Abstract
Aneuploidy has long been recognized as one of the hallmarks of cancer. It nonetheless remains uncertain whether aneuploidy occurring early in the development of a cancer is a primary cause of oncogenic transformation, or whether it is an epiphenomenon that arises from a general breakdown in cell cycle control late in tumorigenesis. The accuracy of chromosome segregation is ensured both by the intrinsic mechanics of mitosis and by an error-checking spindle assembly checkpoint. Many cancers show altered expression of proteins involved in the spindle checkpoint or in proteins implicated in other mitotic processes. To understand the role of aneuploidy in the initiation and progression of cancer, a number of spindle checkpoint genes have been disrupted in mice, most through conventional gene targeting (to create germ-line knockouts). We describe the consequence of these mutations with respect to embryonic development, tumor progression and an unexpected link to premature aging; readers are referred elsewhere [1] for a discussion of other cell cycle regulators.
Keywords: Spindle checkpoint, mouse models, aneuploidy, chromosome instability
Introduction
Cancer is the result of multiple genetic alterations working in tandem to override control mechanisms that prevent inappropriate cell proliferation and restrict division to particular biological niches [2]. In many tumors, accumulation of mutations is accompanied by numerical and structural chromosomal instability (CIN). By numerical CIN we mean changes in chromosome ploidy that arise when cells gain or lose whole chromosomes during division. Structural CIN involves changes in the organization of one or more chromosomes, and is a result of translocations, inversions and deletions; these are generally thought to arise from chromosome breakage followed by inappropriate rejoining events (“bridge-fusion” events [3]). At least three phenomena account for the oncogenic potential of CIN (i) increases in the copy number of oncogenes via localized gene amplification and altered chromosome ploidy (ii) LOH (loss of heterozygosity) of tumor suppressor genes through gene deletion and whole chromosome loss (iii) creation of oncogenic fusions between normally separate regulatory and coding sequence, such as fusion of BCR on chromosome 22 to c-Abl on chromosome 9 to create BCR-Abl [4, 5]). To the first order of approximation, numerical CIN is expected to arise from errors in mitotic chromosome segregation whereas structural CIN arises from errors in DNA metabolism and repair. The two processes interact however, because mitotic non-disjunction fragments chromosomes creating DNA damage and promoting fusion whereas replication errors can create dicentric chromosomes which do not disjoin normally during anaphase and often suffer additional damage.
Despite the fact that karyotypic abnormalities have been linked to cancer for over 100 years [6, 7] and are extremely common in human solid tumors, it remains largely unknown whether numerical CIN is a cause or consequence of oncogenic transformation and thus, whether it plays a critical “mutator” role in human disease [8]. Why this continuing uncertainty? First, it is not yet entirely clear where to look for genes whose mutation might predispose cancer cells to CIN. Proteins involved in the mechanical and regulatory steps of mitosis have been identified only recently and, in most cases, their functions are much less well understood than those of classic oncogenes such as Ras or Src. Thus, while it is known that most tumor cell lines have aberrant checkpoint responses and mis-segregate chromosomes, the genes whose mutations are responsible for checkpoint abnormality remain largely unidentified (despite some early successes [9]). Second, among mitotic regulators that have been sequenced in panels of human tumors, no “smoking guns” have emerged. For example, it is known that correct operation of the spindle assembly (“mitotic”) checkpoint is necessary for accurate chromosome segregation [4], but loss of function mutations in mitotic checkpoint genes appear to be infrequent in human cancers [8, 10]. Third, targeted inactivation of mitotic checkpoint genes in the mouse has been found to cause embryonic lethality rather than cancer, and targeted or partial loss of function generally results in very mild tumorigenic phenotypes. In some cases, premature senescence rather than cancer has been observed (as discussed in detail below). Fourth, functional assays for CIN are primitive and quantitative measures of chromosome non-disjunction, which have proven so valuable in yeast [11], are not yet available for animal cells. This has impeded systematic determination of the consequences of mutating known tumor suppressor genes or oncogenes on CIN. The possibility that tumor suppressor genes identified by other means might play a role in CIN is suggested by experiments in cultured cells demonstrating that chromosome segregation is disrupted by mutations in the Adenomatous Polyposis Coli (APC) gene product, a gene that plays a key role in hereditary and sporadic colorectal cancer [12–15]. APC truncations commonly found in human cancer are known to promote tumorigenesis by disrupting regulation of β-catenin, but the significance of mitotic errors caused by the same APC truncations is less clear: conclusive demonstration that CIN is involved will require separation-of-function mutations. In a similar vein, it has been proposed that the tumor suppressors Rb and REST can act as CIN-promoting tumor suppressor genes by altering the expression of the Mad2 mitotic checkpoint protein, but the significance of Mad2-Rb and Mad2-REST connections in cancer has not as yet been established experimentally [16–19].
Mechanisms that ensure the accuracy of chromosome segregation
Correct chromosome segregation depends on the assembly of a metaphase spindle whose geometry is compatible with the mechanical events of sister chromatid separation as well as on a series of error-sensing checkpoint pathways. Because it is reasonable to search among these mitotic genes for mutations that might cause CIN, we briefly review the key events of mitotic chromosome segregation starting with spindle assembly and chromosomes-spindle attachment and concluding with checkpoints.
Centrosome duplication and establishing spindle bipolarity
A fundamental geometric requirement for mitosis is spindle bipolarity. Redundant mechanisms are involved in ensuring bipolarity, and centrosome duplication is tightly controlled during S-phase, ultimately resulting in segregation of one centrosome into each daughter cell during mitosis (see for detailed review: [20–23]). Depletion or mutation of proteins involved in centrosome biogenesis leads to the formation of multi-polar mitotic spindles, gross defects in chromatid disjunction and numerical CIN. Many solid tumors have abnormal centrosome numbers [24, 25], and a wide range of oncogenic alterations result in centrosome abnormalities, including inactivation of p53, p21CIP1, BRCA1 or BRCA2, oncogenic Ras mutations and deregulation of Survivin [26]. Although mouse knockouts exist for all these proteins, no data are available on the consequences for cancer development of disrupting structural components of the centrosome, such as γ-tubulin or pericentrin; as with other spindle components, the role of centrosomes in cancer remains poorly understood. These issues are discussed in more detail on pages XXXX (King and XXX).
Attaching chromosomes to microtubules via kinetochores
The second geometric requirement for chromosome segregation is bi-orientation of paired chromatids: the first chromatid in a pair of sisters must bind to microtubules emanating from one spindle pole while the sister chromatid must bind to microtubules emanating from the opposite pole [27]. Attachment of chromatids to microtubules is mediated by kinetochores, large multi-protein complexes that assemble on centromeric DNA. By electron microscopy kinetochores are visible as distinctive trilaminar structures that lie at the central constriction of chromosomes, a region of DNA that spans many megabases. A number of kinetochore components (CEN binding proteins or CENPs; e.g. CENPA, CENPF and CENPH) exhibit altered expression in human cancer, implying that abnormal kinetochore composition may accompany tumorigenesis [28], but no structural kinetochore proteins have as-yet been identified among known oncogenes and tumor suppressors. However, regulators of kinetochore function, such as Aurora B or Plk1 are frequently altered in cancer [29, 30]. Aurora B is a critical protein for sister bi-orientation and is thought to act by correcting erroneous attachments [31]. When both sister chromatids are attached to MTs emanating from the same centrosome (a state of syntellic attachment), the kinase activity of Aurora B remains high, thereby down-regulating kinetochore-MT binding and promoting rearrangements in MT attachment that eventually lead to the acquisition of bipolarity [31, 32]. While the mechanisms involved in the process are only now being elucidated, drugs targeting Aurora B (and a related kinase, Aurora A, involved in centrosome biogenesis) are already in clinical trials for cancer [33].
