Abstract
This report introduces a gonadotrope-specific cre transgenic mouse capable of ablating floxed genes in mature pituitary gonadotropes. Initial analysis of this transgenic line, Tg(Lhb-cre)1Sac, reveals expression is limited to the pituitary cells that produce luteinizing hormone beta, beginning appropriately at e17.5. Cre activity is detectable by a reporter gene in nearly every LHβ-producing cell, but the remaining hormone producing cell types and other organs exhibit little to no activity. We used the Tg(Lhb-cre)1Sac strain to assess the role Pitx2 in gonadotrope function. The gonadotrope specific Pitx2 knockout mice exhibit normal expression of LHβ, sexual maturation, and fertility, suggesting that Pitx2 is not required for gonadotrope maintenance or for regulated production of gonadotropins.
Gonadotropes are one of five mature hormone-producing cell types of the anterior pituitary gland. Upon release of GnRH from the hypothalamus, gonadotropes secrete heterodimeric hormones composed of a β-subunit of luteinizing hormone (LHβ) and follicle-stimulating hormone (FSHβ), in combination with a common α-subunit (CGA). These glycoprotein hormones act on the gonads to promote sexual maturity and fertility (Kendall et al., 1995). Transgene ablation of gonadotropes results in near-elimination of the gonadotrope population, hypogonadism, infertility, reduced circulating LH, and reduced levels of PRL (Kendall et al., 1991; Seuntjens et al., 1999).
Identifying the factors that regulate gonadotrope differentiation is a critical step towards understanding fertility at the molecular level. Transcription factors and signaling pathways important for gonadotrope specification and function include Gata2, Pitx2, Nr5a1 (SF1), Egr1, Inhibin, ActRII and ActivRI (Burns and Matzuk, 2002; Charles et al., 2006; Charles et al., 2005; Kumar et al., 2003; Suh et al., 2002; Vesper et al., 2005; Yoshikawa et al., 2000). Examining the roles of some individual genes can be difficult because they are important in many physiological systems essential for embryonic development.
To overcome this problem, cre transgenic lines have been developed with activity in the pituitary gland such as Cga-cre, Nr5a1-cre, and a tetracycline inducible Cga-cre. The Cga-cre transgene targets anterior pituitary cells effectively, although it also exhibits activity in muscle (Charles et al., 2006; Cushman et al., 2000; Zhao et al., 2001). Nr5a1-cre drives cre expression in pre-gonadotropes early in development of the anterior pituitary. Nr5a1 is also expressed in the gonads, adrenal cortex, spleen and hypothalamus, making it difficult to distinguish between the loss of gene function in gonadotropes and the other steroidogenic tissues (Bingham et al., 2006). A transgenic line with tetracycline-inducible Cga-cre activity is effective for gonadotrope-specific excision, but the requirement for drug administration is not compatible with all applications (Naik et al., 2006).
Pitx2 is expressed in the developing and adult anterior pituitary gland (Charles et al., 2005; Gage and Camper, 1997; Semina et al., 1996). Analysis of Pitx2 null mice established the role of Pitx2 in the development of many organs, including the pituitary gland, but mutants die at e14.5 due to severe heart defects (Gage et al., 1999a; Kitamura et al., 1999; Lin et al., 1999; Lu et al., 1999). To study the role of Pitx2 in later pituitary development, a hypomorphic allele (Pitx2neo) was generated. Mice homozygous for the hypomorphic allele live until postnatal day 1 (P1) allowing for pituitary cell specification studies, (Gage et al., 1999a; Suh et al., 2002). Pitx2neo/neo pituitaries lack gonadotropes, and have a decrease in somatotropes and thyrotropes. There is little or no detectable expression of important gonadotrope transcription factors Gata2, Egr1, and Nr5a1 in the hypomorphic pituitaries, demonstrating that the dosage of Pitx2 is critical for the differentiation of the gonadotropes (Suh, et. al., 2002).
In adult mouse pituitaries, the majority of Pitx2-positive cells co-express the gonadotropins or thyrotropin, suggesting that Pitx2 also plays a role in the maintenance of these cell types (Charles, et. al., 2005). To test this hypothesis, we have developed a gonadotrope-specific cre transgenic line to ablate Pitx2 from mature pituitary gonadotropes.
