Abstract
To perceive and produce music accurately, the brain must represent, categorize, plan, and execute pitched information in response to environmental stimuli. Convergent methods from psychophysics, voxel-based morphometry, and diffusion tensor imaging with normal and tone-deaf (TD) subjects have shown that neural networks controlling pitch perception and production systems include bilateral frontotemporal networks. Although psychophysical and neuroimaging results are suggestive of a superior temporal and inferior frontal network responsible for pitch perception and production, active intervention of these areas is necessary to establish a causal connection between superior temporal and inferior frontal areas and pitch production ability. We sought to reverse-engineer the pitch perception-production network by noninvasive brain stimulation. Transcranial direct current stimulation (tDCS), a noninvasive brain-stimulation technique that is optimal for auditory research, was applied over superior temporal and inferior frontal regions. Pitch matching ability was assessed using an individually optimized pitch matching task administered after each stimulation session. Results showed diminished accuracy in pitch matching after cathodal stimulation over inferior frontal and superior temporal areas compared to sham control. Results demonstrate that intact function and connectivity of a distributed cortical network, centered around bilateral superior temporal and inferior frontal regions, are required for efficient neural interactions with musical sounds.
Introduction
The present paper is a brief report of a study on inducing pitch production deficits using the brain stimulation approach. Results from this paper represent a subset of the data presented in a talk by Psyche Loui, entitled “Imaging and Inducing Disorders in Pitch Perception and Production.” Substantial contributors to this study are Anja Hohmann and Gottfried Schlaug.
Musical systems around the world consist of pitches and durations of sounds, and the systematic and hierarchically organized combinations of pitches and durations give rise to melody, harmony, rhythm, and meter. Although the rules that dictate the way pitches and durations are organized may differ across cultures, the existence of these elements in music is universal. Every culture around the world makes music and celebrates the making of music. From the earliest point in infancy, humans have some knowledge of musical structure, broadly defined: evidence for understanding musical attributes, such as beat induction, has been observed even in newborn infants (Winkler et al., 2009), and the knowledge of pitches, keys, and harmony are known to emerge from infancy to childhood (Olsho et al., 1982, Trainor and Trehub, 1994). There is even some evidence for musical knowledge in animals, as evidenced by rhythmic synchronization in the parrot (Patel et al., 2009) and in vocal-mimicking species (Schachner et al., 2009), absolute pitch sensitivity in zebra finches and budgeriars (Weisman et al., 2004), and octave generalization in monkeys (Wright et al., 2000), to name just a few.
Despite the pervasive evidence for competence in pitch and rhythm, some human individuals have an unusual lack of the musical expertise, especially in the perception and production of pitches. Tone-deafness is an abnormal lack of musical ability, especially in the domain of pitch perception and production. It is characterized by the inability to sing in tune, but the screening procedures for tone-deafness include the perceptual tests Montreal Battery for the Evaluation of Amusia (MBEA, (Peretz et al., 2003)) and the psychophysical pitch-discrimination threshold of more than half a semitone (Foxton et al., 2004, Loui et al., 2008). Although the perceptual tasks are sensitive to dysfunctions in pitch perception, the majority of tone-deaf individuals are unaware of their inability to perceive pitches, but only of their inability to sing – perhaps resulting from being discouraged from singing by those around them. In previous studies we had identified that these abilities to perceive and to produce pitches are not necessarily the same amongst all individuals: tone-deaf individuals, who cannot consciously perceive pitch differences smaller than a semitone, can nevertheless produce these pitch intervals in the right direction (Loui et al., 2008). Furthermore, the threshold difference in frequency at which tone-deaf subjects can reliably reproduce pitches is smaller than their threshold of perception, suggesting that tone-deaf individuals have some ability to produce pitches even in the absence of conscious pitch perception, resulting in a mismatch between pitch perception and production abilities.
