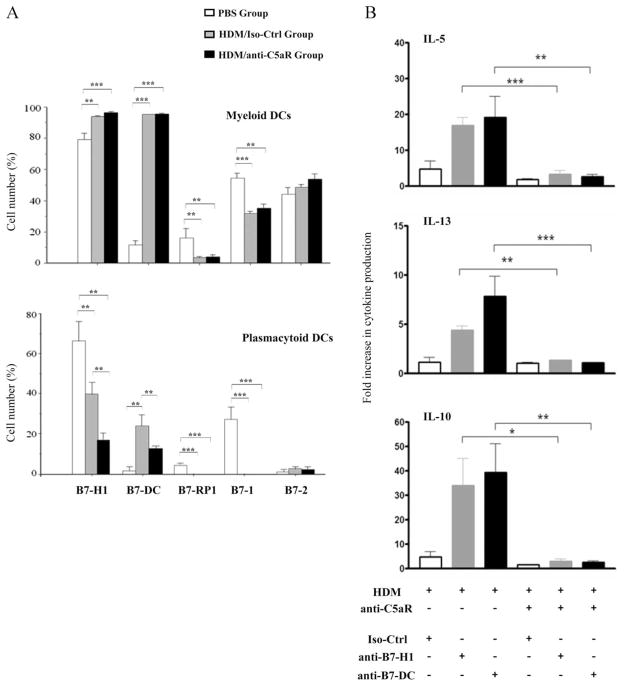
Our previous results suggest that pDCs inhibit myeloid DC-driven activation of Th2 cytokine production from T effector cells after pulmonary HDM exposure (2). Based on these data we hypothesized that the protective impact of C5aR signaling on the development of Th2 immunity results from regulation of costimulatory activity of mDCs and/or pDCs. We evaluated the expression of B7-1, B7-2, B7-H1, B7-DC and B7-RP1 molecules on mDCs and pDCs isolated from WT mice that had been exposed to HDM in the presence or absence of a neutralizing anti-C5aR mAb during allergen sensitization and challenge. HDM exposure increased the frequency of pulmonary mDCs expressing B7-H1 and B7-DC but decreased the frequency of mDCs expressing B7-RP1 and B7-1. B7-2 expression was virtually unchanged. More importantly, we found no difference in B7 molecule expression in the presence or absence of C5aR blockade (Fig. 5A, upper panel).
FIGURE 5.
C5aR targeting during allergen sensitization regulates Th2 cytokine production through its impact on the expression of B7-H1 and B7-DC costimulatory molecules on pulmonary pDCs. A, Expression of B7 costimulatory molecules on pulmonary mDCs (upper panel) and pulmonary pDCs (lower panel) isolated from mice 16 h after the final in vivo HDM exposure. B, Cytokine levels in supernatants of pulmonary CD11c+ DC and CD4+ T cell cocultures. Pulmonary CD11c+ DCs were isolated and incubated in vitro with specific neutralizing anti-B7-H1 or anti-B7-DC Abs for 2 h. DCs were then cocultured with pulmonary CD4+ T cells for 72 h before cytokine levels were measured. All groups were subjected to in vivo HDM treatment. The y-axes shows the fold increase in cytokine levels as compared with in vitro PBS controls. Values shown are the mean ± SEM. n = 10 –25 per group. ±, p < 0.05; 33, p < 0.01; 333, p < 0.001.
The frequency of pDCs expressing B7 molecules was much lower than that of mDCs, indicating a relatively immature status of pDCs under steady state conditions (PBS group). Of note, the frequency of B7-H1+ mDCs and pDCs was similar. Ag exposure decreased the frequency of B7-H1+, B7-RP-1+, and B7-1+ pDCs. In contrast, the frequency of B7-DC+ pDCs increased substantially. As with mDCs, the B7-2+ pDC population remained unchanged after allergen exposure.