The Tg(Lhb-cre)1Sac transgenic construct (Figure 1a) was generated using a 776 bp sequence from the bovine Lhb promoter (Virgin et al., 1985) fused to eGFP (Kaspar et al., 2002); however, we were unable to detect eGFP in transgenic mice. Thus, founder mice were mated to the RosaGFP cre-reporter mice (B6;129-Gt(ROSA)26Sortm2Sho/J), generating Tg(Lhb-cre)1Sac;RosaGFP progeny. Cre activity was estimated from GFP fluorescence in whole pituitaries (Figure 1b). In the highest expressing line, only the anterior lobe had detectable fluorescent signals. Furthermore, the proportion of discrete RosaGFP positive cells in the anterior lobe is consistent with the gonadotrope population size (Figure 1e, h). We assessed cell specificity by examining colocalization of GFP with LHβ using immunohistochemistry (Figure 1f, i). We detected colocalization of GFP with over 80% of LHβ immunoreactive cells and with little or no LHβ negative cells, indicating that the Tg(Lhb-cre)1Sac line is gonadotrope specific in the pituitary (Figure 1g, j).
Figure 1. Gonadotrope-specific cre transgenic construct and expression.
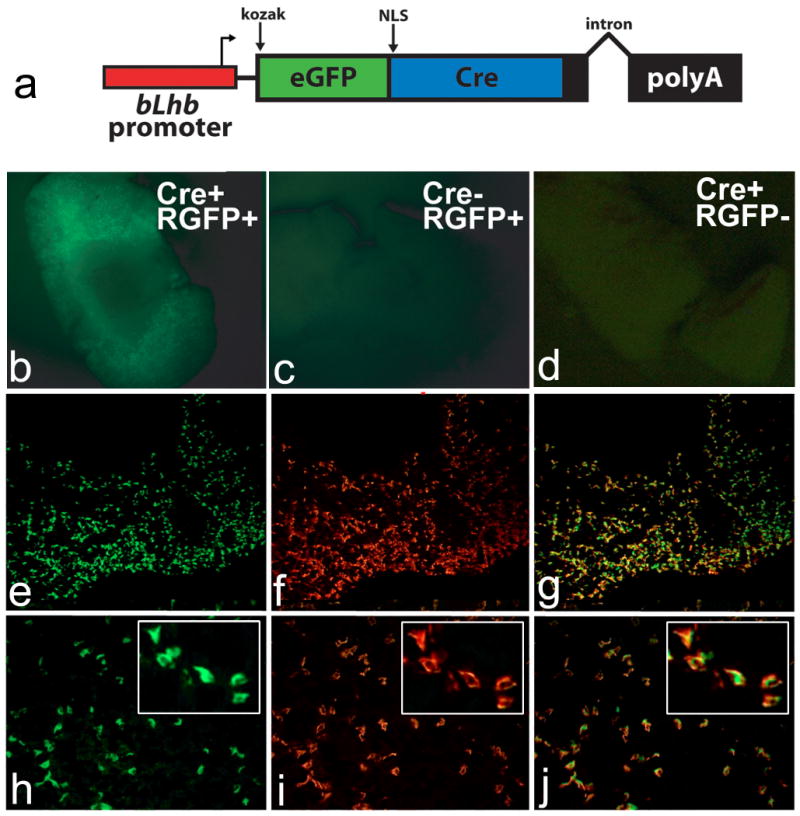
The Tg(Lhb-cre)1Sac transgene (a) is controlled by the bovine Lhb promoter, containing a KOZAK translation initiation site, eGFP and cre recombinase fusion gene with a nuclear localization signal (NLS), and a poly adenylation signal and intron from the rabbit β-globin gene. Pituitaries from mice with the Tg(Lhb-cre)1Sac transgene (Cre+) and the Rosa26-GFP reporter allele (RGFP+) (b, e-j) were compared with pituitaries from Cre-, RGFP+ (c) and Cre+, RGFP- (d). GFP fluorescence is detected in Cre+, RGFP+ mice (b), but not in mice carrying only the reporter (cre-, RGFP+) (c) or only the transgene (Cre+, RGFP-) (d). Frozen pituitary sections of a mouse carrying the Tg(Lhb-cre)1Sac transgene and the Rosa26-GFP reporter allele (e-j) at 10× (e-g) and 20× (h-j) power objective lenses, with high magnification insets. Colabeling with antibody for GFP (e, h) and LHβ (f, i) is detected by yellow color in merged picture (g, j) in over 80% of anterior pituitary cells.