Neuroimaging research is underway to examine the neural underpinnings of pitch perception and production, specifically in tone-deaf individuals. Voxel-based morphometry and cortical thickness studies (Hyde et al., 2006, Hyde et al., 2007, Mandell et al., 2007) have found tone-deaf related differences in the superior temporal and inferior frontal areas. These two islands of structural abnormality in the inferior frontal gyrus and superior and middle temporal gyri are connected in white matter of the human brain by the arcuate fasciculus. Using diffusion tensor tractography, the arcuate fasciculus was found to be diminished in volume among tone-deaf individuals compared to matched controls (Loui et al., 2009). These neuroimaging results, in combination with electrophysiological results showing abnormal electrical brain responses to pitch deviations among tone-deaf individuals (Peretz et al., 2005, Peretz et al., 2009), combine to suggest that there are major differences between the brain structures of tone-deaf and non-tone-deaf individuals, especially within and between regions that subserve pitch perception and production processes.
Results so far have been correlational rather than causal – by observing behavioral and neural differences between a group of individuals with a “musical disorder” and a matched control group, previous studies concluded that these differences must result from the neural underpinnings of the musical disorder. However, to establish a claim of unidirectional causality between a brain region and a behavioral index, we need to go beyond observational studies to the realm of intervention. By actively intervening with the function of specifically targeted brain areas, and showing resultant changes in behavior, we can establish the claim of a causal relationship between a brain region and its behavioral output. Thus, techniques of neural intervention are important for understanding the brain by reverse engineering.
Studies in cognitive neuroscience have focused on two main tools to intervene with brain function in humans: transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Both TMS and tDCS allow the modulation of functions in brain regions underlying the scalp which is stimulated. While TMS uses the principle of electromagnetic induction to change the polarization of neurons (Wagner et al., 2007, Williams et al., 2009), tDCS uses low-amplitude electrical currents to modulate the firing rate of individual neurons, thus modulating cortical excitability (Wagner et al., 2007). For the investigation of auditory functions, tDCS may be preferable for several reasons: it is silent, it has a reliable “placebo” mode of sham stimulation, and the stimulation is administered in a gradual manner (with slow onsets and offsets) such that the participant does not feel pulses over temporal muscles during stimulation, thus minimizing discomfort.
To investigate the role of the inferior frontal and superior temporal regions in pitch perception and production, we investigated effects of brain stimulation on pitch perception and production. In the present study, a pitch production task that is optimized for each individual subject was conducted before and after cathodal tDCS over the superior temporal and inferior lobes.
Methods
Subjects
Nine right-handed volunteers (5 female, mean age 25.3, range 21–28), unselected for musical training (mean = 6.5 yrs, range = 0 – 17), participated after written informed consent was obtained from each subject as approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center. Subjects had a mean of 7.5 years of musical training (range: 0–21 years), but none of them was a trained singer or a professional musician. At the start of the first session for each subject, the subject was asked to hum tones that are within their vocal range in order to assess each subject's comfortable vocal range. The center of each subject's vocal range ranged from 151 to 262 Hz.
