Abstract
The qualitative and quantitative measurements of protein abundance and modification states are essential in understanding their functions in diverse cellular processes. Typical Western blotting, though sensitive, is prone to produce substantial errors and is not readily adapted to high-throughput technologies. Multistrip Western blotting is a modified immunoblotting procedure based on simultaneous electrophoretic transfer of proteins from multiple strips of polyacrylamide gels to a single membrane sheet. In comparison with the conventional technique, Multistrip Western blotting increases the data output per single blotting cycle up to ten-fold, allows concurrent monitoring of up to nine different proteins from the same loading of the sample, and substantially improves the data accuracy by reducing immunoblotting-derived signal errors. This approach enables statistically reliable comparison of different or repeated sets of data, and therefore is beneficial to apply in biomedical diagnostics, systems biology and cell signaling research.
Keywords: Western blotting, electrophoretic transfer, gel cutting, quantitative protein analysis, high-throughput, blotting errors, systems biology, cell signaling
1. Introduction
Qualitative measurement of protein abundance is one of the common tasks in biomedical diagnostics in the search for therapeutic targets and biomarkers of various diseases and disorders (1-8). Quantitative analysis of protein phosphorylation states, recruitment to the specific subcellular compartments and interaction with other proteins is a paramount goal in systems biology, which explores, predicts and explains how signaling networks govern cellular behavior by exploiting experimental data-driven mathematical models. To this aim, the cellular response to external stimuli in vivo is usually compared to the response obtained under one or more perturbations (e.g. pharmacological inhibitors, exposure to physiochemical stresses or the down- or up-regulation of protein expression etc). In addition, the variations in the concentration of a ligand and stimulation time provide deeper insight into the spatio-temporal functioning of a specific cell signaling pathway (9). Apparently, these tasks require the generation of large amounts of high-quality data points.
Western blotting used for the immunodetection of the expression levels and/or the modification status of electrophoretically resolved proteins, is sensitive and widespread technique, which however has several drawbacks (1, 10). It is low-throughput, time-consuming and sample layout-unfriendly multi-step procedure. Each step (sampling, loading, electrophoretical separation and transfer of proteins, immunoblotting and signal detection) is performed under slightly differing conditions in sequential blotting cycles. This eventually increases data variability, which makes difficult to quantitatively compare the signals obtained from different series of samples (11). Therefore the improvements of typical Western blotting procedure are in great request (12-18).
Multistrip Western blotting is a modified immunoblotting procedure based on simultaneous electrophoretic transfer of proteins from multiple strips of polyacrylamide gels to a single membrane sheet. The proposed modification has several advantages over a classic Western blotting procedure (19). First, the transfer and the sequential procedures with the blot such as membrane washing, incubation with antibodies and protein detection are performed under similar conditions. This significantly improves the data accuracy by reducing immunoblotting-derived signal errors. Second, instead of detection of a single protein, many additional proteins of interest that differ in their molecular weight (e.g. ErbB family, GAB1/2, Raf, Shc, ERK1/2, GAPDH and Grb2) can be synchronously detected in the same sample per each blotting cycle. Such approach also eliminates the need of stripping and reprobing blots for the detection of house-keeping proteins such as actin, tubulin, GAPDH etc. The analytical power of Western blotting is increased, since one-step analysis of numerous signaling proteins is more productive, saves time as well as costly materials. Third, when the number of samples to be analyzed exceeds the number of wells in a gel, the comparative quantitative protein analysis can be readily achieved by applying Multistrip Western blotting technique, which increases the data output per single blotting cycle up to ten-fold. As a consequence, a large number of data points can be integrated and analyzed on the same graph.
Although the protocol presented here is developed for NuPAGE 4-12% gradient Bis-Tris 10-well mini-gels using the XCell devices (Invitrogen), Multistrip Western blotting has wide potential for further uses since it does not require any additional tools and is compatible with any conventional gel electrophoresis as well as protein transfer systems.
2. Materials
2.1. Sample preparation
Sample buffer: 4× NuPAGE LDS Sample Preparation Buffer (pH 8.4) and 10× NuPAGE Sample Reducing Agent (both from Invitrogen, Carlsbad, CA) (see Note 1).
2.2. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
XCell SureLock Mini-Cell units (Invitrogen) (see Note 2).
