Abstract
Previous studies have shown that dermal fibroblast cell lines derived from young adult mice of the long-lived Snell dwarf (dw/dw), Ames dwarf (df/df) and growth hormone receptor knockout (GHR-KO) mouse stocks are resistant, in vitro, to the cytotoxic effects of hydrogen peroxide, cadmium, ultraviolet light, paraquat, and heat. Here we show that, in contrast, fibroblasts from mice on low-calorie (CR) or low methionine (Meth-R) diets are not stress resistant in culture, despite the longevity induced by both dietary regimes. A second approach, involving induction of liver cell death in live animals using acetaminophen (APAP), documented hepatotoxin resistance in the CR and Meth-R mice, but dw/dw and GHR-KO mutant mice were not resistant to this agent, and were in fact more susceptible than littermate controls to the toxic effects of APAP. These data thus suggest that while resistance to stress is a common characteristic of experimental life span extension in mice, the cell types showing resistance may differ among the various models of delayed or decelerated aging.
Keywords: Stress resistance, Caloric restriction, Methionine restriction, Snell dwarf, Growth hormone receptor knockout
1. Introduction
The ability to mount an effective response to environmental and cellular stressors may play an important role in determining the onset and progression of late-life disease and aging. For example, in nematodes (Caenorhabitis elegans) and fruit flies (Drosophila melanogaster) long-lived mutant strains are often significantly more resistant to multiple forms of stress (Larsen, 1993; Lithgow et al., 1995; Lin et al., 1998; Cheng et al., 2003; de Castro et al., 2004). Recent studies have shown similar phenomena in long-lived mice. For example, the homozygous deletion of p66shc, which codes for an essential adapter protein in the insulin-like growth factor I (IGF-I) signaling pathway, a heterozygous knock-down mutation of the IGF-I receptor (IGF1R +/−), and the overexpression of the klotho protein all lead to significant life span extension while conferring an increased resistance to paraquat-induced oxidative stress (Migliaccio et al., 1999; Holzenberger et al., 2003; Kurosu et al., 2005). In addition, antioxidant defense systems are upregulated in long-lived Ames dwarf mice, and numerous indices of in vivo oxidative damage are significantly lower in these mice relative to their control littermates (Bartke and Brown-Borg, 2004). Caloric restriction (CR) has a similar augmenting effect on antioxidant defense systems (Yu and Chung, 2001; Liang et al., 2003) and leads to lower indices of oxidation in multiple tissue types (Dubey et al., 1996; Lass et al., 1998; Forster et al., 2000). Mice undergoing CR are also more resistant to the lethal effects of the hepatotoxins thioacetamide (Apte et al., 2003) and bleomycin (Aidoo et al., 1999). Finally, studies have suggested that fibroblast cell lines derived from long-lived mouse stocks, and from long-lived species, may show cellular resistance to a variety of cytotoxic agents in culture (Migliaccio et al., 1999; Kapahi et al., 1999; Holzenberger et al., 2003).
Acetaminophen (APAP) is a widely used analgesic that causes acute liver necrosis when administered in large doses. Injury requires the metabolic conversion of APAP to the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI) via cytochrome P450 detoxification pathways (Ruepp et al., 2002). NAPQI depletes glutathione in hepatocytes and covalently binds to intracellular proteins resulting in mitochondrial dysfunction and oxidant-induced stress (Masubuchi et al., 2005). There are significant strain-specific differences in the susceptibility of mice and rats to APAP-induced injury (Tarloff et al., 1989;Mehendale, 2005), and susceptibility to injury can increase with advancing age (Beierschmitt et al., 1986, 1989; Tarloff et al., 1991, 1996).
In previous work, we have shown that dermal fibroblast cell lines from long-lived Ames dwarf (df/df), Snell dwarf (dw/dw), and growth hormone receptor knockout (GHR-KO) mice exhibit increased resistance to multiple cytotoxic agents (Murakami et al., 2003; Salmon et al., 2005). Furthermore, we have shown that a low methionine (Meth-R) diet, which leads to life span extension in mice (Miller et al., 2005) and rats (Orentreich et al., 1993; Richie et al., 1994), increases resistance of young adult mice to the hepatotoxic effects of APAP exposure (Miller et al., 2005). Overall, these data suggest that increased stress resistance at both the cellular and whole animal level is important in determining the onset and progression of late-life disease and life span in rodents. To see if both forms of stress resistance are invariably found in multiple models of delayed or decelerated aging in mice, we have now evaluated stress resistance in dermal fibroblast cell lines derived from CR and Meth-R mice, and measured APAP hepatotoxicity in CR, Snell dwarf, and growth hormone receptor knockout (GHR-KO) mice.
2. Methods
2.1. Animal subjects
2.1.1. Snell dwarf mice
Snell dwarf animals were dw/dw mice bred as the progeny of (DW/J × C3H/HeJ)-dw/+ females and (DW/J × C3H/HeJ)F1-dw/dw males. The sires of our test mice had been treated with growth hormone and thyroxine to increase their body size and fertility. Littermates with the (dw/+) genotype were used as controls. Resistance to the hepatotoxin acetaminophen (APAP) was determined in 5–9 month old females of each genotype.
2.1.2. Growth hormone receptor knockout (GHR-KO) mice
GHR-KO mice and normal littermate controls were produced by mating heterozygous (+/−) carriers of the disrupted GHR/GHBP gene or homozygous knockout (−/−) males with (+/−) females. The genetic background of these animals is derived from 129/Ola embryonic stem cells and from BALB/c, C57BL/6, and C3H inbred strains (Zhou et al., 1997). APAP resistance was assessed in 10-month-old mice of both sexes.
