Abstract
Background
Hepatitis C virus (HCV) causes chronic liver disease that often leads to cirrhosis and hepatocellular carcinoma. In animal studies, chimpanzees were protected against chronic infection following experimental challenge with either homologous or heterologous HCV genotype 1a strains which predominates in the USA and Canada. We describe a first in humans clinical trial of this prophylactic HCV vaccine.
Methods
HCV E1E2 adjuvanted with MF59C.1 (an oil-in-water emulsion) was given at 3 different dosages on day 0 and weeks 4, 24 and 48 in a phase 1, placebo-controlled, dose escalation trial to healthy HCV-negative adults.
Results
There was no significant difference in the proportion of subjects reporting adverse events across the groups. Following vaccination subjects developed antibodies detectable by ELISA, CD81 neutralization and VSV/HCV pseudotype neutralization. There was no significant difference between vaccine groups in the number of responders and geometric mean titers for each of the three assays. All subjects developed lymphocyte proliferation responses to E1E2 and an inverse response to increasing amounts of antigen was noted.
Conclusions
The vaccine was safe and generally well tolerated at each of the 3 dosage levels and induced anti-body and lymphoproliferative responses. A larger study to further evaluate safety and immunogenicity is warranted.
Keywords: HCV, MF59, E1E2, vaccine
BACKGROUND
There is an urgent need for an effective vaccine to protect against infection and/or disease due to hepatitis C virus (HCV). In the USA alone, the Centers for Disease Control and Prevention estimates there were 17,000 new cases in 2007 and there is a global prevalence of 170 million carriers worldwide.
Most acute HCV infections are either asymptomatic or mild and approximately 25% of acute HCV infections resolve spontaneously.[1, 2] Most individuals develop chronic infection, and 20% of these slowly progress to cirrhosis of the liver.[3, 4] Chronic HCV infection is the leading cause of liver transplantation in the US.[5] Currently there is no vaccine to prevent chronic infection; therapeutic treatments are only partially effective and have significant side effects[6]. While preventing acute infection would be ideal, a vaccine that prevents chronic infection is expected to prevent clinically significant sequelae including cirrhosis and hepatocellular carcinoma.[7, 8, 9, 10, 11]
HCV, an enveloped virus belonging to the flaviviridae family, contains a single stranded plus-sense RNA genome with a single open reading frame encoding both structural and nonstructural proteins.[12] At least six distinct genotypes and many subtypes of HCV exist[13, 14, 15, 16, 17] and infected individuals have complex mixtures of related viruses circulating as a quasi-species.[17,18] Although E1 is thought to be variable, the E2 surface glycoprotein contains a hypervariable region,[19, 20, 21] and is under marked selective pressure to evolve into antigenic variants.[22, 23, 24] Because glycoproteins E1 and E2 are expressed on the surface of the virion, the proteins are expected to elicit neutralizing antibodies. Therefore recombinant HCV E1and E2 glycoproteins were constitutively expressed from the same RNA in permanent Chinese hamster ovary cell lines and purified under native conditions for use as a candidate vaccine. The antigen sequence was derived from the HCV genotype 1a, a predominant genotype in the US [16] and Canada, and was passaged in chimpanzees.
In chimpanzees, vaccination with the E1E2 produced superior immune responses compared to E2 administered alone. Vaccination of chimpanzees followed by intravenous HCV challenge demonstrated that an adjuvanted prototype vaccine containing E1E2 modified the natural course of infection and appeared to sterilize against acute infection following homologous virus challenge[25, 26] in animals which developed high antibody titers; whereas chimpanzees with low anti-E1E2 antibody titers became acutely infected but generally resolved their infections. Vaccination of chimpanzees did not sterilize against acute infection following experimental challenge with heterologous HCV genotype 1a strain but importantly the majority did not develop chronic infection unlike the unimmunized controls. Interestingly, protection against chronic infection did not correlate strongly with anti-E1E2 antibody titers induced by vaccination, suggesting that such protection may be mediated by a combination of anti-E1E2 antibodies and cellular immune responses to vaccine.[26, 27, 28] It is also important to note that in small animals, this vaccine induced anti-E1E2 antibodies capable of cross-neutralizing HCV pseudoparticles (HCVpp) derived from diverse HCV genotypes. [29]
The present study extended the testing of an adjuvanted HCV E1E2 envelope glycoproteins vaccine to humans. Based on dose escalation studies in chimpanzees, vaccine was administered in this study on Day 0, and Weeks 4, 24 and 48. A dose ranging study with 4 μg, 20 μg and 100 μg of HCV E1E2 adjuvanted with MF59C.1 was expected to yield a dose response curve for selecting an optimal dose and schedule for future studies.
METHODS
Vaccine and Placebo
The HCV E1E2/MF59C.1 (Novartis Vaccines and Diagnostics) vaccine contained envelope glycoproteins gpE1 and gpE2 and the adjuvant, MF59C.1. Recombinant HCV E1E2 glycoproteins derived from the sequence of an HCV genotype 1a strain were constitutively expressed in a Chinese hamster ovary (CHO) cell line and purified for vaccine use. The adjuvant, MF59C.1, a sterile, oil (squalene)-in-water emulsion, was the same as used in Novartis’ licensed influenza vaccine, FLUAD®. The antigen and adjuvant were combined prior to injection. Sterile saline was used as the placebo. The volume administered was 0.5 mL intramuscularly for all the vaccine doses and placebo.
