Abstract
Oxidative stress causes mitochondrial dysfunction and metabolic complications through unknown mechanisms. Cardiolipin (CL) is a key mitochondrial phospholipid required for oxidative phosphorylation. Oxidative damage to CL from pathological remodeling is implicated in the etiology of mitochondrial dysfunction commonly associated with diabetes, obesity, and other metabolic diseases. Here we show that ALCAT1, a lyso-CL acyltransferase up-regulated by oxidative stress and diet-induced obesity (DIO), catalyzes the synthesis of CL species which are highly sensitive to oxidative damage, leading to mitochondrial dysfunction, ROS production, and insulin resistance. These metabolic disorders were reminiscent of those observed in type 2 diabetes, and were reversed by rosiglitazone treatment. Consequently, ALCAT1 deficiency prevented the onset of DIO and significantly improved mitochondrial complex I activity, lipid oxidation, and insulin signaling in ALCAT1−/− mice. Collectively, these findings identify a key role of ALCAT1 in regulating CL remodeling, mitochondrial dysfunction, and susceptibility to DIO.
INTRODUCTION
Diabetes and obesity are associated with oxidative stress and mitochondrial dysfunction which are implicated in the etiology of metabolic complications (Anderson et al., 2009; Bonnard et al., 2008; Houstis et al., 2006). A particularly destructive aspect of oxidative stress is the production of reactive oxygen species (ROS) which have been demonstrated to cause insulin resistance under different settings (Houstis et al., 2006; Meigs et al., 2007), impair glucose uptake in insulin sensitive tissues (Tirosh et al., 1999), and inhibit insulin-stimulated Akt signaling (Houstis et al., 2006). Employment of mitochondrial-targeted antioxidants has also identified mitochondria as a major source of oxidative stress and insulin resistance (Anderson et al., 2009; Houstis et al., 2006), which is corroborated by transgenic overexpression of antioxidant enzyme targeted to the mitochondria (Schriner, et al., 2005). Additionally, ROS are highly damaging to biological molecules such as proteins, nucleic acids and lipids, like CL. Indeed, ROS have been shown to cause mitochondrial dysfunction by impairing electron transport complex I and III activity through oxidative damage of CL, a process also known as CL peroxidation (Paradies et al., 2004).
CL is a polyglycerophospholipid exclusively localized in the mitochondria where it regulates mitochondrial function and oxidative stress in species from yeast to mammals (Chen et al., 2008; Chicco and Sparagna, 2007). This role is mediated by the acyl composition of the side chains of CL, which is dominated by linoleic acid in insulin sensitive tissues (Schlame et al., 2000). This unique acyl composition is not derived from de novo synthesis of CL, rather from a remodeling process that involves phospholipases and acyltransferase-transacylases (Cao et al., 2004; Taylor and Hatch, 2009; Xu et al., 2003). Additionally, CL remodeling is believed to replace damaged acyl chains under normal conditions. However, this remodeling process is also capable of generating CL species that are highly sensitive to oxidative damage by ROS under pathological conditions, further exacerbating CL peroxidation and oxidative stress. CL is highly sensitive to damage of its double bonds by oxidative stress due to its rich content in linoleic acid and its location near the site of ROS production in the inner mitochondrial membrane. Consequently, CL has been shown to be the only phospholipid in mitochondria that undergoes early oxidation during apoptosis (Kagan et al., 2005). Therefore, pathological CL remodeling has been implicated in the etiology of mitochondrial dysfunction associated with a host of pathophysiological conditions including diabetes, obesity, cardiovascular diseases and aging, all of which are characterized by increased oxidative stress, CL deficiency, and enrichment of docosahexaenoic acid (DHA) content in CL (Han et al., 2007; Sparagna and Lesnefsky, 2009). However, little is known about the molecular mechanisms governing the pathological remodeling of CL and its relevance to mitochondrial dysfunction in metabolic diseases.
ALCAT1 is the first lyso-CL acyltransferase identified that catalyzes the reacylation of lyso-CL to CL, a key step in CL remodeling (Cao et al., 2004). In comparison to an isoform of the enzyme recently identified from liver, ALCAT1 lacks preference for linoleic acid as substrate, suggesting a possible role in the pathological remodeling of CL (Cao et al., 2004; Taylor and Hatch, 2009). This is corroborated by recent reports that ALCAT1 expression is up-regulated in mammalian cells exhibiting tetralinoleoyl-CL deficiency and in heart and liver of mice suffering from oxidative stress induced by hyperthyroidism (Cao et al., 2009; Van et al., 2007). The present investigation sought to advance our understanding of a regulatory role of ALCAT1 in pathological remodeling of CL and in mitochondrial dysfunction associated with DIO. These studies implicate ALCAT1 as a major regulator of abnormal remodeling of CL in DIO, leading to oxidative stress, mitochondrial dysfunction, and insulin resistance.
RESULTS
ALCAT1 Catalyzes Remodeling of CL with Abnormal Acyl Composition, Leading to Mitochondrial Dysfunction
The recombinant ALCAT1 protein was previously shown to be localized in the endoplasmic reticulum (ER) by immunohistochemical analysis (Cao et al., 2004). This localization seemed to contradict with the exclusive localization of CL in mitochondria. To resolve this issue, we carried out subcellular fractionation analysis of the recombinant ALCAT1 protein overexpressed in COS-7 cells, and analyzed its lyso-CL acyltransferase activity in each of the subcellular fractions. The results showed that ALCAT1 protein was predominantly localized in MAM as confirmed by Western blot analysis of flag-tagged ALCAT1 protein (Fig 1A lower panel) and by enzyme activity assay (Fig 1A upper panel, and quantified in Fig 1B). MAM is a membrane bridge between the ER and mitochondria and a major site for phospholipid synthesis and traffic. A significant portion of the ALCAT1 protein was also detected from the mitochondrial fraction where CL is synthesized. The results are supported by a recent report on DGAT2 which was initially identified as an ER-associated acyltransferase, but has recently been re-localized in the MAM (Stone et al., 2009).
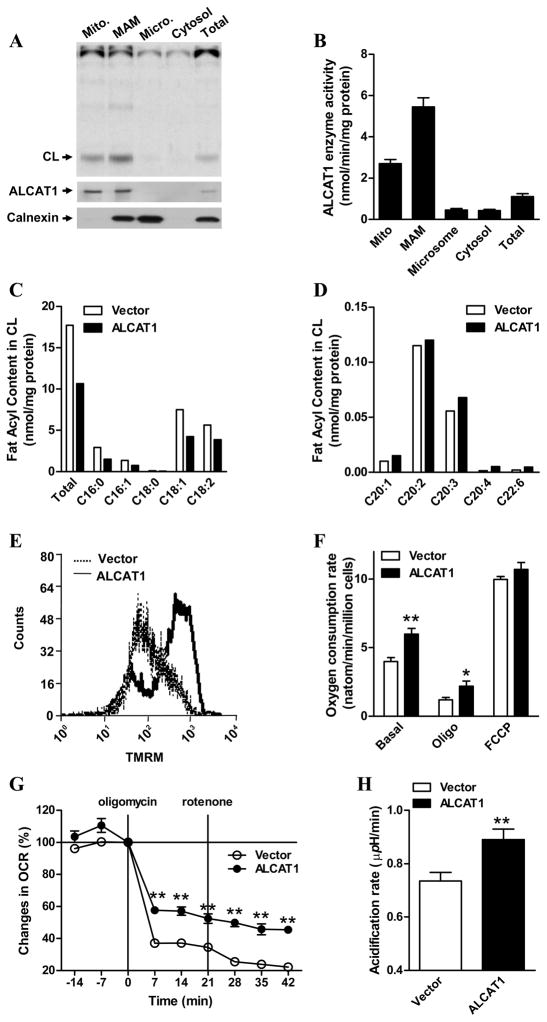
Figure 1. ALCAT1 is localized at the mitochondria-associated membrane (MAM) where it catalyzes remodeling of CL that leads to mitochondrial dysfunction.