2.1.3. Methionine and calorically restricted mice
Female (BALB/cJ × C57BL/6J)F1 mice purchased from the Jackson Laboratories (Bar Harbor, ME) were used. At 6 weeks of age the mice were divided into one of four dietary groups: (1) a diet containing 0.15% methionine (methionine restricted, or Meth-R mice); (2) a diet containing 0.43% methionine (methionine control, or Meth-C mice); (3) a standard lab chow diet (Purina 5001) administered ad libitum (ad libitum control, or AL-C mice); or (4) a standard lab chow diet administered in diminished amounts (80% of ad libitum food intake for 2 weeks, then 70% for 2 weeks, then 60% for the remaining period; calorie restricted, or CR mice). These diets are described in more detail elsewhere (Miller et al., 2005). CR and AL-C mice were tested for APAP resistance at 10 months of age. In addition, fibroblast cell lines were established from 4 to 18-month-old CR and AL-C mice for the assessment of fibroblast resistance to cytotoxicity. Cell lines from 4-month-old Meth-R to Meth-C mice were also evaluated.
2.2. Establishment of fibroblast cell lines
Tail skin biopsies approximately 3–5 mm in length were obtained from the last half of the intact tail of isofluorane-anesthetized mice after skin sterilization with 70% ethanol. Biopsies were further washed in 70% ethanol, placed in Dulbecco’s modified Eagle medium (DMEM, high-glucose variant, Gibco-Invitrogen, Carlsbad, CA), diced to less than 0.5 mm and digested overnight with collagenase type II (400 U/ml, 1000 U total per tail, Gibco-Invitrogen, Carlsbad, CA) dissolved in DMEM supplemented with 20% heat-inactivated fetal bovine serum, antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; Sigma, St. Louis, MO) and 0.25 µg/ml of fungizone (Biowhittaker-Cambrex Life Sciences, Walkersville, MD) at 37° in a humidified incubator with 5% CO2 in air. After collagenase treatment, cells were dislodged, passed through sterilized nylon netting into sterile 14 ml centrifuge tubes (BD Dickenson, Bedford, MA) and were centrifuged for 5 min at 200 × g. After centrifugation the collagenase solution was drawn off the cell pellet and the cells were resuspended in DMEM with 20% heat-inactivated fetal bovine serum, antibiotics and fungizone as indicated previously. Approximately 2.5 × 105 cells in 3 ml media were seeded into tissue culture flasks of 25 cm2 surface area (Corning Costar, Corning, NY). After 3 days, approximately 2/3 total volume of medium was removed and replaced with fresh DMEM with 20% heat-inactivated fetal bovine serum, antibiotics and fungizone. Six-to-seven days after seeding, initial cultures (designated as first passage, or P1, cells) were split and seeded at a density of 1 × 105 cells/cm2 flask surface area into tissue culture flasks of 75 cm2 (Corning Costar). Cells were split by first washing flasks with 1 × phosphate buffered saline solution (PBS, 8.8 g NaCl, 2.25 g Na2HPO4 and 0.26 g NaH2PO4 per 1 l distilled H2O, pH 7.3), followed by incubation with 3 ml trypsin/100 cm2 surface area of flask using Trypsin-EDTA (Gibco-Invitrogen) for 5 min at 37° in a humidified incubator with 5% CO2 in air. At the end of the incubation period, trypsin activity was inhibited with an equal volume of DMEM with 20% heat-inactivated fetal bovine serum, antibiotics and fungizone. Subsequent passages were split at 6 day intervals, with approximately 2/3 total volume of media removed at day 3 and replaced with fresh DMEM with 20% heat-inactivated fetal bovine serum with antibiotics and fungizone. At the end of the third passage (6 days after seeding), confluent cells were harvested for assessment of stress resistance.
2.3. Assessment of cytotoxicity
Six days after seeding, third passage cells were trypsinized as described. Cells were counted by hemocytometer and diluted to a concentration of 3 × 105 ml−1 in DMEM with 20% fetal bovine serum with antibiotics and fungizone and seeded into a 96-well tissue culture-treated microtiter plate at a volume of 100 µl per well. After an 18-h overnight incubation, cells were washed with PBS and incubated in DMEM supplemented with 2% bovine serum albumin (BSA, Sigma), antibiotics and fungizone for approximately 24 h. Previous work has shown (Murakami et al., 2003) that this period of incubation in serum-free conditions is critical for showing differences between mutant Snell dwarf and control cell lines because the presence of serum greatly increases the stress resistance of cells from both genotypes. Cells were then exposed to a range of doses of one of the cytotoxic stressors. For UV light testing, cells were washed, then irradiated with UV light (254 nm at 5.625 J/m2/s) in 100 µl of Dulbecco’s PBS (Biowhittaker-Cambrex Life Sciences). Cells were then incubated in DMEM supplemented with 2% BSA, antibiotics and fungizone, and their survival was measured 18 h later by a test based on reductive cleavage of the tetrazolium dye WST-1 (Roche Applied Science, Indianapolis, IN) to a formazan product, using the protocol suggested by the manufacturer. For assessment of resistance to hydrogen peroxide (H2O2), paraquat (methyl viologen), or cadmium (Sigma), the cells in the 96-well plates were incubated with a range of doses for 6 h in DMEM. Cells were then washed and incubated with DMEM supplemented with 2% BSA, antibiotics and fungizone, and survival was measured 18 h later by the WST-1 test. All incubations were at 37° in a humidified incubator with 5% CO2 in air.