Study Design and Subjects
The Phase I randomized, double-blind, placebo-controlled dose-escalation study assessed the safety and immunogenicity of HCV E1E2/MF59C.1. Sixty adults were enrolled into three groups of twenty. Sixteen subjects in each group received 4μg, 20μg or 100μg doses of HCV E1E2/MF59C.1 vaccine administered intramuscularly into the deltoid area at 0, 4, 24 and 48 weeks; four subjects per group received saline placebo as a nonimmunogenic control. Each subject’s participation lasted approximately 64 weeks (15 months). Local and systemic reactogenicity was captured on a diary card for 15 days (Study Days 0–14) post each vaccination. Subjects graded reactogenicity as mild, moderate or severe. Mild was defined as awareness of symptom but easily tolerated and did not keep the subject from normal, daily activities; moderate was defined as the subject acting like something was wrong and resulted in some limitation in the subject’s normal daily activities; and severe was defined as being extremely distressed or unable to do normal daily activities. Reactogenicity data was summarized as the maximum severity grading experienced for each symptom after each vaccination. Reactogenicity data was reviewed prior to enrolling each successive group.
The study was conducted at the National Institute of Allergy and Infectious Diseases’ Vaccine Treatment and Evaluation Unit at Saint Louis University after approval by the University’s Institutional Review Board. All subjects provided informed consent. Enrollment occurred from August 25, 2003 to June 1, 2004. Healthy HCV antibody and PCR RNA negative subjects between the ages of 18–45 years were eligible if they had an acceptable total white blood cell count, hemoglobin, renal and liver functions, and negative serum cryoglobulin, hepatitis B surface antigen (HBsAg) and HIV ELISA test results. Exclusion criteria were identifiable high-risk behavior for HCV infection including injection drug use or cocaine snorting within the last year, a tattoo or body piercing within the previous 6 months prior to enrollment. Safety labs including CBC, serum chemistries and cryoglobulin, and urinalysis were assessed at 4, 8, 28 and 52 weeks.
Immunogenicity Assays
HCV E1E2 Antibody ELISA
Enzyme-linked immunosorbent assay (ELISA) using purified E1/E2 antigen was used to determine serum IgG levels to HCV E1/E2.[30] Serum samples were diluted and incubated for 1 hour in the antigen-coated plates. The plates were washed five times with washing buffer before adding Horseradish peroxidase conjugated goat anti-human IgG F(ab′)2. Ophenylenediamine substrate was used to develop the plates and the color reaction was stopped after 30 minutes by the addition of 4N H2SO4. The titers of the antibodies are expressed as the reciprocal of the sample dilution, in which the optical density of the samples is between 1.0 and 0.5 as read by a plate reader using dual wavelengths of 492nm and 620 nm. A value of ≥ 20 was considered a positive response to vaccination.
EIA measuring antibodies that inhibit the binding of HCV gpE2 to the HCV receptor component, CD81
Human CD81, a member of the tetraspanin protein superfamily, is an essential component of the HCV receptor and binds recombinant HCV gpE2 envelope glycoprotein.[31, 32] The binding site of HCV gpE2 has been mapped to the major extracellular loop of CD81 (EC2) that is conserved in both humans and chimpanzees. A recombinant fusion molecule comprising EC2 fused to the C-terminal end of thioredoxin was cloned, expressed and purified from E. coli. In brief, this purified protein was then used to develop a surrogate virus neutralization EIA assay.[32] The CD81 recombinant EC2 protein diluted in sodium borate buffer was coated in 96 well medium binding Costar plates (Plate A) overnight. In a dilution plate (Plate B), 55 ul CHO E2 715 antigen in working reagent together with 55 ul monoclonal non-neutralizing anti-E2 antibody (5E5/H7) labeled with Europium (Eu3+) in working reagent were added to each well and shaken for 15 minutes at 40°C. Next to each well (Plate B), 110 ul of various dilutions of test or control sera (four fold dilutions in PBS/BSA buffer) were added and shaken for 45 minutes at 40°C. 200 ul of the contents from each well of plate B was then transferred to the CD81 coated plate (Plate A), shaken for another 45 minutes at 40°C and washed 5 times with wash buffer (1X PBS, 0.1% Tween-20). 200 ul of Enhancement Solution (Wallac) was added to each well and shaken for 5 minutes at room temperature. The plate was placed in a Wallac 1420 Multilabel Counter and read using the Europium protocol. After subtracting background readings from control sera, the dilution of test sera producing 50% inhibition of binding of gpE2 to CD81 was then determined.
