Abstract
Objective
Administered in supra-physiologic doses, the hormone melatonin may reduce blood pressure, particularly nocturnal blood pressure. However, whether lower physiologic levels of melatonin are an independent risk factor for the development of hypertension has never been reported.
Methods
We examined the association between first morning urine melatonin levels and the risk of developing hypertension among 554 young women without baseline hypertension who were followed for 8 years. Cox proportional hazards models were adjusted for age, body mass index, physical activity, alcohol intake, smoking status, urinary creatinine, and family history of hypertension.
Results
During 8 years of follow-up, a total of 125 women developed hypertension. The relative risk for incident hypertension among women in the highest quartile of urinary melatonin (>27.0 ng per mg creatinine) compared to the lowest quartile (<10.1 ng per mg creatinine) was 0.49 (95% confidence intervals, 0.28-0.85; p-trend<0.001).
Conclusions
First morning melatonin levels are independently and inversely associated with incident hypertension; low melatonin production may be a pathophysiologic factor in the development of hypertension.
Keywords: risk factors, hypertension, epidemiology, melatonin
Introduction
Many biologic functions in humans follow 24-hour circadian patterns, including blood pressure, which is normally lower during the night and increases in the morning.1-3 Secretion of melatonin by the pineal gland also follows a circadian rhythm; it is secreted exclusively during the dark phase of a light-dark cycle.4 In animals, melatonin receptors are found in the central nervous system and on endothelial cells.5 In these animals, melatonin leads to relaxation of the aorta and pulmonary circulation, a decrease in sympathetic outflow, and an increase in nitric oxide production.5-7 In humans, short-term physiologic studies demonstrated relaxation of carotid and axillary arteries, as well as reductions in circulating norepinephrine, after administration of oral melatonin;8, 9 furthermore, some short-term interventional studies comparing supra-physiologic doses of melatonin to placebo demonstrated reductions in either nocturnal or 24-hour blood pressure.8-12
Whether physiologic differences in melatonin levels can predict long-term risk of developing hypertension among non-hypertensive individuals, however, has never been examined. Nocturnal plasma melatonin levels are accurately reflected by first morning urinary levels of 6-sulphatoxy-melatonin (aMT6s).13, 14 In order to determine the independent association between urinary aMT6s levels and the risk of incident hypertension, we conducted a prospective cohort study of 554 premenopausal women from Nurses’ Health Study II (NHS II).
Methods
Study population
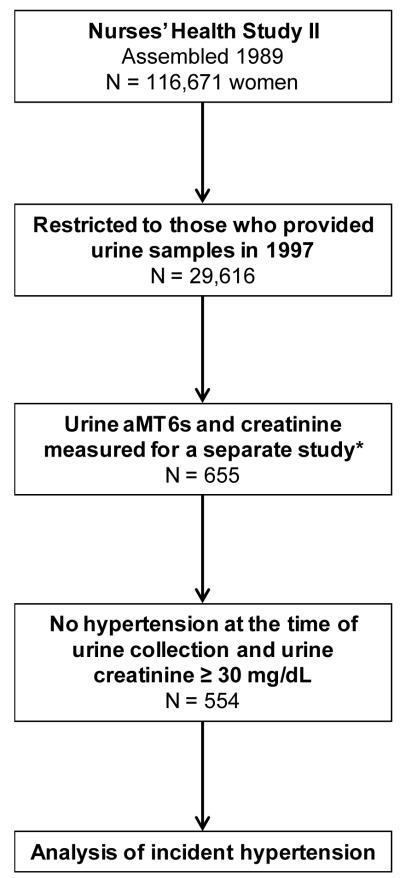
The derivation of the study population for the current analysis is summarized in the Figure. The second Nurses’ Health Study (NHS II) is a prospective cohort of 116,671 female registered nurses who were 25 to 42 years of age when they returned an initial questionnaire in 1989. Subsequent questionnaires have been mailed every 2 years to update information on health-related behaviors and medical events. From 1997 through 1999, 29,613 women agreed to submit urine samples, which were returned by courier and stored in liquid nitrogen freezers. The women who provided urine specimens were similar to those who did not except that they were somewhat less likely to be current smokers. Of the women who provided first morning urine specimens, 576 were selected for a previous case-control study of urinary melatonin and breast cancer risk (192 breast cancer cases and 384 controls).15 In addition to this breast cancer case-control study, 79 women who were part of a study of hormone stability also had urine melatonin measurements and were included. The urine specimens from these women were assayed for creatinine and for aMT6s, the major metabolite of melatonin which is highly correlated with blood and saliva melatonin levels.13, 16-21
Figure. Assembly of the Study Population.
