Abstract
Neuropeptides modulate neuronal function in hippocampus, but the organization of hippocampal sites of peptide release and actions is not fully understood. The stress-associated neuropeptide corticotropin releasing hormone (CRH) is expressed in inhibitory interneurons of rodent hippocampus, yet physiological and pharmacological data indicate that it excites pyramidal cells. Here we aimed to delineate the structural elements underlying the actions of CRH, and determine whether stress influenced hippocampal principal cells also via actions of this endogenous peptide.
In hippocampal pyramidal cell layers, CRH was located exclusively in a subset of GABAergic somata, axons and boutons, whereas the principal receptor mediating the peptide’s actions, CRH receptor 1 (CRF1), resided mainly on dendritic spines of pyramidal cells. Acute ‘psychological’ stress led to activation of principal neurons that expressed CRH receptors, as measured by rapid phosphorylation of the transcription factor cyclic AMP responsive element binding protein. This neuronal activation was abolished by selectively blocking the CRF1 receptor, suggesting that stress-evoked endogenous CRH release was involved in the activation of hippocampal principal cells.
Keywords: neuropeptide, CRH, CRF, synapse, CREB, learning and memory
Stress influences hippocampal learning and memory functions (Conrad et al., 1999; McEwen, 1999; McGaugh and Roozendaal, 2002; Pavlides et al., 2002; Roozendaal et al., 2003). At the functional/behavioral level, acute stress, particularly that evoked by restraint, pain or fear, modulates performance of experimental animals in hippocampus-dependent tasks including the Morris water-maze and object recognition tests (Kim et al., 2001). At the cellular level, acute stress has been shown to impair long-term potentiation (LTP) in several hippocampal regions (Blank et al., 2002; Kim and Diamond, 2002).
Logical candidates for mediating these effects of stress include endogenous molecules that are activated and/or released by stressful stimuli. These include glucocorticoids that are secreted from the adrenal gland and penetrate the blood–brain barrier to act upon cognate hippocampal receptors (Reul and de Kloet, 1985; McEwen, 1999; Pavlides and McEwen, 1999), and the stress neurohormone corticotropin releasing hormone (CRH; Vale et al., 1981; see Avishai-Eliner et al., 2002 for a recent review). Significant populations of CRH-expressing neurons have recently been demonstrated in hippocampus, within the pyramidal cell layers of juvenile rats (Chen et al., 2001a). In addition, CRH receptors, particularly the type 1 receptor, CRF1, have been found in the majority of hippocampal pyramidal cells in CA1 and CA3 (Chalmers et al., 1995; Chen et al., 2000; Van Pett et al., 2000).
The complex functional role of glucocorticoids in influencing stress-provoked alteration of LTP and of learning and memory performance has been well documented (Conrad et al., 1999; Alfarez et al., 2003; Korz and Frey, 2003). In parallel, evidence has been mounting for direct actions of CRH on hippocampal neurons (Aldenhoff et al., 1983; Hollrigel et al., 1998; Wang et al., 1998; Blank et al., 2002) and for involvement of this peptide in hippocampal LTP during stress (Wang et al., 1998; Blank et al., 2002). However, the role of native CRH (vs. systemic or distantly released peptide, Bittencourt and Sawchenko, 2000) in stress-evoked enhancement of hippocampal synaptic function has not been clarified. This is interesting also because CRH has been localized to inhibitory interneurons, yet the synthetic peptide functions as an excitatory neuromodulator (Aldenhoff et al., 1983; Smith and Dudek, 1994; Baram and Hatalski, 1998).
Here we studied the architecture of sites of stress-evoked release of native hippocampal CRH, and of the loci of the peptide’s action on the CRF1 receptor. We demonstrate stress-induced rapid activation of CRF1 bearing pyramidal cells, as determined by phosphorylation of the transcription factor cyclic AMP responsive element binding protein (CREB). This rapid phosphorylated CREB (pCREB) induction was abolished by intracranial pre-stress treatment with selective CRF1 antagonists in doses that did not interfere with peripheral CRH receptors. These data suggest that the mechanisms by which stress activates hippocampal pyramidal cells involve secretion of endogenous CRH, likely of hippocampal origin.
EXPERIMENTAL PROCEDURES
Animals and tissue handling
Brains of immature Sprague–Dawley-derived rats (approximately P18, when density of CRH-expressing neurons in hippocampal pyramidal cell layers is maximal; Chen et al., 2001a) and young adult mice (C57/BL6) were used. Animals were born and maintained in a quiet, uncrowded, NIH-approved facility on a 12-h light/dark cycle, and brains were harvested under relatively stress-free conditions (Yan et al., 1998). Animals were undisturbed for 24 h prior to experiments, and were then deeply anesthetized with sodium pentobarbital (100 mg/kg intra-peritoneally) within 45 s of entry into the animal facility. For light and confocal microscope studies, rats were perfused with fresh 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB; pH 7.4, 4 °C). Brains were cryoprotected and stored as described previously (Chen et al., 2001a), then sectioned coronally into 20 µm thick slices using a cryostat. For electron microscopy (EM), mice received pentobarbital at 300 mg/kg, and perfusates consisted of saline followed by a solution of 0.1% glutaraldehyde, 4% paraformaldehyde and 0.2% picric acid in 0.1 M PB. Brains were removed and postfixed in the same fixative overnight, then washed in PB. All experiments were in compliance with National Institutes of Health guidelines and were approved by Institutional Animal Care and Use Committees of University of California at Irvine and University of Freiburg.