CD4+ T Cell Lymphocyte Proliferation Assay (LPA) and Cytokine Production
E1E2 specific CD4+ T cell proliferation at multiple timepoints was assessed by 3H thymidine incorporation[33] using total peripheral blood mononuclear cells. Phytohemagglutinin (PHA) was used as the positive control. Day 5 (Day 3 for IL-2 detection) culture supernatant was used for cytokine analysis as described below. The data are expressed as Stimulation Index (SI), the mean counts per minute (cpm) of duplicate stimulated cell cultures divided by mean cpm of duplicate unstimulated cell cultures. SI corresponding at value 1 is the lower limit of LPA. SI of greater than or equal to 3 is considered a positive response to vaccination [34].
Induction of secreted cytokine [35] (GM-CSF, IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-10, IL-12 and IL-13) was measured at two weeks post third vaccination and 16 weeks post fourth vaccination. Cytokine concentrations (pg/ml) were determined using the Bio-Plex Suspension Array System (Bio-Rad Laboratories) following incubation with supernatant fluids secreted by peripheral blood mononuclear cell cultures stimulated in vitro with E1E2 vaccine antigen.
VSV/HCV Pseudotype Assay for Neutralization Antibody
VSV derived pseudotypes were generated using both the envelope glycoproteins from HCV genotype 1a (Accession No. M62321) and used as surrogates for virus neutralization.[36] NMSO3 was used in the VSV/HCV pseudotype assay to inhibit any potential residual uptake of parental G glycoprotein to the VSVts045 backbone used in pseudotype generation. Serial dilutions of antibodies were added to a predetermined titer of VSV/HCV (100 pfu/reaction) and NMSO3, and incubated for 1 h at 37° C before addition to the Huh7 cell monolayer to determine neutralizing activity. Cells were washed and infectivity was determined as described previously. Untreated virus was used for comparison. An inhibitory concentration 50% (IC50) of plaque number for VSV/HCV pseudotype was considered as neutralization titer of the test sera. A cut-off of ≥ 10 was considered a positive result.
Statistical Analysis
All results are presented as intent to treat aggregating placebo recipients into one group. Reactogenicity data for each post-vaccination period are summarized in terms of each symptom and its maximum severity grade. The solicited symptoms were analyzed by taking the most severe response over the follow-up period and dichotomizing into a binary variable: none versus mild/moderate/severe, and separately, none/mild/moderate versus severe. The Cochran-Armitage test was used to evaluate trends in the proportion of subjects experiencing underlying symptoms.
CD81 neutralization, ELISA antibody, LPA and VSV/HCV pseudotype neutralization results were summarized using geometric mean titers (GMT) and the proportion of responders. GMTs were compared using an analysis of variance (anova) at each relevant time point, using Tukey’s method to control for p-values for multiple pair-wise comparisons. The 95% percent confidence intervals (CI) for proportions are Clopper-Pearson exact confidence intervals. Proportions of responders in vaccine and control groups are compared using Fisher’s Exact Test. Cytokine induction was summarized using geometric mean concentration (pg/mL), 95% CI, and descriptive statistics for each cytokine observed.
RESULTS
Characteristics of Subjects
A total of sixty subjects were enrolled (Table 1). There were no significant differences noted between the four groups for race, gender and age. Fifty-three (88%) subjects were white, 6 (10 %) were black and 1 (1.7 %) was Asian. Thirty-six subjects (60 %) were females. The mean age (range) of the subjects was 33 (19–45). Ten subjects were withdrawn from the study after enrollment: four subjects from the placebo group and two subjects in each of the vaccine dose groups (Table 1). None of the withdrawals were due to study related to adverse events.
TABLE 1.
Number of Vaccinations Completed by Dosage Group
| Placebo (N=12) | 4 μg (N=16) | 20 μg (N=16) | 100 μg (N=16) | Total (N=60) | ||
|---|---|---|---|---|---|---|
| Vaccination number completed1 (Vaccination timepoints) | Dose 1 (Day 0) | 12 (100%) | 16 (100%) | 16 (100%) | 16 (100%) | 60 (100%) |
| Dose 2 (Week 4) | 11 (91.7%) | 15 (93.7%) | 14 (87.5%) | 16 (100%) | 56 (93.3%) | |
| Dose 3 (Week 24) | 8 (66.7%) | 14 (87.5%) | 14 (87.5%) | 16 (100%) | 52 (86.7%) | |
| Dose 4 (Week 48) | 8 (66.7%) | 14 (87.5%) | 14 (87.5%) | 14 (87.5%) | 50 (83.3%) | |
Ten subjects were withdrawn from the study after enrollment. The reasons for withdrawal included breast cancer, mental illness not reported at the time of enrollment, steroid use for contact dermatitis and tendonitis (2 subjects), fear of contracting cancer due to vaccine receipt, moved out of the area, noncompliance (4 subjects).
Vaccine Safety
A total of 10 serious adverse events (SAEs) occurred in 7 subjects; 2 in the placebo group, 3 in the HCV E1E2 4μg group and 1 each in the 20 μg and 100 μg groups. None were considered related to vaccine. The events included breast cancer; hospitalization for depression; surgical repair of deviated septum; vaginal hysterectomy; schizophrenia not revealed at screening; and emergency room visits for sinusitis with headache, muscle spasms of the back, fall with ankle and back injury, back pain, and puncture wound to the leg.