*The 655 woman who already had aMT6s and creatinine measurements included 576 women from a nested case-control study of breast cancer (192 women who ultimately developed breast cancer, and 384 who did not), plus 79 women who were included in a study of hormone stability.
Our study population for this particular analysis of urinary melatonin and hypertension risk included the subset of these 655 women who had available urinary aMT6s and creatinine levels, and who did not already have hypertension when they submitted their urine specimens. In addition, we further excluded women with very low urinary creatinine concentrations (< 30 mg/dL), leaving 554 women in the analysis.22 All 554 women were pre-menopausal at the time of urine collection. A spot urine with <30 mg/dL of creatinine is currently defined by the World Health Organization as too dilute for adequate analysis.22 The institutional review board at Brigham and Women’s Hospital reviewed and approved this study.
Ascertainment of Normalized Urinary 6-Sulphatoxy-Melatonin
Urine aMT6s was assayed in the Endocrine Core Laboratory of Dr. M. Wilson (Emory University, Atlanta) using an enzyme-linked immunosorbent assay (ALPCO, Windham, NH); the coefficient of variation (CV) for this assay using blinded quality control samples was 13.9%. Urine creatinine (CV=9.2%) was assayed in the same laboratory using a modified Jaffe method. For each participant, urine aMT6s was divided by urine creatinine to obtain a normalized urine aMT6s, expressed as ng/mg.
Ascertainment of hypertension
The baseline and biennial follow-up questionnaires inquired about physician-diagnosed hypertension and the year of diagnosis. Self-reported hypertension was found to be highly reliable in the Nurses’ Health Study.23 In a subset of women who reported hypertension, medical record review confirmed a documented systolic and diastolic BP, respectively, higher than 140 mmHg and 90 mmHg in 100% and higher than 160mmHg and 95 mmHg in 77% of participants.23 Women were considered to have prevalent hypertension at the time of urine collection if they reported a diagnosis of hypertension on any previous biennial questionnaire or reported use of antihypertensive medications on the questionnaire that preceded urine collection. To analyze incident hypertension, women with prevalent hypertension were excluded. Among those without prevalent hypertension at baseline, women were considered to have incident hypertension if they reported, after the date of urine collection, an initial diagnosis of hypertension or new use of antihypertensive medications.
Ascertainment of other factors
Age, body mass index (BMI, kg/m2), smoking status were obtained from a supplemental questionnaire that accompanied the urine collection. Physical activity (metabolic equivalent task scores, METS) and alcohol intake (g/d) were self-reported on the main biennial questionnaire just preceding urine collection. Women reported the amount of time they spent doing various light to vigorous physical activities (in minutes per week), including walking, jogging, running, swimming, racquet sports, bicycling, or other aerobic activity; questionnaire derived information about these activities has been validated in comparison to physical activity diaries (r=0.79).24 Alcohol intake was computed from a validated food frequency questionnaire; information about alcohol intake on this questionnaire also compares favorably to alcohol intake recorded in dietary diaries (r=0.90).25 Information about intakes of sodium, potassium, calcium, and magnesium were also available from the food frequency questionnaire. Information on history of hypertension in a first degree relative was available on the 1989 questionnaire.