Importantly, in vivo ablation of C5aR signaling dramatically reduced the frequency of B7-H1+ pDCs and diminished the increased frequency of B7-DC+ pDCs (Fig. 5A, lower panel). To determine whether the change in the frequency of B7-H1+ and B7-DC+ pDCs has an impact on T cell function, we cocultured pulmonary CD11c+ DCs and CD4+ T cells 16 h after the final in vivo HDM exposure and measured their Th2 cytokine production in vitro. Some DCs were also treated with specific neutralizing anti-B7-H1 or anti-B7-DC Abs to block the function of these molecules. In the presence of C5aR signaling (HDM group), in vitro blockade of B7-H1 or B7-DC resulted in enhanced Th2 cytokine production by cocultured T cells as compared with the isotype Ab-treated group. In contrast, when C5aR signaling was already ablated in vivo, blockade of B7-H1 and B7-DC had no effect on Th2 cytokine production (Fig. 5B). Taken together, these data suggest that B7-H1 and B7-DC are critical for the pDC-driven regulation of Th2 cytokine production from HDM-stimulated T effector cells. Further, our data indicate that C5aR signaling protects from the development of Th2 immunity in allergic asthma through its regulatory impact on B7-H1 and B7-DC expression on pulmonary pDCs.
Discussion
The contribution of C3a to the development of Th2 immunity in experimental allergic asthma is still controversial. C3aRKOs on the C57BL/6 background experience reduced Th2 cytokine production as well as diminished AHR, eosinophilic inflammation, IgE, and mucus production in a model of mixed OVA- and As-pergillus-mediated pulmonary allergy (9). In contrast, Th2 cytokine and IgE production from C3aR knockouts (BALB/c background) is indistinguishable from WT mice in a model of OVA-induced allergic asthma (8). These data suggest that the allergic phenotype depends on the nature of the allergen and/or the background of the mouse strain. Our data suggest a critical role for the allergen but not for the strain background. In a well established model of HDM-induced allergic asthma, we found reduced Th2 cytokine production, AHR, and cellular inflammation in BALB/c C3aRKOs as compared with WTs. In contrast to OVA, Aspergillus conidia and HDM are strong activators of complement, driven by either lectin pathway activation (18) or protease activity leading to C3a and C5a generation from C3 and C5 (19).
The reduced Th2 immunity in C3aRKOs suggests that it might result from a regulatory impact of C3a on DCs, which have been shown to play critical roles in the initiation and perpetuation of maladaptive T cell responses (20). In support of a role for C3a during allergen sensitization, DCs (21) and activated T cells express C3aR (22), and C3/C3a have been shown to regulate DC/T cell interactions in experimental models of atopy (23) and allospecific T cell responses (21).
In contrast to C3aR deficiency, targeting of C5aR signaling during allergen sensitization (2) or genetic deficiency of C5 (4, 5) enhances adaptive Th2 immune responses and the allergic phenotype. In accordance with our previous observations (2), we found increased Th2 cytokine production, eosinophilic inflammation, and IgE response in C5aRKOs upon airway HDM challenge, whereas the induction of AHR was indistinguishable from WT controls.
The opposing effects of C3a and C5a on Th2 cytokine production and eosinophilic inflammation may result from independent activation of C3aR and C5aR pathways, or a cross-talk between both receptors. In support of the latter, we have previously shown that C5a negatively regulates C3aR internalization (24). Here we found substantial C3aR up-regulation in pulmonary WT and C5aR-deficient DCs. Importantly, the up-regulation in C5aR-deficient DCs was ~7-fold higher than that in WT DCs. In contrast, C5aR expression was markedly down-regulated in WT DCs after HDM exposure, which was less pronounced in C3aR-deficient DCs. These data suggest cross-talk between the two AT receptors resulting in their reciprocal regulation.
Our data further suggest that the elevated allergic phenotype in response to allergen exposure is associated with loss of protective C5aR signaling at the DC/T cell interface, and that C3aR signaling modulates C5aR expression as a potential mechanism to enhance the development of Th2 immunity in allergic asthma. In support of this view, C5aR targeting in C3aRKOs before allergen contact not only reverted the impaired Th2 responses but promoted a “C5aR-deficient like” allergic phenotype with increased AHR, eosinophilic airway inflammation, HDM-specific IgE, and Th2 cytokine production. Our findings suggest an important protective role for C5aR signaling during allergen sensitization, which is independent of C3aR signaling. Indeed, recent studies have explored several mechanisms for the regulatory role of C5aR signaling in asthma sensitization. C5aR signaling controls the accumulation of pulmonary DCs and keeps the ratio of immunogenic mDCs to tolerogenic pDCs low, which is crucial for the development of the allergic phenotype (2, 25). In addition, C5aR signaling in pulmonary mDCs suppresses the secretion of Th2 effector cell-homing chemokines CCL17 and CCL22, thus inhibiting the recruitment of Th2 effector cells into the lungs (2). Furthermore, data obtained with C5-deficient mice indicate that C5 is required to keep mDCs susceptible to the suppression of naturally occurring regulatory T cells, thereby controlling the immunogenic effect of mDCs (26).