Of the subjects reporting unsolicited AEs, 7 subjects had 9 events determined to be definitely (5), or possibly (4) related to vaccine including itching at the injection site (5), palpable lymph node (1), elevated monocyte count (1), red blood cells in the urine (1), and fatigue (1). There was no association between dose and proportion of subjects reporting severe unsolicited adverse events (p > 0.05, Fischer’s exact test).
The most frequently reported moderate or severe symptoms on the Diary Card across all vaccinations included fever, discomfort, headache, myalgia, and pain/tenderness at the vaccination site. A similar number of moderate symptoms followed each vaccination; severe symptoms occurred after the third and fourth vaccinations (Figure 1). Overall, significant dose-related trends (Cochran-Armitage p<0.05) in the occurrence of any severity following any vaccination were found with pain/tenderness, rash, warmth, chills, fever, myalgia, weakness and discomfort. Discomfort was the only severe symptom significantly associated with dose following any vaccination (p<0.03).
Figure 1.
A. Percent of Subjects with Maximum Local Reactogenicity by Dose and Vaccination. Reactogenicity data was summarized as maximum severity for each symptom after each vaccination. The most common solicited reaction is pain/tenderness at the injections site. A similar number of moderate events followed each vaccination. Severe reactions occurred in subjects receiving the 20 μg dose following the third vaccination. The proportion of subjects experiencing mild or greater severity of reactogenicity is not associated with assigned dosage level (p < 0.05, exact test).
B. Maximum Systemic Reactogenicity by Dose and Vaccination. Systemic reactogenicity data was solicited for 15 days (Study days 0–14) after each vaccination and was recorded on a diary card. Subjects graded reactogenicity as mild, moderate or severe. Mild was defined as awareness of symptom but easily tolerated and did not keep the subject from normal, daily activities; moderate was defined as the subject acting like something was wrong and resulted in some limitation in the subject’s normal daily activities; and severe was defined as being extremely distressed or unable to do normal daily activities. Reactogenicity was summarized as maximum severity for each symptom after each vaccination. A similar number of moderate events followed each vaccination. Severe reactions occurred in subjects receiving the 20 μg dose following the second and third vaccinations. The proportion of subjects experiencing mild or greater severity of reactogenicity is not associated with assigned dosage level (p < 0.05, exact test).
Immunogenicity
HCV E1E2 ELISA Assay
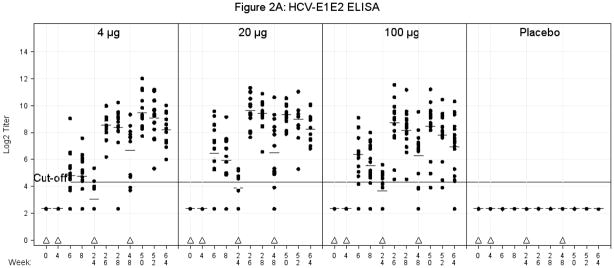
Differences for responses by dose (dose treated as categorical) were compared on a logarithmic scale (equivalent to comparing ratios of GMTs) in a pairwise fashion at every time point using an anova. The only statistically significant differences were between the placebo and vaccine groups. These differences were significant for all comparisons with the exception of the 4μg vaccine group at Week 24 (time of 3rd vaccination). Similarly significant differences were noted between the proportion of responders for all post-vaccination visits between the placebo group and all three vaccine groups (Fisher’s Exact Test) after week 4 (time of second vaccination) except the 4μg group vaccine group at Week 24 (Figure 2A). Overall, the largest number of subjects responded two weeks post 3rd vaccination for the 4 μg dose [14/14 (100%)], the 20μg dose [14/14 (100%)] and the 100μg dose [16/16 (100%)].
Figure 2.
A. E1E2 Binding Antibody ELISA. Serum was obtained for E1E2 binding antibody at multiple time points as indicated. There were no significant differences in comparisons among the 4μg, 20μg, or 100μg vaccine groups for Geometric Mean Titers or number of responders at any time points post vaccination. The values are expressed as Log2 titers. The titers of the antibodies are expressed as the reciprocal of the sample dilution, in which the optical density of the samples is between 1.0 and 0.5 (1.0 > OD > 0.5) as read by a plate reader using dual wavelengths of 492nm and 620 nm. A value of ≥20 (log2 4.32) was considered a positive response to vaccination. Triangles represent vaccination time points. Plus signs represent mean values. The solid line represents a cut-off value of 20 (log2 4.32).
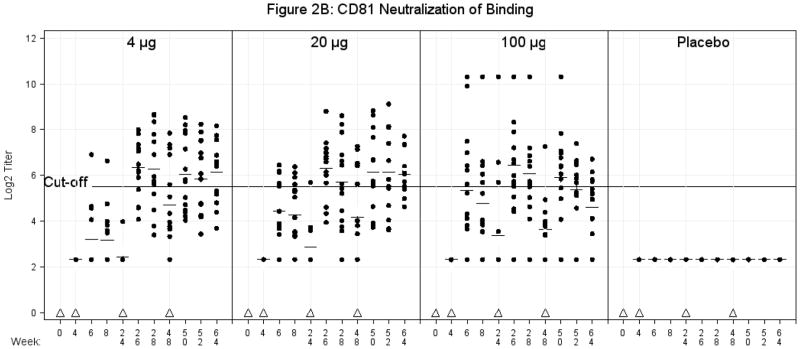
B. CD81 Neutralization of Binding Antibody. Serum was obtained for CD81 neutralization of binding antibody (geometric mean titers) at multiple time points as indicated. The values are expressed as Log2 titers. A titer of ≥ 45 (log2 4.32) or greater was considered a positive response to vaccination. Triangles represent vaccination time points. Plus signs represent mean values. The solid line represents the cut-off value of 45 (log2 5.49).