Transcranial Direct Current Stimulation
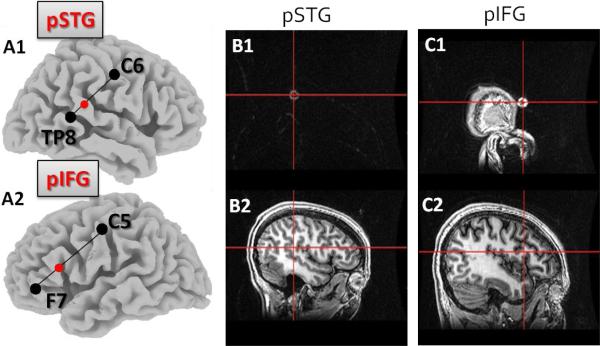
A battery-driven direct current stimulator (Phoresor, Iomed Inc, Salt Lake City) was used to deliver current in tDCS sessions. One saline-dampened electrode (oval in shape, area = 16 cm2) was placed over the target area, serving as the active electrode for cathodal stimulation, and another electrode was placed over the contralateral supraorbital region, serving as the anodal reference. Cathodal tDCS was applied on the scalp over four possible target regions: posterior superior temporal gyrus (pSTG) and posterior inferior frontal gyrus (pIFG) in each hemisphere. Target regions were identified using the International 10–20 system for EEG electrode placement: the pSTG was identified as 1/3 of the distance from TP7 and C5 in the left hemisphere, and 1/3 of the distance from TP8 to C6 in the right hemisphere. The pIFG was identified as 1/3 of the distance from F7 to C5 (left hemisphere) and 1/3 of the distance from F8 to C6 (right hemisphere). These locations were predicted using LORETA (Pascual-Marqui, 1994), a tool which allowed visualization of the 10–20 locations over the model brain. The accuracy of the target locations were verified by placing markers on the scalp over the target area in a subset of subjects (n = 3), and then obtaining anatomical MRI (T1 images) of these same subjects to observe accurate correspondence between marker location and anatomical regions of interest in the brain (see Fig. 1). 2mA of stimulation was delivered for 20 minutes over four target regions (Left pSTG, Left pIFG, Right pSTG, Right pIFG) on four separate days. In a fifth session, sham stimulation was applied over a region that was randomly selected among the four target areas. During sham stimulation, all procedures were the same as the cathodal stimulation sessions, except that 30 seconds after the2mA of current was applied, the current was gradually turned off by the experimenter, resulting in no stimulation being delivered unbeknownst to the subject. Subjects reported the same tingling sensation at the start of cathodal as well as sham stimulation sessions, and were unable to distinguish between cathodal and sham stimulation conditions. Subjects read a book or magazine during the stimulation.
Figure 1.
Theoretical and actual target locations of tDCS. A1: The right pSTG as identified in the model brain. A2: The left pIFG as identified in the model brain. B1: Marker placed on scalp over targeted pSTG. B2: Sagittal slice showing crosshairs over the region underlying the marker, showing correspondence between marker location and pSTG. C1: Marker placed on scalp over targeted pIFG. C2: Sagittal slice showing crosshairs over the region underlying the marker, showing accurate correspondence between marker location and pIFG.
Stimuli & Procedure
Testing was done after each stimulation session. Pure tones within each subject's vocal range were presented. After the presentation of each pure tone, subjects' task was to reproduce the tone by humming. All tones were equal in amplitude (70 dB) and duration (1000ms, smooth envelopes with rise and decay times of 50 ms each) and were presented through Altec Lansing headphones (AHP512i). Target tones were −3, −2, −1, −0.5, 0, 0.5, 1, 2, and 3 semitones away from the center of each subject's comfortable vocal range. Each vocal production was recorded on an IBM laptop computer with a microphone (Logitech 980186-0403 USB), using Praat software (Boersma and Sweenink, 2005).
Data analysis
Data analysis was performed on recorded sound files in Praat. The mean pitch of each sound production was extracted and its deviation from target (in cents of a semitone) was calculated. In addition to the cents deviation of each trial, the variance in cents deviation was also computed for each subject.
Results
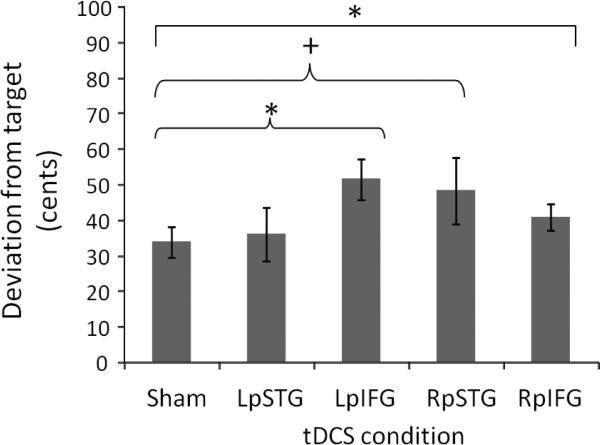
A two-way ANOVA was run on the dependent variable of deviation from target (in cents of a semitone) with the within-subjects factors of tDCS condition (Sham, LpSTG, LpIFG, RpSTG, RpIFG) and trial (−3, −2, −1, −0.5, 0, 0.5, 1, 2, and 3 semitones from center frequency). This omnibus ANOVA showed significant effects of tDCS condition (F(4, 484) = 2.95, p = 0.020) and trial (F(8,484) = 6.10, p < 0.001), but no significant interaction between condition and trial (F(32,484) = 0.83, p = 0.731), suggesting that stimulation affected pitch matching ability equally regardless of the actual pitch being produced (Fig. 2).