NuPAGE Novex 4-12% gradient Bis-Tris Mini-gels (Invitrogen) (see Note 3).
Running buffer: 50 mM 3-(N-morpholino)-propanesulfonic acid (MOPS), 50 mM Tris Base, 0.1% (w/v) sodium-dodecyl sulphate (SDS), 1 mM ethylene-diamine-tetraacetic acid (EDTA, pH 7.7) (available from Invitrogen and Boston Bioproducts, Worcester, MA). Store at room temperature. Supplement the running buffer in the upper chamber of XCell SureLock Mini-Cell with 0.5 ml NuPAGE Antioxidant (Invitrogen) before electrophoresis (see Note 1).
Prestained molecular weight markers: Precision Plus Protein standards (Bio-Rad, Hercules, CA). Store at −20°C.
2.3. Western Blotting
XCell II Blot module (Invitrogen) (see Note 2).
Setup buffer: 20 mM Tris Base, 154 mM glycine, 0.02 % SDS, 20% (v/v) methanol.
Transfer buffer: 25 mM Bicine, 25 mM Bis-Tris, 1.0 mM EDTA, 0.05 mM Chlorobutanol, pH 7.2 (available from Invitrogen), 10% (v/v) methanol. Store at 4°C. Supplement with 0.1% (v/v) NuPAGE Antioxidant (Invitrogen) in the transfer apparatus before electrophoretic transfer (see Note 1).
Nitrocellulose membrane from Bio-Rad (see Note 4).
Blotting filter paper 2.45 mm thickness, 320 grade is available from E&K Scientific (Santa Clara, CA) or Colonial Scientific (Richmond, VA).
Tris-buffered saline with Triton X-100 (TBS-T) buffer (1X): 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% (v/v) Triton X-100. Store at room temperature.
Blocking buffer: dissolve 4% (w/v) heat inactivated bovine serum albumen (BSA) (Roche Diagnostics, Indianapolis, IN) in TBS-T buffer.
Primary and secondary antibodies diluted in TBS-T buffer.
Chemiluminescent reagent: SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology, Rockford, IL).
Square Dishes with Grid (Fisher).
Imaging system: Image Station 440CF (Eastman Kodak Scientific Imaging Systems, New Haven, CT)
3. Methods
3.1. Preparation of Samples for Multistrip Western Blotting
Immediately after cell lysis, the supernatant of each cell lysate is mixed with 4× NuPAGE LDS Sample Buffer and supplemented with 10× NuPAGE Sample Reducing Agent in a ratio of 65:25:10 in labeled Eppendorf tubes. The tubes with samples are then heated for 5 min at 75°C. After cooling to room temperature, they are ready for separation by SDS-PAGE or can be stored for further use at −80°C.
3.2. Sample loading and SDS-PAGE
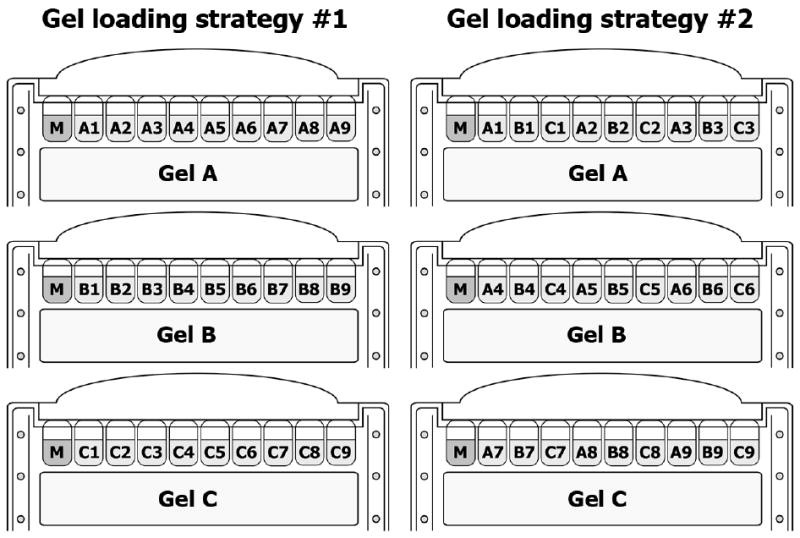
These instructions assume the use of XCell SureLock Mini-Cell apparatus for SDS-PAGE of 10-well mini-gels (see Note 3). The number of gels to be loaded depends on the number of data series and the number of samples within each series to be analyzed. For example, when the time-course (e.g. 0, 1, 3, 5, 7, 10, 20, 30 and 60 minutes) of protein X activation in control cells (A) is compared to that in the presence of first perturbation (e.g. inhibitor of protein X) (B) and the second perturbation (e.g. the suppression of protein Y by siRNA) (C), one will have to load three data series (A, B and C) consisting of 9 time-points each (A1, A2…A9 etc.) into three gels. There are two alternative ways of loading such array of samples (Fig. 1).