Mitotic checkpoints
Chromosome bi-orientation and the consequent generation of MT-dependent pulling forces generates tension between sister chromatids that, upon dissolution of sister cohesion at anaphase, causes chromosomes to move to opposite ends of the spindle. The mitotic checkpoint appears to monitor both kinetochore-microtubule attachment per se and the imposition of tension. Only when tension is present and bipolarity ensured, is the checkpoint silenced, the anaphase promoting complex activated, and progress from metaphase into anaphase possible. The mitotic checkpoint involves a set of ~10 interacting proteins (including Mad1, Mad2, Bub1, BubR1, Bub3, Mps1,TAO1, Rod and ZW10) that are highly conserved through evolution [10, 32]. These proteins localize to the kinetochores of unattached or maloriented chromosomes and send a signal that acts both locally and at a distance to inhibit CDC20, a member of a family of specificity factors that regulate the anaphase promoting complex (Cyclosome-APC), an E3 ubiquitin ligase [34, 35]. CDC20-dependent activation of Cyclosome-APC is necessary for degrading proteins such as Securin and Cyclin B1. Securin degradation permits Separase activation, causing cleavage of the cohesin complexes that glue sister chromatids together and cohesion cleavage, in turn, causes physical separation of sister chromatids. In parallel, Cyclin B1 degradation results in the inactivation of CDK1 and allows cells to exit from a mitotic state [4, 32, 36]. The precise biochemical events involved in generating and transmitting checkpoint signals are under intensive investigation but remain poorly understood. It is thought that conformational changes in Mad2 catalyzed by unattached kinetochores generate an active Mad2 conformation that binds to and blocks Cdc20 activity. A complex set of phosphorylation events mediated by the Bub1, BubR1, Mps1 and other kinases collaborate with Mad2 in restraining Cdc20 until bipolar attachment of all sisters is achieved.
Chromosome instability, mitotic errors and checkpoint defects in human cancer
The majority of human cancer cell lines exhibit an altered response to drugs that provoke the spindle assembly checkpoint, such as paclitaxel or Vinca alkaloids. Many studies have explored the basis of selective killing of human cancers cells by anti-microtubule drugs. From those studies, it appears that mitotic checkpoint function is retained in most human cancer cell lines, as measured by the ability of cells to arrest transiently following spindle disruption, but that cells vary in the duration of cell cycle arrest [4, 9, 37–42] and the extent to which mitotic blockade induces apoptosis. The consequences of slipping through the checkpoint also vary, depending on the p53 status of cells [43]. Some human cancers exhibit altered expression of mitotic regulators and checkpoint proteins, including Bub1, BubR1, Aurora-A, Incenp, Securin and Mad2 [28], and it is probable that deregulation of these genes causes subtle changes in checkpoint function. Recent sequencing of human cancers has also uncovered several previously unknown spindle checkpoint mutations present in a subset of aneuploid cancers [44–46]. Nonetheless, it remains unclear whether identified missense mutations in coding sequences and mutations that alter gene expression actually play a role in either CIN or cancer. Moreover, in at least some aneuploid tumor cell lines the spindle checkpoint response is seemingly normal [15]. In these cases, CIN is unlikely to involved checkpoint defects.
A further complication in thinking about the roles of CIN in tumorigenesis is that chromosome instability might be intermittently important. When would tumors benefit the most from a potential window of instability: at the start of tumorigenesis, when initial growth-promoting mutations must be generated, or later when multiple mutations involved in metastasis etc. accumulate? Insights into this question are provided by the study of colorectal carcinomas, all of which exhibit genomic instability; approximately 85% display CIN whereas the remaining 15% exhibit the MIN (microsatellite instability) phenotype associated with defective mismatch repair [47, 48]. Two distinct types of dysplasia can be distinguished in early adenomas: hyperplastic crypts, which seldom develop into carcinomas and unicryptal adenomas. The latter show APC mutations and frequently develop into colorectal carcinomas. CIN is observed relatively early in the development of these colorectal carcinomas, at a stage following the formation of unicryptal adenomas when a few hundred cells are present [48–52]. Some of these tumors harbor small chromosome changes, including allelic losses or small karyotypic alterations [53]. However, with progression from adenoma to cancer aneuploidy becomes increasingly apparent, implying that CIN is continuous and involved in the creation of increasingly malignant phenotypes [47, 48]. Based on these observations, it seems likely that colorectal tumors start out having normal chromosome segregation and that CIN arises midway between tumor initiation and the carcinoma stage. Mice models in which CIN is induced at various stages of tumor development should provide more insight in these intriguing observations.
Mouse models of chromosome instability
As a means to study the consequences of mitotic checkpoint loss on development and tumorigenesis, conventional gene knockouts have been constructed for almost all known mitotic checkpoint genes as well as for several mitotic regulators and kinetochore components (Table 1). A much more limited set of conditional mutations has also been created, but very few compound mutations have been examined as yet.
| Spindle checkpoint genes | Phenotypes +/− animals | |||||||
|---|---|---|---|---|---|---|---|---|
| Genes | −/− | +/− | Cancer prone? | Chemical induced cancer? | Other Phenotypes |
Aneuploidy in tissue? |
Aneuploidy in MEFs? |
Reference |
| Bub1 | Embryonic lethal at E6.5 | Viable, no overt developmental defects |
No | DMBA-induced | No | ND | ND | [62] |
| Bub1 hypomorph |
NA | NA | 50% have developed tumors by 20 months (lymphomas, lung and liver tumors) |
ND | No | ND | 15% of the hypomorphic cells show segregation defects |
[62] |
| Bub3 | Embryonic lethal at E6.5 | Viable, no overt developmental defects |
No | DMBA-induced | No | 10% of splenocytes are aneuploid |
20% of cells are aneuploidy |
[59, 60, 66, 67] |
| Bub3; Rae1 |
ND | Viable, no overt developmental defects |
No | DMBA-induced | Premature aging | 40% of the splenocytes are aneuploid |