Statistical Analyses
Because they were not normally distributed, urinary aMT6s/creatinine ratios were examined in quartiles, using the lowest quartile as the reference group. Person-time was counted from the date of urine collection to the date the last biennial questionnaire was returned (2005), and allocated according to exposure status. Person-time was truncated when an event occurred. Participants were censored at the date of death, or, if they did not return a subsequent questionnaire, they were censored at the date the subsequent questionnaire was mailed. Associations between quartiles of normalized urinary aMT6s and incident hypertension were analyzed using Cox proportional hazards regression. We computed hazard ratios (reported as relative risks, RR) for age-adjusted models, as well as multivariable-adjusted models; all multivariable RR reported were adjusted for established hypertension risk factors including age (continuous),26 BMI (continuous),27 the square of BMI ([BMI]2, continuous), physical activity (quintiles),27 smoking (never, past, current), urinary creatinine (continuous), family history of hypertension (yes/no), and alcohol intake (7 categories).27 Tests for linear trend were assessed using log-transformed normalized aMT6s levels as a continuous variable.
In the principal analysis, all 554 participants (including both breast cancer cases and controls, as well as women from the hormone stability study) were included. Because breast cancer diagnosis may in some way have impacted hypertension risk, we performed a secondary analysis after excluding breast cancer cases (N=401). Other secondary analyses included: analysis of non-normalized aMT6s levels (without dividing by creatinine), analysis of log-transformed aMT6s/creatinine ratio as a continuous variable, adjustment for night shift work, adjustment for baseline blood pressure, and exclusion of current smokers from the analyses.
In order to provide an effect size estimate demonstrating the potential clinical relevance of melatonin in the development of hypertension, we calculated a hypothetical attributable fraction using the highest three quartiles of urine aMT6s/creatinine ratios as the “unexposed” group and the lowest quartile as the “exposed” group. The adjusted RR for the exposed group was used in the following equation to calculate an attributable fraction, where Pe is equal to 25% (the prevalence of the exposed):
The interpretation of the hypothetical attributable fraction is the % of all cases of incident hypertension that conceivably could have been prevented if all women in the population had aMT6s/creatinine ratios that fell in the “unexposed” range.
For all RRs, we calculated 95% confidence intervals (95% CI). All p-values are two-tailed. Statistical tests were performed using SAS statistical software, version 9 (SAS Institute Inc, Cary, NC).
Results
Baseline Characteristics
The median aMT6s/creatinine ratio was 17.0 ng/mg (interquartile range [IQR], 10.1-27.0). The median age was 44 years (IQR, 41-48), and the median BMI was 23.6 kg/m2 (IQR, 21.8-26.6). Baseline characteristics are shown in Table 1 stratified by quartile of aMT6s/creatinine ratio. With increasing quartile, median values for age, BMI, and current smoking all were lower. Alcohol intake, physical activity, urinary creatinine, and family history of hypertension did not appear to differ consistently with differences in the aMT6s/creatinine ratio.
Table 1.