We utilized a separate cre-reporter strain to analyze expression of the transgene in other pituitary cell types because colocalization with other pituitary hormones requires co-immunohistochemistry with antibodies generated in the same species. Transgenic mice were mated to the Rosa26LacZ cre reporter mouse strain (officially B6;129S4-Gt(ROSA)26Sortm1Sor/J) (Friedrich and Soriano, 1991; Zambrowicz et al., 1997). Progeny of the cross, Tg(Lhb-cre)1Sac;Rosa26LacZ, yielded varying degrees of lacZ expression, although the penetrance of expression in the majority of pituitaries (n=14) was comparable to expectations for gonadotropes (Figure 2a, c). For each pituitary, 3-6 slides were examined for each hormone. Little to no colocalization is detectable between the lacZ-positive cells and GH, ACTH, PRL or TSHβ in most pituitaries (Figure 2d-g). Rarely, in the Tg(Lhb-cre)1Sac;Rosa26LacZ pituitaries, overlapping expression is detectable with a GH- or TSHβ-producing cell, which may represent normal dual-hormone producing cells (Burrows et al., 1999). The penetrance and specificity of cre reporter activity within LHβ positive cells suggests this line will be an effective tool in the deletion of pituitary transcription factors in gonadotropes.
Figure 2. Tg(Lhb-cre)1Sac transgene is active in late gestation and is specific to pituitary gonadotropes.
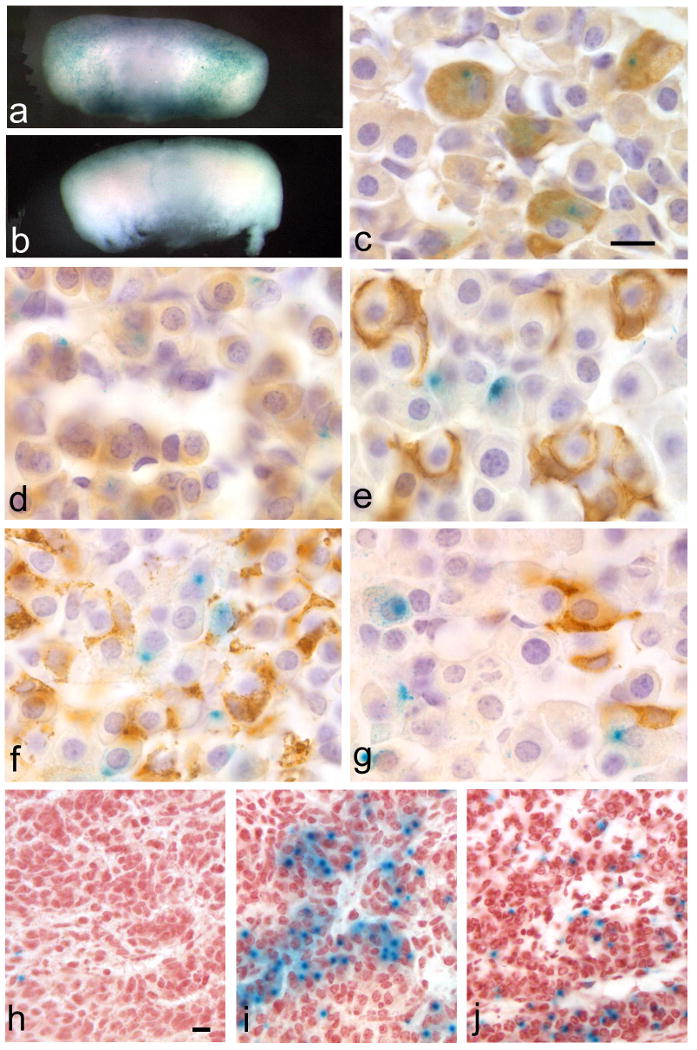
Adult pituitaries of Tg(Lhb-cre)1Sac; Rosa26LacZ reporter progeny (a) and non-transgenic (b) stained with X-gal. Staining is detectable in the adult anterior lobe of the Tg(Lhb-cre)1Sac;Rosa26LacZ reporter progeny (a, c-g) but not in the non-transgenic (b). Immunohistochemical staining of pituitary hormones shows colocalization of the X-gal staining with LHβ immunoreactivity (c), but very little or no colocalization with GH (d), POMC (e), PRL (f) or TSHβ immunoreactivity (g). Tg(Lhb-cre)1Sac; Rosa26LacZ embryos reveal the onset of transgene activity by X-gal staining in only one or two cells at e16.5 (h), and increased penetrance of X-gal staining comparable to normal LHβ expression at e17.5 (i) and e18.5 (j). Scale bars represent 10μm for panels c-g (100× magnification) and panels h-j (40× magnification).