A, COS-7 cells overexpressing the flag-tagged ALCAT1 protein were fractionated into mitochondria (Mito), MAM, microsomes (micro), and cytosolic fractions. Monolyso-CL acyltransferase activity was analyzed from each fraction by TLC analysis, and quantified (B). ALCAT1 and calnexin (an ER marker) levels in each fraction were analyzed by western blot analysis (bottom panels). C, lipidomic analysis of total CL and the C16–18 acyl content in CL, or D, the long chain polyunsaturated acyl content in CL, from C2C12 cells stably overexpressing ALCAT1 (filled bar) or an empty vector (open bar) by negative-ion electrospray ionization mass spectrometry. The C2C12 cells stably overexpressing ALCAT1 or vector were also analyzed for E, mitochondrial membrane potentials by flow cytometry; F, oxygen consumption rate (OCR) by oxygraph; and G, proton leakage as measured by changes in OCR after sequential treatment with oligomycin and rotenone, which was profiled by Seahorse XF-24 analyzer and expressed in % of basal line. H, up-regulation of glycolytic activity by ALCAT1 overexpression in C2C12 cells as evidenced by higher acidification rate of the culture medium measured by the XF-24 analyzers. N=3. *P < 0.05, ** P < 0.01.
To identify a role of ALCAT1 in regulating CL remodeling and mitochondrial activity, we generated several C2C12 cell lines stably transfected with ALCAT1 cDNA expression vector or an empty vector. C2C12 is a mouse myoblast cell line commonly used for metabolic research. Using the stable cell lines as a cell-based model, we first analyzed the effect of ALCAT1 overexpression on changes in the acyl profile of CL and other phospholipids including phosphotidylinositol (PI) and phosphotidylglycerol (PG) by mass spectrometric analysis. ALCAT1 overexpression in C2C12 cells caused a 40% reduction in total CL levels (Fig 1C, 2.74 vs. 4.56 nM/mg protein, ALCAT1 vs. vector) by selectively decreasing the content of C16-C18 fatty acids in CL, including linoleic acid, the dominant acyl form of CL in metabolically active tissues. In contrast, the level of CL species that contain long chain polyunsaturated fatty acid (PUFA) was either unchanged or significantly increased (Fig 1D). In particular, the level of CL species that contain DHA (C22:6n3) increased by more than 300%. These changes in CL acyl profile are similar to those observed in the etiology of various pathological conditions, including diabetes, obesity, heart failure, hyperthyroidism, and aging (Gredilla et al., 2001; Han et al., 2007; Sparagna and Lesnefsky, 2009). In comparison, there were no significant changes in either acyl composition or total levels of phosphatidylinositol (PI) and and phosphatidylglycerol (PG) in C2C12 cells overexpressing ALCAT1 relative to the vector control (Supplemental Fig S1).
Increased DHA content in CL has been shown to stimulate mitochondrial membrane potential, proton leakage, and ROS production in cultured cells (Ng et al., 2005; Watkins et al., 1998). Consistent with increased DHA content in CL, ALCAT1 overexpression caused mitochondrial dysfunction in C2C12 cells by significantly increasing mitochondrial membrane potential (Fig 1E), oxygen consumption rate (OCR) (Fig. 1F), and proton leakage as evidenced by significantly higher OCR in ALCAT1-expressing C2C12 cells in response to treatment of oligomycin, a mitochondrial ATP synthase inhibitor (Fig 1F). The effect of ALCAT1 overexpression on mitochondrial leakage was further confirmed by a Seahorse XF24 analyzer. As shown by the OCR profile in Fig 1G, C2C12 cells overexpressing ALCAT1 exhibited a significantly higher OCR than empty-vector control cells when treated with oligomycin, which was exacerbated by treatment with rotenone, a mitochondrial complex I inhibitor. As a compensatory response to the increased levels of uncoupling respiration and ROS production, ALCAT1 overexpression significantly increased glycolytic activity in C2C12 cells as evidenced by significantly higher acidification rate of the culture medium analyzed by the XF-24 analyzer (Fig 2H).
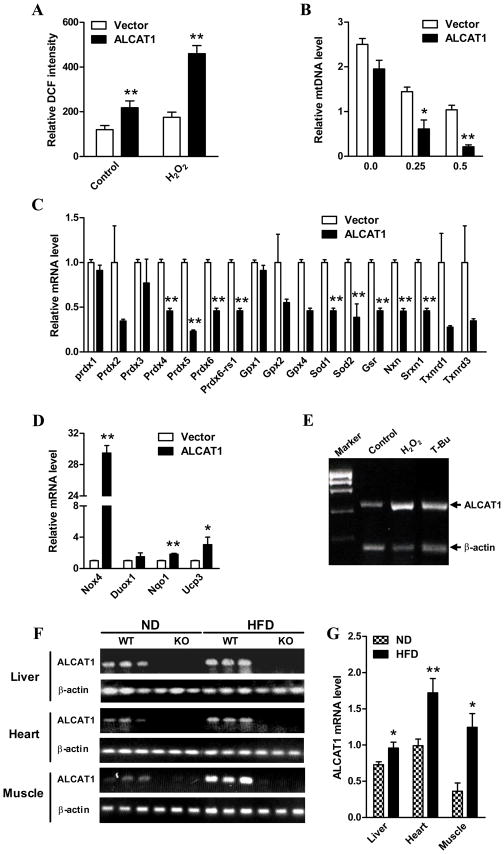
Figure 2. ALCAT1 causes oxidative stress in C2C12 cells, and is up-regulated by ROS and by DIO.
The C2C12 cells stably overexpressing ALCAT1 or vector used in Fig 1 were analyzed for A, intracellular level of ROS before (control) and after treatment with H2O2; B, mtDNA copy number in response to H2O2 treatment; C, the expression of genes encoding antioxidant enzymes, including peroxiredoxin (Prdx), peroxiredoxin-6 related sequence (Prdx6-rs1), glutathione peroxidases (Gpx), superoxide dismutases (Sod), glutathione reductase (Gsr), nucleoredoxin (Nxn), sulfiredoxin 1 homolog (Srxn1), and thioredoxin reductase (Txnrd); and D, expression of genes encoding oxidant enzymes, including NADPH oxidase-4 (Nox4), dual oxidase-1 (Duox1), NAD(P)H quinone oxidoreductase-1 (Nqo1), and uncoupling protein-3 (Ucp3) by using an oxidative stress pathway real-time PCR microarray kit. E, ALCAT1 mRNA expression was up-regulated in isolated mouse cardiomyocytes in response to treatment with tert-butylhydroperoxide (T-Bu), an oxidant, and H2O2, as measured by RT-PCR analysis using β-actin as an internal control. F, ALCAT1 mRNA expression was analyzed in liver, heart, and skeletal muscle of ALCAT1−/− mice (KO) and the wild type control (WT) mice fed a normal diet (ND) or high-fat diet (HFD) by RT-PCR analysis using β-actin as internal control, and quantified (G). SEM, *P<0.05, **P<0.01, compared to vector controls. N=3. *P < 0.05, ** P < 0.01.