Baseline Characteristics
| Quartiles of aMT6s/creatinine ratio, ng/mg (median, range) | ||||
|---|---|---|---|---|
| 5.5 (<10.1) |
13.8 (10.1-16.9) |
21.0 (17.0-27.0) |
36.9 (>27.0) |
|
| No. of participants Characteristic |
138 | 139 | 139 | 138 |
| Median (IQR) | ||||
| Age, y | 46 (42-49) | 45 (42-48) | 44 (40-47) | 44 (41-47) |
| BMI, kg/m2 | 25.5 (22.3-29.0) | 23.8 (22.1-26.6) | 23.4 (21.4-26.5) | 22.6 (20.8-24.6) |
| Physical activity, METS | 11.9 (4.6-30.8) | 15.1 (5.5-26.1) | 11.5 (5.4-23.5) | 15.6 (5.7-29.9) |
| Alcohol intake, g/d | 1.8 (0-6.0) | 1.3 (0-5.7) | 0.9 (0-4.1) | 1.8 (0-3.7) |
| Urine creatinine, mg/dL | 97 (63-142) | 102 (70-155) | 100 (71-150) | 88 (58-126) |
| % | ||||
| Current smoking | 11.6 | 7.9 | 4.3 | 6.5 |
| Past smoking | 29.7 | 21.6 | 18.7 | 25.4 |
| Family history of hypertension | 46.4 | 50.4 | 50.4 | 42.8 |
Urinary 6-Sulphatoxy-Melatonin and Risk of Hypertension
Among 554 women without prevalent hypertension at the time of urine collection, there were 125 cases of incident hypertension identified through 8-years of follow-up. Compared to women whose urine aMT6s/creatinine ratio was in the lowest quartile (<10.1 ng/mg), the multivariable RR (adjusting for age, BMI, BMI2, physical activity, smoking status, urinary creatinine, family history of hypertension, and alcohol intake) for incident hypertension among those in the highest quartile (>27.0 ng/mg) was 0.49 (95% CI, 0.28-0.85; p-trend<0.001; Table 2).
Table 2.
Normalized Urinary Melatonin and Risk of Incident Hypertension
| Quartiles of aMT6s/creatinine ratio, ng/mg | |||||
|---|---|---|---|---|---|
| 5.5 (<10.1) |
13.8 (10.1-16.9) |
21.0 (17.0-27.0) |
36.9 (>27.0) |
p-trend | |
| No. of participants | 138 | 139 | 139 | 138 | |
| No. of cases | 49 | 33 | 22 | 21 | |
| Age-adjusted RR (95% CI) | 1.0 (ref) | 0.63 (0.40-0.98) | 0.42 (0.25-0.70) | 0.38 (0.23-0.65) | <0.001 |
| Multivariable RR (95% CI) | 1.0 (ref) | 0.66 (0.42-1.05) | 0.42 (0.25-0.72) | 0.49 (0.28-0.85) | <0.001 |
Multivariable models adjusted for age, BMI, (BMI)2, physical activity, alcohol intake, smoking, status, urine creatinine, and family history of hypertension.
We also performed various secondary analyses. First, we adjusted for the number of night shifts worked in the two week prior to submitting the urine sample in addition to our full multivariable model; the RR for the highest compared to lowest quartile of melatonin creatinine ratio was 0.51 (95% CI, 0.29-0.89). Addition of dietary intakes of sodium, potassium, calcium, and magnesium to the multivariable models likewise had little impact on the results; the RR for the highest aMT6s/creatinine ratio was 0.48 (95% CI, 0.27-0.83). Exclusion of current smokers from the analysis did not substantially impact the results (multivariable RR=0.54 comparing the highest to lowest quartile, 95% CI, 0.31-0.97; p-trend=0.001). Urine aMT6s/creatinine ratios were inversely related to both baseline systolic blood pressure (correlation coefficient [r] = −0.12, p=0.005) and baseline diastolic blood pressure (r = −0.12, p=0.004). Even though blood pressure is presumably on the causal pathway, we analyzed the association between melatonin and incident hypertension after also controlling for baseline blood pressure. The RR comparing the highest to lowest quartile was 0.57 (95% CI, 0.32-1.01).
We also analyzed our data after excluding women who were selected based upon developing breast cancer (N=401 with 89 cases). Although with less statistical power in this analysis the confidence intervals were wider, the multivariable RR in the highest compared to lowest quartile of urine aMT6s/creatinine was not different from the analysis that included breast cancer cases and was still significant (RR=0.49, 95% CI, 0.26-0.93; p-trend=0.01).
In addition, we analyzed quartiles of urinary aMT6s that were not normalized to the urine creatinine concentration. In this analysis, women in the highest compared to lowest quartile of non-normalized aMT6s had a multivariable RR for incident hypertension of 0.40 (95% CI, 0.20-0.78). Furthermore, we analyzed the urinary aMT6s/creatinine ratio as a continuous variable after log-transformation (log-transformation resulted in a distribution resembling normal). The RR for a one unit higher log-transformed aMT6s/creatinine ratio was 0.68 (95% CI, 0.55-0.84).