2.4. Assessment of acetaminophen resistance
All mice were deprived of food for approximately 18 h prior to APAP administration to reduce hepatic stores of glutathione (Neff et al., 2003). Female CB6F1 mice undergoing CR received a single intraperitoneal injection of APAP (Sigma) at 250 mg/kg body weight (20 ml/kg) in warm (37 °C) sterile PBS. Female Snell dwarf mice received a single intraperitoneal injection at one of three doses: 125, 200, or 250 mg APAP/kg body weight in warm PBS. GHR-KO and WT control mice of both sexes were each challenged with 175 mg/kg. Pilot studies indicated that doses greater than 175 mg/kg were often lethal on this genetic background. Food (Purina 5001 rodent chow) was made available to the mice immediately after APAP injection, and venous blood samples were withdrawn at 6–8, 24, and 48 h for comparison to blood withdrawn just prior to APAP administration. Serum alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) activity in serum samples (1–5 µl) collected from the APAP-exposed mice were used to assess the degree of resistance of each stock. ALT and LDH activities were determined using either an in-house assay (for the AL-C and CR mice) or commercially available kits (Catachem, Inc.) modified to fit a 96-well microtiter plate format (for the study of the dw/dw, dw/+, GHR-KO and GHR wild-type mice).
2.5. Statistical analyses
2.5.1. Resistance to cytotoxins
Stress resistance of each cell line was expressed as the LD50, i.e. the dose of agent that reduced WST-1 signal for 50%, as calculated by linear regression of probit-transformed data using triplicate wells at each tested dose. Differences between groups in mean LD50 levels were evaluated using Student’s t-test.
2.5.2. APAP resistance
Differences between groups of mice in mean APAP-induced ALT and LDH activity were assessed using Student’s t-test at each of the indicated times. Differences in the proportion of animals alive at the end of the 48-h observation period were calculated using Fisher’s exact test.
3. Results
3.1. Resistance to cytotoxicity
We previously showed that fibroblast cell lines isolated from long-lived mouse stocks harboring mutations in the GH/IGF axis (i.e., Snell dwarf, Ames dwarf, and GHR-KO mice) were significantly more resistant than cells from littermate controls to multiple cytotoxic and DNA-damaging agents in vitro (Murakami et al., 2003; Salmon et al., 2005). Hence, we hypothesized that cell lines isolated from mice exposed to diets that extend life span by decelerating aging, such as CR or Meth-R diets (Weindruch and Walford, 1988; Orentreich et al., 1993; Miller et al., 2005), would be similarly resistant.
Unexpectedly, however, cell lines from 4-month-old mice that had been on the 60% CR diet for 5 weeks (Fig. 1, top) as well as 4-month-old Meth-R mice (Fig. 1, bottom) exhibited no difference in their resistance to the cytotoxic effects of ultraviolet radiation, H2O2, or cadmium relative to control mice receiving a normal diet ( p > 0.08 for all). In most cases, the mean LD50 was indistinguishable between treatments and are consistent with those seen in previous studies (Murakami et al., 2003; Salmon et al., 2005). Some studies have suggested that caloric restriction can have both immediate and long-term effects on the expression of specific hepatic genes (Cao et al., 2001), thus 5 weeks of restriction might have been insufficient to induce significant differences in cellular stress resistance between fully fed and CR mice. To evaluate this idea, dermal fibroblast cell lines from a different group of mice that had been on a CR diet for 15 months were tested for their resistance to cytotoxic agents in culture. Results of these experiments also showed no evidence of an effect of CR on cellular resistance to UV, H2O2, cadmium (Fig. 1, middle), or paraquat (data not shown) relative to AL-C mice (p > 0.2 for all).
Fig. 1.
Mean (±S.E.) LD50 for ultraviolet radiation, hydrogen peroxide, and cadmium in fibroblast cell lines derived from: young (top panels) and old (middle panels) calorie restricted (CR) CB6F1 females, as well as young (bottom panels) methionine-restricted (Meth-R) CB6F1 females. There are no significant differences relative to the controls (AL-C, Meth-C) by t-test (p > 0.08 for all). N = 6–9 mice per group.
3.2. Acetaminophen resistance