40% of the double heterozygous cells are aneuploidy |
[60, 67] |
| BubR1 | Embryonic lethal at E6.5 | Viable, no overt developmental defects |
No | DMBA-induced and azoxymethane induced |
Hematopoietic defect |
Polyploidy in megakaryocytes |
15% of the cells are aneuploid |
[56, 57, 82] |
| BubR1 hypomorph |
NA | NA | No | DMBA-induced | Premature aging | Up to 30% of splenocytes are aneuploid at 12 months of age (hypomorph animals) |
35% of the hypomorphic cells are aneuploid |
[57] |
| CENPE | Embryonic lethal before E7.5 |
Viable, no overt developmental defects |
20% develop tumors by 19–21 months of age (both lung and spleen) |
Tumor suppression upon DMBA treatment |
Tumor suppression in a p19Arf−/− background |
40% of splenocytes are aneuploid |
20% of the cells are aneuploid (increasing to 70% upon prolonged passaging) |
[61, 114] |
| Mad1 | Embryonic lethal at day 6.5 |
Viable, no overt developmental defects |
20% develop tumors within 18–20 months of age, mostly lung tumors |
Vinicristine-induced | No | ND | 10% of the cells are aneuploid |
[58] |
| Mad2 | Embryonic lethal at E6.5 | Viable, no overt developmental defects |
30% develop lung tumors at 18 months of age |
ND | No | ND | 55% of the cells are aneuploid |
[55, 65] |
| Mad2 over- expresser |
NA | NA | 50% have developed tumors by 20 months (lymphomas, lung and liver tumors) |
DMBA-induced | No | Massive chromosomal instability as determined by CIN in tumors |
50% of the over- expressing cells are aneuploid |
[17] |
| Rae1 | Embryonic lethal at E6.5 | Viable, without developmental defects |
No | DMBA-induced | No | 10% of the splenocytes are aneuploid |
20% of the cells are aneuploid |
[60, 67] |
| Inner plate proteins | Phenotypes +/− animals | |||||||
| −/− | +/− | Cancer prone? | Chemical induced cancer? | Other Phenotypes |
Aneuploidy in embryos? |
Aneuploidy in MEFs? |
Reference | |
| CENPA | Embryonic lethal at E6.5 | Viable, without developmental defects |
ND | ND | NA | Chromosomal missegregation in −/− embryos E6.5) |
NA | [77] |
| CENPB | Viable, no phenotype | Viable, no phenotype |
ND | ND | Lower body and testis weight |
ND | ND | [74–76] |
| CENPC | Embryonic lethal at E3.5 | Viable, without developmental defects |
ND | ND | NA | Aberrant mitosis and formation of micronuclei in early embryos |
NA | [78] |
|
Chromosomal passenger genes/ mitotic spindle binding proteins |
Phenotypes +/− animals | |||||||
| −/− | +/− | Cancer prone? | Chemical induced cancer? | Other Phenotypes |
Aneuploidy in embryos/ tissues? |
Aneuploidy in MEFs? |
Reference | |
| APC/MIN | Embryonic lethal before E8.5 |
Viable | Develop intestinal tumors within 3 months |
ND | Anemia, presumably due to intestinal bleeding |
Aneuploidy and aberrant mitoses in crypt cells |
Yes, and is increased by mono-allelic BubR1 loss |
[13, 97, 98, 105] |
| Incenp | Embryonic lethality in between E3.5 and 8E.5 |
Viable, no overt developmental defects |
ND | ND | NA | Abnormal nuclear morphology and higher than normal chromosomal content in E3.5 embryos |
NA | [80] |
| Survivin | Embryonic lethal at E6.5 | Viable, no overt developmental defects |
ND | ND | NA | Formation of giant nuclei in early embryos |
NA | [80] |
| Genes involved in mitosis otherwise | Phenotypes −/− animals | |||||||
| −/− | +/− | Cancer prone? | Chemical induced cancer? | Other Phenotypes |
Aneuploidy in tissue? |
Aneuploidy in MEFs? |
Reference | |
| Ltzs1 | Viable, no developmental defects |
Viable, no overt develop- mental defects |
All the −/− and 60% of the +/− mice developed tumors in between 8–24 months of age (lymphomas, breast, liver and liver tumors) |
NMBA-induced | No | ND | 25% of the cells show lagging chromosomes during mitosis |
[99] |
| Chfr | Viable, no developmental defects |
Viable, no develop- mental defects |
Yes, 50% of the −/− animals develop tumors within 20 months |
DMBA-induced | No | ND | 25% of the cells are aneuploid |
[103] |
Inactivation of spindle checkpoint genes causes embryonic cell death in the mouse
The first spindle checkpoint gene to be knocked out in mouse was Mad2, which unexpectedly proved to be cell-essential (based on studies in yeast, it had been assumed that checkpoint genes were nonessential [54]). Homozygous Mad2 loss in mice results in embryonic lethality by E8.5 [55], although a small number of embryos survive beyond this point. BubR1, Bub1, Mad1, Bub3, Rae1 and CENPE are also essential genes, with homozygous knockouts causing embryonic lethality at E6.5-E8.5 in all cases. When E8.5 blastocysts are cultured in vitro, cells of the inner cell mass (ICM) are observed to undergo apoptosis although non-cycling trophectodermal cells survive. Death of rapidly proliferating ICM is presumably a consequence of frequent missegregation [56–63]. Intriguingly, cell death is dramatically suppressed in Mad2−/−; p53−/− blastocysts and viable cell lines can be recovered from the small subset of embryos that survive to E10.5. Thus, a significant fraction of the cell death in Mad2−/−embryonic cells appears to be a result of p53-dependent apoptosis [64].
Effect of reduced mitotic checkpoint protein expression on tumorigenesis
Many cancers exhibit decreased expression of spindle checkpoint proteins implying that partial checkpoint inactivation, rather than complete loss of function as generated by conventional mouse knockouts, may be associated with tumorigenesis [8]. Because inactivation of one copy of Mad2, Mad1, CENPE, Bub1, BubR1, Bub3 or Rae1 decreases protein expression, without compromising embryonic development (heterozygotes are born at a normal Mendelian ratio and lack overt developmental defects, except for a mild hematopoietic defect in BubR1+/− animals [56, 58, 60–62, 65–67]), they afford an opportunity to examine the effects on tumorigenesis of a subtly comprised checkpoint response. Mad2, Mad1 and CENPE heterozygous knockout animals have been observed to develop cancer in substantial numbers (20–30%), albeit relatively late in life (at 18–20 months of age). In Mad2+/− and Mad1+/− animals, lung tumors predominate but in CENPE +/− animals, malignancies of the spleen also occur [58, 61, 65]. Thus, Mad1, Mad2 and CENPE function as haplo-insufficient tumor suppressors in the mouse. In contrast, heterozygous deletion of BubR1, Bub1, Bub3 and Rae1 and double deletion of Bub3+/− Rae1 +/− does not lead to increased malignancy (relative to wild type) in animals up to 15–20 months of age (Table 1 and Figure 1) [56, 57, 60, 62].
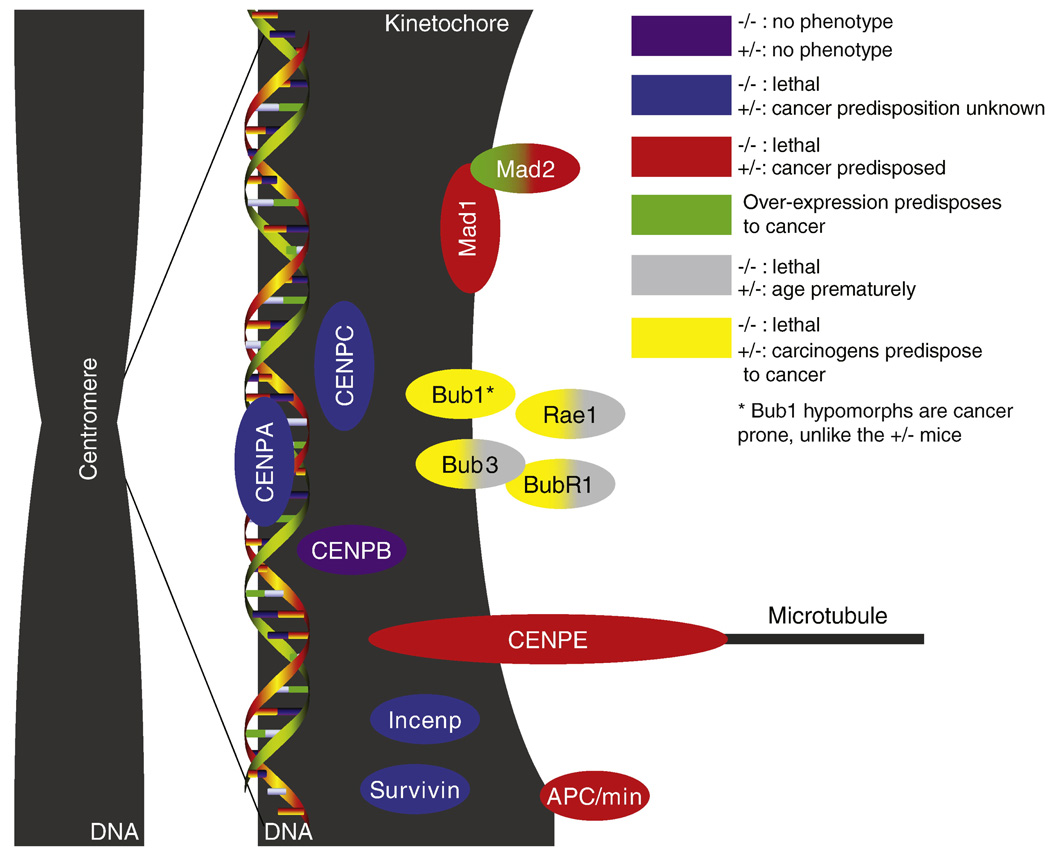
Figure 1.