Fig. 1. Gel loading strategies when the number of samples exceeds the number of gel wells.
The upper chamber is filled with 200 ml of running buffer to completely cover the sample wells of a gel. 600 ml of running buffer is poured into the lower chamber.
A pipette equipped with prolonged gel loading tip (Fisher) is used to underlay 7 μl of prestained protein molecular weight marker (M) into the first and/or the last gel well.
Equal volume of each sample (e.g. 20 μl) (see Note 5) is loaded into the rest of gel wells. If there are empty wells without loaded sample left, fill them with similar amount of sample buffer. Mark the sequence of loaded samples in a laboratory notebook.
Lock the gel tension lever. The electrophoresis unit is then completely assembled by adding the lid on the buffer core, and connected to a power supply. The proteins are electrophoretically separated at 140 V, until the blue dye front reaches the bottom of a gel. If running more than 2 gels, make an interval of 5 minutes before loading the next tandem of gels and powering on the electrophoresis unit. This will reserve enough time for follow-up steps.
At the end of gel run, a gel cassette is removed out of apparatus and gently opened with a gel knife. Note that upon opening the cassette, the gel can be adhered on either side. If the gel remains on a notched side, the sequence of sampling should be rewritten in the laboratory notebook in a reversed order. Discard the plate of gel cassette without the gel.
Perform steps 1-5 with gel cassettes from other electrophoresis units.
3.3. Gel cutting
Fig. 2 illustrates the plate with attached gel after protein separation according to their molecular weight by SDS-PAGE. The prestained marker is visibly separated into the bands corresponding to protein molecular weights of 250, 150, 100, 75, 50, 37, 25, 20, 15 and 10 kDa (Fig. 2, M).
A millimeter-scaled transparent ruler is firmly positioned near the edge lane with separated marker so that zero (0 cm) aligns with the middle of the blue dye front (Fig. 2, BDF). The distance from the blue dye front to the center of each marker band (Fig. 2, H) is measured in millimeters and registered in a table (see Note 6).
The distance between two electrophoretically separated marker bands corresponds to the migration range of certain molecular weight proteins. For instance, the distance between H250 and H150 defines a migration range of electrophoretically separated proteins with molecular sizes between 150 and 250 kDa. This range is termed zone ➀ in the Fig. 2. Each sample provides up to nine protein-containing zones that may be simultaneously cut out from a single gel.
A regular gel knife is used to cut out the strip, which covers an area with a protein of interest located in the middle, from the gel across its entire width (Fig. 2, scissors symbol).
The number of strips to be cut out from the gel depends on the number of distinct proteins to be detected. Most frequently studied signal transduction proteins migrating in various zones are listed in Table 1, which also indicates the appropriate areas that can be cut out of the gel in order to detect these proteins later. For example, it is convenient to separate the activated EGF receptor (165 kDa, zone ➀), from the phosphorylated PLD1 (116 kDa) and the phosphorylated 90 kDa ribosomal S6 kinase (RSK) that migrate in the zones ➁ and ➂, respectively. Then it is easy to separate the phosphorylated Akt (60 kDa), which is found in the zone ➃, from both the activated ERK1 (44 kDa) and ERK2 (42 kDa) kinases that co-migrate in the zone ➄, and from the phosphorylated S6 Ribosomal Protein (32 kDa, zone ➅). In this case, according to the Table 1, the gel is cut into six strips at 12 mm and 22 mm (for phospho-S6 Ribosomal Protein), at 29 mm (for ERK1/2), at 38 mm (for phospho-Akt), at 44 mm (for phospho-RSK), at 49 mm (for phospho-PLD1) and at 54 mm (for phospho-EGFR) from the BDF (see Note 7).