Finally, we repeated our analyses after stratifying by BMI (<25 kg/m2 vs. ≥25 kg/m2). The multivariable RR for incident hypertension comparing the highest to lowest quartile of urine aMT6s/creatinine was 0.39 (95% CI, 0.18-0.85) among those women whose BMI was <25 kg/m2, and was 0.45 (95% CI, 0.18-1.11) among women whose BMI was >25 kg/m2 (p-value for interaction = 0.20).
To estimate a hypothetical attributable fraction, we defined the lowest aMT6s/creatinine quartile (<10.1 ng/mg) as the “exposed” group, and the highest three quartiles (≥10.1 ng/mg) as the “unexposed” group. The adjusted RR comparing the exposed to unexposed group was 1.88 (95% CI, 1.27-2.77). Using the aforementioned equation, we calculated the hypothetical attributable fraction (the percent of new cases of hypertension that could conceivably have been avoided if all women had aMT6s/creatinine ratios ≥10.1 ng/mg) as 18% (95% CI, 5-31%).
Discussion
Among 554 non-hypertensive young women higher morning urinary aMT6s levels, and by implication higher nocturnal plasma melatonin levels, were independently associated with a decreased risk of developing hypertension. To our knowledge, this is the first prospective study to demonstrate an association between melatonin levels with long-term hypertension risk.
The existing literature dealing with melatonin and hypertension in humans consists of eight small, short-term (1-4 weeks duration) interventional studies of supra-physiologic melatonin doses (ranging from 1-10 mg per dose).8-12, 28-30 While the mean physiologic nocturnal melatonin concentrations range from 7.8 to 158 pg/mL (depending on study and hour of measurement),31-34 a single 1 mg dose of melatonin can lead to a 570-fold increase in plasma melatonin levels within 90 minutes.9 Of these eight studies, two were performed in adolescent type 1 diabetic patients, and demonstrated no significant effect on blood pressure.28, 29 A third study randomized 40 treated hypertensive patients in a crossover design to 4 weeks of melatonin (5 mg nightly) or placebo and observed a 6.5/4.9 mmHg increase in average 24-hour blood pressure among treated individuals.30 The remaining five studies demonstrated significant reductions in at least one blood pressure outcome. Two studies of hypertensive individuals documented decreases of 6/4 mmHg and 6/3 mmHg in nocturnal blood pressure, without any change in daytime blood pressure.10, 12 Another randomized crossover study among normotensive individuals noted a 6.4 mmHg decrease in 24-hour average systolic blood pressure.11 The remaining two studies conducted in young healthy men and women measured only acute changes in blood pressure 90 minutes after dosing and found reductions of 9.5/7.5 mmHg and 9/4 mmHg.8, 9
In addition to melatonin, rotating shift work has been demonstrated in some studies to impact blood pressure. Yamasaki et al demonstrated that women working evening and night-shifts were 6-times less likely to appropriately “dip” their blood pressure during the subsequent sleep period compared to women working a day shift.35 In addition, women working night or evening shifts had a paradoxical increase in urine catecholamines during sleep.35 Similar findings were reported by Lo et al and Kitamura et al, who observed a switch from dipping to non-dipping status following a night shift,36,37 although 4 days of continuous night shift work appeared to restore the normal dipping pattern, suggesting that cycle disruption (rather than night work per se) is key to disruption of normal blood pressure cycles.36 In our study, however, controlling for the number of night shifts worked in two weeks prior to urine collection did not substantially attenuate the association; thus, rotating night work cannot fully explain the association between melatonin and hypertension.