Genes discussed in this review. A simplified overview of the spindle checkpoint and centromeric proteins for which mouse models exist with their respective phenotype.
As an alternative means to achieve partial inactivation of the spindle checkpoint in mice, hypomorphic BubR1 and Bub1 alleles have been generated. These alleles express less protein as a result of a neomycin gene insertion that functions as a cryptic exon and lowers mRNA expression. Mice carrying a Bub1 hypomorph (in which expression of Bub1 is observed to be ~20% of wildtype levels) develop lymphomas, and lung and liver tumors when 18–20 months old [62]. Reduction of BubR1 levels to 10% of wildtype levels results in massive aneuploidy in several tissues, but does not induce tumors [57]. Instead, animals exhibit premature aging, as evidenced by decreased subcutaneous fat, spinal deformation (spinal kyphosis) and muscle atrophy. The median lifespan of animals is six months and none live for longer than 15 months [57]. A similar but less severe phenotype is observed in Bub3+/−Rae1+/− compound heterozygotes (Table 1) [62]. Intriguingly, BubR1 levels are observed to decline with age in the tissues of wildtype animals [57]. Together these data suggest a role for BubR1, Bub3 and Rae1 in aging, presumably as a consequence of the widespread aneuploidy that their mutation causes. It remains unclear, however, why aneuploidy should cause premature aging in some circumstances and cancer in others; also unclear is why checkpoint mutation should differ in this regard (Mad2+/− vs. BubR1+/−). Perhaps, differing roles in checkpoint and kinetochore-microtubule attachment [68] and mitotic timing [69] are involved.
To better study the connection between a compromised checkpoint and aneuploidy, studies have been performed in MEFs isolated from heterozygous animals. Mutant MEFs exhibit significantly elevated numbers of aneuploid cells compared to wildtype control MEFs, ranging from 10% aneuploid cells in Mad1+/− MEFs to 50% aneuploid cells in Mad2 heterozygotes and Mad2 over-expressing cells (and <<10% aneuploidy in wildtype MEFs; Table 1) [17, 58, 65]). Intriguingly BubR1 hypomorphic and Bub3-Rae1 compound heterozygous MEFs grown in culture exhibit premature senescence and increased levels of senescence-associated proteins such as p19Arf, p21Cip1 and p16Ink4a [57, 60]. These CDK inhibitors promote activation of p53 and the Rb family proteins, thereby restricting the proliferative capacity and increasing senescence in MEFs [70–72]. From these experiments, it is clear that levels of aneuploidy in mice are not predictive of either cancer predisposition or premature aging phenotypes (Table 1). However, it is important to realize that determining levels of aneuploidy remains difficult and highly sensitive to experimental conditions and cell passage number [61]. Thus, new assays that follow the development and progression of aneuploidy in vivo will be crucial to better understand the causes and consequences of chromosome missegregation.
Consequence of CENP and chromosomal passenger genes deletion
CENPA, B and C were among the first kinetochore proteins to be identified [73]; they bind to CEN DNA throughout the cell cycle. CENPA is a specialized histone H3 thought to be involved in establishing an epigenetic mark involved in centromere specification. CENPB is a sequence-selective DNA binding protein that associates preferentially with alpha-satellite arrays found at centromeres; CENPC is less well understood, but is probably also a DNA binding protein. Whereas homozygous CENPB knockout is associated with a very mild phenotype including lower testis and body weight [74–76], disruption of CENPA or CENPC causes early embryonic lethality (E3.5-E6.5). CENPA and CENPC knockout embryos contain micronuclei, have a lower mitotic index and exhibit enlarged nuclei (suggestive of polyploidy; Table 1). Heterozygous CENPA+/− or CENPC+/− mice develop normally and are fertile. Currently these animals are being tested for spontaneous and carcinogen-induced cancer predisposition [77, 78] Andy Choo, personal communication].
Incenp and Survivin form a complex with the Aurora B kinase and function as a “chromosome passenger” complex characterized by localization to kinetochores early in mitosis and subsequently, during anaphase, to the spindle midzone. The chromosomal passenger complex is essential for generating correct bipolar attachment between spindle microtubules and kinetochores, for sensing tension across the metaphase plate and for the correct execution of late events in cytokinesis [79]. Knockouts of Survivin or Incenp in the mouse result in embryonic lethality prior to E8.5, consistent with their essential cellular functions [80, 81]. Heterozygous animals are indistinguishable from their wild-type littermates, but it is not yet known whether heterozygotes develop cancer at a higher rate than littermate controls [Andy Choo, personal communication].
Interaction of checkpoint mutations with carcinogens and tumor suppressor genes
Several spindle checkpoint defects have been tested for their ability to act additively or synergistically with carcinogen treatment and with classical tumor suppressor mutations. When treated with a single application of DMBA on the dorsal skin of a mouse, Bub1+/−, Bub3+/−, Rae1+/− and Bub3+/−Rae1 +/− neonates developed more tumors, primarily in the lung, than similarly treated wildtype mice even though none of the mutations exhibit increased tumor loads in the absence of a DMBA [60, 62]. DMBA-treated BubR1 hypomorphs are also predisposed to tumors [57] and BubR1+/− develop colonic tumors in response to subcutaneous injection with the carcinogen azoxymethane [82]. Finally, 40% of Mad1 heterozygous mice treated with the microtubule-depolymerizing drug Vinicristine developed tumors as compared to no tumor formation in wildtype controls [58]. Together, these data (summarized in Table 1) suggest that partial checkpoint loss is tumor-promoting in combination with classical chemical carcinogens.
In the cases in which it has been examined, germline heterozygosity in checkpoint genes does not appear to cooperate with classical tumor suppressor genes in oncogenesis. For example, tumors were no more frequent in compound heterozygous mice in which Bub3+/− was combined with either p53+/− or Rb+/− , as compared to mice carrying mutations in one gene alone [66]. This is unexpected since both Rb and p53 heterozygotes develop tumors in which the wildtype allele is frequently lost [83–85], and it seemed reasonable to expect that LOH would be accelerated by mutations that promote aneuploidy. Even more striking is the recent finding that CENPE heterozygosity prevents tumorigenesis in p19Arf deficient animals. p19Arf−/− animals develop lymphomas and sarcomas with an average latency of 6–7 months [86] but tumor latency increases to 12 months in CENPE+/− - p19Arf−/− compound mice, implying that aneuploidy might actually suppress tumorigenesis. Additionally, it has been observed that DMBA-induced tumorigenesis in CENPE+/− - p19Arf proficient animals is delayed or prevented: liver cancers are dramatically reduced in size and 3-fold in number [61]. These unexpected findings shed a completely different light on the role of aneuploidy in tumorigenesis.