The gel pieces outside the strips are discarded.
The first plate with prepared multiple gel strips is covered with a sheet of moistened filter paper (CFP, for covering filter paper) and placed on the bench top. Similarly, the second gel is cut, covered with another sheet of moistened CFP and placed next to the previously laid plate on the bench top. Repeat above procedure with the rest of the gels.
Fig. 2. The identification and cutting of protein migration zones in Multistrip Western blotting procedure.
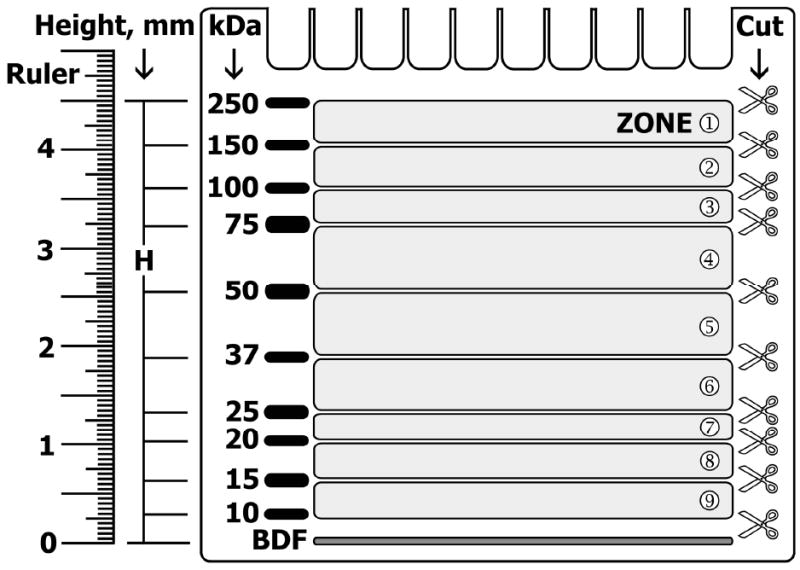
M, prestained protein molecular weight marker; BDF, blue dye front; H, the distance from BDF to the center of particular marker band. Scissor symbols indicate the cutting lines, which would separate the entire gel into nine strips. Protein migration zones are enumerated by numbers in circles (modified from ref. 19 with permission from WILEY-VCH Verlag GmbH & Co. KG aA).
Table 1. Migration of various signal transduction proteins in Novex 4-12% gradient NuPAGE mini-gel and their cutting areas, based on the migration of prestained Precision Plus Protein marker (modified from ref. 19 with permission from WILEY-VCH Verlag GmbH & Co. KG aA).
| Zone | Protein MW (kDa) | Cut area, H (mm from BDF) | Migration range of signal transduction proteins in a gel | |||
|---|---|---|---|---|---|---|
| 1 | 150 – 250 | 49 ± 1 – 54 ± 1 | EGFR, ErbBs, PDGFR, CSFR | c-Met, PLCs, FGFR, c-Kit, SHIP, JAK/Tyk2 | SOS, IRS1/4, Filamin | VEGFR, c-Ret, ASK1, RhoGAP |
| 2 | 100 – 150 | 44 ± 1 – 49 ± 1 | RasGAP, FAK, c-Cbl, Cas, PLD1, PTPα, Vinculin | PI3K-p110, Gab1/2, Pyk2, Vav, STATs, MLK3, Catenin | Cadherin, cPLA2, PKD, eNOS, ERK5 | |
| 3 | 75 – 100 | 38 ± 1 – 44 ± 1 | PI3K-p85, FRS-2, GRK, APS | IR, PKCs, RSK, IKKα, IGF-1R | Raf, Grb10, PAK, p70S6K, FKHR, Calpain, Gab3 | |
| 4 | 50 – 75 | 29 ± 1 – 38 ± 1 | SHP1/2, Src family, PTEN, Akt, Csk, Grb14, Grb7 | SHC, α-Tubulin, SAPK/JNK, SGK, ILK1, PP2A, CaMKII, GSK-3, PTP1B, β-Arrestin | Dok-R, Paxillin, p53, PDK1, Sam68, c-Fos, AMPKα/γ | |
| 5 | 37 – 50 | 22 ± 1 – 29 ± 1 | Crk, ERK, MEK, Nck, CREB, AMPKβ | β-Actin, PKA, MAPKAPK2, c-Jun, MKKs | Sprouty, p38 MAPK, GAPDH, LAT | |
| 6 | 25 - 37 | 12 ± 1 – 22 ± 1 | 14-3-3, Bic | Bcl-2, PP1, S6RP | Bak, GRB2 | |
| 7 | 20 - 25 | 9 ± 1 – 12 ± 1 | Bad, Rac1/cdc42 | Caveolin-1, Ras, DAP1, RKIP | BID | |
| 8 | 15 - 20 | 5 – 9 ± 1 | Survivin, Bmf | TCL1 | ||
| 9 | 10 - 15 | 2 – 5 | ||||
3.4. Assembly of gel strips