ALCAT1 Causes Oxidative Stress in C2C12 Cells, and is Up-regulated by ROS, DIO, and Type 2 Diabetes
ROS are mainly produced when electrons leak from the mitochondrial electron transport chain (Kokoszka et al., 2001). Consistent with increased proton leakage, overexpression of ALCAT1 in C2C12 cells significantly increased the intracellular level of ROS (Figure 2A), which was further exacerbated after treatment with H2O2. Furthermore, treatment with H2O2 resulted in significant depletion of mtDNA in C2C12 cells overexpressing ALCAT1 relative to the vector control cells (Fig 2B). To provide further evidence for a causative role of ALCAT1 in oxidative stress, we analyzed the expression of genes involved in ROS production and oxidative response by real-time RT-PCR analysis. As shown by Fig 2C & 2D, ALCAT1 overexpression significantly increased expression of genes that cause oxidative stress, such as NAD(P)H quinone oxidoreductase 1 (Nqo1) and NADPH oxidase 4 (Nox4), and decreased expression of genes encoding antioxidative enzymes, including glutathione peroxidases (Gpx), peroxiredoxins (Prdx), thioredoxin reductases (Txnrd), and superoxide dismutases (Sod) in C2C12 cells. The Nqo1 enzyme is essential for oxidative stress in mice and humans (Voynow et al., 2009), whereas Nox4 is an NAD(P)H oxidase homolog that stimulates the production of ROS in insulin sensitive tissues (Mahadev et al., 2004). The expression of Nox4 was up-regulated by more than 29 fold by ALCAT1 overexpression in C2C12 cells (Fig 2D). Furthermore, the mRNA expression of Ucp3 was also elevated by more than three folds, which is consistent with the increased state 4 respiration caused by ALCAT1 overexpression (Fig 1G).
To provide direct evidence that links ALCAT1 expression to oxidative stress, we next analyzed ALCAT1 mRNA expression in isolated primary cardiomyocytes from C56/Bl6 mice in response to oxidative stress. As shown in Fig 2E, ALCAT1 mRNA expression was significantly up-regulated in isolated cardiomyocytes in culture by tert-butylhydroperoxide (T-Bu), an oxidant that causes mitochondrial dysfunction, and by H2O2 treatment. The results suggest a causative role of ALCAT1 in mitochondrial dysfunction in response to oxidative stress in metabolic diseases, which was supported by up-regulation of ALCAT1 mRNA expression in liver, heart, and skeletal muscle in response to the onset of DIO in C56/Bl6 mice (Fig 2F, quantified in 2G). Likewise, both ALCAT1 mRNA expression and enzyme activity were up-regulated by the onset of type 2 diabetes in db/db mice (Supplemental Fig S2).
ALCAT1−/− Mice Are Protected from DIO and Insulin Resistance
To investigate a regulatory role of ALCAT1 in regulating CL remodeling and mitochondrial dysfunction associated with metabolic diseases, we have generated mice with targeted deletion of the ALCAT1 gene (Supplemental Fig S3). ALCAT1−/− mice were born with predicted Mendelian ratios without any obvious phenotypic abnormality when fed a standard mouse chow for 12 weeks. After feeding a high-fat diet which contained 60% calories from animal fat for 8 consecutive weeks, the weight gain in ALCAT1−/− mice was significantly lower in male (Fig 3A), compared to wild type mice. Female mice were unaffected (data not shown). The body weight difference were due to decreased fat mass in ALCAT1−/− mice as measured by 1H-nuclear magnetic resonance (Fig 3B). The total body fat content was 8.7% higher in wild type mice relative to the ALCAT1−/− mice.
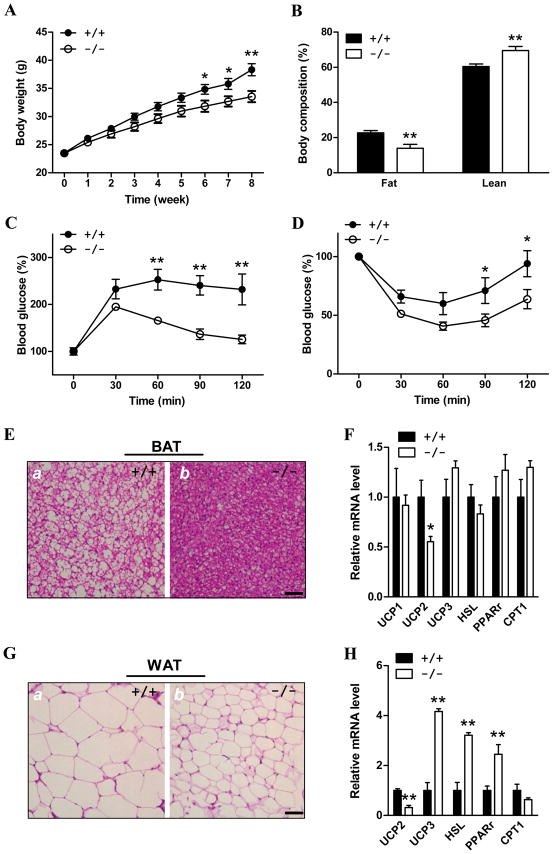
Figure 3. ALCAT1−/− mice are protected from the onset of DIO and its related insulin resistance.
Male ALCAT1−/− mice and the wild type control, 8 weeks old, were fed a diet with 60% fat for 8 consecutive weeks, and were analyzed for A, weight gain; B, body composition; C, glucose tolerance test; and D, insulin tolerance test, n=8–10. E, ALCAT1 deficiency decreased the size of lipid droplets in brown adipose tissue (BAT), and G, in white adipose tissue (WAT) of ALCAT1−/− mice as analyzed by hematoxylin and eosion (H/E) staining. F, analysis of expression of genes involved in energy metabolism in BAT, and H, in WAT, including uncoupling proteins (UCP), hormone-sensitive lipase (HSL), peroxisome proliferator-activated receptor-γ (PPARγ), and carnitine palmitoyltransferase I (CPT1), by RT-PCR analysis. N=5–10. *P < 0.05, ** P < 0.01.
We next analyzed changes in glucose homeostasis and insulin sensitivity in the ALCAT1−/− mice relative to the wild type sibling controls. After an overnight fast, blood glucose levels were indistinguishable between the ALCAT1−/− and the wild type controls. However, blood glucose concentrations were significantly lower in the ALCAT1−/− mice than wild type during a glucose tolerance test (Fig 3C). The improvement in glucose tolerance in ALCAT1−/− is most likely caused by enhancement in insulin sensitivity, as evidenced by the results of the insulin tolerance tests (Fig 3D). In comparison to wild type mice, the ALCAT1−/− mice were more sensitive to hypoglycemia induced by insulin injection (Fig 3D), which is supported by lower serum insulin level (Table S2). Since both genders of the heterozygous ALCAT1 knockout mice and the female ALCAT1−/− mice were not significantly different from the wild type controls in weight gain and insulin/glucose tolerance (data not shown), subsequent studies were carried out only in male mice.
Consistent with the finding of reduced fat content, brown adipose tissue (BAT) and white adipose tissue (WAT), the ALCAT1−/− mice exhibited smaller fat droplets compared to those from the wild type mice when tissue sections were examined with H/E staining (Fig 3E). ALCAT1 deficiency caused dramatic changes of genes involved in energy homeostasis in WAT, including uncoupling protein 2 and 3 (Ucp2, Ucp3), hormone sensitive lipase (HSL), and peroxisome proliferator-activated receptor-γ (PPARγ, but not carnitine palmitoyltransferase I (CPT1). Up-regulated mRNA expression of Ucp3, HSL, and PPARγ in WAT was consistent with improved insulin sensitivity and higher energy expenditure in ALCAT1−/− mice.