The mechanisms underlying the C5aR-mediated regulation of DC functions are unclear at this point. To better understand the effect of C5a on DCs, we assessed the impact of C5aR signaling on the expression and function of costimulatory B7-molecules on pulmonary mDCs and pDCs. The B-7 family comprises B7-1 (CD80), B7-2 (CD86), B7-H1 (PD-L1), B7-DC (PD-L2), B7-RP1 (ICOS-L), B7-H3, and B7-H4 molecules. Previously, we have shown that B7-2 signaling is crucial for the development of in vivo allergic responses to inhaled allergen exposure (27). More recently, B7-DC but not B7-H1 signaling has been implicated in allergic response during the allergic effector phase in an OVA-model of asthma (28). Our data indicate that C5aR signaling specifically regulates the accumulation of pulmonary pDCs expressing B7-H1 and B7-DC molecules. Moreover, our ex vivo studies suggest critical functional roles for B7-H1 and B7-DC signaling in pDCs to control Th2 cytokine production from T effector cells. Importantly, we found reciprocal regulation of B7-H1 and B7-DC on pDCs, in contrast to mDCs where B7-H1 and B7-DC were up-regulated. pDCs have been shown to regulate mDC as well as regulatory T cell function. To this end, the target cell of B7-H1 and B7-DC signaling as well as the receptors are unknown. The recent discovery of B7-1 as a second receptor on T cells in addition to PD-1 suggests substantial plasticity of B7 signaling (29). Further studies are required to define the mechanisms underlying the regulatory role of B7-H1 and B7-DC signaling in pDCs and their regulation by C5a.
In summary, we provide novel insights into the role of C3a and C5a in the development of maladaptive Th2 immunity in experimental allergic asthma. Our data suggest cross-regulation of AT receptor expression in pulmonary DCs associated with a protective function of C5aR signaling in vivo in the absence of C3aR. Our data further indicate that regulation of B7-H1 and B7-DC on pDCs is one important mechanism underlying the protective effect of C5a during allergen sensitization.
Acknowledgments
We thank Craig Gerard (Harvard University College of Medicine) for providing C5aRKOs and C3aRKOs.
Footnotes
This work was funded by National Institutes of Health Grant AI057839 (to J. K.).
Abbreviations used in this paper: AHR, airway hyperresponsiveness; AT, anaphylatoxin; BAL, bronchoalveolar lavage; C3aR, C3a receptor; C5aR, C5a receptor; C3aRKO, C3aR-deficient mice; C5aRKO, C5aR-deficient mice; DC, dendritic cell; HDM, house dust mite; i.t., intratracheal; mDC, myeloid dendritic cell; pDC, plasmacytoid dendritic cell; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 2.Köhl J, Baelder R, Lewkowich IP, Pandey MK, Hawlisch H, Wang L, Best J, Herman NS, Sproles AA, Zwirner J, et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J Clin Invest. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Köhl J, Wills-Karp M. A dual role for complement in allergic asthma. Curr Opin Pharmacol. 2007;7:283–289. doi: 10.1016/j.coph.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Karp CL, Grupe A, Schadt E, Ewart SL, Keane-Moore M, Cuomo PJ, Köhl J, Wahl L, Kuperman D, Germer S, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000;1:221–226. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 5.Drouin SM, Sinha M, Sfyroera G, Lambris JD, Wetsel RA. A protective role for the fifth complement component (c5) in allergic airway disease. Am J Respir Crit Care Med. 2006;173:852– 857. doi: 10.1164/rccm.200503-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinley L, Kim J, Bolgos GL, Siddiqui J, Remick DG. Allergens induce enhanced bronchoconstriction and leukotriene production in C5 deficient mice. Respir Res. 2006;7:129. doi: 10.1186/1465-9921-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bautsch W, Hoymann HG, Zhang Q, Meier-Wiedenbach I, Raschke U, Ames RS, Sohns B, Flemme N, Meyer zu Vilsendorf A, Grove M, Klos A, Köhl J. Cutting edge: guinea pigs with a natural C3a-receptor defect exhibit decreased bronchoconstriction in allergic airway disease: evidence for an involvement of the C3a anaphylatoxin in the pathogenesis of asthma. J Immunol. 2000;165:5401–5405. doi: 10.4049/jimmunol.165.10.5401. [DOI] [PubMed] [Google Scholar]