The potential mechanisms mediating melatonin’s impact on blood pressure have been studied extensively in laboratory experiments. In cultured aortic rings, melatonin increases nitric oxide levels and decreases levels of reactive oxygen species;38 additionally, the vasoactive effects of norepinephrine and phenylephrine are blunted by melatonin.38, 39 Treatment of spontaneously hypertensive rats with melatonin decreases blood pressure while increasing nitric oxide activity, decreasing the levels of reactive oxygen species and catecholamines, and causing relaxation of vascular smooth muscle cells.6, 39-41 By comparison, few mechanistic studies have been performed in humans. In two studies of healthy young women and men, treatment with melatonin led to relaxation of carotid and axillary arteries, and decreased circulating levels of norepinephrine.8, 9
Alternative explanations exist for the inverse association between melatonin and hypertension risk. It is well established that sleep disordered breathing is a risk factor for the development of hypertension,42 presumably through activation of the sympathetic nervous system.43, 44 Moreover, there is some evidence that sleep disordered breathing leads to abnormal patterns in melatonin secretion,33, 45 and individuals with obstructive sleep apnea have lower overall nocturnal plasma melatonin levels.33 Therefore, lower melatonin levels may simply be one mechanism linking sleep disordered breathing with hypertension. On the other hand, rats are nocturnal animals with peak melatonin levels occurring during periods of wakefulness; therefore, the blood pressure lowering effect of melatonin (at least in rats) cannot be explained as being mediated by disrupted sleep. Another possible explanation for the inverse association we observed is 25-hydroxyvitamin D (25[OH]D), which has emerged as an independent risk factor for hypertension.46 It is conceivable that individuals with lower nocturnal melatonin production, potentially because of more nocturnal wakefulness, happen to also have less daytime sun exposure and lower 25(OH)D levels.
Our study has limitations that deserve mention. First, each participant submitted a single morning urine specimen rather than multiple specimens or an overnight collection. Because of potential night to night within-person variation in melatonin production, some participants in our study likely had their melatonin status misclassified. However, the correlation between two urinary aMT6s measurements collected 3 years apart among 80 participants of this cohort was 0.72, suggesting that profound misclassification was unlikely. Furthermore, this type of misclassification, if it occurred, would likely be random and therefore tend to bias the results toward an underestimation of the true association. Second, we did not directly measure our participants’ blood pressure. Nevertheless, all participants are trained health professionals, and self-reporting of hypertension has been validated in this cohort. Third, we lacked information about sleep disordered breathing in these participants, and also lacked plasma 25(OH)D measurements; therefore, we could not analyze these factors as either potential mechanisms or confounders. Fourth, the prospective association between urine melatonin and incident hypertension fell just below the significance threshold when we adjusted for baseline blood pressure, so it is conceivable through some unrecognized mechanism that higher blood pressures suppress melatonin production or that a third, yet unknown, factor is present which influences both melatonin and blood pressure. Finally, our study population is predominately white and entirely female, so the results may not be generalizable to other racial groups or to men.
Conclusion
In conclusion, higher melatonin excretion is associated with a decreased risk of developing hypertension. Low melatonin levels may be a pathophysiologic factor in the development of hypertension. Experimental studies are required to further explore potential mechanisms, and additional clinical studies are needed to confirm this association. Melatonin may hold promise as a novel risk factor for developing hypertension, paving the way for long-term interventions to prevent high blood pressure.
Acknowledgments
Sources of Funding: This work was funded by the American Heart Association grant 0535401T, and NIH grants HL079929-01A2 and CA50385.