Demonstration of endogenous CRH-mediated activation of CRH receptor-bearing neurons
Endogenous CRH release was provoked using a ‘psychological stress’ consisting of 30 min of crowding (four to five animals per cage; cage dimensions 25×15×10 cm) and jostling (Laboratory Rotator; model 1314; Laboratory-Line Instruments, Inc., Melrose Park, IL, USA), because restraint is not a powerful stressor in juvenile rats (Avishai-Eliner et al., 2001). Control groups consisted of stress-free littermates. For rapid neuronal activation studies, animals were anesthetized within 2 min of the end of the stress, then perfused, or killed 60 min after the stress. To study the potential role of endogenous CRH in stress-evoked pCREB induction, a group of stressed animals were pre-infused with the selective CRF1 blocker R121919 (15 µg in 1 µl), into the lateral cerebral ventricle, 30 min prior to stress onset, as described in detail previously (Brunson et al., 2001). This dose was chosen because it did not result in blocking of peripheral CRH receptors (see below). For this experiment, plasma corticosterone was obtained within 2 min after the end of this moderate ‘psychological’ stress.
Immunocytochemistry (ICC)
CRH-ICC was performed on free-floating sections (20 µm) using standard avidin–biotin complex methods as described previously (Chen et al., 2001a). Briefly, after several washes with 0.01 M PB-saline (PBS) containing 0.3% Triton X-100 (PBS-T; pH 7.4), sections were treated for 30 min in 0.3% H2O2/PBS, followed by blockade of nonspecific sites with 5% normal goat serum in PBS for 30 min. After rinsing, sections were incubated for 36 h at 4 °C with rabbit anti-CRH antiserum (1:40,000; a gift from Dr. W. W. Vale, Salk Institute, La Jolla) in PBS containing 1% bovine serum albumin, and washed in PBS-T (3×5 min). Sections were incubated in biotinylated goat-anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA, USA) in PBS for 1 h at room temperature. After washing (3×5 min), sections were incubated in the avidin–biotin–peroxidase complex solution (1:100; Vector) for 2 h, rinsed (3×5 min), and the reaction product visualized by incubating the sections in 0.04% 3,3′-diaminobenzidine (DAB) containing 0.01% H2O2. For visualization of endogenous CRH release, matched sections from control and stimulated groups were incubated and developed in the same wells to ascertain identical conditions.
Combined in situ hybridization (ISH) and ICC were performed as described previously (Chen et al., 2001a). Briefly, free-floating sections were first processed for GAD67-ISH followed by CRH-ICC. For hybridization, DIG-labeled RNA probes were added and sections were incubated at 55 °C. Following hybridization, sections were washed, most stringently in 0.1× SSC at 65 °C for 30 min, and hybrid molecules were detected using an anti-DIG-alkaline phosphatase. For visualization of CRH-immunoreaction, sections were rinsed and processed for CRH-ICC as above with the exception that decreased concentrations of DAB (0.02%) and H2O2 (0.005%) were used. The specificity of the hybridization reaction was verified by substituting labeled sense probe for the antisense probe and by omitting either the antisense probe or alkaline phosphatase-conjugated antibody. No labeling was observed under these conditions. To evaluate the possibility of altered sensitivity or specificity due to combined ISH/ICC, sections processed only for ICC or ISH were compared with matched sections subjected to the dual procedure.
Double-labeling ICC
Sections were processed for concurrent immuno-labeling of CRF1 and pCREB, as described in detail previously (Chen et al., 2001b), using goat anti-CRF1 polyclonal antiserum (1:10,000; Santa Cruz, CA, USA). The specificity of this antiserum has been previously established (Chen et al., 2000). Rabbit anti-pCREB antiserum (1:4000; Upstate Biotech, Lake Placid, NY, USA) and benzidine dihydrochloride yielded granular blue deposits that contrasted with the brown DAB products of CRH immunoreactivity. For co-labeling of CRH and GAD65 (Fukuda et al., 1998; Esclapez and Houser, 1999) or syntaxin (Bennett et al., 1992), sections were treated first with anti-CRH (1:20,000), then with mouse anti-GAD65 (1:1000; Boehringer Mannheim, Indianapolis, IN, USA), or anti-syntaxin (1:6000; Sigma, St. Louis, MO, USA). GAD65 delineates GABAergic proximal and distal axons and syntaxin marks presynaptic sites. Immunoreactive sites were visualized using second antibody mixtures containing goat anti-rabbit IgG conjugated to Alexa Fluor 568 and goat anti-mouse IgG conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA).