- 8.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998 –1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 9.Drouin SM, Corry DB, Hollman TJ, Kildsgaard J, Wetsel RA. Absence of the complement anaphylatoxin C3a receptor suppresses Th2 effector functions in a murine model of pulmonary allergy. J Immunol. 2002;169:5926 –5933. doi: 10.4049/jimmunol.169.10.5926. [DOI] [PubMed] [Google Scholar]
- 10.Milian E, Diaz AM. Allergy to house dust mites and asthma. P R Health Sci J. 2004;23:47–57. [PubMed] [Google Scholar]
- 11.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384 – 6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 12.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258 –2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 13.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Köhl J. C5a negatively regulates Toll-like receptor 4-induced immune responses. Immunity. 2005;22:415– 426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunol Lett. 2003;88:47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 15.Godau J, Heller T, Hawlisch H, Trappe M, Howells E, Best J, Zwirner J, Verbeek JS, Hogarth PM, Gerard C, et al. C5a initiates the inflammatory cascade in immune complex peritonitis. J Immunol. 2004;173:3437–3445. doi: 10.4049/jimmunol.173.5.3437. [DOI] [PubMed] [Google Scholar]
- 16.Baelder R, Fuchs B, Bautsch W, Zwirner J, Köhl J, Hoymann HG, Glaab T, Erpenbeck V, Krug N, Braun A. Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J Immunol. 2005;174:783–789. doi: 10.4049/jimmunol.174.2.783. [DOI] [PubMed] [Google Scholar]
- 17.Köhl J, Wills-Karp M. Complement regulates inhalation tolerance at the dendritic cell/T cell interface. Mol Immunol. 2007;44:44–56. doi: 10.1016/j.molimm.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Kozel TR, Wilson MA, Farrell TP, Levitz SM. Activation of C3 and binding to Aspergillus fumigatus conidia and hyphae. Infect Immun. 1989;57:3412–3417. doi: 10.1128/iai.57.11.3412-3417.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J Allergy Clin Immunol. 1997;100:253–260. doi: 10.1016/s0091-6749(97)70233-1. [DOI] [PubMed] [Google Scholar]
- 20.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 21.Zhou W, Peng Q, Li K, Sacks SH. Role of dendritic cell synthesis of complement in the allospecific T cell response. Mol Immunol. 2007;44:57– 63. doi: 10.1016/j.molimm.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Werfel T, Kirchhoff K, Wittmann M, Begemann G, Kapp A, Heidenreich F, Götze O, Zwirner J. Activated human T lymphocytes express a functional C3a receptor. J Immunol. 2000;165:6599 – 6605. doi: 10.4049/jimmunol.165.11.6599. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto S, Yalcindag A, Laouini D, Brodeur S, Bryce P, Lu B, Humbles AA, Oettgen H, Gerard C, Geha RS. The anaphylatoxin C3a downregulates the Th2 response to epicutaneously introduced antigen. J Clin Invest. 2004;114:399 – 407. doi: 10.1172/JCI19082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Settmacher B, Bock D, Saad H, Gartner S, Rheinheimer C, Köhl J, Bautsch W, Klos A. Modulation of C3a activity: internalization of the human C3a receptor and its inhibition by C5a. J Immunol. 1999;162:7409 –7416. [PubMed] [Google Scholar]
- 25.De Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89 –98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549 –1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane-Myers AM, Gause WC, Finkelman FD, Xhou XD, Wills-Karp M. Development of murine allergic asthma is dependent upon B7-2 costimulation. J Immunol. 1998;160:1036 –1043. [PubMed] [Google Scholar]
- 28.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, et al. B7-DC regulates asthmatic response by an IFN-γ -dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 29.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]