Hyperphagia, Hyperactivity, and Hypermetabolism in ALCAT1−/− Mice
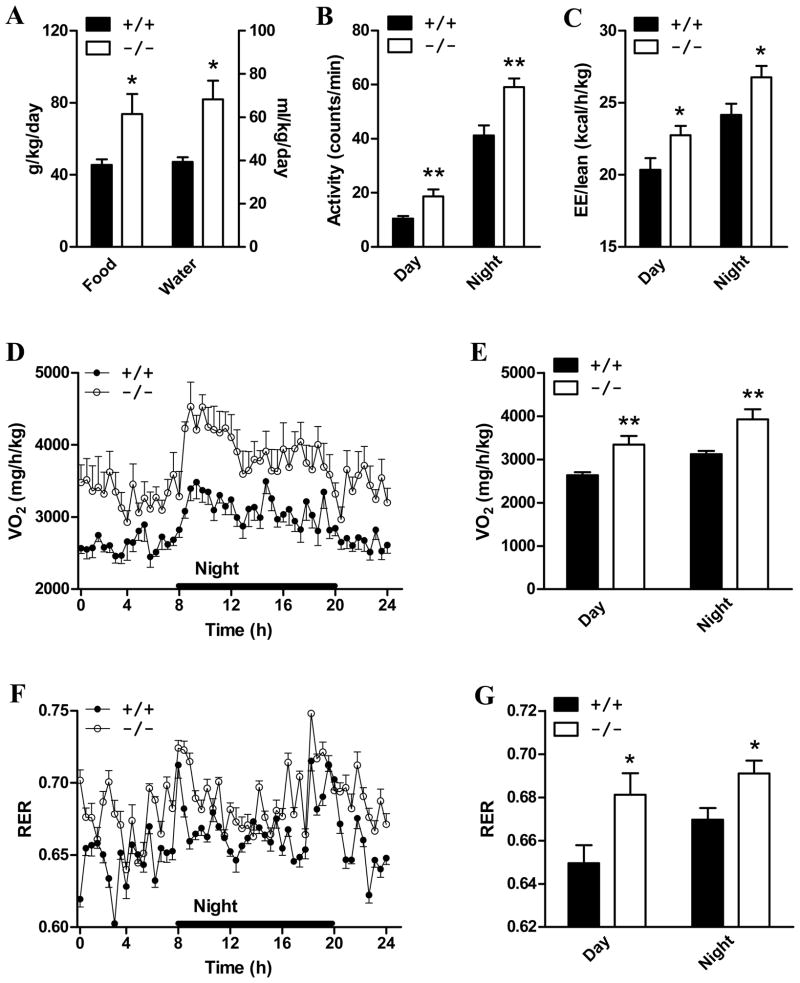
To determine the mechanisms accounting for the resistance to DIO in ALCAT1−/− mice, water intake, physical activity, and energy expenditure were analyzed over 72 hours in mice acclaimed to cages of comprehensive lab animal monitoring system (CLAMS). As shown by Fig 4A, the lean phenotype of ALCAT1−/− was not secondary to reduced food intake. Acctually, ALCAT1−/− mice consumed 62% more food and 73% more water (Fig 4A), respectively, per kg of body weight than wild type controls during the 24 hour period. The ALCAT1−/− mice were also hyperactive, as evidenced by enhanced physical activity relative to the wild type mice (Fig 4B). Consequently, the energy expenditure (EE) rate was significantly higher in ALCAT1−/− mice (Fig 4C) due to higher metabolic rate, as evidenced by the increased oxygen consumption rate (Fig 4D, quantified in 4E). Respiratory exchange ratio (RER) during the 24 hour period was significantly elevated in the ALCAT1−/− mice (Fig 4F, quantified in 4G), suggesting that the null mice burned more carbohydrate as a fuel.
Figure 4. ALCAT1−/− mice exhibits hyperphagia, hyperactivity, and hyper-metabolism.
Male ALCAT1−/− mice (−/−) and the controls (+/+) from Fig 3 were analyzed for A, food intake and water intake; B, physical activity; C, energy expenditure; D, oxygen consumption rate (quantified in E); and F, respiratory exchange ratio (RER) (quantified in G) by TSE systems. N=6–8. *P < 0.05, ** P < 0.01.
ALCAT1 Deficiency Improves Insulin Signaling in ALCAT1−/− Mice
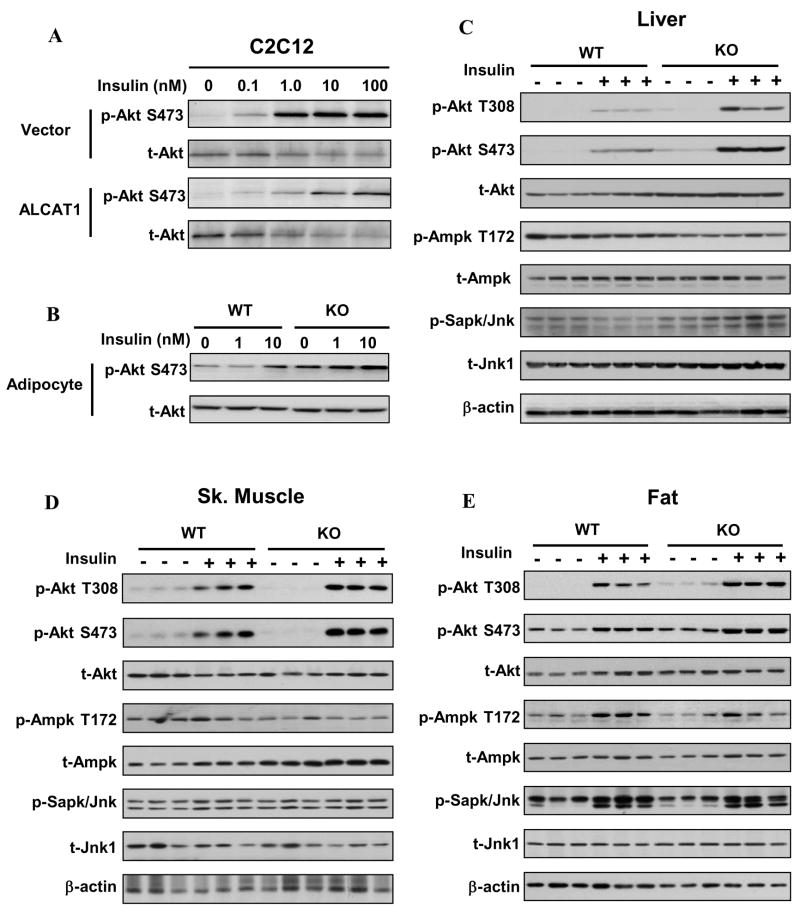
To gain further insight into the molecular mechanisms underlying the improved insulin sensitivity in ALCAT1−/−, we next analyzed the effect of ALCAT1 overexpression and deficiency on insulin signaling in C2C12 cell lines, isolated adipocytes from the male ALCAT1−/− and wild type mice, and in various tissues of male ALCAT1−/− and wild type mice, respectively. As shown in Fig 5A, overexpression of ALCAT1 in C2C12 cells significantly decreased insulin-stimulated Akt phosphorylation. Conversely, isolated adipocytes in culture from the ALCAT1−/− mice exhibited a significant increase in Akt phosphorylation in response to stimulation by insulin (Fig 5B). The negative effect of ALCAT1 on insulin signaling was further corroborated from the analysis of whole body insulin sensitivity in response to acute insulin treatment. As shown in Fig 5C-5E, insulin-stimulated Akt phosphorylation was significantly enhanced in liver, adipose tissue, and skeletal muscle of ALCAT1−/− mice relative to that from the wild type controls. The expression of Akt protein was also significantly up-regulated in liver of the ALCAT1−/− mice (Fig 5C). Consistent with improved insulin sensitivity, ALCAT1 deficiency suppressed the phosphorylation of AMPK at theronine-172, a major activation site. Furthermore, the expression of AMPK protein was up-regulated in skeletal muscle of the ALCAT1−/− mice (Fig 5D), without any effect on JNK expression or phosphorylation.
Figure 5. Effects of ALCAT1 overexpression and deficiency on insulin signaling in C2C12 cell lines and in mouse tissues.