Tg(Lhb-cre)1Sac;Rosa26LacZ mice were used to determine when cre excision begins (Figure 2h-j). At e16.5, very little to no cre activity is detected by X-gal staining (n=3/3). At e17.5, when endogenous LHβ is detectable by immunohistochemistry, cre activity is detectable (n=3/3). X-gal staining is also present at e18.5 (n=5/5), and in the adult pituitary (Figure 2a).
Adult tissues were examined from Tg(Lhb-cre)1Sac;Rosa26LacZ mice (n=4-8) to ascertain the degree of transgene leakiness in non-pituitary tissues (see Figure 3). There was no evidence of transgene activity in the heart, lung, liver, pancreas, spleen, or skeletal muscle (data not shown). Trophoblast-derived placental tissues are negative, and the visceral yolk sac and chorioallantoic plate are positive at e16.5 (n=3/4, data not shown). No transgene activity is detected in the hypothalamus at e17.5 (a). Transgene activity is detected at very low levels in a few cells in the cortex of the brain (b) and the kidney (c), but not in the adrenal gland (d). Faint X-gal staining is detectable in some corpora lutea and follicles of the ovary in Tg(Lhb-cre)1Sac;Rosa26LacZ mice (e) as well as in non-transgenic mice (f), suggesting background β-galactosidase activity. Activity is evident in some seminiferous tubules of the testes in Tg(Lhb-cre)1Sac;Rosa26LacZ mice (g) but not in non-transgenics. Because active Lhβ transcription has been found in both the rat and human testis, it is possible that the lacZ staining in the testis results from appropriate activity of the Lhβ promoter (Berger et al., 1994; Zhang et al., 1995a; Zhang et al., 1995b).
Figure 3. Limited ectopic activity of Tg(Lhb-cre)1Sac transgene.
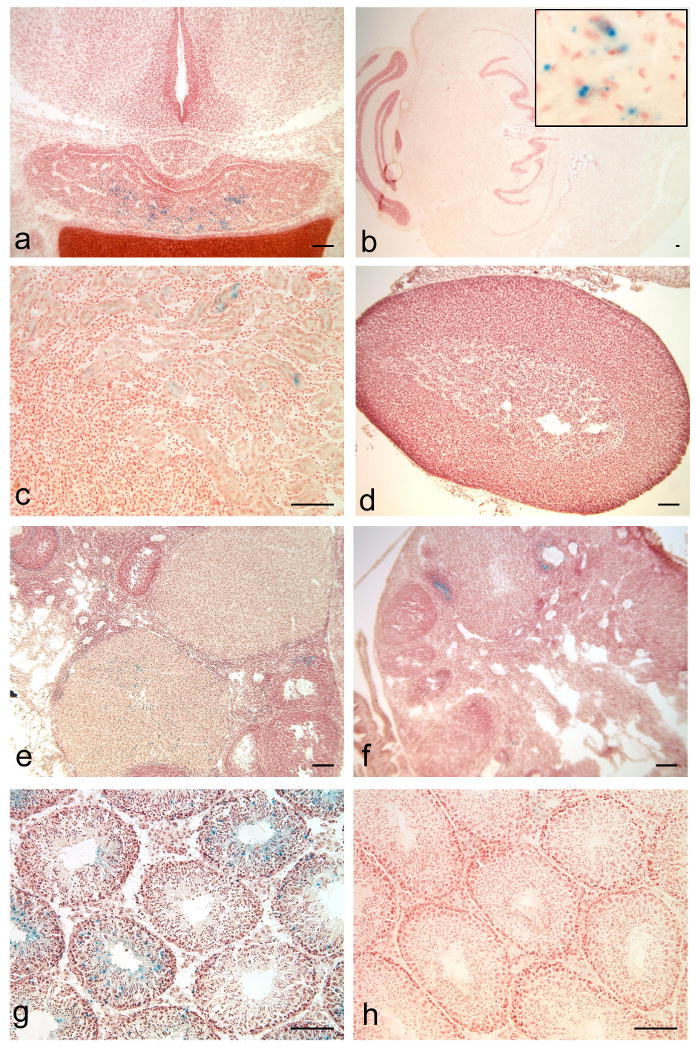
Tissues from Tg(Lhb-cre)1Sac;Rosa26-LacZ reporter animals stained with X-gal reveal that cre activity is high in the e17.5 anterior pituitary, but is absent from the hypothalamus (a). Limited evidence of cre activity is detected by X-gal staining in rare cells in the adult brain (b) and kidney (c), but not in the adrenal gland (d). Low levels of X-gal staining are detected in a few ovarian follicles and corpora lutea of from Tg(Lhb-cre)1Sac;Rosa26LacZ double transgenic mice (e) above background X-gal staining in Rosa26LacZ mice lacking the cre transgene (f). X-gal staining is present in some seminiferous tubules of the double-transgenic mice (g), but not in wild-type testis (h). Scale bars represent 100μm for all panels. Inset (b) width represents 100μm.