To determine whether presynaptic CRH-containing terminals were GABAergic, CRH was co-localized with VGAT (vesicular inhibitory amino acid transporter) that is located in GABAergic terminals and is involved in loading of GABA within synaptic vesicles (McIntire et al., 1997). Sections were first immunostained for CRH using a tyramide signal amplification technique. Briefly, CRH antiserum incubation (1:160,000, overnight) was followed by horseradish peroxidase-conjugated anti-rabbit IgG (1:1000; Perkin Elmer, Boston, MA, USA), followed by incubation in the dark with tyramide conjugated to Cy3, diluted 1:150 in amplification buffer (Perkin Elmer). After CRH-detection, sections were exposed to rabbit anti-VGAT antiserum (1:500; Chemicon) overnight at 4 °C, and immunoreactivity was visualized using goat anti-rabbit IgG conjugated to Alexa Fluor 488 (1:400; Molecular Probes). To exclude the possibility of cross-reaction due to the two primary antisera raised in the same species, sections processed for this combined procedure were compared with matched sections processed for CRH or VGAT alone. No cross-reaction was observed. In addition, no signal was found when sections incubated in CRH antiserum were exposed to goat anti-rabbit IgG conjugated to Alexa Fluor 488. For all fluorophore methods, sections were viewed on a Nikon Eclipse E400 epi-fluorescence microscope equipped with fluorescein, rhodamine, and DAPI/FITC/TRITC filter sets. Images were obtained using a Olympus Fluoview confocal microscope (FV300-IX) and acquired into the Adobe Photoshop format.
EM
Post-embedding immunogold labeling was performed using vibratome sections (300 µm) from which the CA1 and CA3 areas of the hippocampus were excised. Tissue blocks were cryoprotected in glycerol, cryofixed in nitrogen-cooled propane, substituted in methanol containing 1.5% uranyl acetate and embedded in Lowicryl HM20. Ultrathin sections were processed for post-embedding immunostaining, employing the CRF1 antiserum described above (1:100). Immunolabelling was visualized using 10 nm gold-coupled secondary antibodies (rabbit anti-goat; 1:20; Aurion).