A, The C2C12 cell lines used in Fig 2 were treated indicated concentrations of insulin, and analyzed for Akt phosphorylation (T308 and S473) and total Akt level on the same blot by Western blot analysis. B, ALCAT1 deficiency improved insulin signaling in isolated adipocytes in culture from ALCAT1−/− mice (KO) vs. wild type mice (WT). C–E, mice from Fig 4 were treated with insulin (1 unit/kg, I.P.) and were analyzed for Akt phosphorylation in the liver, skeletal muscle, and adipose tissues by Western blot analysis. The same blots were also analyzed for phosphorylation of AMPK and JNK and total protein level of each kinase (t-Akt, t-AMPK, and t-JNK) using β-actin as an internal control for protein loading.
ALCAT1 Deficiency Suppresses ATP Production Rate Analogous to the Effects of an Insulin Sensitizer
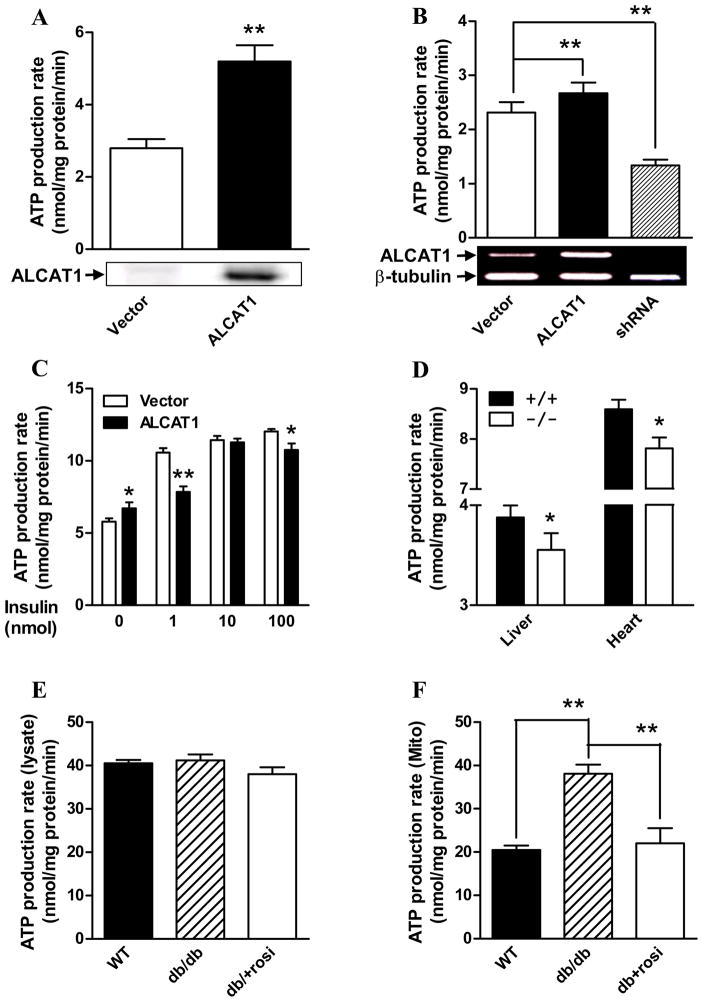
Enhanced ATP production rate is associated with severe insulin resistance in American Indians, whereas insulin stimulation of ATP production is impaired in type 2 diabetic patients and their insulin resistant offspring (Asmann et al., 2006; Petersen et al., 2005). Consistent with a causative role of ALCAT1 in insulin resistance, overexpression of ALCAT1 in COS-7 or C2C12 cells increased ATP production rate by 50% in isolated mitochondria when compared to vector control (Fig 6A & B). Conversely, targeted inactivation of endogenous ALCAT1 mRNA expression in C2C12 cells by RNA interference (shRNA) significantly decreased the ATP production rate (Fig 6B). ALCAT1 overexpression also impaired the stimulating effect of insulin on ATP production rate as observed in diabetic patients. As shown in Fig 6C, ATP production rate was stimulated by insulin in a dose-dependent manner in control C2C12 cells overexpressing an empty plasmid vector. In contrast, ATP production rate was blunted in C2C12 cells overexpressing the ALCAT1 cDNA.
Figure 6. Regulation of mitochondrial ATP production rate by ALCAT1 and type 2 diabetes.
A, isolated mitochondria from COS-7 cells overexpressing ALCAT1 were analyzed for ATP production rate. The expression level of the flag-tagged ALCAT1 was verified by western blot analysis (blow). B, ATP production rate was analyzed from stable C2C12 cell lines used in Figure 1, and C2C12 cells with ALCAT1 deficiency mediated by shRNA (shRNA), The mRNA level of ALCAT1 was verified by RT-PCR analysis (blow). C, ATP production rate was analyzed in isolated mitochondria isolated from the stable C2C12 cell lines used in panel B pretreated with indicated doses of insulin. D, ATP production rate was analyzed in isolated mitochondria from liver and heart of the ALCAT1−/− mice. E, ATP production rates were unchanged in liver protein lysate of db/db mice. F, ATP production rate was analyzed in mitochondria isolated from liver of 10-week old db/db diabetic mice, the non-diabetic controls (WT), and the db/db diabetic mice pretreated with 0.375 mg/day of rosiglitazone for 4 consecutive weeks (db+rosi). N=5. *P < 0.05, ** P < 0.01.
To understand how ATP production was regulated by obesity and type 2 diabetes, we next analyzed the effect of hyperglycemia and an anti-diabetic drug on ATP production rate in isolated mitochondria from the liver of db/db mice, a mouse model of obesity and type 2 diabetes. As shown by Fig 6E, the rate of ATP production by liver lysates was not different between the db/db mice and the non-diabetic control mice. In contrast, ATP-production rate was significantly higher in isolated mitochondria from liver of db/db mice relative to non-diabetic control mice (Fig 6F). However, this defect was completely normalized by 4 weeks of treatment of the db/db mice with rosiglitazone, an insulin sensitizer, suggesting that enhanced ATP production rate is a major defect in diabetes. In further support of a role of ALCAT1 in linking mitochondrial dysfunction to insulin resistance, ATP production rates were significantly lower in isolated mitochondria from liver and heart of ALCAT1−/− mice that exhibits improved insulin sensitivity (Fig 6D).