Footnotes
Conflicts of Interest / Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 2.Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. discussion 130-132. [PubMed] [Google Scholar]
- 3.Parati G, Valentini M. Prognostic relevance of blood pressure variability. Hypertension. 2006;47(2):137–138. doi: 10.1161/01.HYP.0000198542.51471.c4. [DOI] [PubMed] [Google Scholar]
- 4.Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998;3(1):13–22. doi: 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- 5.Paulis L, Simko F. Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol Res. 2007;56(6):671–684. doi: 10.33549/physiolres.931236. [DOI] [PubMed] [Google Scholar]
- 6.Weekley LB. Melatonin-induced relaxation of rat aorta: interaction with adrenergic agonists. J Pineal Res. 1991;11(1):28–34. doi: 10.1111/j.1600-079x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 7.Weekley LB. Effects of melatonin on isolated pulmonary artery and vein: role of the vascular endothelium. Pulm Pharmacol. 1993;6(2):149–154. doi: 10.1006/pulp.1993.1019. [DOI] [PubMed] [Google Scholar]
- 8.Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol. 1999;83(9):1417–1419. doi: 10.1016/s0002-9149(99)00112-5. [DOI] [PubMed] [Google Scholar]
- 9.Cagnacci A, Arangino S, Angiolucci M, Maschio E, Melis GB. Influences of melatonin administration on the circulation of women. Am J Physiol. 1998;274(2 Pt 2):R335–338. doi: 10.1152/ajpregu.1998.274.2.R335. [DOI] [PubMed] [Google Scholar]
- 10.Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006;119(10):898–902. doi: 10.1016/j.amjmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Lusardi P, Preti P, Savino S, Piazza E, Zoppi A, Fogari R. Effect of bedtime melatonin ingestion on blood pressure of normotensive subjects. Blood Press Monit. 1997;2(2):99–103. [PubMed] [Google Scholar]
- 12.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43(2):192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 13.Baskett JJ, Cockrem JF, Antunovich TA. Sulphatoxymelatonin excretion in older people: relationship to plasma melatonin and renal function. J Pineal Res. 1998;24(1):58–61. doi: 10.1111/j.1600-079x.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Graham C, Cook MR, Kavet R, Sastre A, Smith DK. Prediction of nocturnal plasma melatonin from morning urinary measures. J Pineal Res. 1998;24(4):230–238. doi: 10.1111/j.1600-079x.1998.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 15.Schernhammer ES, Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst. 2005;97(14):1084–1087. doi: 10.1093/jnci/dji190. [DOI] [PubMed] [Google Scholar]
- 16.Arendt J, Bojkowski C, Franey C, Wright J, Marks V. Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24-hour rhythm with atenolol. J Clin Endocrinol Metab. 1985;60(6):1166–1173. doi: 10.1210/jcem-60-6-1166. [DOI] [PubMed] [Google Scholar]
- 17.Cook MR, Graham C, Kavet R, Stevens RG, Davis S, Kheifets L. Morning urinary assessment of nocturnal melatonin secretion in older women. J Pineal Res. 2000;28(1):41–47. doi: 10.1034/j.1600-079x.2000.280106.x. [DOI] [PubMed] [Google Scholar]
- 18.Lang U, Kornemark M, Aubert ML, Paunier L, Sizonenko PC. Radioimmunological determination of urinary melatonin in humans: correlation with plasma levels and typical 24-hour rhythmicity. J Clin Endocrinol Metab. 1981;53(3):645–650. doi: 10.1210/jcem-53-3-645. [DOI] [PubMed] [Google Scholar]
- 19.Leibenluft E, Feldman-Naim S, Turner EH, Schwartz PJ, Wehr TA. Salivary and plasma measures of dim light melatonin onset (DLMO) in patients with rapid cycling bipolar disorder. Biol Psychiatry. 1996;40(8):731–735. doi: 10.1016/0006-3223(95)00488-2. [DOI] [PubMed] [Google Scholar]
- 20.Nowak R, McMillen IC, Redman J, Short RV. The correlation between serum and salivary melatonin concentrations and urinary 6-hydroxymelatonin sulphate excretion rates: two non-invasive techniques for monitoring human circadian rhythmicity. Clin Endocrinol (Oxf) 1987;27(4):445–452. doi: 10.1111/j.1365-2265.1987.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 21.Wetterberg L. Melatonin in humans physiological and clinical studies. J Neural Transm Suppl. 1978;13:289–310. [PubMed] [Google Scholar]