Figure 2.
Averaged deviation from target after different conditions of tDCS stimulation, in cents of a semitone (100 cents = 1 semitone). * = p < 0.05; + = p < 0.10.
Paired t-tests were used to test for pairwise contrasts between sham condition and each tDCS region. These pairwise comparisons revealed significantly higher deviations from sham following Left pIFG stimulation (t(8) = 2.33, p < 0.05) and a trend toward significantly higher deviations from sham following right pSTG stimulation (t(8) = 2.19, p = 0.06, Fig, 2). No other pairwise contrasts were significant.
Discussion
The present results show disrupted pitch matching following tDCS, providing preliminary evidence for the induction of a musical disorder through the reverse-engineering approach of noninvasive brain stimulation. Pitch matching, an ability that is impaired in tone-deaf individuals, was disrupted following cathodal tDCS relative to sham tDCS. Specifically, produced deviations from target pitches were significantly higher following stimulation over the left posterior inferior frontal gyrus, and marginally higher following stimulation over the right posterior superior temporal gyrus. These results establish the causal role of the superior temporal gyrus and the inferior frontal gyrus in pitch perception and production.
Pitch matching is a fundamental aspect of musical ability. To be able match pitches accurately, the brain must be able to perceive and extract pitch information from sound input, to form an accurate mental representation of the target pitch, and to select and execute the motor plan based on the mental representation, and then to perceive feedback information from one's pitch output so as to fine-tune the output in the next iteration. Our preliminary data suggest that the sequence of perceptual, cognitive, and motor processes involved in pitch matching recruits a distributed cortical network including superior temporal and inferior frontal areas. Although an omnibus test showed an overall effect of tDCS, pairwise comparisons revealed that left pIFG and right pSTG were the only two nodes that were affected, as indicated by increased deviation from target after stimulation in these regions.
While it is unclear why the other regions, i.e. right pIFG and left pSTG, were unaffected by stimulation, effects observed may be due to the multi-stage nature of the pitch matching task: in particular, we expect that left pIFG may be more involved in selecting and executing the motor plan, whereas the right pSTG may be more involved in perceiving and maintaining a mental representation of the target. As the present study employed only unilateral stimulation, the contralateral pSTG and pIFG might have been able to take over some components of the pitch matching task during the selective disruption of stimulated regions.
The observed increases in produced deviations from target pitch parallel results obtained from tone-deaf individuals, who show obvious impairments in pitch matching as well as pitch perception (Hutchins et al., Dalla Bella et al., 2009, Loui et al., 2008). By demonstrating mild disruptions in pitch matching ability following modulation of neural excitability in superior temporal and inferior frontal regions, results simulate the induction of tone-deafness, providing evidence for a causal role of bilateral superior temporal and inferior frontal networks in pitch perception and production.
Acknowledgements
Supported by NIDCD (R01 DC009823-01) and SSHERC (AIRS).