Enhanced Mitochondrial Complex I Activity and Fatty Acid Oxidation Rate in ALCAT1−/− Mice
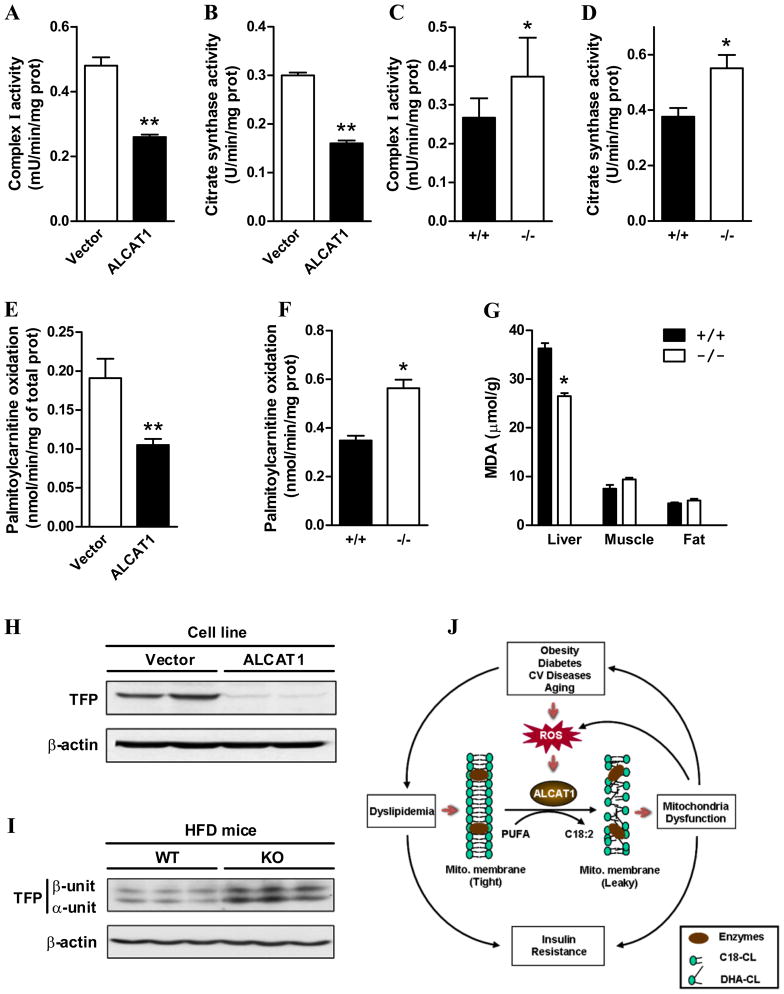
Oxidative stress impairs mitochondrial complex I activity through CL peroxidation (Paradies et al., 2002). We next analyzed the effect of ALCAT1 overexpression and deficiency on mitochondrial complex I activity and oxygen consumption in response to different substrates. In further support of a causative role of ALCAT1 in mitochondrial dysfunction, ALCAT1 overexpression significantly impaired mitochondrial complex I and citrate synthase activity (Fig 7A–B), which was reversed by ALCAT1 deficiency in ALCAT−/− mice (Fig 7C–D). Furthermore, ALCAT1 deficiency also improved mitochondrial respiratory coupling, as evidenced by significantly lower oxygen consumption rate in isolated mitochondrial from ALCAT1−/− mice in response to treatment with oligomycin (Supplemental Fig S4). Mitochondrial fatty acid overload and impaired fatty acid oxidation is a contributing factor to insulin resistance in rodents and humans (Bandyopadhyay et al., 2006; Koves et al., 2008). Consistent with improved hepatic insulin sensitivity in ALCAT1−/− mice as shown by Fig 5C, isolated liver mitochondria from the ALCAT1−/− mice oxidized palmitoylcarnitine faster than mitochondria from the control mice on a high-fat diet (Fig 7F). The use of palmitoylcarnitine bypasses regulation by CPT1, allowing direct assessment of mitochondrial respiratory function. Conversely, fatty acid oxidation rates were significantly decreased by the ALCAT1 overexpression in C2C12 cells (Fig 7E).
Figure 7. Enhanced mitochondrial complex I activity, fatty acid oxidation, and trifunctional protein (TFP) expression in liver of ALCAT1−/− mice.
A, mitochondrial complex I activity and B, citrate synthase activity were analyzed in C2C12 cells as used in Figure 1. C, mitochondrial complex I activity and D, citrate synthase activity were analyzed in isolated liver mitochondria from ALCAT1−/− (−/−) and wild type control (+/+) mice. E, palmitoylcarnitine oxidation rate was analyzed from C2C12 cells used in Figure 1, and F, from isolated mitochondria from the liver of the ALCAT1−/− mice (−/−) and the wild type controls (+/+) mice. G, lipid peroxidation levels, as indicated by the level of malonaldehyde (MDA), were analyzed in tissue lysate from liver, skeletal muscle, and fat of the ALCAT1−/− mice and the controls by a TBARS kit, N=4. D, western blot analysis of TFP from C2C12 cells used in Figure 1, or D, from liver lysates of wild type (WT) and the ALCAT1−/− (KO) mice on high fat diet (HFD), using anti-β-actin antibodies (internal control). J, a schematic diagram depicts a causative role of ALCAT1 in mitochondrial dysfunction and insulin resistance. N=6–8. *P < 0.05, ** P < 0.01.
Obesity increases the level of lipid peroxidation by oxidative stress (Olusi, 2002). Since ALCAT1 overexpression stimulated ROS production in C2C12 cells, we questioned whether ALCAT1 deficiency would suppress oxidative stress in ALCAT1−/− mice. This was done by analyzing changes in the level of thiobarbituric acid reactive substances (TBARS), an indicator of lipid peroxidation from oxidative stress. Consistent with increased ROS in C2C12 cells overexpressing ALCAT1 (Fig 2C), the TBARS level was significantly lower in liver of ALCAT1−/− mice relative to the wild type mice (Fig 7G). In contrast, ALCAT1 deficiency had no significant effect on oxidative stress in fat and skeletal muscles.
Up-regulated Expression of Mitochondrial Trifunctional Protein (TFP), Cardiac Tetralinoleoyl-CL, and Hepatic PG in ALCAT1−/− Mice
To identify molecular mechanisms accounting for a causative role of ALCAT1 in pathological remodeling of CL, we next analyzed the effect of ALCAT1 on the expression of TFP which was recently reported to encode MLCL AT, a mitochondrial isoform of ALCAT1. As shown in Fig 7H, TFP protein expression was completely suppressed by ALCAT1 overexpression in H9c2 cell lines. Conversely, TFP expression was significantly up-regulated by ALCAT1 deficiency in liver of ALCAT1−/− mice (Fig 7I). Consistent with a previously reported role of MLCL AT in catalyzing beneficial CL remodeling (Taylor and Hatch, 2003), cardiac CL from ALCAT1−/− mice demonstrated a significant increase in linoleic acid content (6.27 vs. 4.35 nmol/mg protein, p<0.001, ALCAT1−/− vs. control) compared to heart from wild type mice (Supplemental Fig S5A). Additionally, total CL concentrations were significantly elevated in the heart of ALCAT−/− mice relative to the wild type controls (19.08 vs.15.05 nmol/mg protein, p<0.05, ALCAT1−/− vs. control). Finally, ALCAT1 deficiency also increased the total concentration of hepatic PG by more 400% (4.74 vs. 0.99 nmol/mg protein, p<0.001, ALCAT1−/− vs. control) without a significant impact on the total CL level in liver of ALCAT1−/− mice (Supplemental Fig S5B).
DISCUSSION
Diabetes and obesity are characterized by CL deficiency and profound remodeling of CL’s acyl composition through unknown mechanisms (Han et al., 2007; Sparagna et al., 2007; Watkins et al., 2002). The current studies identified for the first time a key role of ALCAT1 in catalyzing the synthesis of CL species that are highly susceptible to oxidative damage. Accordingly, ALCAT1 overexpression in C2C12 cells led to CL deficiency and alterations of acyl composition of CL reminiscent of those observed in diabetes without major effects on other phospholipids. Conversely, ALCAT1 deficiency significantly increased the levels of tetralinoleoyl-CL in heart and PG in the liver of ALCAT1−/− mice. PG is an immediate biosynthetic precursor of CL and can substitute for some essential functions of CL (Jiang et al., 2000). Both CL and PG levels are substantially depleted by the onset of diabetes (Han et al., 2005). The results are further corroborated by a major role of ALCAT1 as a negative regulator of TFP, a newly identified ALCAT1 isoform also known as MLCL AT (Taylor and Hatch, 2009). In contrast to ALCAT1, MLCL AT catalyzes CL remodeling by increasing the content of linoleic acid, leading to an increase of the “good” CL (Cao et al., 2004; Taylor and Hatch, 2003). Thereby, TFP expression was significantly down-regulated by ALCAT1 overexpression in C2C12 cells, and up-regulated by ALCAT1 deficiency in the liver of ALCAT1−/− mice.