Mouse models of naturally occurring checkpoint mutations
More recently, mouse models for CIN have been generated that more closely simulate the types of genetic alterations that might occur in real cancers. As mentioned above, mutations that alter Mad2 sequence do not occur very frequently in human cancer [38, 87, 88] but increases in Mad2 expression are quite frequent [16, 89, 90]. One explanation for the observed over-expression of Mad2 in tumors is altered regulation of E2F transcription factors. The Rb – E2F pathway is mutated in more than 80% of human tumors [91] and deregulation of Rb has been associated with CIN [92–94]. Recently, Mad2 was shown to be an E2F target gene, and its expression to be elevated in Rb−/− mouse embryonic fibroblasts. Moreover, Rb−/− MEFs display increased aneuploidy as compared to wildtype MEFs, but partial RNAi-mediated Mad2 knockdown in Rb−/− MEFs (to levels that approximate wild-type) reduced the number of aneuploid cells. Conversely, over-expression of either E2F or Mad2 in wildtype MEFs increased aneuploidy [16]. It has also been observed that expression of oncogenic REST leads to elevated Mad2 levels and to aneuploidy [18, 19]. These results suggest that abnormal activation of E2F or REST may promote aneuploidy, at least partially via elevated Mad2 expression. Indeed, over-expression of Mad2 in mice from a tetracycline-regulable promoter predisposes animals to a wide spectrum of tumors including lung adenomas, lymphomas, hepatocellular carcinomas and fibrosarcomas) with a similar latency (~20 months), but higher frequency (~50% at 20 months) than the lung tumors arising in Mad2 heterozygous mice (Table 1, Figure 1) [17]. At the first glance, these results seem somewhat counterintuitive: why would increasing the levels of a checkpoint protein lead to checkpoint failure? One possibility is that the delayed degradation of Securin and Cyclin B1 at the metaphase-anaphase transition in Mad2-overexpressing cells might promote chromosome nondisjunction and thereby promote CIN. However, this needs to be proven experimentally in animals.
As noted above, the Adenomatous polyposis coli (APC) gene is a tumor suppressor often mutated in human colorectal cancers. In addition to APC’s well-known role in regulating β-catenin transcriptional activity and cell proliferation, multiple lines of evidence suggest that mutated APC contributes to chromosome missegregation and CIN. First, cell culture studies show that APC localizes to kinetochores and microtubule ends. Perturbing APC by mutation, over-expression or depletion causes CIN and aneuploidy [15, 95, 96]. Second, mice expressing one copy of truncated APC display multiple intestinal tumors with aberrant mitosis and polyploidy [13, 14, 97, 98]. A further hint of this link is that mice mutated for APC exhibit increased expression of BubR1 and Mad2, as had been observed previously in human adenomas and colorectal carcinomas [14]. Together these data suggest a connection between deregulation of APC and checkpoint errors in cancer progression. However, to prove a role for APC in CIN, it will be necessary to generate separation of function mutations that discriminate between the roles of APC in mitosis and β-catenin regulation.
Other mitotic genes involved in CIN and tumorigenesis
Mitotic defects other than spindle checkpoint failure are also known to perturb chromosome segregation and induce aneuploidy. Lzts1 for example, was discovered as a gene that is frequently lost in breast, lung, gastric, esophageal, prostate, and bladder cancers. Lzts1 deletion impairs Cyclin B1 activation, resulting in lowered Cyclin B1-Cdk1 activity in mitosis and premature mitotic exit. Indeed, Lzts1-deficient mice develop a wide spectrum of tumors by 19 months with high penetrance. Lzts1 loss also increases carcinogen-induced tumorigenesis, as intragastric NMBA treatment generates forestomach tumors in 100% of Lzts1+/− and Lzts1−/− animals, but in only 15% of wildtype controls (Table 1). Thus, premature mitotic exit triggered by decreased Cyclin B1 levels may also be tumor promoting [99].
Entry into mitosis is guarded by a prophase checkpoint that can be activated by chromosome damage or disruption of microtubules [100, 101]. Chfr has recently been identified as a tumor suppressor gene and an E3 ubiquitin ligase that functions in at prophase by ubiquinating Aurora A and thereby blocking progression into metaphase [102]. Homozygous deletion of Chfr in the mouse revealed that it is dispensable for normal development: null animals are born at normal Mendelian frequencies and exhibit no major developmental defects. However, half of the Chfr-deficient animals develop tumors (lymphomas, lung, liver and intestinal tumors) by 20 months and exhibit accelerated tumor development following DMBA treatment [103].
Summary
Since the discovery, nearly a century ago that aneuploidy is frequent in cancer [6, 7], many genes involved in mitotic checkpoints and in the mechanical events of chromosome segregation have been identified. However, loss-of-function mutations in these genes appear uncommon in human cancer. Genetic analysis in the mouse unequivocally shows that deletion of checkpoint genes results in early embryonic lethality, aneuploidy and apoptosis at a time coincident with maximal rates of cell division during development. RNAi in human cell lines emphasize the likelihood that spindle checkpoint genes are cell-essential, and thus, that rather than increasing the mutability of tumor cells, complete loss of checkpoint function kills them [104].
How might checkpoint mutations contribute to CIN and to cancer? One possibility is that the lethality associated with checkpoint loss can be overcome by deficiency in p53 (and possibly also by loss of other genes). Mad2−/− p53−/− double knockout embryonic cells are inviable in utero from day E10.5, but they can be passaged in culture, occasionally giving rise to immortal cell lines, despite the absence of a checkpoint response and the presence of extremely high rates of CIN [64]. This implies that inactivation of both Mad2 and p53 in adult tissue might result in aneuploidy and cancer in these tissues, an issue we have been exploring experimentally. Another possibility is that cancer cells inactivate the checkpoint only partly. However, the types of partial inactivation explored to date in mice result in a mild cancer predisposition and only in a subset of circumstances. Moreover, the level of aneuploidy in mutant animals does not appear to be predictive of cancer risk. For example, in both CENPE+/− and Bub3+/− Rae1+/− mice, 40% of splenocytes are observed to be aneuploid but whereas CENPE+/− animals develop malignancies Bub3+/− Rae1+/− mice do not. Thus, even in those cases in which a partially compromised checkpoint leads to cancer, tumorigenesis is slow and infrequent.
A particularly surprising result from studies in mice is that partial loss of function of some checkpoint genes, such as BubR1, Bub3 and Rae1, leads to premature aging rather than transformation. Premature aging in animals is presumably attributable to premature senescence of somatic cells, a phenomenon that can be observed in cell culture. Premature senescence could also explain why CENPE+/− p19Arf−/− mice are less susceptible to tumors than parental p19Arf−/− animals and why CENPE+/− animals are resistant to carcinogenesis [61]. These findings highlight the importance of studying the poorly understood relationship between senescence, aneuploidy and cancer.
Conclusion and future directions
Taken together, mouse models have demonstrated that alterations including under- and over-expression of spindle checkpoint genes can promote tumorigenesis, albeit with late onset. Partial loss-of-function mutations in some checkpoint genes cause aneuploidy whereas mutations in other spindle checkpoint genes cause senescence. We currently lack assays with which to assay CIN in living tissue, making it difficult to determine when and how specific mutations promote apoptosis, premature senescence and cellular transformation. We know that complete spindle checkpoint deficiency leads to massive aneuploidy and cell death within six cell divisions in human cell lines [104], but we have no idea what the direct consequences of such an event might be in the context of intact tissue in an animal. Better assays should also lead to a more thorough understanding of the roles of known tumor suppressor genes and oncogenes (such as APC and Rb) in aneuploidy and its prevention. A greater understanding of aneuploidy-induced apoptosis in animals will inevitably lead to new drugs that can specifically kill aneuploid cancers but does raise the question whether directly attempting to block checkpoint genes, with the aim of predisposing cells to killing with anti-mitotic drugs, is necessary a wise therapeutic strategy.