During this step, the gel strips that are derived from different gels, are assembled onto a single sheet of filter paper (AFP, for assembling filter paper) for the subsequent electrophoretic protein transfer onto the same piece of nitrocellulose membrane (see Note 8). The strategy of assembly depends on the quantity of gels used as well on the number of analyzable proteins per lane (i.e. the number of precut gel strips comprising of appropriate zones). Here we provide two exemplar cases of gel strip assembly:
when six gels (A, B, C, D, E and F) are run and five proteins of interest from each sample (e.g. phospho-EGFR from zone ➀, phospho-GAB1 migrating between zones ➁ and ➂, phospho-SHP2 from zone ➃, phospho-ERK1/2 from zone ➄ and Grb2 as house-keeping protein, which migrates between zones ➅ and ➆) are subsequently detected under equal conditions. Guidance for cutting of one out of six gels is provided in left panel of Fig. 3;
when three gels (A, B and C) are run and four proteins from each sample (phospho-IRS1 from zone ➀, phospho-IR from zone ➂, phospho-Akt from zone ➃ and GAPDH as house-keeping protein, which migrates between zones ➄ and ➅) are analyzed. Guidance for cutting of one out of three gels is provided in right panel of Fig. 3.
Fig. 3. Example of single gel cutting into five (left panel) or four (right panel) strips containing distinct protein zones.
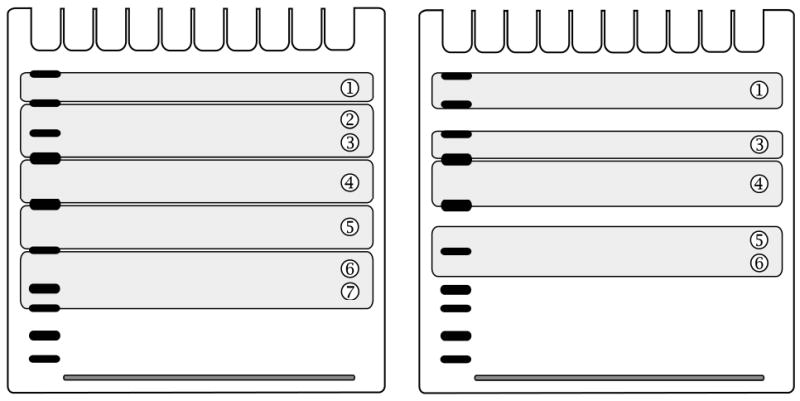
The strips will be subsequently transferred onto assembling filter papers together with similar strips derived from other gels.
In case (i), the plate containing gel A strips is flipped and gently lifted so that all strips would stick to the moistened CFP. Use gel knife if the strips do not independently detach from the plate.
The first gel strip from the top (possessing zone ➀ proteins) is lifted with a gloved hand and carefully transferred onto the AFP #1. The CFP with remaining gel strips is returned onto the plate by flipping it back.
Similarly, top gel strips derived from gels B to F are sequentially transferred onto the AFP #1 so that the strips would lay side by side and parallel to each other. AFP #1 is now ready for immediate protein transfer (see Note 9).
Steps 1-3 are repeated with the strips derived from gels A to F that possess the proteins migrating in zones ➁ ➂, then ➃, ➄ and finally ➅ ➆. This procedure will yield six AFPs (AFP #1 through #6) with collected five gel strips on each (Fig. 4A). Now they are ready for electrophoretic protein transfer onto the same membrane.