Methionine restriction leads to an increased resistance to the hepatotoxic effects of acetaminophen (Miller et al., 2005), and CR protects against the hepatotoxic effects of bleomycin and thioacetamide (Aidoo et al., 1999; Apte et al., 2003). This led us to consider the hypothesis that increased resistance to hepatotoxins in vivo is also associated more broadly with increased life span.
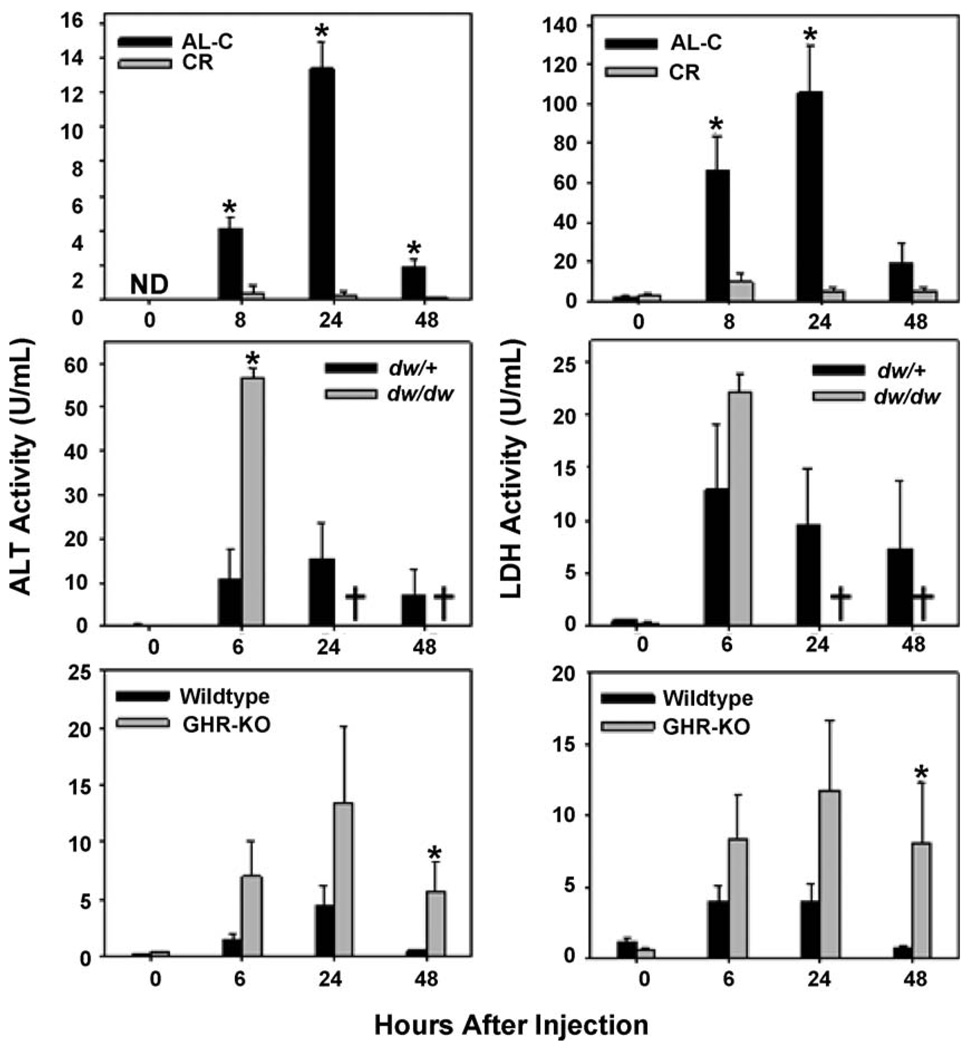
Consistent with this idea, we found that female CB6F1 mice that had been exposed to the CR diet for 8 months exhibited dramatically less liver damage than AL-C mice after APAP challenge (250 mg/kg), as indicated by negligible increases of serum ALT and LDH (Fig. 1, top). In contrast, however, Snell dwarf mice do not show an increased resistance to APAP-induced liver injury relative to their control littermates; but instead appear to be more sensitive than littermate controls. Six hours after intraperitoneal injection of APAP (250 mg/kg body weight), serum ALT and LDH activities were significantly (p < 0.001) increased in both mutant dw/dw and control dw/+ mice (Fig. 2, middle panels); however, ALT activity was significantly (p = 0.003) higher in the dw/dw mice relative to the dw/+ controls. Serum LDH activity was similarly affected, although the difference at 6 h post-injection did not reach statistical significance (p = 0.22; Fig. 2, middle). Each of the three dw/dw mice tested at this dose died within the first 24 h after inoculation, preventing measurements of ALT and LDH after the 6 h time point. A second experiment (not shown), using 200 mg/kg, resulted in death of one of the two dw/dw mice tested. None of the five control mice treated with 200– 250 mg/kg APAP died during the 48 observation period. The difference in survival between dwarf and control mice was significant at p = 0.02. Table 1 presents these survival statistics. Lower doses of APAP (125 mg/kg) induced equal, low levels of LDH and ALT in dwarf and control mice, and did not kill mice in either group (not shown).
Fig. 2.
Serum alanine aminotransferase (ALT; left) and lactate dehydrogenase (LDH; right) activity in response to a single acetaminophen (APAP challenge) in AL-C vs. CR female CB6F1 mice (top panels), female Snell dwarf (dw/dw) vs. normal littermate control mice (dw/+; middle panels), and male GHR-KO vs. wild-type control mice (WT; bottom panels). Each point represents the mean (±S.E.) for each group at each of the indicated times. *Indicates a significant difference at p < 0.05 vs. the respective control group. †Indicates all individuals died as a result of APAP toxicity. N = 8 per group for AL-C and CR mice, three per group for dw/dw and dw/+ mice, and eight per group for GHR-KO and WT mice. Note that N = 7 at the 48 h time point for GHR-KO mice due to APAP-induced mortality. See text for details.
Table 1.
Proportion of mice still alive 48 h after receiving a single intraperitoneal APAP challenge
| Genotype | Sex | Dose (mg/kg) | # Alive (T = 0) | # Alive (T = 48) | Proportion alive (T = 48) | P-valuea (vs. control) |
|---|---|---|---|---|---|---|
| dw/+ | F | 200–250 | 5 | 5 | 1.0 | – |
| dw/dw | F | 200–250 | 5 | 1 | 0.20 | 0.02 |
| WT | F | 175 | 8 | 8 | 1.0 | – |
| GHR-KO | F | 175 | 8 | 3 | 0.375 | 0.03 |
| WT | M | 175 | 8 | 8 | 1.0 | – |
| GHR-KO | M | 175 | 8 | 7 | 0.875 | 0.99 |
The proportion of mice alive was compared using Fisher’s exact test.
A similar study of APAP toxicity was conducted using male and female mice of the long-lived GHR-KO stock. Male GHR-KO mice challenged with 175 mg of APAP/kg body weight were, like the Snell dw/dw mice, relatively sensitive to APAP-induced liver injury when compared to wild-type controls (Fig. 2, bottom). Repeated measures ANOVA indicated a significant main effect of genotype on serum ALT and LDH activity (p < 0.01) after APAP challenge; however, despite being two- to five-fold higher at 6 and 24 h after injection, the difference in serum ALT and LDH activities between GHR-KO and control mice was only marginally significant (t-test, 0.06 > p < 0.10); most likely due to significant inter-individual variation in the degree of APAP-induced liver injury. Nonetheless, at 48 h after APAP injection both serum ALT and LDH activities were significantly higher (p ≤ 0.04 for all) in the GHR-KO mice. One (of eight) of the APAP-treated male GHR-KO mice died within 48 h of injection (Table 1); all male control mice survived. When female GHR-KO mice were tested at this dose, four of eight died within 6 h, and only three of eight survived the first 48 h (see Table 1); none of the control females died. Thus both male and female GHR-KO mice were sensitive to APAP-mediated toxicity.