Statistical analyses
All values are presented as means±standard errors, and the effects of stress on plasma corticosterone was determined using Student’s t-test with significance levels set at P<0.05.
RESULTS
Presumed releasable pools of hippocampal CRH are located in axon terminals of GABAergic interneurons
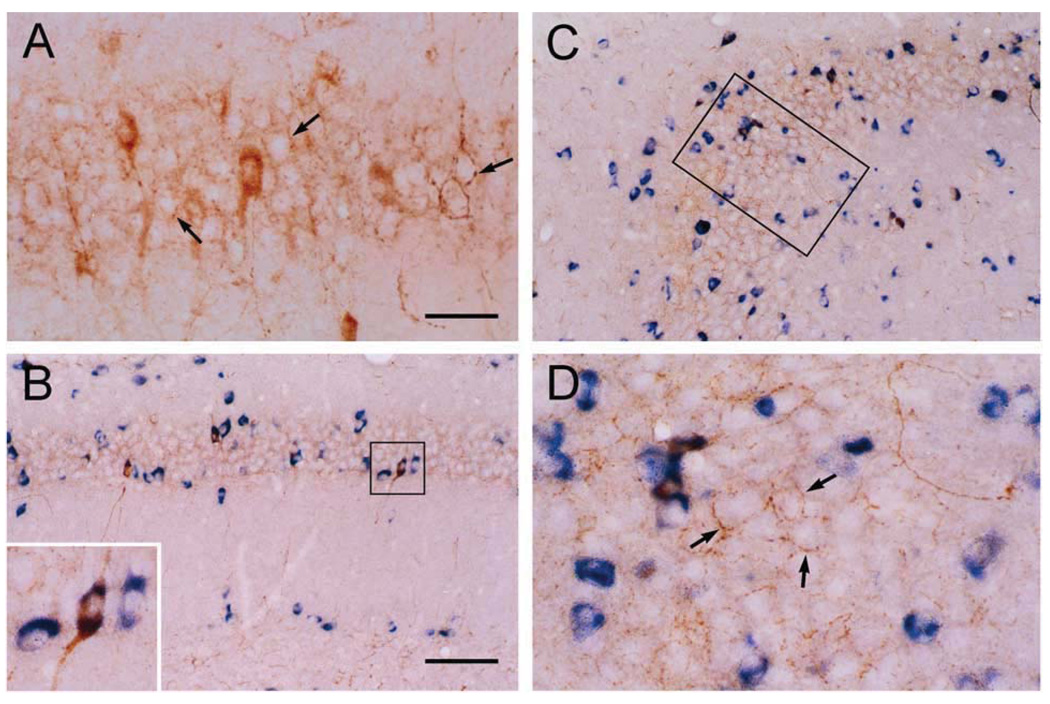
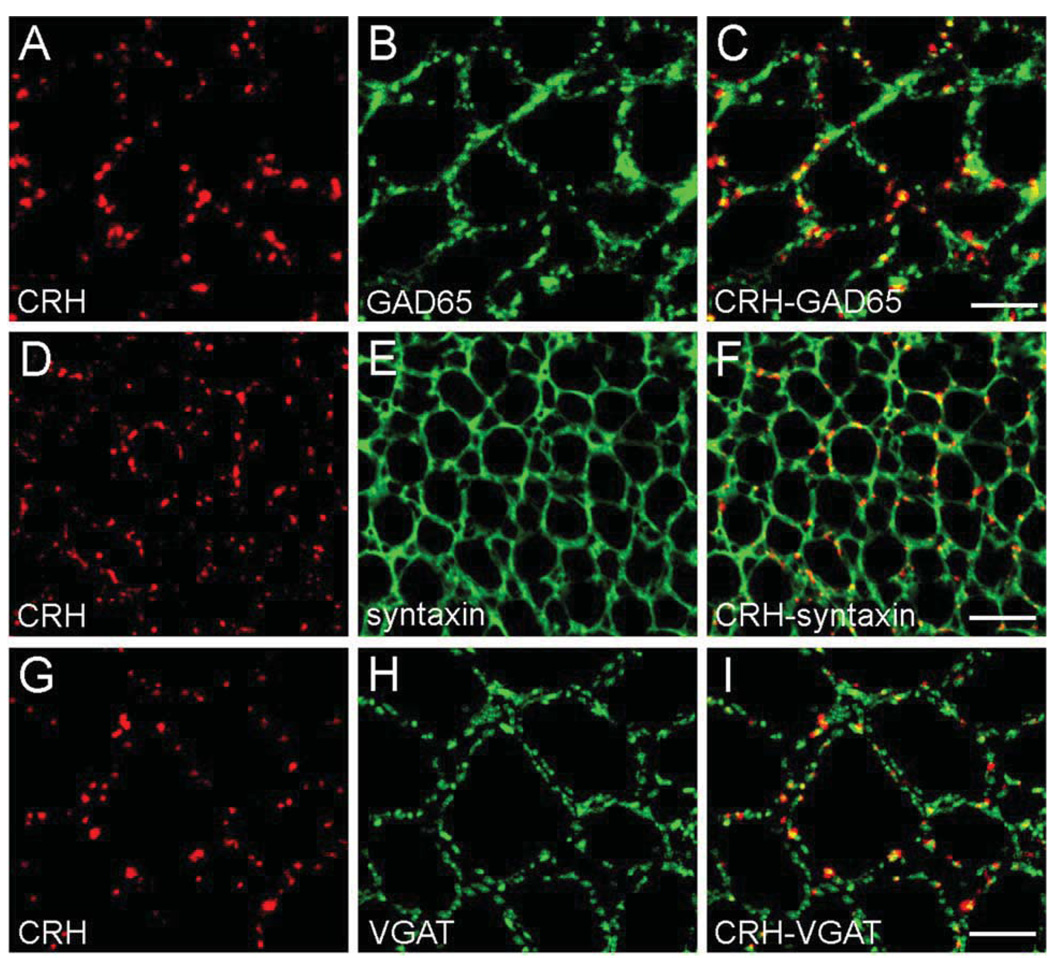
Consistent with earlier studies (Sakanaka et al., 1987; Yan et al., 1998; Chen et al., 2001a), CRH was expressed in basket-type interneurons within the hippocampal pyramidal cell layer (Fig. 1A). These CRH-immunoreactive somata co-expressed mRNA for the GABA synthesizing enzyme glutamic acid decarboxylase (GAD67; Fig. 1B–D). Robust CRH-immunoreactivity was apparent within the perisomatic axonal ‘baskets’ surrounding principal cells (Fig. 1A, arrows), and double-labeling confirmed that CRH-immunoreactive axons belonged to GABAergic interneurons and formed perisomatic baskets exclusively around GAD negative pyramidal cells (Fig. 1D). Transport of CRH to axon terminals and the nature of these release sites was suggested by the co-distribution of CRH and GAD65 (the isoform preferentially targeted to neuronal processes; Esclapez and Houser, 1999) in interneuronal axons and terminals (Fig. 2A–C). CRH was stored in en passant synapses and in terminals, as apparent from significant co-localization of the peptide with the pre-synaptic marker syntaxin, a protein involved in synaptic anchoring (Bennett et al., 1992; Fig. 2D–F). In addition, analysis of the co-distributions of the GABA synaptic vesicle transporter, VGAT (McIntire et al., 1997) and CRH suggested that a fraction of the CRH that resided in interneuronal axon terminals was located in close proximity to GABA-containing vesicles (Fig. 2G–I). This overlap of CRH-immunoreactivity with both syntaxin and VGAT, confirmed by EM (not shown, also see Yan et al., 1998) suggested that releasable CRH pools occupied presynaptic regions of GABAergic interneurons.
Fig. 1.