References
- Dalla Bella S, Giguere JF, Peretz I. Singing in congenital amusia. J Acoust Soc Am. 2009;126:414–24. doi: 10.1121/1.3132504. [DOI] [PubMed] [Google Scholar]
- Foxton JM, Dean JL, Gee R, Peretz I, Griffiths TD. Characterization of deficits in pitch perception underlying `tone deafness'. Brain. 2004;127:801–10. doi: 10.1093/brain/awh105. [DOI] [PubMed] [Google Scholar]
- Hutchins S, Zarate JM, Zatorre RJ, Peretz I. An acoustical study of vocal pitch matching in congenital amusia. J Acoust Soc Am. 127:504–12. doi: 10.1121/1.3270391. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch JP, Zatorre RJ, Griffiths TD, Evans AC, Peretz I. Cortical thickness in congenital amusia: when less is better than more. J Neurosci. 2007;27:13028–32. doi: 10.1523/JNEUROSCI.3039-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I. Morphometry of the amusic brain: a two-site study. Brain. 2006;129:2562–70. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
- Loui P, Alsop D, Schlaug G. Tone-Deafness: a Disconnection Syndrome? Journal of Neuroscience. 2009;29:10215–10220. doi: 10.1523/JNEUROSCI.1701-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loui P, Guenther FH, Mathys C, Schlaug G. Action-perception mismatch in tone-deafness. Current Biology. 2008;18:R331–2. doi: 10.1016/j.cub.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell J, Schulze K, Schlaug G. Congenital amusia: An auditory-motor feedback disorder? Restorative Neurology and Neuroscience. 2007;25:323–334. [PubMed] [Google Scholar]
- Olsho LW, Schoon C, Sakai R, Turpin R, Sperduto V. Auditory frequency discrimination in infancy. Developmental Psychology. 1982;18:721–726. [Google Scholar]
- Patel A, Iversen JR, Bregman MR, Schulz I. Experimental Evidence for Synchronization to a Musical Beat in a Nonhuman Animal. Current Biology. 2009;19:1–4. doi: 10.1016/j.cub.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Peretz I, Brattico E, Jarvenpaa M, Tervaniemi M. The amusic brain: in tune, out of key, and unaware. Brain. 2009:awp055. doi: 10.1093/brain/awp055. [DOI] [PubMed] [Google Scholar]
- Peretz I, Brattico E, Tervaniemi M. Abnormal electrical brain responses to pitch in congenital amusia. Ann Neurol. 2005;58:478–82. doi: 10.1002/ana.20606. [DOI] [PubMed] [Google Scholar]
- Peretz I, Champod AS, Hyde K. Varieties of musical disorders. The Montreal Battery of Evaluation of Amusia. Ann N Y Acad Sci. 2003;999:58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- Schachner A, Brady TF, Pepperberg IM, Hauser MD. Spontaneous Motor Entrainment to Music in Multiple Vocal Mimicking Species. Current Biology. 2009;19:1–6. doi: 10.1016/j.cub.2009.03.061. [DOI] [PubMed] [Google Scholar]
- Trainor L, Trehub SE. Key membership and implied harmony in Western tonal music: developmental perspectives. Perception & Psychophysics. 1994;56:125–32. doi: 10.3758/bf03213891. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive Human Brain Stimulation. Annual Review of Biomedical Engineering. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Weisman RG, Njegovan MG, Williams MT, Cohen JS, Sturdy CB. A behavior analysis of absolute pitch: sex, experience, and species. Behav Processes. 2004;66:289–307. doi: 10.1016/j.beproc.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Williams JA, Imamura M, Fregni F. Updates on the use of non-invasive brain stimulation in physical and rehabilitation medicine. J Rehabil Med. 2009;41:305–11. doi: 10.2340/16501977-0356. [DOI] [PubMed] [Google Scholar]
- Winkler I, Haden GP, Ladinig O, Sziller I, Honing H. Newborn infants detect the beat in music. Proc Natl Acad Sci U S A. 2009;106:2468–71. doi: 10.1073/pnas.0809035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA, Rivera JJ, Hulse SH, Shyan M, Neiworth JJ. Music perception and octave generalization in rhesus monkeys. J Exp Psychol Gen. 2000;129:291–307. doi: 10.1037//0096-3445.129.3.291. [DOI] [PubMed] [Google Scholar]