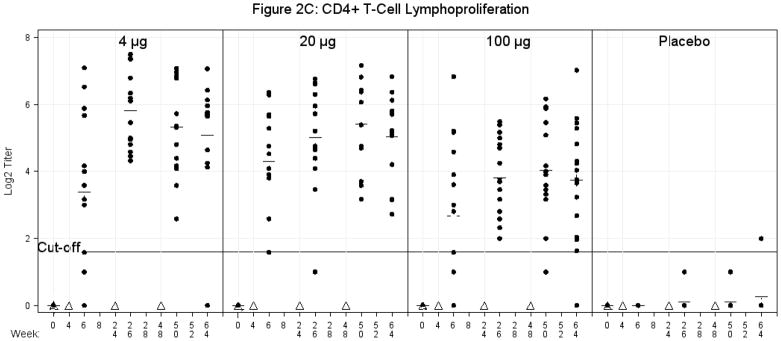
C. Lymphoproliferation of CD4+ T-cell assay using E1E2 antigen. Cells were obtained for a CD4+ T-cell lymphoproliferation assay at multiple time points as indicated. Stimulation index (SI) = mean counts per minute (cpm) of duplicate stimulated cell cultures divided by mean cpm of duplicate unstimulated cell cultures. Stimulation index corresponding to a value of 1 is the lower limit of lymphoproliferation (LPA). SI of greater than or equal to 3 is considered a positive response to vaccination. Triangles represent vaccination time points. Plus signs represent mean values. The solid line represents a cut-off of 3 (log2 1.58).
Inhibition of Binding of HCV gpE2 to HCV Receptor Component, CD81
Pair-wise comparisons similar to those for the HCV E1E2 ELISA assay GMTs were conducted at all time points between the placebo group and vaccine groups starting with Week 4 (time of 2nd vaccination). Prior to 26 weeks, significant differences were also observed between placebo and the 20μg and 100μg groups two and four weeks after the second vaccination. Starting at Week 26 (two weeks post third vaccination), in all instances the vaccine recipients had significantly higher titers than placebo recipients with the exception of Weeks 48, (time of 4th vaccination) when only the 4μg group was statistically superior to placebo (Figure 2B). The only statistically significant difference between non-placebo groups was the improved response for the 100 μg group relative to the 4 μg group at weeks 6 (two weeks post the 2nd vaccination) and 8 (four weeks post the 2nd vaccination), and the opposite by week 64 (16 weeks post 4th vaccination), when the 4 μg group titers were significantly higher than the 100 μg group. Fisher’s Exact Test found significant differences in the proportion of responders between the placebo group and all vaccine groups after Week 24 except Week 48 where there were no significant differences, and at Week 64 (16 weeks post 4th vaccination) where only the 4μg and 20 μg had better response rates than placebo. Comparisons of proportions among the vaccine groups were only significant two weeks after the second dose where the 4μg was inferior to the higher dose groups, and at week 64 where the 20μg group had a higher response rate than the 100μg group. Overall, the largest number of subjects responded two weeks post 3rd vaccination for the 4 μg dose [10/14 (71%)], the 20μg dose [11/14 (79%)] and the 100μg dose [12/16 (75%)]; the proportions decreased after the 4th dose.
HCV E1E2-specific CD4+ T-Cell Proliferation
In all subjects, vaccine produced a strong proliferation to recombinant E1E2. Of interest, there was a general inverse proliferative response to increasing amount of antigen. An anova found a significant difference between the placebo group and each of the vaccine groups two weeks after the first, second and third vaccinations and at Week 64 (16 weeks post 4th vaccination). Comparisons between vaccine groups were significant at Weeks 26 (two weeks post 3rd vaccination) and 50 (2 weeks post 4th vaccination) with the 100μg group having lower responses than both the 4 μg and the 20 μg groups, respectively. The Fisher’s Exact Test found a significant difference in proportion of responders for the placebo group versus all the vaccine groups post first vaccination. Among vaccine groups, the 20 μg group had a higher response rate than the 100 μg group at 28 days (prior to 2nd vaccination) (Figure 2C). Overall, the largest number of subjects responded two weeks post 3rd vaccination for the 4 μg dose [14/14 (100%)], the 20μg dose [13/14 (93%)] and the 100μg dose [16/16 (100)]; the proportions decreased after the 4th dose. All subjects responded to PHA except one placebo recipient at baseline (data not shown).