- 22.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 24.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133(8):810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 26.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 27.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavallo A, Daniels SR, Dolan LM, Bean JA, Khoury JC. Blood pressure-lowering effect of melatonin in type 1 diabetes. J Pineal Res. 2004;36(4):262–266. doi: 10.1111/j.1600-079X.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 29.Cavallo A, Daniels SR, Dolan LM, Khoury JC, Bean JA. Blood pressure response to melatonin in type 1 diabetes. Pediatr Diabetes. 2004;5(1):26–31. doi: 10.1111/j.1399-543X.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- 30.Lusardi P, Piazza E, Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: a 24-hour study. Br J Clin Pharmacol. 2000;49(5):423–427. doi: 10.1046/j.1365-2125.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345(8962):1408. doi: 10.1016/s0140-6736(95)92600-3. [DOI] [PubMed] [Google Scholar]
- 32.Hallam KT, Olver JS, Chambers V, Begg DP, McGrath C, Norman TR. The heritability of melatonin secretion and sensitivity to bright nocturnal light in twins. Psychoneuroendocrinology. 2006;31(7):867–875. doi: 10.1016/j.psyneuen.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez C, Abreu J, Abreu P, Castro A, Jimenez A. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J. 2007;30(3):496–500. doi: 10.1183/09031936.00051906. [DOI] [PubMed] [Google Scholar]
- 34.Luboshitzky R, Ophir U, Nave R, Epstein R, Shen-Orr Z, Herer P. The effect of pyridoxine administration on melatonin secretion in normal men. Neuro Endocrinol Lett. 2002;23(3):213–217. [PubMed] [Google Scholar]
- 35.Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32(3):417–423. doi: 10.1161/01.hyp.32.3.417. [DOI] [PubMed] [Google Scholar]
- 36.Kitamura T, Onishi K, Dohi K, Okinaka T, Ito M, Isaka N, Nakano T. Circadian rhythm of blood pressure is transformed from a dipper to a non-dipper pattern in shift workers with hypertension. J Hum Hypertens. 2002;16(3):193–197. doi: 10.1038/sj.jhh.1001328. [DOI] [PubMed] [Google Scholar]
- 37.Lo SH, Liau CS, Hwang JS, Wang JD. Dynamic Blood Pressure Changes and Recovery Under Different Work Shifts in Young Women. Am J Hypertens. 2008;21(7):759–764. doi: 10.1038/ajh.2008.186. [DOI] [PubMed] [Google Scholar]
- 38.Anwar MM, Meki AR, Rahma HH. Inhibitory effects of melatonin on vascular reactivity: possible role of vasoactive mediators. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130(3):357–367. doi: 10.1016/s1532-0456(01)00261-7. [DOI] [PubMed] [Google Scholar]
- 39.KL A, Wu L, Foucart S, de Champlain J. Impaired basal sympathetic tone and alpha1-adrenergic responsiveness in association with the hypotensive effect of melatonin in spontaneously hypertensive rats. Am J Hypertens. 1998;11(2):219–229. doi: 10.1016/s0895-7061(97)00401-9. [DOI] [PubMed] [Google Scholar]
- 40.Girouard H, Denault C, Chulak C, de Champlain J. Treatment by n-acetylcysteine and melatonin increases cardiac baroreflex and improves antioxidant reserve. Am J Hypertens. 2004;17(10):947–954. doi: 10.1016/j.amjhyper.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Pechanova O, Zicha J, Paulis L, Zenebe W, Dobesova Z, Kojsova S, Jendekova L, Sladkova M, Dovinova I, Simko F, Kunes J. The effect of N-acetylcysteine and melatonin in adult spontaneously hypertensive rats with established hypertension. Eur J Pharmacol. 2007;561(1-3):129–136. doi: 10.1016/j.ejphar.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 42.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 43.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103(6):1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90(4):1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 45.Ulfberg J, Micic S, Strom J. Afternoon serum-melatonin in sleep disordered breathing. J Intern Med. 1998;244(2):163–168. doi: 10.1046/j.1365-2796.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- 46.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]