4. Discussion
We and others have shown that dietary and genetic mouse models of extended longevity, as well as cells derived from those animals, often exhibit enhanced resistance to various forms of stress. Some reports, comparing mice (or species) that differ genetically, have documented cellular stress resistance in primary cultures of dermal (Kapahi et al., 1999; Murakami et al., 2003; Salmon et al., 2005) and embryonic (Migliaccio et al., 1999; Holzenberger et al., 2003) fibroblasts. Other studies have evaluated the resistance of intact animals to hepatotoxins (Aidoo et al., 1999; Apte et al., 2003; Miller et al., 2005), myotoxins (Usuki et al., 2004), neurotoxins (Bruce-Keller et al., 1999), and the pulmonary toxin paraquat (Migliaccio et al., 1999; Holzenberger et al., 2003; Kurosu et al., 2005). Here we sought to measure both fibroblast stress resistance in vitro and hepatotoxic resistance in vivo in parallel studies of two anti-aging diets, and two mutations that extend maximal life span. We saw fibroblast resistance to stress in dw/dw and GHR-KO mutants, but not in the CR or Meth-R mice. Conversely, CR and Meth-R mice showed dramatic resistance to hepatoxicity, while dw/dw and GHR-KO mice were more sensitive than controls to this agent. Overall, the results of this study suggest that these models of delayed aging may differ in the spectrum of cell types and tissues affected.
The life prolonging effect of CR has been repeatedly documented in multiple species of animal, both vertebrate and invertebrate (Weindruch and Walford, 1988). Previous studies had also shown that CR confers an increased resistance to the hepatotoxins thioacetamide and bleomycin (Aidoo et al., 1999; Apte et al., 2003), the excitatory neurotoxin kainic acid (Bruce-Keller et al., 1999), and the heavy metal cadmium (Shaikh et al., 1999). The toxicity of each of these compounds is mediated, at least in part, by oxidative stress. Our results show that CR mice, like the Meth-R mice previously studied (Miller et al., 2005), are resistant to this oxidative hepatotoxin, consistent with earlier reports using other agents in intact animals (Fig. 2, top). Fibroblast cultures derived from CR donors, however, did not show an increase in resistance to any of the cytotoxic agents tested in vitro (Fig. 1), whether the cells were taken from mice exposed to 60% CR for 5 weeks or 15 months. Similarly, a diet with a reduced level of the essential amino acid methionine (Meth-R), also failed to alter responses of dermal fibroblast cell lines to in vitro cytotoxic stress resistance (Fig. 1) despite its ability to extend life span and to enhance resistance to APAP toxicity (Miller et al., 2005).
In a previous study (Salmon et al., 2005), we noted that fibroblast cell lines from mice less than 1 week old were not resistant to UV or cadmium stress compared to cells from control mice, and showed only a minimal increase in their resistance to hydrogen peroxide in vitro. Importantly, the prenatal environment is identical for both Snell dwarf and their littermate controls, and mice of the two genotypes are indistinguishable in size at birth, after which dwarf mice grow more slowly than normal mice (Brown-Borg and Rakoczy, 2000). Hence, it may be that stress resistance in this model is regulated by differences in hormone exposure during early postnatal development, prior to the age of tissue biopsy (Yu and Chung, 2001), and that the epigenetic changes induced in dermal fibroblasts by this environment are manifested by the observed increase in stress resistance in vitro. In our own study, CR was not begun until the mice were 6 weeks old. It is possible that by this age dermal fibroblasts may have reached a stage of differentiation at which changes relevant to stress resistance can no longer be induced. In this context it would be of interest to evaluate properties of dermal fibroblasts derived from mice exposed to nutritional deprivation starting at an earlier stage of the life span.
Alternatively, any hypothetical effects of these dietary interventions on the stress resistance properties of fibroblasts may be transient in nature, or mediated by pathways that are sensitive to changes induced by cell division in vitro. For example, skin-derived primary fibroblasts from CR mice have been shown to have enhanced colony formation (Pendergrass et al., 1995), but show no difference in population doublings or replicative life span in long-term culture (Pignolo et al., 1992). Similarly, lens epithelial cells isolated from CR mice are resistant to H2O2-induced cell death (Li et al., 1997) and show enhanced proliferative capacity, though only for a short time when isolated using standard cell culture techniques (Li et al., 1998). Further, CR in rats reduces the production of reactive oxygen species (ROS) from liver mitochondria in vivo (Lambert and Merry, 2004); however this difference is not maintained in hepatocyte cultures derived from these same animals (Lambert and Merry, 2005). There is some data to suggest that serum from rodents exposed to a CR diet can increase the stress resistance of cell lines in vitro (de Cabo et al., 2003; Cohen et al., 2004), but such systemic factors cannot be involved in our own system, in which both control and experimental cell lines are maintained in identical culture conditions through multiple weeks, using fetal bovine serum as a source of growth factors.
We do not know why the Snell dwarf mice and the GHR-KO mice are, contrary to our initial hypothesis, even more sensitive than control animals to APAP-mediated toxicity. The pathogenesis of liver cell death in response to acetaminophen (APAP) is multifaceted, and in addition to intrinsic hepatocyte responses to APAP metabolites, is influenced by proinflammatory cytokine cascades (Laskin and Laskin, 2001), heat shock proteins (Hsp) (Tolson et al., 2006), and peroxisome proliferator activated receptor (PPAR)-α activity (Nguyen et al., 1999; Shankar et al., 2003). The hepatic level of reduced glutathione is also an important factor in APAP-induced damage (Oz et al., 2005) and may contribute to some of the animal-to-animal variation in the response to APAP. However, fasting causes a dramatic reduction in hepatic levels of reduced glutathione (Langley and Kelly, 1992; Jenniskens et al., 2002), and although the overnight fast (18 h) used in our APAP-toxicity protocol was expected to minimize differences in basal glutathione levels between mice, we do not have any direct evidence that levels of this protective factor were indeed similar in mutant and control mice after fasting.