HCV E1E2-specific Cytokine Production
Of those subjects evaluated in the 4μg, 20μg and 100μg groups, there was a 100, ≥92 and ≥56 percent response rate, respectively, for the induction of cytokines to E1E2 for GM-CSF, IFN-γ, TNF-α, IL-2, IL-5, IL-10, and IL-13 at two weeks post third vaccination and a 100, 100 and ≥56 percent response rate, respectively, 16 weeks post fourth vaccination. The corresponding placebo response rates were ≤ 57 and ≤ 33. There was no induction of IL-12 in any vaccine dose group and a range of 25% to 91% of subjects produced IL-4. One placebo recipient had an IL-4 and an IL-2 response.
VSV/HCV Pseudotype Neutralization Assay
VSV/HCV pseudotype neutralization testing (Table 2) was performed on fifty subjects prevaccination and 16 weeks post 4th vaccination serum samples. Of these, one subject each from the placebo, 20μg and 100μg group did not have a prevaccination sample available. No subjects had a positive response at the prevaccination timepoint, however two subjects in the placebo group had a positive response post vaccination. There is no significant difference for a 2-fold rise between the vaccine dosage groups post vaccination. At the 16 week post 4th vaccination time point, 9 (69%), 6 (46%) and 5 (31%) subjects had VSV/HCV neutralizing titers ≥ 10 in the 4μg, 20μg, 100μg groups, respectively. Although there appears to be a trend for an inverse relationship between dosage groups, it is not significant.
Table 2.
The Number and Proportion of Subjects with ≥10 VSV/HCV Pseudotype Neutralizing Titers 4 months (Study Week 64) Post Fourth Vaccination
| Titer | Placebo N=9 (% responders) | 4 μg N=12 (% responders) | 20 μg N=13 (% responders) | 100 μg N=16 (% responders) | ||||
|---|---|---|---|---|---|---|---|---|
| Day 0* | Wk 64 | Day 0 | Wk 64 | Day 0* | Wk 64 | Day 0* | Wk 64 | |
| # ≥10 | 0 (0.00) | 2 (0.22) | 0 (0.00) | 9 (0.75) | 0 (0.00) | 6 (0.46) | 0 (0.00) | 5 (0.31) |
| # <10 | 8 (1.00) | 7 (0.78) | 12 (1.00) | 3 (0.25) | 12 (1.00) | 7 (0.54) | 15 (1.00) | 11 (0.69) |
The cut-off value is 5. In the placebo group, 1 subject each had a titer of 10 and 20. In the 4μg group, 3 subjects had a titer of 20 and 1 had a titer of 40. In the 20μg group, 3 subjects had a titer of 40. In the 100μg group 2 subjects had a titer of 20 and 1 had a titer of 40
One subject’s sample from the group was not tested.
DISCUSSION
The primary objective of this study was to evaluate the safety and tolerability of three doses of HCV E1E2/MF59C.1 vaccine administered intramuscularly to healthy adults. The vaccine was well-tolerated at each of the three dosage levels tested. Pain at the injection site was typically mild to moderate and systemic reactions were infrequent. There was no statistically significant association between dose and proportion of subjects experiencing adverse events related to vaccine. There was evidence for an increasing proportion of subjects experiencing reactogenicity as reported on the diary card as the dosage level increased.
The secondary objective of the study was to compare the immune responses to HCV E1E2 vaccine given at 4μg, 20μg, or 100μg in MF59C.1 adjuvant. HCV E1E2/MF59C.1 vaccine was able to stimulate significant humoral and cell-mediated immune responses however, a dose-response effect was not evident when measuring antibody responses. This finding is similar to findings with other antigens and may be due to the adjuvant exhibiting significant dose sparing effects.[37, 38] Additionally, all subjects had a strong proliferative response to E1E2 but with a general trend for an inverse proliferative response. It is plausible that the higher antigen concentration reduced priming T cell responses or had a prozone-like effect which was not predicted by in vitro studies. Nor is it clear what effect the adjuvant had on the immunogenicity measured in the trial since antigen alone was not administered to the subjects in this study. However, preclinical animal studies have shown that inclusion of the adjuvant significantly increases anti-E1E2 EIA titers.
CD81 is one essential host component facilitating HCV cell entry and so antibodies that block the binding of HCV gpE2 to CD81 contribute to virus neutralization.[32, 33] CD81-blocking antibody titers correlated well with antibody levels as detected by HCV E1E2 ELISA. Using Log(titers), the CD81 and ELISA titers showed significant correlation at all time points with the Pearson correlation of 0.40 at Week 24 (prior to 3rd vaccination) and ranging from 0.72 to 0.91 for all other time points. There was a higher seroconversion rate in the 20μg versus 4μg HCV E1E2MF59C.1 group in the CD81 NOB assay, a strong surrogate marker for viral neutralization antibodies. A rapid and strong immune response at priming is considered an advantage when vaccinating persons at risk for acquiring HCV such as injection drug users and health care workers.
Although VSV/HCV pseudotype neutralizing antibody titers were generally not high nor significantly different between the 3 dosage groups, the titers for all groups were at least 2-fold higher than the prevaccination titers. Possible reasons for not seeing very high titers with the VSV/HCV pseudotype system is that the vaccine may not be strongly immunogenic for virus neutralization, there are unrecognized mechanisms of HCV entry and escape from antibody mediated neutralization (Ray, et al, JID, in press).