In case (ii), steps 1-2 are performed with gel A, followed by sequential transfer of gel strips derived from gel B and gel C onto the AFP #1. Then, gel strips that contain the proteins migrating in zone ➂ are sequentially placed onto the AFP #1 below previously laid triplet of strips. Leave a small gap between triplets.
AFP #2 is processed in the same manner so that it would contain triplet of strips with zone ➃ and triplet of strips with zone ➄ ➅ (Fig. 4B). After protein transfer, the resulting nitrocellulose membrane is cut into two pieces across the gap between triplets. The pieces are then treated in separate dishes (see Note 10).
Fig. 4. Assembly of multiple gel strips onto assembling filter paper.
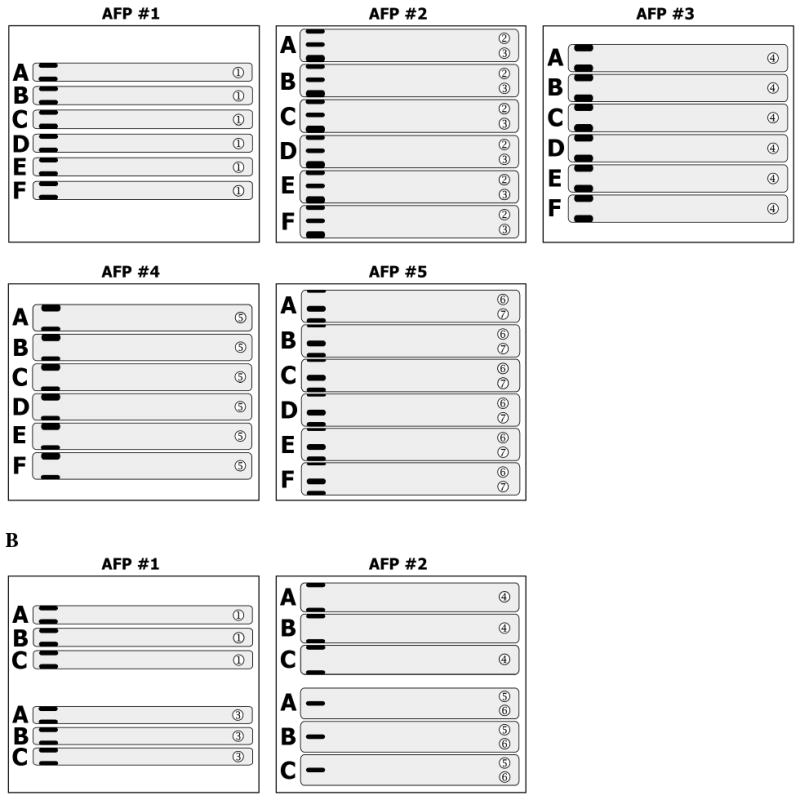
A. SDS-PAGE of six gels (A through F) was performed. Five strips were cut out of each gel and combined onto appropriate assembling filter papers (AFP, #1 through #6). B. SDS-PAGE of three gels (A through C) was performed. Four strips were cut out of each gel and combined onto two assembling filter papers (AFP #1 and #2).
3.4. Western blotting
Instructions provided below assume the use of XCell II Blot module that is used for protein transfer from one AFP.
One side of assembly tray is filled with 500 ml of setup buffer, while another side – with 400 ml of transfer buffer.
Four sponge pads are presoaked in setup buffer. Remove air bubbles by squeezing the pads while they are submerged in buffer. A nitrocellulose membrane is cut to the dimensions of AFP and presoaked in transfer buffer for 5 minutes before using. Three additional sheets of filter paper are briefly moistened in setup buffer immediately before using.
Two soaked sponge pads are placed into the cathode (-) core of the blot module and covered with one sheet of moistened filter paper. The AFP with collected gel strips is placed on the top. Subsequently, the surface of gel strips is covered with a sheet of nitrocellulose membrane. Remove any trapped air bubbles by rolling a blotting roller over the membrane surface. Two moistened filters are then placed onto the surface of the membrane followed by tandem of soaked sponge pads (see Note 11).
The anode (+) core is placed on the top of the pads. Slide the blot module into the rails on the lower chamber. Lock the gel tension lever.
The blot module is filled with transfer buffer until the blotting sandwich is completely submerged. The outer chamber is filled with cold deionized water.