The effect of CR diets on Hsps, inflammatory cascades and PPAR activity have been well documented (Nguyen et al., 1999; Yu and Chung, 2001; Shankar et al., 2003; Tsuchiya et al., 2005), and are consistent with the increased ability of these mice to resist xenobiotic insults. In dwarf mouse models, it has been shown that constitutive PPAR-α activity and its related gene products is increased (Stauber et al., 2005; Masternak et al., 2005), and that Ames dwarf mice exhibit an enhanced resistance to paraquat-induced lethality (Bartke et al., 2001a) while GHR-KO mice are relatively sensitive to paraquat-induced death (Hauck et al., 2002); however little else is known about xenobiotic metabolism in these mouse models. To what degree nutrient versus genetic models of delayed aging differ in these key pathways (i.e., Hsps, cytokines) needs to be further evaluated.
In addition, the interpretation of APAP toxicity is further complicated by the need for the hepatic conversion of APAP, via cytochrome P450 activity, to NAPQI, the proximal cause of liver cell damage. Although CR can improve cytochrome P450-mediated detoxification in multiple tissues in rodents (Manjgaladze et al., 1993; Seng et al., 1996), there are no documented reports of P450 activities in Snell dwarf or GHR-KO mice, and inter-individual variation in cytochrome P450 activity can have a dramatic influence on the degree of toxin-induced liver damage (Mathijssen and van Schaik, 2006). In addition, tests using APAP do not provide information about oxidation resistance in other tissues or to toxins that act through other pathways, such as the direct liver toxin, diquat (Burk et al., 1980) or the nephrotoxin gentamicin (Nakajima et al., 1994).
Sex-specific differences are well described in both rat and mouse models of APAP toxicity, and are the result of variation in biotransformation pathways (Galinsky et al., 1990; Hoivik et al., 1995; Tarloff et al., 1996; Mugford and Tarloff, 1997) and the abundance of specific target proteins in cellular membranes (Mattow et al., 2006). Although males are typically more susceptible to APAP-toxicity, in this study we found that GHR-KO females exhibited a higher degree of mortality after APAP injection relative to males. What accounts for this effect remains unknown; however in the absence of normal GH signaling the sex-specific expression pattern of hepatic cytochrome P450 enzymes is significantly disrupted (Ahluwalia et al., 2004; Jarukamjorn et al., 2006).
The observation that the life span of Ames dwarf mice can be further extended by a CR diet (Bartke et al., 2001b), as well as evidence for differences in hepatic gene expression profiles in dwarf and in CR rodents (Miller et al., 2002), suggest that the pathways by which the pituitary dwarf mutations lead to longevity overlap only partly with those induced by CR. Our data provide further points of contrast between dwarf mice, and mice whose longevity reflects either a low-calorie or a methionine-restricted diet: the former show stress resistance in tests of skin cell fibroblasts, and the latter show resistance to APAP toxicity, but not vice versa. Work in worms and flies has suggested that resistance to multiple forms of stress is characteristic of mutants that confer extended life span (Larsen, 1993; Sorensen and Loeschcke, 2001; Arking, 2001; de Castro et al., 2004), leading to the plausible idea that stress resistance itself brings about the retardation of aging and postponement of death, at least in these invertebrate systems. We take, as our working hypothesis, the idea that stress resistance in one or more cell types may play a role in the anti-aging effects of dwarf, CR and Meth-R mice, but acknowledge that much more is still to be learned about the cells and tissues involved in each of these models, the ways in which stress resistance is induced by nutritional and hormonal deviations, and the pathways by which altered cellular properties delay or decelerate age-related injuries in critical tissues.
Acknowledgements
We thank Gretchen Buehner, Maggie Vergara, Jessica Sewald and Alexis Xu for their technical assistance. This work was supported by NIH Grants AG16699, AG11687, and AG08808.
References
- Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol. Endocrinol. 2004;18:747–760. doi: 10.1210/me.2003-0138. [DOI] [PubMed] [Google Scholar]
- Aidoo A, Desai VG, Lyn-Cook LE, Chen JJ, Feuers RJ, Casciano DA. Attenuation of bleomycin-induced Hprt mutant frequency in female and male rats by calorie restriction. Mutation Res. 1999;430:155–163. doi: 10.1016/s0027-5107(99)00197-9. [DOI] [PubMed] [Google Scholar]
- Apte UM, Limaye PB, Desaiah D, Bucci TJ, Warbritton A, Mehendale HM. Mechanisms of increased liver tissue repair and survival in diet-restricted rats treated with equitoxic doses of thioacetamide. Toxicol. Sci. 2003;72:272–282. doi: 10.1093/toxsci/kfg021. [DOI] [PubMed] [Google Scholar]
- Arking R. Gene expression and regulation in the extended longevity phenotypes of Drosophila. Ann. N.Y. Acad. Sci. 2001;928:157–167. doi: 10.1111/j.1749-6632.2001.tb05645.x. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C. Prolonged longevity of hypopituitary dwarf mice. Exp. Gerontol. 2001a;36:21–28. doi: 10.1016/s0531-5565(00)00205-9. [DOI] [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Longevity: extending the lifespan of long-lived mice. Nature. 2001b;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Beierschmitt WP, Brady JT, Bartolone JB, Wyand DS, Khairallah EA, Cohen SD. Selective protein arylation and the age dependency of acetaminophen hepatotoxicity in mice. Toxicol. Appl. Pharmacol. 1989;98:517–529. doi: 10.1016/0041-008x(89)90180-4. [DOI] [PubMed] [Google Scholar]