A significant increase in the immune response was not noted beyond three doses of HCV E1E2MF59C.1 vaccine. The kinetics of the antibody response as measured by both ELISA and CD81 blocking antibody assay following the 3rd immunization suggests an anamnestic response. In addition, the evaluation of cytokines (IFN-γ, IL-2, IL-4, and IL-10) revealed few significant differences between the placebo group and the 100 μg group.
Conclusions
The vaccine is safe and induced significant lymphoproliferative and antibody responses at all three doses. The results suggest that the 20μg dose may be superior; the 100μg did not increase immune responses. A higher seroconversion rate occurred in the 20μg versus 4μg group in the CD81-blocking antibody assay after the second dose and correlated with the ELISA results. An anamnestic antibody response occurred following the 3rd immunization with no substantial increase after the 4th dose. As no further, substantial increase of the specific humoral immune response was noted after the 4th dose, we conclude that the primary immunization schedule for the HCV E1E2MF59C.1 vaccine should comprise of 3 rather than 4 doses of 20μg. In addition the 20μg dose would be more economically attainable. Finally, we plan to further evaluate the neutralizing and cross neutralizing capability of antibody against E1E2 with HCVpp and HCVcc neutralization assays. The observed safety and immunization of this vaccine in humans combined with the efficacy data derived previously from chimpanzee protection studies support further testing in the human population.
Acknowledgments
We would like to thank the volunteers who participated in the trial and Janice Tenant, Keith Meyer and Patricia Osmack and the staff of the Saint Louis University Vaccine Trials and Evaluation Unit, and the National Institutes of Health, Division of Microbiology and Infectious Diseases including Leslye Johnson, Leigh Sawyer, Holli Hamilton, Wendy Fanarof-Ravick, Robert Johnson, Stephen Heyse, Elaine Matzen, Carol Ostrye and Rajen Koshy and Chiron staff including Andria Langenberg, Amy Bolten, Deborah Novick, Lisa Wisniewski, Kevin Crawford and Yiu-lian Fong.
This research was funded by NIH Grant Number N01-AI-25464.
Footnotes
Clinical Trials Registration Number: NCT00500747
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. N Engl J Med. 1992;327(27):1906–11. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ, Margolis HS, Krawcynski K, Judson FN, Mares A, Alexander WJ, et al. The natural history of community-acquired hepatitis C in the United States. N Engl J Med. 1992;327(27):1899–905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 3.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–6. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterol. 1997;112(2):463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 5.Araya V, Rakela J, Wright T. Hepatitis C after orthotopic liver transplantation. Gastroenterol. 1997;112(2):575–82. doi: 10.1053/gast.1997.v112.agast970575. [DOI] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336(5):347–56. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 7.Kew MC. Hepatitis C virus and hepatocellular carcinoma. FEMS Microbiol Rev. 1994;14(3):211–20. doi: 10.1111/j.1574-6976.1994.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 8.Simonetti RG, Cammà C, Fiorello F, Cottone M, Rapicetta M, Marino L, et al. Hepatitis C virus infection as a risk factor for hepatocellular carcinoma in patients with cirrhosis: a case-control study. Ann Intern Med. 1992;116(2):97–102. doi: 10.7326/0003-4819-116-2-97. [DOI] [PubMed] [Google Scholar]
- 9.Jeng J-E, Tsai J-F. Hepatitis C virus antibody in hepatocellular carcinoma in Taiwan. J Med Virol. 1991;34(1):74–7. doi: 10.1002/jmv.1890340113. [DOI] [PubMed] [Google Scholar]
- 10.Castells L, Vargas V, Gonzalez A, Esteban J, Esteban R, Guardia J. Long interval between HCV infection and development of hepatocellular carcinoma. Liver. 1995;15(3):159–63. doi: 10.1111/j.1600-0676.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka K, Hirohata T, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, et al. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991;51(11):2842–47. [PubMed] [Google Scholar]
- 12.Choo Q-L, Richman KK, Han JH, Berger K, Lee C, Done C, et al. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci. 1991;88(6):2451–55. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Peralta RP, Qian K, She JY, Davis GL, Ohno T, Mizokami M, et al. Clinical implications of viral quasispecies heterogeneity in chronic hepatitis C. J Med Virol. 1996;49(3):242–47. doi: 10.1002/(SICI)1096-9071(199607)49:3<242::AID-JMV14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Stuyver L, Van Arhem W, Wyseur A, Hernandez F, Delaporte E, Maertens G. Classification of hepatitis C viruses based on phylogenetic analysis of the envelope I and nonstructural 5B regions and identification of five additional subtypes. Proc Natl Acad Sci. 1994;91(21):10134–38. doi: 10.1073/pnas.91.21.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McOmish F, Yap PL, Dow BC, Follett EAC, Seed D, Keller AJ, et al. Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative survey. J Clin Microbiol. 1994;32(4):884–92. doi: 10.1128/jcm.32.4.884-892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zein NN, Rakela J, Krawitt EL, Reddy R, Tominaga T, Persing DH, et al. Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Ann Intern Med. 1996;125(8):634–39. doi: 10.7326/0003-4819-125-8-199610150-00002. [DOI] [PubMed] [Google Scholar]
