Dr. Haurani, et al present a prospective study on the treatment of problematic scars with 5-FU. Keloids were treated with 5-FU following excision, and hypertrophic scars were treated without excision. Both patient groups were followed using questionnaires about scar symptomatology and by measuring the scar volume. The use of scar volume as a quantitative measure is a good objective approach to studying treatment efficacy. The authors report a recurrence rate of only 19% when keloids are resected and then treated with 5-FU, and a 50% median decrease in scar volume in the hypertrophic scars during the period of the study. As with any treatment for problematic scars, there were patients who failed to respond although the numbers reported are low relative to reports of other treatments in the literature.1
One important feature of this study is the separation of the patients with pathologic scars into those with hypertrophic scars and those with keloids. The clinical behavior of keloids, with persistent growth over years, is clearly distinct from hypertrophic scars that can regress spontaneously.2, 3 When the biology is better known, we think that it may reveal a different molecular mechanism for the pathogenesis of each requiring specific treatments.
Collagen deposition following wound healing is important for normal repair, but treatment with 5-FU could have the unwelcome side effects. Their results in keloids are very good with treatment beginning at 2 weeks, but it would be interesting to test whether changing the time of treatment initiation affects healing or efficacy. In hypertrophic scarring, it is known that hypertrophic scars can regress on their own over time, and it might be worthwhile to perform a randomized prospective trial of a control injection compared to 5-FU.
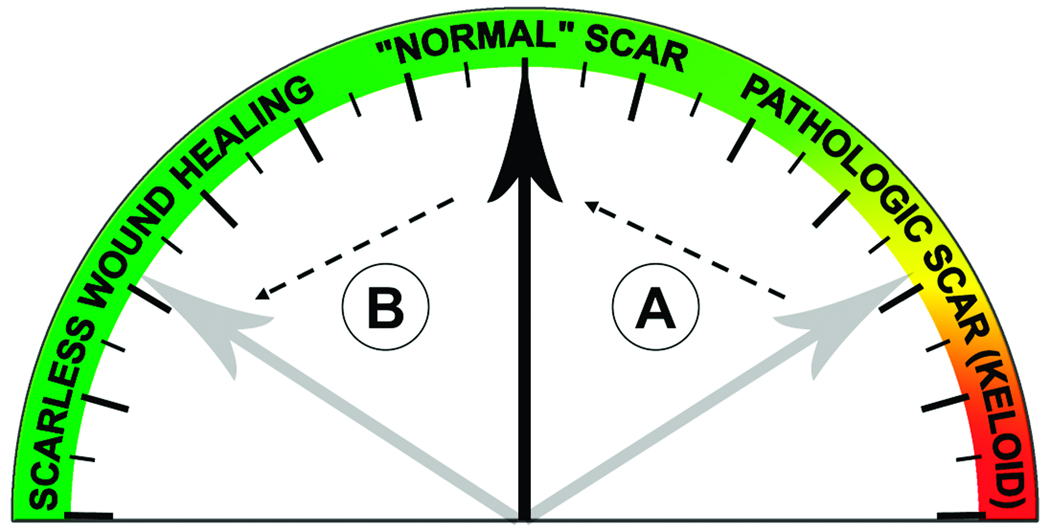
Scar formation can be viewed along a continuum from scarless to normal to pathologic wound healing (see Figure 1). Most people heal with a “normal,” fine scar. Treatments for pathologic wounds are aimed at getting thickened scars to heal like a normal wound (depicted as “A” in Figure 1). Optimistically, the goal is to allow patients to heal with no scar at all (depicted as “B” in Figure 1) as is seen during early gestation fetal skin wound healing. There are a number of efforts aimed at understanding how fetal wounds heal differently from adults, and how we might apply those lessons to allow for scarless wound healing in adults.4
Figure 1.
The continuum of wound healing from scarless repair to pathologic scars.
Treatments such as 5-FU and corticosteroids rely upon generalized suppression of the normal inflammatory and proliferative processes that are exacerbated in problematic wounds. In pathologic scars, there is evidence of excessive production of the ECM5 and 5-FU would function to broadly decrease this. A theory of keloid formation is that there is unchecked inflammation leading to excessive production of growth factors and cytokines with a subsequent increase in cell proliferation and ECM production6, and corticosteroids act by suppressing the inflammatory process. The negative side effects are related to interference with the normal metabolism as seen in the two patients who developed ulcerations in this study. A parallel clinical condition is malignancy where drugs such as 5-FU target the faster growing cells that comprise the tumor but have unwanted effects on normal cells that have higher proliferation rates, such as the digestive epithelium.
The treatment of malignancy is undergoing a marked change due to therapies that target discrete behaviors specific to the cancer cells. Vascular endothelial growth factor (VEGF) is the primary factor involved in tumor angiogenesis. Avastin is a monoclonal antibody directed against VEGF preventing it from stimulating angiogenesis, and has gained wide use in the treatment of metastatic colon, lung and breast cancer.7 Study of the basic biology of gastrointestinal stromal tumors (GISTs) demonstrated that overactivation of c-kit, a transmembrane tyrosine kinase receptor, was a primary factor in their growth. Gleevec targets c-kit by inhibiting signaling, and has shown tremendous efficacy giving an effective chemotherapeutic treatment where there previously was none.8
Lack of knowledge about their basic biology has prevented development of a targeted approach to the treatment of keloids and hypertrophic scars. Numerous papers describe how keloids and hypertrophic scars differ in their biologic behavior from normal scars, but no clear triggering mechanism has been identified.9–14 Unlike cancer, where specific mutations or deletions of oncogenes or tumor suppressors have been identified and targeted for treatment, there is no gene identified whose mutation leads to keloid formation. In the study of keloid pathogenesis, we are still waiting for the first transgenic mouse that forms keloids.