To determine the importance of Pitx2 in gonadotrope maintenance, we crossed the Tg(Lhb-cre)1Sac mice to Pitx2tm2Sac (Pitx2+/-). The resulting Pitx2+/-;Tg(Lhb-cre)1Sac progeny were mated to Pitx2flox/flox mice to delete Pitx2 post-germ cell development, generating gonadotrope-specific Pitx2-deficient offspring (Pitx2flox/-; Tg(Lhb-cre)1Sac). To verify that Pitx2 deletion was effective in the gonadotropes of the knock-out mice, double-immunohistochemistry was used to detect the colocalization of PITX2 and LHβ in adult pituitaries. In the wild type mouse pituitary, nearly all LHβ cells clearly express PITX2 (Figure 4a). As expected, not all PITX2-positive cells express LHβ since PITX2 is also expressed in thyrotropes (Charles et al., 2005). In the knock-out pituitaries, no PITX2 immunoreactivity was detected in the majority of LHβ cells. The remaining PITX2 expression is likely coming from thyrotropes (Figure 4b). Hence, the Tg(Lhb-cre)1Sac line effectively knocked out Pitx2 in gonadotropes.
Figure 4. Normal LH expression in gonadotrope-specific Pitx2 knockout mice.

(a) Most LHβ immunostained cells (red) co-stain with PITX2-specific antibodies (green) in Pitx2flox/+ adult mouse pituitary (white arrows), but about half of the PITX2 immuno-positive cells do not co-stain with LHβ-specific antibodies (yellow arrows). (b) Pitx2flox/-; Tg(Lhb-cre)1Sac adult mouse pituitary lacks cells that co-stain with antibodies for PITX2 and LHβ. Cells immunostained for PITX2 only (yellow arrow), or LHβ only (pink arrows) predominate, while PITX2 and LHβ co-stained cells are quite rare (white arrow).
To assess the effect of gonadotrope-specific Pitx2 deletion on fertility, four Pitx2flox/-;Tg(Lhb-cre)1Sac mice were each mated with four 6-week old C57BL/6J female mice. All the females mated to the Pitx2flox/-;Tg(Lhb-cre)1Sac males presented a copulation plug and carried full-term pregnancies. Four Pitx2flox/-;Tg(Lhb-cre)1Sac female mice were mated with adult C57BL/6J male mice, and all females were able to carry a full term pregnancy, give live births, and care for pups.
A thorough comparison of the physical phenotype of gonadotrope-specific Pitx2 knock-out mice and wild type mice was performed. Gonadotropin deficiency can cause males to exhibit a diminished growth spurt, resulting in adult males and females of equivalent weight (Davey et al., 1999). Average weights of gonadotrope-specific Pitx2 knock-out mice and their normal, cre-negative litter mates (Pitx2flox/+) were recorded for males and females aged 2-12 weeks. There was no evidence of growth differences in the mutant and normal mice, and males grew larger than females (data not shown). Gonadotropin deficiency can cause striking reductions in the weight of the testes and seminal vesicles (Cunha, 1972). The size and weight of the gonads from both sexes of knock-out and wild type mice were similar. Misregulation of gonadotropins can cause precocious puberty or delayed sexual maturation (Cushman et al., 2001; Kendall et al., 1991; Vesper et al., 2006). The vaginal openings in female knock-out and wild type mice occurred at the same time, as did the detection of major urinary proteins (MUPs) in the urine of male mice (data not shown). Taken together, these data suggest that Pitx2 expression in gonadotropes is dispensable for pituitary-gonadal axis function after birth.
It was surprising that deletion of Pitx2 in pituitary gonadotropes had no apparent effect on gonadal development, puberty, or fertility. Many studies have shown that Pitx2 transactivates the genes encoding gonadotropin subunits Cga, Lhb and Fshb (Suszko et al., 2003; Tremblay et al., 2000). It is possible that Pitx2 is dispensable in differentiated gonadotropes because Pitx1 is capable of compensation. Pitx1 and Pitx2 have overlapping functions in the activation of Lhx3 in Rathke's pouch development at e10.5 and in establishing a normally sized pituitary anlage (Charles et al., 2005; Gage et al., 1999a; Suh et al., 2002). Pitx1 and Pitx2 also have overlapping functions in other organs (Gage et al., 1999a, 1999b; Lanctot et al., 1999; Marcil et al., 2003; Suh et al., 2002). Because Pitx1 mutant pituitaries appear minimally affected, and Pitx2 null pituitaries are extremely underdeveloped, Pitx2 is more important than Pitx1 in pituitary development (Suh et al., 2002). Pitx1 might be more critical in adult pituitary function than Pitx2. This prediction could be tested with a floxed allele of Pitx1 and the Tg(Lhb-cre)1Sac line that we report here.