The unit is completely assembled by adding the lid on the buffer core, and connected to a power supply. The proteins are electrophoretically transferred at 30 V constant for 90 minutes.
After transfer is stopped, the nitrocellulose membrane is removed out of the blot module and placed into a square Petri dish. Used filter papers and gel strips are discarded.
After 3 minute rinsing with deionized water, the membrane is incubated with 20 ml of blocking buffer for 1 hour at room temperature on a rotating platform.
After the blocking buffer is discarded, the membrane is briefly rinsed with deionized water and blotted with appropriate primary antibody at dilution ratio as recommended by a manufacturer overnight at 4°C on a rotating platform (see Note 12).
The membrane is extensively rinsed with deionized water and washed five times for 7 minutes each with TBS-T buffer at room temperature on a rotating platform.
The membrane is incubated with appropriate secondary antibody at dilution ratio as recommended by a manufacturer for 1 hour at room temperature on a rotating platform followed by step 10 once again.
The membrane is incubated with a working solution of chemiluminescent reagent for 5 minutes and the signal is captured by Imaging system and quantified using KODAK Digital Science software (see Note 13). To enable side-by-side comparison, the capture time and number of frames should be equal for each separately exposed membrane.
Acknowledgments
The authors gratefully acknowledge Drs. Boris N Kholodenko and Jan B Hoek for support. This work was supported by National Institutes of Health Grants GM59570 and AA0125311.
Footnotes
Laemmli instead of LDS sample buffer can be used with appropriate running and transfer buffers. Electrophoresis can be performed under reducing as well as non-reducing conditions.
Choose the type of apparatus for electrophoresis and protein transfer according to the size of your gels (e.g. mini-, midi- or maxi-gels).
This protocol can be adapted for gels of any percentage, composition, size and with any number of wells.
If desired, PVDF or nylon membranes can be also used.
The samples to be loaded can be different or repetitive.
The table is designed to track the statistics of marker migration in the gel of selected percentage. In addition, different tables can be created according to the marker type used. The statistics is required for successive Multistrip Western blotting procedures if one needs to cut out the gel strip with the protein of interest (with known molecular weight), but no prestained marker has been loaded.
If a protein of interest migrates in an intersection of marker-defined zones (e.g. 100 kDa GAB1, which migrates between zones ➁ and ➂; 25 kDa Grb2, which migrates between zones ➅ and ➆; 74 kDa c-Raf, which migrates very close to the zone ➂ etc.), the cutting area must include both zones or at least should be wider. For example, Shc protein has three isoforms of 46 kDa, 52 kDa and 66 kDa migrating in the zones ➃ and ➄, so it can be separated from the proteins that lay in the zone ➂ (e.g. p85, a regulatory subunit of PI3K) and between zones ➅ and ➆ (e.g. GRB2) by cutting the gel into three strips at 9, 22, 38 and 49 mm (see Table 1).
The maximal number of gel strips that can be combined onto a single AFP depends on the overall dimension of the transfer unit, hence on the size (height and width) of AFP. Routinely we use 7 × 10 cm filter, which provides space for maximum of twelve gel strips of 0.6 cm height each. However, regularly we place fewer amounts of gel strips (e.g. six), especially when they are wider and/or the membrane should be cut into two or more pieces after electrophoretic protein transfer.
If some pauses occur, regularly wet the surface of gel strips by dropping deionized water.
Alternatively, the whole piece of nitrocellulose membrane can be treated with blocking reagent and then incubated with the mixture of primary antibodies (be sure that they do not cross-react) in a single dish.
The pads should rise at least 0.5 cm over the rim of the cathode core. If not, place an additional filter paper of sponge pad in the tank.
The primary antibodies can be collected into the tube and reused several times if supplemented with 0.1% sodium azide. If precipitation occurs, filter the solution through 0.22 μm filter unit.
The chemiluminescent signal can be visualized by another imaging instrument and quantified using an appropriate software. Alternatively, the signal can be captured on the film followed by densitometric quantification.