- 17.Martell M, Esteban JI, Quer J, Genesca J, Weiner A, Esteban R, et al. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66(5):3325–29. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bréchot C. Hepatitis C virus genetic variability: clinical implications. Am J Gastroenterol. 1994;89(8):S41–47. [PubMed] [Google Scholar]
- 19.Kurosaki M, Enomoto N, Marumo F, Sato C. Rapid sequence variation of the hypervariable region of hepatitis C virus during the course of chronic infection. Hepatology. 1993;18(6):1293–99. [PubMed] [Google Scholar]
- 20.Kao J-M, Chen P-J, Lai M-Y, Wang T-H, Chen D-S. Quasispecies of hepatitis C virus and genetic drift of the hypervariable region in chronic type C hepatitis. J Infect Dis. 1995;172(1):261–64. doi: 10.1093/infdis/172.1.261. [DOI] [PubMed] [Google Scholar]
- 21.Weiner AJ, Brauer MJ, Rosenblatt J, Richman KH, Tung J, Crawford K, et al. Variable and hypervariable domains are found in the regions of HCV corresponding to the flavivirus envelope and NS I proteins and the pestivirus envelope glycoproteins. Virology. 1991;180(2):842–48. doi: 10.1016/0042-6822(91)90104-j. [DOI] [PubMed] [Google Scholar]
- 22.Kato N, Ootsuyama Y, Tanaka T, Nakagawa M, Nakazawa T, Muraiso K, et al. Marked sequence diversity in the putative envelope proteins of hepatitis C viruses. Virus Res. 1992;22(2):107–23. doi: 10.1016/0168-1702(92)90038-b. [DOI] [PubMed] [Google Scholar]
- 23.Kato N, Sekiya H, Ootsuyama Y, Nakazawa T, Hijikata M, Ohkoshi S, et al. Humoral immune response to hypervariable region I of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67(7):3923–30. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato N, Oostuyama Y, Ohkoshi S, Nakazawa T, Sekiya H, Hijikata M, et al. Characterization of hypervariable regions in the putative envelope protein of hepatitis C virus. Biochem Biophys Res Comm. 1992;189(1):119–27. doi: 10.1016/0006-291x(92)91533-v. [DOI] [PubMed] [Google Scholar]
- 25.Choo Q-L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci. 1994;91(4):1294–98. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houghton M, Choo QL, Chein, et al. Development of an HCV Vaccine. Viral Hepatitis and Liver Disease. 1997:656. [Google Scholar]
- 27.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436(18):961–66. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 28.Coates S, Choo Q-L, Kuo G, Crawford K, Dong C, Wininger M, et al. Protection of chimpanzees against heterologous 1a viral challenge using a gpE1/gpE2 heterodimer vaccine. In: Jilbert AR, Gragacic EVL, Vickery K, Burrell C, Cossart YE, editors. Proceedings of the 11th International Symposium on Viral Hepatitis and Liver Disease, Australian Centre for Hepatitis Virology; Sydney, Australia. April 6–10, 2003.pp. 118–23. [Google Scholar]
- 29.Stamataki Z, Coates S, Evans MJ, Wininger M, Crawford K, Dongb C, et al. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine. 2007;25 (45):7773–84. doi: 10.1016/j.vaccine.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, McHutchinson J, Francis B, DiNello R, Polito A, Quan S, et al. Improved detection of antibodies to hepatitis C virus using a second generation ELISA. Adv Exp Med Biol. 1992;312:183–89. doi: 10.1007/978-1-4615-3462-4_19. [DOI] [PubMed] [Google Scholar]
- 31.Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, et al. A quantitative test to estimate neutralizating antibodies to the hepatitis C virus: Cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci. 1996;93(5):1759–63. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chien DY, Arcangel P, Kuo G, Pileri P, Coates S, Baumeister M, Houghton M, Abrignani S. Design of a quantitative human CD81-HCV envelope antigen binding assay to evaluate envelope binding and antibody titers in vaccinated animals. 6th International Symposium on Hepatitis C & Related Viruses, Molecular Virology and Pathogenesis; June 6–9, 1999; Bethesda, MD: NIH; p. 15. proceedings. [Google Scholar]
- 33.Wack A, Soldaini E, Tseng C-TK, Nuti S, Klimpel GR, Abrignani S. Binding of hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur J Immunol. 2001;31(1):166–75. doi: 10.1002/1521-4141(200101)31:1<166::aid-immu166>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nature Med. 2000;6(5):578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 35.de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10(1):133–39. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer K, Basu A, Beyene A, Bowlin TL, Basu A, Ray R. Coexpression of hepatitis C virus E1 and E2 chimeric envelope glycoproteins display separable ligand sensitivity and increases pseudotype infectious titer. J Virology. 2004;78(23):12838–47. doi: 10.1128/JVI.78.23.12838-12847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59®-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE [Electronic Resource] 2009;4(2):e4384, 1–10. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HKB, Graham IL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 2008;197(5):667–75. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]