In this study, we characterize a cre transgenic line that is a valuable tool for deleting genes in gonadotropes and demonstrate its effectiveness by using it to assess Pitx2 function in mature gonadotropes.
Methods
Generation of Transgene Construct
Tg(Lhb-cre)1Sac mice were generated using a 776 bp sequence from the bovine Lhb promoter. The gonadotrope specificity of the promoter was demonstrated using a herpes simplex virus thymidine kinase reporter gene (Keri et al., 1994; Virgin et al., 1985). We generated a construct for microinjection that uses the bovine LHβ subunit gonadotrope-specific promoter to drive the expression of an enhanced GFP-cre recombinase fusion gene (Kaspar et al., 2002). We engineered a consensus KOZAK sequence (GCCGCCACCATGG) to encourage efficient translation of the transgene mRNA transcript into protein and inserted a poly adenylation signal and intron from the rabbit β-globin gene for efficient nuclear processing of the transcript (Figure 1a). The complete construct was linearized and microinjected into F2 zygotes from (C57BL/6J × SJL) F1 (Hogan, 1994). Live progeny were genotyped via PCR for the presence of cre recombinase in from genomic DNA prepared from a tail biopsy.
Mice
Cre mice were maintained by crossing with C57BL/6J mice from The Jackson Laboratory. All mice were maintained at the University of Michigan under the guidelines of the Unit for Laboratory Animal Medicine and the University Committee for Care and Use of Animals. Cre mice were identified by PCR amplification of genomic DNA with primers 5′-gcataaccagtgaaacagcattgctg-3′ and 5′-ggacatgttcagggatcgccaggcg-3′ under the following conditions: 94°C for 3 minutes, followed by 32 cycles of 94°C for 30 seconds, 60°C for 60 seconds and 72°C for 90 seconds, and a final 10 minute extension at 72°C. For transgene analysis, experimental animals carried one allele of the cre transgene and one allele of the reporter gene, while controls were negative for the cre transgene but positive for a reporter gene. B6;129-Gt(ROSA)26Sortm2Sho/J and B6;129S4-Gt(ROSA)26Sortm1Sor/J reporter mice were obtained from The Jackson Laboratory and maintained as homozygotes. Genotyping of B6;129-Gt(ROSA)26Sortm2Sho/J mice was performed by PCR using primers 5′-ggcttaaaggctaacctgatgtg-3′, 5′gcgaagagtttgtcctcaacc-3′ and 5′-ggagcgggagaaatggatatg-3′ under the following conditions: 94°C for 3 minutes, followed by 35 cycles of 94°C for 30 seconds, 64°C for 60 seconds and 72°C for 60 seconds, and a final 10 minute extension at 72°C. The B6;129-Gt(ROSA)26Sortm2Sho/J band is 1,146 bp and wild-type band is 374 bp. Pitx2flox/-;Tg(Lhb-cre)1Sac mice were generated by mating B6-Pitx2+/- mice with Tg(Lhb-cre)1Sac positive mice. The Pitx2+/-;Tg(Lhb-cre)1Sac offspring were mated to B6-Pitx2flox/flox mice, and genotyping was performed as previously described (Gage et al., 1999a).