The obvious next step in the field is to generate inducible knockouts of spindle checkpoint genes that are directed to specific tissues and that therefore overcome the organismal lethality associated with embryonic gene inactivation. We can then ask whether checkpoint loss in adult cells accelerates tumorigenesis to a greater extent that germ line heterozygosity. It will also be valuable to induce checkpoint gene mutation at specific stages of a multi-step tumorigenic process, in the colon for example [105]. Ideally, these mutations should be subtle than simple deletion. Transcriptional profiling of numerous cancers suggests that over-expression of spindle checkpoint genes is more frequent than loss (as determined from the Oncomine database [106]) and conditional over-expression is easy to engineer [17]. Some checkpoint genes have also been found to harbor point mutations or truncations in human cancers [9, 45, 87, 107–109], and these mutations should now be reengineered into cells and then animals to see if they are CIN- and tumor-promoting. Moreover, although deletions of Mad and Bub genes been examined in mice, several important checkpoint kinases have not been studied, including Mps1 and TAO1 [110, 111]. Generating conditional truncation and kinase-dead versions of these proteins is undoubtedly worthwhile: it is already known that kinase-dead mutations and RNAi of Bub1 and BubR1 are associated with different phenotypes in cultured cells. Appropriate mouse models will also be useful in studying small molecule kinases inhibitors that target checkpoint kinases, several of which are in development [112]. Finally, the generation and analysis of compound mutations, involving p53 and spindle checkpoint lesions for example, is an obvious way to see if lethality associated with checkpoint deficiency can be suppressed [64]. Perhaps p53 loss will allow the creation of cells in which spindle checkpoint function is completely abrogated in specific tumor cells. Tissue and stage specific mutagenesis of adult tissues is now standard in mouse models of human cancer, (e.g. [113]) but has not yet been pursued as a strategy for studying CIN and spindle checkpoint genes. Its application to spindle checkpoint genes, in combination with better assays for CIN should help to dramatically increase our understanding of the role aneuploidy plays in tumor initiation and progression and also in cellular senescence and aging.
Acknowledgements
We are very grateful to Sophia Bruggeman, Laura Sontag-Kleiman and Ying Yue for their valuable comments on the manuscript. FF is a recipient of a Dutch Cancer Society (KWF-Kankerbestrijding) postdoctoral fellowship; this work was funded by CA84179 and GM51464.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ciemerych MA, Sicinski P. Cell cycle in mouse development. Oncogene. 2005;24:2877–2898. doi: 10.1038/sj.onc.1208608. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Murnane JP. Telomeres and chromosome instability. DNA repair. 2006;5:1082–1092. doi: 10.1016/j.dnarep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 5.Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1:109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- 6.Boveri T. Uber mehrpolige Mitosen als Mittel zur Analyse des Zellkerns (Multipolar mitoses as means of the analysis of the nucleus) Vehr. d. phys. med. Ges. zu Wurzburg NF35. 1902:67–90. [Google Scholar]
- 7.Boveri T. Zur frage der Entsehung maligner Tumoren (The origin of malignant tumors) 1914 [Google Scholar]
- 8.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Current opinion in cell biology. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 10.Draviam VM, Xie S, Sorger PK. Chromosome segregation and genomic stability. Current opinion in genetics & development. 2004;14:120–125. doi: 10.1016/j.gde.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.McAinsh AD, Tytell JD, Sorger PK. Structure, function,and regulation of budding yeast kinetochores. Annual review of cell and developmental biology. 2003;19:519–539. doi: 10.1146/annurev.cellbio.19.111301.155607. [DOI] [PubMed] [Google Scholar]
- 12.Abal M, Obrador-Hevia A, Janssen KP, Casadome L, Menendez M, Carpentier S, Barillot E, Wagner M, Ansorge W, Moeslein G, Fsihi H, Bezrookove V, Reventos J, Louvard D, Capella G, Robine S. APC inactivation associates with abnormal mitosis completion and concomitant BUB1B/MAD2L1 up-regulation. Gastroenterology. 2007;132:2448–2458. doi: 10.1053/j.gastro.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell CM, Green RA, Kaplan KB. APC mutations lead to cytokinetic failures in vitro and tetraploid genotypes in Min mice. The Journal of cell biology. 2007;178:1109–1120. doi: 10.1083/jcb.200703186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K, Sansom O, Jamieson TJ, Meniel V, Clarke A, Nathke IS. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. The Journal of cell biology. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. Journal of cell science. 2004;117:6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- 16.Hernando E, Nahle Z, Juan G, Diaz-Rodriguez E, Alaminos M, Hemann M, Michel L, Mittal V, Gerald W, Benezra R, Lowe SW, Cordon-Cardo C. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 17.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guardavaccaro D, Frescas D, Dorrello NV, Peschiaroli A, Multani AS, Cardozo T, Lasorella A, Iavarone A, Chang S, Hernando E, Pagano M. Control of chromosome stability by the beta-TrCP-REST-Mad2 axis. Nature. 2008;452:365–369. doi: 10.1038/nature06641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, Shi Y, Harper JW, Elledge SJ. SCF[bgr]-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends in cell biology. 2005;15:303–311. doi: 10.1016/j.tcb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Tsou MF, Stearns T. Controlling centrosome number: licenses and blocks. Current opinion in cell biology. 2006;18:74–78. doi: 10.1016/j.ceb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer letters. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Nigg EA. Centrosome duplication: of rules and licenses. Trends in cell biology. 2007;17:215–221. doi: 10.1016/j.tcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, Doxsey SJ. Centrosome defects and genetic instability in malignant tumors. Cancer research. 1998;58:3974–3985. [PubMed] [Google Scholar]
- 25.Nigg EA. Origins and consequences of centrosome aberrations in human cancers. International journal of cancer. 2006;119:2717–2723. doi: 10.1002/ijc.22245. [DOI] [PubMed] [Google Scholar]
- 26.Duensing S, Munger K. Centrosome abnormalities, genomic instability and carcinogenic progression. Biochimica et biophysica acta. 2001;1471:M81–M88. doi: 10.1016/s0304-419x(00)00025-1. [DOI] [PubMed] [Google Scholar]
- 27.Biggins S, Walczak CE. Captivating capture: how microtubules attach to kinetochores. Curr Biol. 2003;13:R449–R460. doi: 10.1016/s0960-9822(03)00369-5. [DOI] [PubMed] [Google Scholar]
- 28.Yuen KW, Montpetit B, Hieter P. The kinetochore and cancer: what's the connection? Current opinion in cell biology. 2005;17:576–582. doi: 10.1016/j.ceb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy cancer,a coincidence or a real link? Trends in cell biology. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 30.van de Weerdt BC, Medema RH. Polo-like kinases: a team in control of the division. Cell Cycle. 2006;5:853–864. doi: 10.4161/cc.5.8.2692. [DOI] [PubMed] [Google Scholar]