References
- 1.Soundy P, Harvey B. Western Blotting as a Diagnostic Method. In: Walker JM, Rapley R, editors. Medical Biomethods Handbook. Totowa: Humana Press; 2005. pp. 43–62. [Google Scholar]
- 2.Omenn GS. Strategies for plasma proteomic profiling of cancers. Proteomics. 2006;6(20):5662–73. doi: 10.1002/pmic.200600331. [DOI] [PubMed] [Google Scholar]
- 3.Hueber W, Robinson WH. Proteomic biomarkers for autoimmune disease. Proteomics. 2006;6(14):4100–4105. doi: 10.1002/pmic.200600017. [DOI] [PubMed] [Google Scholar]
- 4.Fardilha M, Wu W, Sa R, et al. Alternatively spliced protein variants as potential therapeutic targets for male infertility and contraception. Ann N Y Acad Sci. 2004;1030:468–478. doi: 10.1196/annals.1329.059. [DOI] [PubMed] [Google Scholar]
- 5.Ducruet AP, Vogt A, Wipf P, Lazo JS. Dual specificity protein phosphatases: therapeutic targets for cancer and Alzheimer's disease. Annu Rev Pharmacol Toxicol. 2005;45:725–750. doi: 10.1146/annurev.pharmtox.45.120403.100040. [DOI] [PubMed] [Google Scholar]
- 6.Gaiger A, Heintel D, Jager U. Novel molecular diagnostic and therapeutic targets in chronic lymphocytic leukaemia. Eur J Clin Invest. 2004;34 2:25–30. doi: 10.1111/j.0960-135X.2004.01367.x. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer TK. Cancer metastasis therapeutic targets and drug discovery: emerging small-molecule protein kinase inhibitors. Expert Opin Investig Drugs. 2004;13(1):1–19. doi: 10.1517/13543784.13.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353(2):172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 9.Kholodenko BN. Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol. 2006;7(3):165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurien BT, Scofield RH. Western blotting. Methods. 2006;38(4):283–293. doi: 10.1016/j.ymeth.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Schilling M, Maiwald T, Bohl S, et al. Quantitative data generation for systems biology: the impact of randomisation, calibrators and normalisers. Syst Biol (Stevenage) 2005;152(4):193–200. doi: 10.1049/ip-syb:20050044. [DOI] [PubMed] [Google Scholar]
- 12.Bergendahl V, Glaser BT, Burgess RR. A fast Western blot procedure improved for quantitative analysis by direct fluorescence labeling of primary antibodies. J Immunol Methods. 2003;277(1-2):117–125. doi: 10.1016/s0022-1759(03)00183-2. [DOI] [PubMed] [Google Scholar]
- 13.Bolt MW, Mahoney PA. High-efficiency blotting of proteins of diverse sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1997;247(2):185–192. doi: 10.1006/abio.1997.2061. [DOI] [PubMed] [Google Scholar]
- 14.Kashino Y, Koike H, Satoh K. An improved sodium dodecyl sulfate-polyacrylamide gel electrophoresis system for the analysis of membrane protein complexes. Electrophoresis. 2001;22(6):1004–1007. doi: 10.1002/1522-2683()22:6<1004::AID-ELPS1004>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Kurien BT, Scofield RH. Heat-mediated, ultra-rapid electrophoretic transfer of high and low molecular weight proteins to nitrocellulose membranes. J Immunol Methods. 2002;266(1-2):127–133. doi: 10.1016/s0022-1759(02)00103-5. [DOI] [PubMed] [Google Scholar]
- 16.Swank MW, Kumar V, Zhao J, Wu GY. A novel method of loading samples onto mini-gels for SDS-PAGE: Increased sensitivity and Western blots using sub-microgram quantities of protein. J Neurosci Methods. 2006;158:224–233. doi: 10.1016/j.jneumeth.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Stockley PG, Martin WJ., 2nd An improved western blotting technique effectively reduces background. Electrophoresis. 2002;23(15):2373–2376. doi: 10.1002/1522-2683(200208)23:15<2373::AID-ELPS2373>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Schilling M, Maiwald T, Bohl S, et al. Computational processing and error reduction strategies for standardized quantitative data in biological networks. Febs J. 2005;272(24):6400–6411. doi: 10.1111/j.1742-4658.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 19.Aksamitiene E, Hoek JB, Kholodenko B, Kiyatkin A. Multistrip Western blotting to increase quantitative data output. Electrophoresis. 2007;28(18):3163–3173. doi: 10.1002/elps.200700002. [DOI] [PMC free article] [PubMed] [Google Scholar]