The TGF-® signaling pathway has been a common focal point of study since TGF-® is normally up-regulated in adult wound healing and has been implicated in a number of fibrotic diseases.15–18 There does appear to be some relationship between TGF-® biology and keloid formation, and it is important to note that increased activation of the TGF-® signaling pathway leads to increased dermal thickness,19 but not keloid formation.
In the continuum of wound healing noted above, TGF-® becomes an inviting target not just to prevent pathologic wound healing, but also to make normal wounds heal with less scar. Specifically, it has been noted that the TGF-®3 isoform appears to counteract the pro-fibrotic activity of the TGF-®1 and -®2 isoforms.20 There are currently clinical trials underway to determine if TGF-®3 can be used to minimize scar formation. It would be interesting if the same drug were directed at treatment of hypertrophic scars and keloids. It remains to be seen if TGF-®3 will become the first targeted therapy for scar prevention in the same manner that Avastin and Gleevec have been used for malignancy.
Multiple laboratories have focused on how cells respond to components of the wound environment at the molecular level. The hypothesis has been that there is a derangement in the cellular response to stress that leads to the differences found between keloid and normal cells. While differences have been shown in the manner in which keloid cells respond to stress compared to normal cells including mechanical stretch, exposure to serum and hypoxia, there is still not a single pathway that is common to all of these stresses. We hypothesize that identification of common crosstalk pathways between all of these cellular stresses might lead to determination of a mutation in a gene or multiple genes that can promote keloid formation.
ACKNOWLEDGMENTS
This work was supported by grants from the Oak Foundation, and the NIH (R01 GM65213 to M.T.L., K08 GM069677 to G.P.Y.).
Financial Disclosure: The authors report that they have no financial conflicts of interest related to this manuscript.
REFERENCES
- 1.Butler PD, Longaker MT, Yang GP. Current progress in keloid research and treatment. J Am Coll Surg. 2008;206(4):731–741. doi: 10.1016/j.jamcollsurg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26(5):379–384. doi: 10.1097/00000372-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Atiyeh BS, Costagliola M, Hayek SN. Keloid or hypertrophic scar: the controversy: review of the literature. Ann Plast Surg. 2005;54(6):676–680. doi: 10.1097/01.sap.0000164538.72375.93. [DOI] [PubMed] [Google Scholar]
- 4.Hantash BM, Zhao L, Knowles JA, Lorenz HP. Adult and fetal wound healing. Front Biosci. 2008;13:51–61. doi: 10.2741/2559. [DOI] [PubMed] [Google Scholar]
- 5.Phan TT, Lim IJ, Bay BH, et al. Differences in collagen production between normal and keloid-derived fibroblasts in serum-media co-culture with keloidderived keratinocytes. J Dermatol Sci. 2002;29(1):26–34. doi: 10.1016/s0923-1811(02)00008-7. [DOI] [PubMed] [Google Scholar]
- 6.Al-Attar A, Mess S, Thomassen JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 7.Reinacher-Schick A, Pohl M, Schmiegel W. Drug insight: antiangiogenic therapies for gastrointestinal cancers--focus on monoclonal antibodies. Nat Clin Pract Gastroenterol Hepatol. 2008;5(5):250–267. doi: 10.1038/ncpgasthep1097. [DOI] [PubMed] [Google Scholar]
- 8.Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)--the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. 2008;5(2):102–111. doi: 10.1038/ncponc1037. [DOI] [PubMed] [Google Scholar]
- 9.De Felice B, Ciarmiello LF, Mondola P, et al. Differential p63 and p53 expression in human keloid fibroblasts and hypertrophic scar fibroblasts. DNA Cell Biol. 2007;26(8):541–547. doi: 10.1089/dna.2007.0591. [DOI] [PubMed] [Google Scholar]
- 10.Smith JC, Boone BE, Opalenik SR, et al. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol. 2008;128(5):1298–1310. doi: 10.1038/sj.jid.5701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Fong KD, Phan TT, et al. Increased transcriptional response to mechanical strain in keloid fibroblasts due to increased focal adhesion complex formation. J Cell Physiol. 2006;206(2):510–517. doi: 10.1002/jcp.20486. [DOI] [PubMed] [Google Scholar]
- 12.Xia W, Kong W, Wang Z, et al. Increased CCN2 transcription in keloid fibroblasts requires cooperativity between AP-1 and SMAD binding sites. Ann Surg. 2007;246(5):886–895. doi: 10.1097/SLA.0b013e318070d54f. [DOI] [PubMed] [Google Scholar]
- 13.Xia W, Longaker MT, Yang GP. P38 MAP kinase mediates transforming growth factor-beta2 transcription in human keloid fibroblasts. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R501–R508. doi: 10.1152/ajpregu.00472.2005. [DOI] [PubMed] [Google Scholar]
- 14.Xia W, Phan TT, Lim IJ, et al. Differential transcriptional responses of keloid and normal keratinocytes to serum stimulation. J Surg Res. 2006;135(1):156–163. doi: 10.1016/j.jss.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Babu M, Diegelmann R, Oliver N. Keloid fibroblasts exhibit an altered response to TGF-beta. J Invest Dermatol. 1992;99(5):650–655. doi: 10.1111/1523-1747.ep12668146. [DOI] [PubMed] [Google Scholar]
- 16.Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98(5):827–833. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 18.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Sarkar P, Mi Q, et al. Overexpression of Smad2 reveals its concerted action with Smad4 in regulating TGF-beta-mediated epidermal homeostasis. Dev Biol. 2001;236(1):181–194. doi: 10.1006/dbio.2001.0332. [DOI] [PubMed] [Google Scholar]
- 20.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGFbeta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]