Tissue preparation and histology
Adult pituitaries were collected at six weeks of age or later and fixed for 1 hour in 4% formaldehyde in PBS. X-gal staining was performed as previously described (Brinkmeier et al., 1998). After staining, pituitaries were fixed in 4% formaldehyde overnight, rinsed in PBS, dehydrated and embedded in a Citadel 1000 (Thermo Electric, Chesire, England) paraffin embedding machine, and sectioned coronally at 5μm thickness. Immunohistochemistry for the pituitary hormones was performed as previously described (Kendall et al., 1991). For embryos, noon of the day of the vaginal plug is designated as embryonic day 0.5. Embryos and adult organs were dissected, frozen on dry ice and stored at -80°C. Embryos were embedded in OCT (Sakura Finetek Co., Torrance, CA) and cryosectioned at 16μm thickness. After X-gal staining, sections were counterstained for 2 minutes with 1% neutral red stain plus 4% sodium acetate:glacial acetic acid. Sections were dehydrated and mounted with xylene:permount 1:2 (Fisher) mounting media. Whole mount capture of GFP fluorescence of freshly dissected Tg(Lhb-cre)1Sac × B6;129-Gt(ROSA)26Sortm2Sho/J progeny was achieved using a Leica MZFL III stereo/dissecting fluorescent microscope. Pituitaries were fixed for 1 hour in 4% formaldehyde and rinsed in 1× PBS. Immunohistochemistry for GFP and fluorescent LHβ expression was performed on 8-10μm pituitary cryosections using a rabbit anti-GFP Alexa Fluor 488 (Molecular Probes, Eugene, OR) antibody overnight at 4°C, diluted in a blocking solution comprised of 3% normal donkey serum, 1% BSA, and 0.5% Triton-X100 in 1× PBS. Slides were washed 3 times for 5 minutes using 0.5% Triton-X100 in 1× PBS. Guinea pig anti-LHβ antibody (NHPP) was diluted 1:100 in the same block and incubated for 1 hour at room temperature. Washing with 0.5% Triton-X100 in PBS was followed by a one hr incubation with biotinylated anti-guinea pig secondary antibody (Jackson Immunoresearch), 0.5% Triton-X100/PBS washes. Streptavidin-TRITC was added for 1 hour at 1:200 dilution. Rabbit-anti-PITX2 antibody was generated by Dr. Tord Hjalt (Lund University, Sweden) and provided by Dr. Philip Gage (University of Michigan, Ann Arbor). PITX2 antibody was diluted 1:100 in the same block described above and 100 μl was placed on each slide over night at 4°C. Secondary detection was performed as described above using biotinylated anti-rabbit antibody (Vector Laboratories) then washed in PBS/Triton, mounted with fluorescent mounting media and images were captured using a Leica DMRB fluorescent microscope.
Acknowledgments
We would like to thank the University of Michigan DNA Sequencing Core, Dr. John Nilson for providing the Lhb promoter, Dr. Parlow at the National Hormone and Pituitary Program for providing the pituitary hormone antibodies, Drs. Philip Gage and Donna Martin for the Pitx2flox/flox mice, Drs. Fred Gage and Hoonkyo Suh for supplying the eGFPcre fusion construct, Kailey Owens for help with genotyping the mice, Galina Gavrilina for preparation of transgenic mice, and the University of Michigan Transgenic Animal Model Core.
Footnotes
Author contributions: MAC prepared the transgene construct and identified the best cre line by breeding with the GFP reporter strain as part of his PhD thesis research. AHM used the Tg(Lhb-cre) line to analyze the role of Pitx2 in gonadotropes. MAP analyzed the developmental, tissue-specific, and cell-specific expression of Tg(Lhb-cre) and played the leading role in preparation of the manuscript.
References
- Berger P, Kranewitter W, Madersbacher S, Gerth R, Geley S, Dirnhofer S. Eutopic production of human chorionic gonadotropin beta (hCG beta) and luteinizing hormone beta (hLH beta) in the human testis. FEBS Lett. 1994;343:229–233. doi: 10.1016/0014-5793(94)80561-x. [DOI] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Gordon DF, Dowding JM, Saunders TL, Kendall SK, Sarapura VD, Wood WM, Ridgway EC, Camper SA. Cell-specific expression of the mouse glycoprotein hormone alpha-subunit gene requires multiple interacting DNA elements in transgenic mice and cultured cells. Mol Endocrinol. 1998;12:622–633. doi: 10.1210/mend.12.5.0103. [DOI] [PubMed] [Google Scholar]
- Burns KH, Matzuk MM. Minireview: genetic models for the study of gonadotropin actions. Endocrinology. 2002;143:2823–2835. doi: 10.1210/endo.143.8.8928. [DOI] [PubMed] [Google Scholar]
- Burrows HL, Douglas KR, Seasholtz AF, Camper SA. Genealogy of the Anterior Pituitary Gland: Tracing a Family Tree. Trends Endocrinol Metab. 1999;10:343–352. doi: 10.1016/s1043-2760(99)00189-7. [DOI] [PubMed] [Google Scholar]
- Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–1903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Epithelio-mesenchymal interactions in primordial gland structures which become responsive to androgenic stimulation. Anat Rec. 1972;172:179–195. doi: 10.1002/ar.1091720206. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Watkins-Chow DE, Brinkmeier ML, Raetzman LT, Radak AL, Lloyd RV, Camper SA. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum Mol Genet. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- Davey HW, Park SH, Grattan DR, McLachlan MJ, Waxman DJ. STAT5b-deficient mice are growth hormone pulse-resistant. Role of STAT5b in sex-specific liver p450 expression. J Biol Chem. 1999;274:35331–35336. doi: 10.1074/jbc.274.50.35331. [DOI] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Camper SA. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet. 1997;6:457–464. doi: 10.1093/hmg/6.3.457. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999a;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. The bicoid-related Pitx gene family in development. Mamm Genome. 1999b;10:197–200. doi: 10.1007/s003359900970. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, et al. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Springs Harbor: Cold Springs Harbor Press; 1994. [Google Scholar]
- Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee KF, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci U S A. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Saunders TL, Jin L, Lloyd RV, Glode LM, Nett TM, Keri RA, Nilson JH, Camper SA. Targeted ablation of pituitary gonadotropes in transgenic mice. Mol Endocrinol. 1991;5:2025–2036. doi: 10.1210/mend-5-12-2025. [DOI] [PubMed] [Google Scholar]
- Keri RA, Wolfe MW, Saunders TL, Anderson I, Kendall SK, Wagner T, Yeung J, Gorski J, Nett TM, Camper SA, et al. The proximal promoter of the bovine luteinizing hormone beta-subunit gene confers gonadotrope-specific expression and regulation by gonadotropin-releasing hormone, testosterone, and 17 beta-estradiol in transgenic mice. Mol Endocrinol. 1994;8:1807–1816. doi: 10.1210/mend.8.12.7708066. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Agno J, Janovick JA, Conn PM, Matzuk MM. Regulation of FSHbeta and GnRH receptor gene expression in activin receptor II knockout male mice. Mol Cell Endocrinol. 2003;212:19–27. doi: 10.1016/j.mce.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Moreau A, Chamberland M, Tremblay ML, Drouin J. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–1810. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Marcil A, Dumontier E, Chamberland M, Camper SA, Drouin J. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130:45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- Naik K, Pittman It, Wolfe A, Miller RS, Radovick S, Wondisford FE. A novel technique for temporally regulated cell type-specific Cre expression and recombination in the pituitary gonadotroph. J Mol Endocrinol. 2006;37:63–69. doi: 10.1677/jme.1.02053. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Seuntjens E, Vankelecom H, Quaegebeur A, Vande Vijver V, Denef C. Targeted ablation of gonadotrophs in transgenic mice affects embryonic development of lactotrophs. Mol Cell Endocrinol. 1999;150:129–139. doi: 10.1016/s0303-7207(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. Regulation of the rat follicle-stimulating hormone beta-subunit promoter by activin. Mol Endocrinol. 2003;17:318–332. doi: 10.1210/me.2002-0081. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Goodyer CG, Drouin J. Transcriptional properties of Ptx1 and Ptx2 isoforms. Neuroendocrinology. 2000;71:277–286. doi: 10.1159/000054547. [DOI] [PubMed] [Google Scholar]
- Vesper AH, Raetzman LT, Camper SA. Role of PROP1 in gonadotrope differentiation and puberty. Endocrinology. 2006 doi: 10.1210/en.2005-1080. [DOI] [PubMed] [Google Scholar]
- Virgin JB, Silver BJ, Thomason AR, Nilson JH. The gene for the beta subunit of bovine luteinizing hormone encodes a gonadotropin mRNA with an unusually short 5′-untranslated region. J Biol Chem. 1985;260:7072–7077. [PubMed] [Google Scholar]
- Yoshikawa SI, Aota S, Shirayoshi Y, Okazaki K. The ActR-I activin receptor protein is expressed in notochord, lens placode and pituitary primordium cells in the mouse embryo. Mech Dev. 2000;91:439–444. doi: 10.1016/s0925-4773(99)00320-2. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FP, Markkula M, Toppari J, Huhtaniemi I. Novel expression of luteinizing hormone subunit genes in the rat testis. Endocrinology. 1995a;136:2904–2912. doi: 10.1210/endo.136.7.7540543. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Rannikko A, Huhtaniemi I. Isolation and characterization of testis-specific cDNAs for luteinizing hormone beta-subunit in the rat. Biochem Biophys Res Commun. 1995b;210:858–865. doi: 10.1006/bbrc.1995.1737. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Hypomorphic phenotype in mice with pituitary-specific knockout of steroidogenic factor 1. Genesis. 2001;30:65–69. doi: 10.1002/gene.1034. [DOI] [PubMed] [Google Scholar]