One of the major unresolved questions in metabolism is how oxidative stress contributes to the onset of mitochondrial dysfunction in metabolic diseases. The results from the current studies suggest CL remodeling by ALCAT1 as a missing link between oxidative stress and mitochondrial dysfunction, which is supported by multiple lines of evidence. First, ALCAT1 overexpression leads to CL deficiency and enrichment of DHA content which is known to increase mitochondrial membrane potential, oxidative stress, and lipid peroxidation (Hong et al., 2002; Watkins et al., 1998). Second, ALCAT1 causes proton leakage as evidenced by up-regulation of UCP3 expression and state 4 respiration in C2C12 cells. The mitochondrial defects are similar to those observed in rodent models of diabetes, obesity, and hyperthyroidism (Boudina et al., 2007; Short et al., 2007). Third, ALCAT1 overexpression significantly increases ROS production in C2C12 cells, which is exacerbated in response to H2O2 treatment. The increased ROS production is explained by up-regulation of genes encoding oxidant enzymes and down-regulation of those encoding antioxidant enzymes. For example, the expression of Nox4, an NAD(P)H oxidase homolog, was increased more than 29 fold. Nox4 stimulates the production of ROS in insulin sensitive tissues (Mahadev et al., 2004), which is a major source of ROS in diabetic animals (Block et al., 2009). Up-regulated Nox4 expression could also contribute to higher oxygen consumption rate observed in C2C12 cells overexpressing ALCAT1. Fourth, ALCAT1 mRNA expression is up-regulated in primary mouse cardiomyocytes and in insulin sensitive tissues in responses to oxidative stress (Fig 2E), DIO (Fig 2F), and hyperthyroidism (Cao et al., 2009) which stimulates CL peroxidation. Finally, ALCAT1 deficiency significantly reduces the level of lipid peroxidation in liver of the ALCAT1−/− mice on HFD.
Mitochondrial dysfunction has been proposed to be a unifying cause of diabetes and obesity (Lowell and Shulman, 2005), yet the underlying molecular mechanisms remain elusive (Turner and Heilbronn, 2008). Several early studies suggested that mitochondrial oxidative function is compromised in diabetic and prediabetic humans (Bandyopadhyay et al., 2006; Mootha et al., 2004) and their offspring (Petersen et al., 2004). However, several recent reports suggest mitochondrial hyperactivity and overload as major defects associated with severe insulin resistance in Asian Indian immigrants in USA (Nair et al., 2008) and animal models of diabetes and obesity (Koves et al., 2008; Pospisilik et al., 2007). Our work on CL remodeling by ALCAT1 suggests an alternative molecular mechanism by which mitochondrial hyperactivity causes insulin resistance. Accordingly, the ATP production rate was stimulated by ALCAT1 overexpression and insulin resistance in C2C12 cells, and was down-regulated in ALCAT1−/− mice that exhibit enhanced insulin sensitivity and signaling. These observations are further corroborated by enhanced mitochondrial ATP production rate in db/db mice, which is normalized by treatment with rosiglitazone. Rosiglitazone reduces oxidative stress (Bagi et al., 2004; Quintanilla et al., 2008) and improves mitochondrial function by increasing levels of total CL, the linoleic acid content in CL (Pan et al., 2006; Watkins et al., 2002), and the expression of enzymes involved in fatty acid metabolism, including TFP (Wilson-Fritch et al., 2003; Wilson-Fritch et al., 2004). TFP is a trifunctional enzyme localized in the inner mitochondrial membrane where it catalyzes three out of the four steps in the β-oxidation cycle. TFP deficiency in humans and rodents causes severe fatty acid oxidation disorder (den Boer et al., 2003), fatty liver, insulin resistance, and oxidative stress (Ibdah et al., 2005; Tonin et al., 2010). Consequently, fatty acid oxidation was impaired by TFP deficiency in C2C12 cells overexpressing ALCAT1, and was enhanced in isolated hepatic mitochondria by up-regulated TFP expression in ALCAT1−/− mice. In further support of a causative role of ALCAT1 in insulin resistance, insulin-stimulated Akt phosphorylation and ATP-production rate were blunted in C2C12 cells overexpressing ALCAT1 analogous to those observed in diabetic humans (Asmann et al., 2006; Petersen et al., 2005), which are reversed by ALCAT1 deficiency.
Importantly, the present findings carry additional implications for future studies on other metabolic diseases, since pathological CL remodeling has been identified as a common defect in diabetes, obesity, cardiovascular diseases, and aging (Gredilla et al., 2001; Han et al., 2007; Pan et al., 2006; Sparagna and Lesnefsky, 2009). Our results prompt speculation that CL remodeling by ALCAT1 may be a common denominator in oxidative stress and mitochondrial dysfunction associated with metabolic diseases and aging (Fig 7J). Accordingly, up-regulation of ALCAT1 in response to ROS causes CL peroxidation which leads to oxidative stress and mitochondrial dysfunction, further exacerbating ROS production, insulin resistance, and other metabolic complications. Consequently, inhibition of ALCAT1 by chemical reagents may provide a potential treatment for obesity and other metabolic diseases.
EXPERIMENTAL PROCEDURES
Shotgun Lipidomics Analysis of CL Species
The lipidomics analysis was carried out using methods as previously described (Han et al., 2007). Briefly, total lipids from C2C12 cells and animal tissues were analyzed by triple-quadruple mass spectrometer (MS) (Thermo Electron TSQ Quantum Ultra) controlled by Xcalibur system software. All the MS spectra and tandem MS spectra were automatically acquired by a customized sequence subroutine operated under Xcalibur software.
Mitochondrial Membrane Potential (ΔΨ) and Intracellular ROS
ΔΨ and intracellular ROS generation in C2C12 cells was investigated using TMRM (T-668, Invitrogen) or 2′,7′-dichlordehydrofluorescein-diacetate (DCFH-DA; Molecular Probes) at a final concentration of 200 nM or 5 μM, respectively. Cells were incubated with dye in culture medium for 30 min at 37 °C and then resuspended in 0.5 ml of PBS, and submitted to flow cytometric analysis using FACS Caliburs Flow Cytometer (Becton Dickinson, San Jose)
Oxygen Consumption in Intact Cells
Oxygen consumption rates were measured in intact cells using a SOM-2 PO2 Clark oxygen electrode. C2C12 cells, 3×106 total were suspended in 1.4ml DMEM culture medium, transferred into the chamber which was maintained at 37°C. After equilibration and stirring, basal respiration was measured as the average oxygen consumption over 5 min, followed by sequential injection of oligomycin (2.5 μg/ml final concentration), and p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (FCCP, 2uM final concentration), respectively.
Mitochondrial Isolation and ATP production Rate Assay
Cells were harvested in 1ml homogenization buffer (20mM HEPES, 140mM KCl, 5mM MgCl2·6H2O, 1mM EDTA, pH 7.4), homogenized for 3 min on ice. The homogenate was then centrifuged at 600 × g 4°C for 5 min, and the supernatant was centrifuged for 10 min at 10,000 × g, 4°C to pellet mitochondria. Then mitochondrial ATP production rate (MAPR) was measured using the ENLITEN ATP Assay system (Promega) with 1420 Multilabel Counter (Perkin Elmer).
Mitochondrial Complex I Activity Assay
The complex I (NADH-CoQ reductase) activity was measured in mitochondria and cell homogenate obtained after sonication in 50 mM phosphate buffer, pH 7.2. The assay mixture contained 2mM of NaCN, 2μg/ml antimycin A, and 0.1mM decylubiquinone. The mitochondrial sample or cell sample (75 μg total protein) was added to 1 ml of the assay mixture and the reaction was started by the addition of 200μM NADH. The reaction was measured by following the decrease in absorbance of NADH at 340 nm with a spectrophotometer. The activity was calculated by an extinction coefficient of 6.22 mM−1cm−1 for NADH. Citrate synthase activity was used as a mitochondrial enzymatic marker. Mitochondria or cell homogenates (50 μg total protein) were added to a buffer with 100 mM Tris·HCl, 1 mM 5,5′-dithiobis-2-nitrobenzoic acid, 3 mM acetyl-CoA, pH 8, and 0.2% Triton X-100 (vol/vol). The reaction was started by the addition of 1 mM oxaloacetate, and the initial rate was measured following the decrease of absorbance at 420 nm wavelength.