- 31.Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the auroras come into view. Current opinion in cell biology. 2003;15:672–683. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature reviews. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 33.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 34.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. The Journal of cell biology. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. The Journal of cell biology. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends in cell biology. 2005;15:386–392. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, Haruki N, Nomoto S, Masuda A, Saji S, Osada H, Takahashi T. Identification of frequent impairment of the mitotic checkpoint molecular analysis of the mitotic checkpoint genes, hsMAD2 p55CDC in human lung cancers. Oncogene. 1999;18:4295–4300. doi: 10.1038/sj.onc.1202807. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Jin DY, Ng RW, Feng H, Wong YC, Cheung AL, Tsao SW. Significance of MAD2 expression to mitotic checkpoint control in ovarian cancer cells. Cancer research. 2002;62:1662–1668. [PubMed] [Google Scholar]
- 39.Ouyang B, Knauf JA, Ain K, Nacev B, Fagin JA. Mechanisms of aneuploidy in thyroid cancer cell lines and tissues: evidence for mitotic checkpoint dysfunction without mutations in BUB1 and BUBR1. Clinical endocrinology. 2002;56:341–350. doi: 10.1046/j.1365-2265.2002.01475.x. [DOI] [PubMed] [Google Scholar]
- 40.Tighe A, Johnson VL, Albertella M, Taylor SS. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO reports. 2001;2:609–614. doi: 10.1093/embo-reports/kve127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saeki A, Tamura S, Ito N, Kiso S, Matsuda Y, Yabuuchi I, Kawata S, Matsuzawa Y. Frequent impairment of the spindle assembly checkpoint in hepatocellular carcinoma. Cancer. 2002;94:2047–2054. doi: 10.1002/cncr.10448. [DOI] [PubMed] [Google Scholar]
- 42.Yoon DS, Wersto RP, Zhou W, Chrest FJ, Garrett ES, Kwon TK, Gabrielson E. Variable levels of chromosomal instability and mitotic spindle checkpoint defects in breast cancer. The American journal of pathology. 2002;161:391–397. doi: 10.1016/S0002-9440(10)64194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Current opinion in genetics & development. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3443–3448. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HS, Park KH, Kim SA, Wen J, Park SW, Park B, Gham CW, Hyung WJ, Noh SH, Kim HK, Song SY. Frequent mutations of human Mad2, but not Bub1, in gastric cancers cause defective mitotic spindle checkpoint. Mutation research. 2005;578:187–201. doi: 10.1016/j.mrfmmm.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Cummins JM, Shen D, Cahill DP, Jallepalli PV, Wang TL, Parsons DW, Traverso G, Awad M, Silliman N, Ptak J, Szabo S, Willson JK, Markowitz SD, Goldberg ML, Karess R, Kinzler KW, Vogelstein B, Velculescu VE, Lengauer C. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer research. 2004;64:2998–3001. doi: 10.1158/0008-5472.can-04-0587. [DOI] [PubMed] [Google Scholar]
- 47.Sieber OM, Heinimann K, Tomlinson IP. Genomic instability--the engine of tumorigenesis? Nat Rev Cancer. 2003;3:701–708. doi: 10.1038/nrc1170. [DOI] [PubMed] [Google Scholar]
- 48.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annual review of cell and developmental biology. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 49.Bardi G, Pandis N, Fenger C, Kronborg O, Bomme L, Heim S. Deletion of 1p36 as a primary chromosomal aberration in intestinal tumorigenesis. Cancer research. 1993;53:1895–1898. [PubMed] [Google Scholar]
- 50.Bomme L, Bardi G, Pandis N, Fenger C, Kronborg O, Heim S. Clonal karyotypic abnormalities in colorectal adenomas: clues to the early genetic events in the adenoma-carcinoma sequence. Genes, chromosomes & cancer. 1994;10:190–196. doi: 10.1002/gcc.2870100307. [DOI] [PubMed] [Google Scholar]
- 51.Bardi G, Parada LA, Bomme L, Pandis N, Willen R, Johansson B, Jeppsson B, Beroukas K, Heim S, Mitelman F. Cytogenetic comparisons of synchronous carcinomas and polyps in patients with colorectal cancer. British journal of cancer. 1997;76:765–769. doi: 10.1038/bjc.1997.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bomme L, Bardi G, Pandis N, Fenger C, Kronborg O, Heim S. Cytogenetic analysis of colorectal adenomas: karyotypic comparisons of synchronous tumors. Cancer genetics and cytogenetics. 1998;106:66–71. doi: 10.1016/s0165-4608(98)00047-8. [DOI] [PubMed] [Google Scholar]
- 53.Sieber O, Heinimann K, Tomlinson I. Genomic stability and tumorigenesis. Seminars in cancer biology. 2005;15:61–66. doi: 10.1016/j.semcancer.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Rudner AD, Murray AW. The spindle assembly checkpoint. Current opinion in cell biology. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- 55.Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Liu T, Fang Y, Xie S, Huang X, Mahmood R, Ramaswamy G, Sakamoto KM, Darzynkiewicz Z, Xu M, Dai W. BUBR1 deficiency results in abnormal megakaryopoiesis. Blood. 2004;103:1278–1285. doi: 10.1182/blood-2003-06-2158. [DOI] [PubMed] [Google Scholar]
- 57.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nature genetics. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 58.Iwanaga Y, Chi YH, Miyazato A, Sheleg S, Haller K, Peloponese JM, Jr, Li Y, Ward JM, Benezra R, Jeang KT. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer research. 2007;67:160–166. doi: 10.1158/0008-5472.CAN-06-3326. [DOI] [PubMed] [Google Scholar]
- 59.Kalitsis P, Earle E, Fowler KJ, Choo KH. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes & development. 2000;14:2277–2282. doi: 10.1101/gad.827500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker DJ, Jeganathan KB, Malureanu L, Perez-Terzic C, Terzic A, van Deursen JM. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. The Journal of cell biology. 2006;172:529–540. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Jeganathan K, Malureanu L, Baker DJ, Abraham SC, van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. The Journal of cell biology. 2007;179:255–267. doi: 10.1083/jcb.200706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Putkey FR, Cramer T, Morphew MK, Silk AD, Johnson RS, McIntosh JR, Cleveland DW. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Developmental cell. 2002;3:351–365. doi: 10.1016/s1534-5807(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 64.Burds AA, Lutum AS, Sorger PK. Generating chromosome instability through the simultaneous deletion of Mad2 and p53. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11296–11301. doi: 10.1073/pnas.0505053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger PK, Murty VV, Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 66.Kalitsis P, Fowler KJ, Griffiths B, Earle E, Chow CW, Jamsen K, Choo KH. Increased chromosome instability but not cancer predisposition in haploinsufficient Bub3 mice. Genes, chromosomes & cancer. 2005;44:29–36. doi: 10.1002/gcc.20215. [DOI] [PubMed] [Google Scholar]
- 67.Babu JR, Jeganathan KB, Baker DJ, Wu X, Kang-Decker N, van Deursen JM. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. The Journal of cell biology. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. The EMBO journal. 2005;24:1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Developmental cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Bruggeman SW, van Lohuizen M. Controlling stem cell proliferation: CKIs at work. Cell Cycle. 2006;5:1281–1285. doi: 10.4161/cc.5.12.2806. [DOI] [PubMed] [Google Scholar]
- 71.Foijer F, te Riele H. Check, double check: the G2 barrier to cancer. Cell Cycle. 2006;5:831–836. doi: 10.4161/cc.5.8.2687. [DOI] [PubMed] [Google Scholar]
- 72.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Earnshaw WC, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91:313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- 74.Hudson DF, Fowler KJ, Earle E, Saffery R, Kalitsis P, Trowell H, Hill J, Wreford NG, de Kretser DM, Cancilla MR, Howman E, Hill L, Cutts SM, Irvine DV, Choo KH. Centromere protein B null mice are mitotically and meiotically normal but have lower body and testis weights. The Journal of cell biology. 1998;141:309–319. doi: 10.1083/jcb.141.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Castro AV, Shamanski FL, Meneses JJ, Lovato TL, Vogel KG, Moyzis RK, Pedersen R. Centromeric protein B null mice are viable with no apparent abnormalities. Developmental biology. 1998;201:135–143. doi: 10.1006/dbio.1998.9005. [DOI] [PubMed] [Google Scholar]