CRH is expressed in hippocampal interneurons within the pyramidal cell layer. Co-expression of GAD67 in virtually all CRH neurons in CA1 (A, B) and CA3 (C, D) neurons is apparent, using coupled ICC (CRH, brown) and ISH (GAD67, blue). Peptidergic axons form dense perisomatic baskets (arrows) around GAD-negative pyramidal cells. Frame in C denotes area magnified in D. Scale bars = 25 µm (A, D, and inset in B) and 75 µm (B, C).
Fig. 2.
CRH is located in GABAergic boutons. In CA3 pyramidal cell layer, confocal micrographs demonstrate co-localization of CRH- and GAD65-immunoreactivities in axon terminals (A–C). (D–F) Double ICC for CRH and the pre-synaptic marker syntaxin demonstrate that the majority of CRH is located within pre-synaptic terminals. (G–I) The GABAergic identity of CRH-containing terminals is indicated by overlapping immunoreactivities of CRH and the GABA synaptic vesicle transporter VGAT. Scale bars = 12.5 µm (A–C), 25 µm (D–F), and 10 µm (G–I).
CRH receptors reside in asymmetric postsynaptic densities (PSDs) on dendritic spines
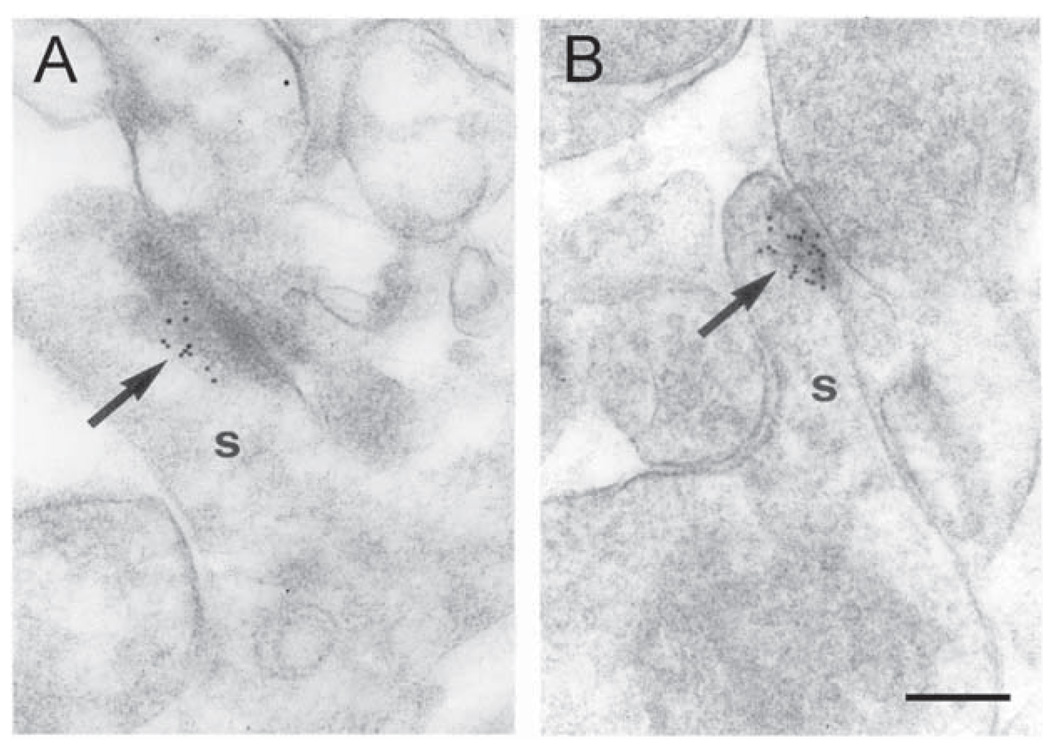
If CRH is involved in stress-evoked modulation of synaptic efficacy of pyramidal cells, then these neurons should express CRH-specific receptors (Chalmers et al., 1995, 1996). Among characterized members of the CRH receptor family, pharmacological and physiological data indicate that CRF1 is responsible for mediating the synaptic actions of this peptide on hippocampal pyramidal cells (Chalmers et al., 1995; Baram et al., 1997; Brunson et al., 2002). Indeed, analysis of CRF1 protein expression revealed its presence in pyramidal cells. Using the finer resolution afforded by EM (Fig. 3), CRF1-immunogold particles were primarily concentrated at asymmetric PSDs of dendritic spines, typical sites for the location of excitatory synapses. This localization of the receptor is consistent with a potentiating role of CRH on hippocampal neurotransmission.
Fig. 3.
The CRH receptor CRF1 is located primarily at post-synaptic densities of synaptic contacts on dendritic spines (s). (A, B) Immunogold particles (arrows) are concentrated at postsynaptic membrane specializations of spines in stratum oriens of hippocampal CA3. Very few gold grains were observed over other tissue components. Scale bar = 0.1 µm.