Fatty Acid Oxidation Assay
Fatty acid oxidation rates were measured in liver mitochondria and C2C12 cells, using sealed vials in a volume of 0.4 ml in a medium (25 mM sucrose, 75 mM Tris-HCl, 10 mM KH2PO4, 5 mM MgCl2, and 1 mM EDTA, pH 7.4) supplemented with 5 mM ATP, 1 mM NAD+, 0.1 mM CoA, 0.5 mM L-carnitine, 0.5 mM L-malate, and 25 μM cytochrome C. For C2C12 cells, they were cultured in 25 cm2 flask and washed 3 times with cold PBS when they reached 80–90% confluence and replaced the medium with 0.4 ml of low glucose DMEM supplemented with 2% BSA. The reaction was started by injecting 0.1 ml of 600 μmol/l of [14C] palmitoylcarnitine, and incubated at 37°C for 15 min for isolated mitochondria or 60 min for cell lysate. The reaction as stopped by the addition of 0.2 ml of 20% perchloric acid. After 90 min of shaking at room temperature, the production level of [14]CO2 was analyzed by liquid scintillation counter (Beckman Instruments).
Body Composition, Energy Expenditure, Activity, and Food Intake
Body fat and lean body mass were measured using an LF90 TD-NMR (Bruker Optics). Measurements of food/water intake, energy expenditure, respiratory exchange ratio, and physical activity were performed using metabolic cages (TSE Systems, Bad Homburg, Germany) for three consecutive days. Constant airflow (0.4 l/min) was drawn through the chamber and monitored by a mass-sensitive flow meter. The concentrations of oxygen and carbon dioxide were monitored at the inlet and outlet of the sealed chambers to calculate oxygen consumption and RER. Each chamber was measured for 1 min at 15 min intervals. Physical activity was monitored by infrared technology (OPT-M3, Columbus Instruments), as the count of three-dimensional beam breaking (X total, X ambulatory, and Z) were measured.
Statistical Analysis
Statistical comparisons were done using two-tailed non-paired t-test to determine difference between the two C2C12 cells lines, and between ALCAT1−/− and wild type mice. Analysis of covariance (ANCOVA) was also used to assess differences between ALCAT1−/− and wild type mice in metabolic parameters relative to total body weight and lean weight. Data are expressed as means ± SEM.
Supplementary Material
Acknowledgments
We thank Drs. Xian Li for help with statistical analysis and Tomas Garner for critically reading the manuscript. This study was supported in part by grants from NIH (DK076685, Y.S.; DK062880, CL; DK15658, LSJ; and P01 HL57278, XH), the Chinese National Natural Science Foundation (#30871786, H.W.), and a scholarship from the Chinese National Scholarship Council (J.L.).
Footnotes
The authors disclose no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JWr, Kang L, Rabinovitch PS, Szeto HH, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(113):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Koller A, Kaley G. PPARgamma activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am J Physiol - Heart & Cir Physiol. 2004;286:H742–748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes. 2006;55:2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG. Mitochondrial energetics in the heart in obesity related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56(10):2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- Cao J, Liu Y, Lockwood J, Burn P, Shi Y. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. Journal of Biological Chemistry. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- Cao J, Shen W, Chang Z, Shi Y. ALCAT1 is a polyglycerophospholipid acyltransferase potently regulated by adenine nucleotide and thyroid status. Am J Physiol Endocrinol Metab. 2009;296:E647–653. doi: 10.1152/ajpendo.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, He Q, Greenberg ML. Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol Microbiol. 2008;68:1061–1072. doi: 10.1111/j.1365-2958.2008.06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- den Boer ME, Dionisi-Vici C, Chakrapani A, van Thuijl AO, Wanders RJ, Wijburg FA. Mitochondrial trifunctional protein deficiency: a severe fatty acid oxidation disorder with cardiac and neurologic involvement. J Pediatr. 2003;142:684–689. doi: 10.1067/mpd.2003.231. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Lopez Torres M, Portero-Otin M, Pamplona R, Barja G. Influence of hyper- and hypothyroidism on lipid peroxidation, unsaturation of phospholipids, glutathione system and oxidative damage to nuclear and mitochondrial DNA in mice skeletal muscle. Mol & Cell Biochem. 2001;221:41–48. doi: 10.1023/a:1010930110382. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 2005;44:16684–16694. doi: 10.1021/bi051908a. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 2007;46:6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang K, Yang J, Cheng H, Gross RW. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. J Lipid Res. 2006;47:864–879. doi: 10.1194/jlr.D500044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME, et al. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381–1390. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem. 2000;275:22387–22394. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/-) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, Larson MG, Fox CS, Keaney JF, Jr, Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diab Care. 2007;30:2529–2535. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, et al. Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes. 2008;57:1166–1175. doi: 10.2337/db07-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, Lupton JR, Chapkin RS. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes Relat Metab Disord. 2002;26:1159–1164. doi: 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- Pan HJ, Lin Y, Chen YE, Vance DE, Leiter EH. Adverse hepatic and cardiac responses to rosiglitazone in a new mouse model of type 2 diabetes: relation to dysregulated phosphatidylcholine metabolism. Vascul Pharmacol. 2006;45:65–71. doi: 10.1016/j.vph.2005.11.011. Epub 2006 Jun 2005. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Cir Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. [see comment] New Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005;2:e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. J Biol Chem. 2008;283:25628–25637. doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Short KR, Nygren J, Nair KS. Effect of T(3)-induced hyperthyroidism on mitochondrial and cytoplasmic protein synthesis rates in oxidative and glycolytic tissues in rats. Am J Physiol - Endo & Metab. 2007;292:E642–647. doi: 10.1152/ajpendo.00397.2006. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–61. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WA, Hatch GM. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J Biol Chem. 2003;278:12716–12721. doi: 10.1074/jbc.M210329200. [DOI] [PubMed] [Google Scholar]
- Taylor WA, Hatch GM. Identification of the human mitochondrial linoleoyl-coenzyme A monolysocardiolipin acyltransferase (MLCL AT-1) J Biol Chem. 2009;284:30360–30371. doi: 10.1074/jbc.M109.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh A, Potashnik R, Bashan N, Rudich A. Oxidative stress disrupts insulin-induced cellular redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. A putative cellular mechanism for impaired protein kinase B activation and GLUT4 translocation. J Biol Chem. 1999;274:10595–10602. doi: 10.1074/jbc.274.15.10595. [DOI] [PubMed] [Google Scholar]
- Tonin AM, Grings M, Busanello EN, Moura AP, Ferreira GC, Viegas CM, Fernandes CG, Schuck PF, Wajner M. Long-chain 3-hydroxy fatty acids accumulating in LCHAD and TFP deficiencies induce oxidative stress in rat brain. Neurochem Int. 2010;56:930–936. doi: 10.1016/j.neuint.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19:324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Van Q, Liu J, Lu B, Feingold KR, Shi Y, Lee RM, Hatch GM. Phospholipid scramblase-3 regulates cardiolipin de novo biosynthesis and its resynthesis in growing HeLa cells. Biochem J. 2007;401:103–109. doi: 10.1042/BJ20060373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Fischer BM, Zheng S, Potts EN, Grover AR, Jaiswal AK, Ghio AJ, Foster WM. NAD(P)H quinone oxidoreductase 1 is essential for ozone-induced oxidative stress in mice and humans. Am J Respir Cell Mol Biol. 2009;41:107–113. doi: 10.1165/rcmb.2008-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Watkins SM, Carter LC, German JB. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J Lipid Res. 1998;39:1583–1588. [PubMed] [Google Scholar]
- Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res. 2002;43:1809–1817. doi: 10.1194/jlr.m200169-jlr200. [DOI] [PubMed] [Google Scholar]
- Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Kelley RI, Blanck TJJ, Schlame M. Remodeling of cardiolipin by phospholipid transacylation. J Biol Chem. 2003;278:51380–51385. doi: 10.1074/jbc.M307382200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.