- 76.Kapoor M, Montes de Oca Luna R, Liu G, Lozano G, Cummings C, Mancini M, Ouspenski I, Brinkley BR, May GS. The cenpB gene is not essential in mice. Chromosoma. 1998;107:570–576. doi: 10.1007/s004120050343. [DOI] [PubMed] [Google Scholar]
- 77.Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KH. Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1148–1153. doi: 10.1073/pnas.97.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalitsis P, Fowler KJ, Earle E, Hill J, Choo KH. Targeted disruption of mouse centromere protein C gene leads to mitotic disarray and early embryo death. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1136–1141. doi: 10.1073/pnas.95.3.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Current opinion in cell biology. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 80.Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 81.Cutts SM, Fowler KJ, Kile BT, Hill LL, O'Dowd RA, Hudson DF, Saffery R, Kalitsis P, Earle E, Choo KH. Defective chromosome segregation, microtubule bundling and nuclear bridging in inner centromere protein gene (Incenp)-disrupted mice. Human molecular genetics. 1999;8:1145–1155. doi: 10.1093/hmg/8.7.1145. [DOI] [PubMed] [Google Scholar]
- 82.Dai W, Wang Q, Liu T, Swamy M, Fang Y, Xie S, Mahmood R, Yang YM, Xu M, Rao CV. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer research. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 83.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 84.Maandag EC, van der Valk M, Vlaar M, Feltkamp C, O'Brien J, van Roon M, van der Lugt N, Berns A, te Riele H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. The EMBO journal. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 86.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 87.Percy MJ, Myrie KA, Neeley CK, Azim JN, Ethier SP, Petty EM. Expression mutational analyses of the human MAD2L1 gene in breast cancer cells. Genes, chromosomes & cancer. 2000;29:356–362. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1044>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 88.Wang X, Jin DY, Wong YC, Cheung AL, Chun AC, Lo AK, Liu Y, Tsao SW. Correlation of defective mitotic checkpoint with aberrantly reduced expression of MAD2 protein in nasopharyngeal carcinoma cells. Carcinogenesis. 2000;21:2293–2297. doi: 10.1093/carcin/21.12.2293. [DOI] [PubMed] [Google Scholar]
- 89.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 90.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 91.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 92.Schaeffer AJ, Nguyen M, Liem A, Lee D, Montagna C, Lambert PF, Ried T, Difilippantonio MJ. E6 and E7 oncoproteins induce distinct patterns of chromosomal aneuploidy in skin tumors from transgenic mice. Cancer research. 2004;64:538–546. doi: 10.1158/0008-5472.can-03-0124. [DOI] [PubMed] [Google Scholar]
- 93.Lentini L, Pipitone L, Di Leonardo A. Functional inactivation of pRB results in aneuploid mammalian cells after release from a mitotic block. Neoplasia (New York N.Y) 2002;4:380–387. doi: 10.1038/sj.neo.7900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lentini L, Iovino F, Amato A, Di Leonardo A. Centrosome amplification induced by hydroxyurea leads to aneuploidy in pRB deficient human and mouse fibroblasts. Cancer letters. 2006;238:153–160. doi: 10.1016/j.canlet.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. The EMBO journal. 2006;25:2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Molecular biology of the cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science (New York N.Y. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 98.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M. Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4482–4486. doi: 10.1073/pnas.92.10.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vecchione A, Baldassarre G, Ishii H, Nicoloso MS, Belletti B, Petrocca F, Zanesi N, Fong LY, Battista S, Guarnieri D, Baffa R, Alder H, Farber JL, Donovan PJ, Croce CM. Fez1/Lzts1 absence impairs Cdk1/Cdc25C interaction during mitosis and predisposes mice to cancer development. Cancer cell. 2007;11:275–289. doi: 10.1016/j.ccr.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pines J, Rieder CL. Re-staging mitosis: a contemporary view of mitotic progression. Nature cell biology. 2001;3:E3–E6. doi: 10.1038/35050676. [DOI] [PubMed] [Google Scholar]
- 101.Mikhailov A, Cole RW, Rieder CL. DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr Biol. 2002;12:1797–1806. doi: 10.1016/s0960-9822(02)01226-5. [DOI] [PubMed] [Google Scholar]
- 102.Matsusaka T, Pines J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. The Journal of cell biology. 2004;166:507–516. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ, Ward IM, Saya H, Fang G, van Deursen J, Chen J. Chfr is required for tumor suppression and Aurora A regulation. Nature genetics. 2005;37:401–406. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 104.Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8699–8704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rao CV, Yang YM, Swamy MV, Liu T, Fang Y, Mahmood R, Jhanwar-Uniyal M, Dai W. Colonic tumorigenesis in BubR1+/−ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4365–4370. doi: 10.1073/pnas.0407822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database integrated data-mining platform. Neoplasia (New York N.Y. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olesen SH, Thykjaer T, Orntoft TF. Mitotic checkpoint genes hBUB1, hBUB1B, hBUB3 and TTK in human bladder cancer, screening for mutations and loss of heterozygosity. Carcinogenesis. 2001;22:813–815. doi: 10.1093/carcin/22.5.813. [DOI] [PubMed] [Google Scholar]
- 108.Scintu M, Vitale R, Prencipe M, Gallo AP, Bonghi L, Valori VM, Maiello E, Rinaldi M, Signori E, Rabitti C, Carella M, Dallapiccola B, Altomare V, Fazio VM, Parrella P. Genomic instability and increased expression of BUB1B and MAD2L1 genes in ductal breast carcinoma. Cancer letters. 2007;254:298–307. doi: 10.1016/j.canlet.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 109.Tsukasaki K, Miller CW, Greenspun E, Eshaghian S, Kawabata H, Fujimoto T, Tomonaga M, Sawyers C, Said JW, Koeffler HP. Mutations in the mitotic check point gene, MAD1L1, in human cancers. Oncogene. 2001;20:3301–3305. doi: 10.1038/sj.onc.1204421. [DOI] [PubMed] [Google Scholar]
- 110.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Current opinion in genetics & development. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 111.Draviam VM, Stegmeier F, Nalepa G, Sowa ME, Chen J, Liang A, Hannon GJ, Sorger PK, Harper JW, Elledge SJ. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nature cell biology. 2007;9:556–564. doi: 10.1038/ncb1569. [DOI] [PubMed] [Google Scholar]
- 112.Goldstein DM, Gray NS, Zarrinkar PP. High-throughput kinase profiling as a platform for drug discovery. Nat Rev Drug Discov. 2008;7:391–397. doi: 10.1038/nrd2541. [DOI] [PubMed] [Google Scholar]
- 113.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, Settleman J, Giovannini M, Jacks T. Differential effects of oncogenic K-Ras N-Ras on proliferation, differentiation and tumor progression in the colon. Nature genetics. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weaver BA, Bonday ZQ, Putkey FR, Kops GJ, Silk AD, Cleveland DW. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. The Journal of cell biology. 2003;162:551–563. doi: 10.1083/jcb.200303167. [DOI] [PMC free article] [PubMed] [Google Scholar]