Stress evokes activation of hippocampal pyramidal cells, including those bearing CRH receptors
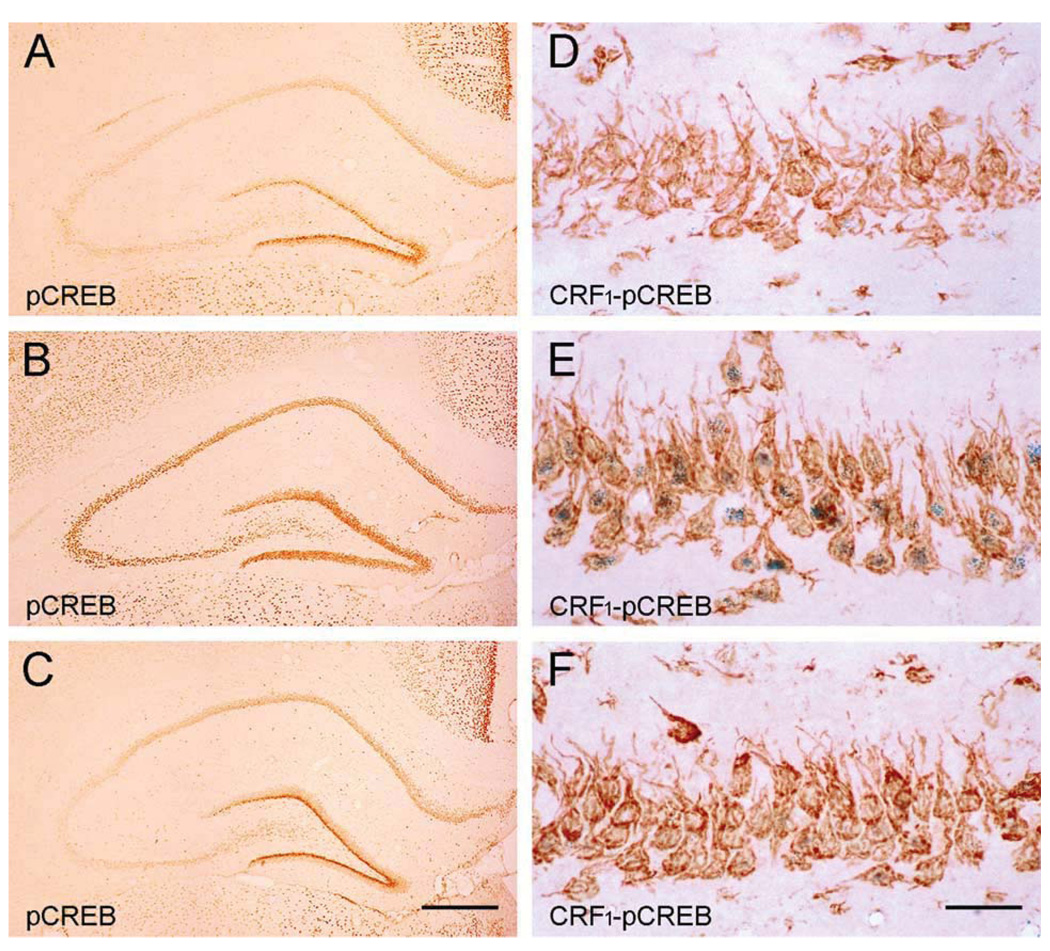
‘Psychological’ stresses activate CRH release-coupled synthesis in hippocampal interneurons of the immature rat (Hatalski et al., 2000). Therefore, we used a moderate psychological stress suitable for the juvenile rat (where restraint is only a modest stressor), to elicit release of native peptide (Brunson et al., 2001; Blank et al., 2002). For analysis of stress-evoked activation of CRH-receptor bearing pyramidal cells, we relied on the rapid phosphorylation of the transcription factor CREB. This marker was chosen because pCREB appears within seconds of stress in mature and developing rat (Kovacs and Sawchenko, 1996; Chen et al., 2001b) and is known to convey signals from synapse to soma (Lonze and Ginty, 2002). As shown in Fig. 4, compared with the non-stressed condition (Fig. 4A, D), pCREB immunoreactivity was abundant in pyramidal neurons, including those expressing the CRH receptor CRF1 (Fig. 4B, E).
Fig. 4.
Binding of endogenous CRH to the CRF1- receptor is required for stress-evoked transcriptional activation of receptor-bearing neurons. (A, D) Little pCREB is found in a stress-free hippocampus. (B, E) Immediately after stress, pCREB is abundant in CRF1 immunoreactive neurons. (C, F) Pre-administration of the selective CRF1 blocker R121919 attenuates stress-evoked pCREB. This ‘dampening’ of CREB phosphorylation is particularly prominent in CRF1 expressing pyramidal neurons. Scale bars = 360 µm (A–C) and 60 µm (D–F).