- Beierschmitt WP, Keenan KP, Weiner M. Age-related increased susceptibility of male Fischer 344 rats to acetaminophen nephrotoxicity. Life Sci. 1986;39:2335–2342. doi: 10.1016/0024-3205(86)90664-8. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp. Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Burk RF, Lawrence RA, Lane JM. Liver necrosis and lipid peroxidation in the rat as a result of paraquat and diquat administration. J. Clin. Invest. 1980;65:1024–1031. doi: 10.1172/JCI109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10630–10635. doi: 10.1073/pnas.191313598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Valmas N, Reilly PEB, Collins PJ, Kopittke R, Ebert PR. Caenorhabditis elegans mutants resistant to phosphine toxicity show increased longevity and cross-resistance to the synergistic action of oxygen. Toxicol. Sci. 2003;73:60–65. doi: 10.1093/toxsci/kfg049. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp. Gerontol. 2003;38:631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- de Castro E, Hegi de Castro S, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic. Biol. Med. 2004;37:139–145. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J. Gerontol. Biol. Med. Sci. 2000;55:B522–B529. doi: 10.1093/gerona/55.11.b522. [DOI] [PubMed] [Google Scholar]
- Galinsky RE, Johnson DH, Kane RE, Franklin MR. Effect of aging on hepatic biotransformation in female Fischer 344 rats: changes in sulfotransferase activities are consistent with known gender-related changes in pituitary growth hormone secretion in aging animals. J. Pharmacol. Exp. Ther. 1990;255:577–583. [PubMed] [Google Scholar]
- Hauck SJ, Aaron M, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm. Metab. Res. 2002;34:481–486. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- Hoivik DJ, Manautou JE, Tveit A, Hart SGE, Khairallah EA, Cohen SD. Gender-related differences in susceptibility to acetaminophen-induced protein arylation and nephrotoxicity in the CD-1 mouse. Toxicol. Appl. Pharmacol. 1995;130:257–271. doi: 10.1006/taap.1995.1031. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Blouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Jarukamjorn K, Sakuma T, Jaruchotikamol A, Ishino Y, Oguro M, Nemoto N. Modified expression of cytochrome P450 mRNAs by growth hormone in mouse liver. Toxicology. 2006;219:97–105. doi: 10.1016/j.tox.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Jenniskens FA, Jopperi-Davis KS, Walters LC, Schorr EN, Rogers LK, Welty SE, Smith CV. Effects of fasting on tissue contents of coenzyme A and related intermediates in rats. Pediatric Res. 2002;52:437–442. doi: 10.1203/00006450-200209000-00022. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic. Biol. Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Merry BJ. Lack of effect of caloric restriction on bioenergetics and reactive oxygen species production in intact rat hepatocytes. J. Gerontol. Biol. Med. Sci. 2005;60:175–180. doi: 10.1093/gerona/60.2.175. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Merry BJ. Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R71–R79. doi: 10.1152/ajpregu.00341.2003. [DOI] [PubMed] [Google Scholar]
- Langley SC, Kelly FJ. Differing response of the glutathione system to fasting in neonatal and adult guinea pigs. Biochem. Pharmacol. 1992;44:1489–1494. doi: 10.1016/0006-2952(92)90462-r. [DOI] [PubMed] [Google Scholar]
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Laskin JD. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology. 2001;160:111–118. doi: 10.1016/s0300-483x(00)00437-6. [DOI] [PubMed] [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yan Q, Pendergrass WR, Wolf NS. Response of lens epithelial cells to hydrogen peroxide stress and the protective effect of caloric restriction. Exp. Cell Res. 1998;239:254–263. doi: 10.1006/excr.1997.3870. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan Q, Wolf NS. Long-term caloric restriction delays age-related decline in proliferation capacity of murine lens epithelial cells in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 1997;38:100–107. [PubMed] [Google Scholar]
- Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp. Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Lithgow GL, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjgaladze M, Chen S, Frame LT, Seng JE, Duffy PH, Feuers RJ, Hart RW, Leakey JE. Effects of caloric restriction on rodent drug and carcinogen metabolizing enzymes: implications for mutagenesis and cancer. Mutation Res. 1993;295:201–222. doi: 10.1016/0921-8734(93)90021-t. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Bartke A. Effects of caloric restriction and growth hormone resistance on the expression level of peroxisome proliferator-activated receptors superfamily in liver of normal and long-lived growth hormone receptor/binding protein knockout mice. J. Gerontol. Biol. Sci. Med. Sci. 2005;60:1394–1398. doi: 10.1093/gerona/60.11.1394. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J. Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Mathijssen RHJ, van Schaik RHN. Genotyping and phenotyping cytochrome P450: perspectives for cancer treatment. Eur. J. Cancer. 2006;42:141–148. doi: 10.1016/j.ejca.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Mattow J, Demuth I, Haeselbarth G, Jungblut PR, Klose J. Selenium-binding protein 2, the major hepatic target for acetaminophen, shows sex differences in protein abundance. Electrophoresis. 2006;27 doi: 10.1002/elps.200500703. [DOI] [PubMed] [Google Scholar]
- Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol. Pathol. 2005;33:41–52. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adapter protein controls oxidative stress response and life span in mammals. Nature. 1999;402:302–309. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chang Y, Galecki A, Al-Regaiey K, Kopchick JJ, Bartke A. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol. Endocrinol. 2002;16:2657–2666. doi: 10.1210/me.2002-0142. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford CA, Tarloff JB. The contribution of oxidation and deacetylation to acetaminophen nephrotoxicity in female Sprague-Dawley rats. Toxicol. Lett. 1997;93:15–22. doi: 10.1016/s0378-4274(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Murakami S, Salmon AB, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Hishida A, Kato A. Mechanisms for protective effects of free radical scavengers on gentamicin-mediated nephropathy in rats. Am. J. Physiol. Renal Physiol. 1994;266:F425–F431. doi: 10.1152/ajprenal.1994.266.3.F425. [DOI] [PubMed] [Google Scholar]
- Neff SB, Neff TA, Kunkel SL, Hogaboam CM. Alterations in cytokine/chemokine expression during organ-to-organ communication established via acetaminophen-induced toxicity. Exp. Mol. Pathol. 2003;75:187–193. doi: 10.1016/s0014-4800(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Carbone JM, Silva VM, Chen C, Hennig GE, Whiteley HE, Manautou JE. The PPAR activator docosahexaenoic acid prevents acetaminophen hepatotoxicity in male CD-1 mice. J. Toxicol. Environ. Health A. 1999;58:171–186. doi: 10.1080/009841099157377. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends lifespan. J. Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJ, Chen TS. Diverse antioxidants protect against acetaminophen hepatotoxicity. J. Biochem. Mol. Toxicol. 2005;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- Pendergrass WR, Li Y, Jiang D, Fei RG, Wolf NS. Caloric restriction: conservation of cellular replicative capacity in vitro accompanies life-span extension in mice. Exp. Cell Res. 1995;217:309–316. doi: 10.1006/excr.1995.1091. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, Masoro EJ, Nichols WW, Bradt CI, Cristofalo VJ. Skin fibroblasts from aged Fischer 344 rats undergo similar changes in replicative life span but not immortalization with caloric restriction of donors. Exp. Cell Res. 1992;201:16–22. doi: 10.1016/0014-4827(92)90343-7. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Ruepp SU, Tonge RP, Shaw J, Wallis N, Pognan F. Genomics and proteomics analysis of acetaminophen toxicity in mouse liver. Toxicol. Sci. 2002;65:135–150. doi: 10.1093/toxsci/65.1.135. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Phyiol. Endocrinol. Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Seng JE, Gandy J, Turturro A, Lipman R, Bronson RT, Parkinson A, Johnson W, Hart RW, Leakey JEA. Effects of caloric restriction on expression of testicular cytochrome P450 enzymes associated with the metabolic activation of carcinogens. Arch. Biochem. Biophys. 1996;335:42–52. doi: 10.1006/abbi.1996.0480. [DOI] [PubMed] [Google Scholar]
- Shaikh ZA, Jordan SA, Tang W. Protection against chronic cadmium toxicity by caloric restriction. Toxicology. 1999;133:93–103. doi: 10.1016/s0300-483x(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Shankar K, Vaidya VS, Corton JC, Bucci TJ, Liu J, Waalkes MP, Mehendale HM. Activation of PPAR-α in streptozotocin-induced diabetes is essential for resistance against acetaminophen toxicity. FASEB J. 2003;17:1748–1756. doi: 10.1096/fj.02-1186fje. [DOI] [PubMed] [Google Scholar]
- Sorensen JG, Loeschcke V. Larval crowding in Drosophila melanogaster induces Hsp70 expression, and leads to increased adult longevity and adult thermal stress resistance. J. Insect. Physiol. 2001;47:1301–1307. doi: 10.1016/s0022-1910(01)00119-6. [DOI] [PubMed] [Google Scholar]
- Stauber AJ, Brown-Borg H, Liu J, Waalkes MP, Laughter A, Staben RA, Coley JC, Swanson C, Voss KA, Kopchick JJ, Corton JC. Constitutive expression of peroxisome proliferator-activated receptor α-regulated genes in dwarf mice. Mol. Pharmacol. 2005;67:681–694. doi: 10.1124/mol.104.007278. [DOI] [PubMed] [Google Scholar]
- Tarloff JB, Goldstein RS, Hook JB. Strain differences in acetaminophen nephrotoxicity in rats: role of pharmacokinetics. Toxicology. 1989;56:167–177. doi: 10.1016/0300-483x(89)90131-5. [DOI] [PubMed] [Google Scholar]
- Tarloff JB, Goldstein RS, Sozio RS, Hook JB. Hepatic and renal conjugation (Phase II) enzyme activities in young adult, middle-aged, and senescent male Sprague-Dawley rats. Proc. Soc. Exp. Biol. Med. 1991;197:297–303. doi: 10.3181/00379727-197-43259. [DOI] [PubMed] [Google Scholar]
- Tarloff JB, Khairallah EA, Cohen SD, Goldstein RS. Sex- and age-dependent acetaminophen hepato- and nephrotoxicity in Sprague-Dawley rats: role of tissue accumulation, nonprotein sulfhydryl depletion, and covalent binding. Fundam. Appl. Toxicol. 1996;30:13–22. doi: 10.1006/faat.1996.0038. [DOI] [PubMed] [Google Scholar]
- Tolson JK, Dix DJ, Voellmy RW, Roberts SM. Increased hepatotoxicity of acetaminophen in Hsp70i knockout mice. Toxicol. Appl. Pharmacol. 2006;210:157–162. doi: 10.1016/j.taap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Higami Y, Komatsu T, Tanaka K, Honda S, Yamaza H, Chiba T, Ayabe H, Shimokawa I. Acute stress response in calorie-restricted rats to lipopolysaccharide-induced inflammation. Mech. Ageing Dev. 2005;126:568–579. doi: 10.1016/j.mad.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Usuki F, Yasutake A, Umehara F, Higuchi I. Beneficial effects of mild lifelong dietary restriction on skeletal muscle: prevention of age-related mitochondrial damage, morphological changes, and vulnerability to a chemical toxin. Acta Neuropathologica. 2004;108:1–9. doi: 10.1007/s00401-004-0844-0. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- Yu BP, Chung HY. Stress resistance by caloric restriction for longevity. Ann. N.Y. Acad. Sci. 2001;928:39–47. doi: 10.1111/j.1749-6632.2001.tb05633.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Larón mouse) Proc. Natl. Acad. Sci. U.S.A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]