Blocking the actions of endogenous CRH prevents stress-evoked activation of CRF1-expressing hippocampal neurons
To investigate whether this stress-induced pCREB induction required CRH receptor activation, we examined hippocampi from similarly stressed animals that were infused intracranially with the selective CRF1 blocker, R121919 30 min prior to the stress. As shown in Fig. 4C and F, stress-provoked CREB phosphorylation was markedly dampened in antagonist-pretreated animals, most prominently in CRF1-expressing neurons, indicating that binding of the native peptide to the receptor was required for stress-induced neuronal activation (Brunson et al., 2001, 2002). Notably, the antagonist did not influence peripheral measures of stress, determined by plasma glucocorticoid levels. Thus, plasma corticosterone of ‘stress-free’ rats, the stressed-vehicle treated group and a stressed-antagonist treated group were 0.77±0.1, 5.3±0.8, and 5.6±0.6 µg/dl, respectively. These data are consistent with a role for central, potentially hippocampal CRH in the activation of CRH-receptor bearing pyramidal cells by psychological stress. Although comprising a seven-fold increased in basal plasma corticosterone, these stress-evoked corticosterone levels are relatively low. This is attributable to several factors: first, the age of the animals (P18), where basal plasma corticosterone levels in general are lower than in adults. In addition, the experiments were performed in the morning, during the nadir of diurnal plasma corticosterone and its responsiveness (Dallman et al., 1987). Notably, we did not aim for a severe stress, but for a moderate ‘psychological’ stress, leading to moderate increase in peripheral stress hormones.
DISCUSSION
The principal findings of these studies are: (1) The hippocampal CRH ‘system’ is organized with CRH expressed in GABAergic interneurons and the mediating receptor on dendritic spines of principal cells, (2) stress induces molecular/ transcriptional activation of hippocampal pyramidal cells, (3) stress-induced activation of a subset of hippocampal neurons requires activation of CRH receptors expressed by these cells, and (4) blocking central CRH receptors without interfering with the peripheral actions of the peptide eliminates neuronal activation, suggesting that the source of native peptide is central. Taken together, these findings suggest that in addition to initiating steroid and neurotransmitter receptor activation, stress influences hippocampal neurons via peptidergic neuromodulation by CRH, and are consistent with the notion that local, hippocampal CRH might be involved.
The current study delineates the fine structural distribution of CRH and its principal hippocampal receptor, CRF1, in hippocampal pyramidal layers. The data indicate that the peptide is stored in GABAergic terminals, while the receptors are found in locations typical of excitatory synapses. The way in which CRH reaches and activates the receptor is not clear. Finding that CRH-immunoreactivity in extracellular spaces adjacent to the pyramidal cells is increased by stress is suggestive of the possibility that local hippocampal CRH is released by stress from interneurons, and that the peptide diffuses to pyramidal cell dendritic spines. However, the ability of this peptide to travel long distances within brain has been previously demonstrated (Bittencourt and Sawchenko, 2000), so that an extra-hippocampal source and rapid transport of the peptide from distant sites cannot be excluded. In addition, the ability of CRH to influence excitatory neurotransmission has been shown using electrophysiological methods (Hollrigel et al., 1998; Blank et al., 2002).
CRH is a stress-activated neuromodulator in a number of CNS regions, including hippocampus (cf. Brunson et al., 2003 for recent review). Stress modulates information flow in the hippocampus in a complex manner, depending—at least in part—on whether the stressful stimulus is acute or chronic, mild or severe (e.g. Shors et al., 1989; McEwen, 1999; Pavlides et al., 2002; Alfarez et al., 2003). These differential effects of stress have been shown to be mediated by steroid hormones as well as a number of neurotransmitters (e.g. Magarinos and McEwen, 2000; Mc-Gaugh, 2002). Much has been learned about the role of glucocorticoids, arriving in the hippocampal formation from their origin in the adrenal, in stress-associated alterations of hippocampal synaptic plasticity (McEwen, 1999; Joels, 2001; Pavlides et al., 2002) as well as learning and memory functions (McGaugh, 2002). Indeed, these hormones have been broadly considered to be major mediators of the effects of stress described above (Korz and Frey, 2003; Roozendaal et al., 2003). The current studies suggest that CRH, expressed and stored within the hippocampus itself (or arriving there rapidly from other CNS sites, such as amygdala), might provide a rapid and efficient additional means for conveying stress-related information to hippocampal neurons. One could speculate that an early step in the actions of stress on hippocampal neurons involves rapid local release of CRH. This is followed by recruitment of peripheral glucocorticoids that reach the hippocampus to bind their abundant receptors (Herman et al., 1989). The latter may exert the more sustained and global impact of stress on hippocampal function (McEwen, 1999).
Thus, stress-evoked peptide release may bridge the temporal and spatial ‘gaps’ between neurotransmitter and steroid hormone effects on hippocampal neurons: because the constituents of the hippocampal CRH ‘peptidergic synapse’ differ from those of classical neurotransmitters, they permit the peptide to function in broader domains of time and space (Agnati et al., 1995; Nicholson, 2000). From the spatial perspective, we find that CRH is located in perisomatic GABAergic terminals, from which it might be released by stress. CRH-receptors are located at the relatively distant dendritic spines, suggesting that transport of the secreted peptide from perisomatic terminals to dendrites might be involved. From the temporal perspective, the peptidergic CRH synapse seems to lack efficient mechanisms to constrain the duration of the peptide’s effects: uptake and transport mechanisms, typical for classical neurotransmitters (e.g. Scanziani, 2002), have not been described for this peptide. In addition, efficient degrading enzymes, such as those for monoamines or GABA have not been delineated. Whereas a CRH-binding protein, putatively sequestering CRH away from its receptors, is found in mature CNS (Potter et al., 1992), its expression in rodent hippocampus is limited, particularly in the juvenile period studied here (Brunson KL and Baram TZ, unpublished observations). In view of the absence of efficient ‘disposal mechanisms’ for CRH, extracellular peptide in appreciable quantities may be present and active for time-periods measured in minutes, bridging neurotransmitter and steroid hormone temporal action profiles.
In summary, binding of CRH, originating in GABAergic interneurons, to the CRF1 receptor (located on dendritic spines) is required for stress-evoked transcriptional activation of subsets of pyramidal cells, and may contribute to the established stress-related alteration of hippocampal synaptic plasticity. This novel mechanism should provide molecular targets for modulating the salubrious as well as the adverse effects of stress on hippocampal learning and memory functions.
Acknowledgements
The authors thank Karin Mews and Aniko Schneider for help with the EM studies, and M. Hinojosa for excellent editorial assistance. Grant number: NS28912, NS39307 (TZB), SFB 505 (MF). Grant sponsor: NIH and Deutsche Forschungsgemeinschaft.
Abbreviations
- CREB
cyclic AMP responsive element binding protein
- CRH
corticotropin releasing hormone
- DAB
3,3′-diaminobenzidine
- EM
electron microscopy
- GAD67
glutamic acid decarboxylase
- ICC
immunocytochemistry
- ISH
in situ hybridization
- LTP
long-term potentiation
- PB
sodium phosphate buffer
- PBS
phosphate-buffered saline
- PBS-T
phosphate-buffered saline containing 0.3% Triton X-100
- pCREB
phosphorylated cyclic AMP responsive element binding protein
- PSD
postsynaptic density
REFERENCES
- Agnati LF, Zoli M, Stromberg I, Fuxe K. Intercellular communication in the brain: wiring versus volume transmission. Neuroscience. 1995;69:711–726. doi: 10.1016/0306-4522(95)00308-6. [DOI] [PubMed] [Google Scholar]
- Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Chalmers DT, Chen C, Koutsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors? an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Grigoriadis DE, Lorang MT, Baram TZ. Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Exp Neurol. 2002;176:75–86. doi: 10.1006/exnr.2002.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: a double-edged sword? Mol Neurobiol. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotrophin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF1)-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Frotscher M, Baram TZ. Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J Neurosci. 2001a;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hatalski CG, Brunson KL, Baram TZ. Rapid phosphorylation of the CRE binding protein precedes stress-induced activation of the corticotropin releasing hormone gene in medial parvocellular hypothalamic neurons of the immature rat. Mol Brain Res. 2001b;96:39–49. doi: 10.1016/s0169-328x(01)00265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Houser CR. Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol. 1999;412:488–505. [PubMed] [Google Scholar]
- Fukuda T, Aika Y, Heizmann CW, Kosaka T. GABAergic axon terminals at perisomatic and dendritic inhibitory sites show different immunoreactivities against two GAD isoforms, GAD67 and GAD65, in the mouse hippocampus: a digitized quantitative analysis. J Comp Neurol. 1998;395:177–194. doi: 10.1002/(sici)1096-9861(19980601)395:2<177::aid-cne3>3.0.co;2-#. [erratum in: J Comp Neurol 399:424#x02013;426] [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Corticosteroid actions in the hippocampus. J Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: involvement of adrenal steroid receptors. J Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Experimental diabetes in rats causes hippocampal dendritic and synaptic reorganization and increased glucocorticoid reactivity to stress. Proc Natl Acad Sci USA. 2000;97:11056–11061. doi: 10.1073/pnas.97.20.11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Volume transmission in the year 2000. Prog Brain Res. 2000;125:437–446. doi: 10.1016/S0079-6123(00)25030-9. [DOI] [PubMed] [Google Scholar]
- Pavlides C, McEwen BS. Effects of mineralocorticoid and glucocorticoid receptors on long-term potentiation in the CA3 hippocampal field. Brain Res. 1999;851:204–214. doi: 10.1016/s0006-8993(99)02188-5. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci USA. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: dependence on the basolateral amygdala. Proc Natl Acad Sci USA. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Scanziani M. Competing on the edge. Trends Neurosci. 2002;25:282–283. doi: 10.1016/s0166-2236(02)02155-0. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Age-related epileptogenic effects of corticotropin-releasing hormone in the isolated CA1 region of rat hippocampal slices. J Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur J Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Yan XX, Toth Z, Schultz L, Ribak CE, Baram TZ. Corticotropin-releasing hormone (CRH)-containing neurons in the immature rat hippocampal formation: light and electron microscopic features and colocalization with glutamate decarboxylase and parvalbumin. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]