Abstract
Stroke in the developing brain is an important cause of chronic neurological morbidities including neurobehavioral dysfunction and epilepsy. Here, we describe a mouse model of neonatal stroke resulting from unilateral carotid ligation that results in acute seizures, long-term hyperactivity, spontaneous lateralized circling behavior, impaired cognitive function and epilepsy. Exploration-dependent induction of immediate early gene, Arc (activity-regulated cytoskeleton associated protein) in hippocampal neurons was examined in the general population of neurons versus neurons that were generated ~ 1week after the ischemic insult and labeled with BrdU. Although Arc was inducible in a network specific manner after severe neonatal stroke, it was impaired, not only in the ipsilateral injured but also in the contralateral uninjured hippocampi when examined at 6 months after the neonatal stroke. Severity of the stroke-injury and the acquired post-stroke epilepsy, both negatively correlated with Arc-induction and new neuron integration into functional circuits in the injured hippocampi.
Keywords: Arc, neonatal stroke, epilepsy, Timm stain, open-field test, behavioral co-morbidities, hyperactivity, lateralized circling
Introduction
Neonatal strokes occur in ~1 per 4000 term births and commonly present with acute seizures [1]. Neonatal strokes and seizures frequently result in long-lasting consequences like epilepsy and cognitive deficits, neurobehavioral impairments like hyperactivity disorders and problematic social skills [2–4]. Perinatal strokes in humans that result in congenital porencephalic cysts are frequently associated with hippocampal atrophy and sclerosis on neuroimaging and neuropathology [5–7] that may be the underlying cause for the learning and memory deficits. Thus stroke in the developing brain is a significant cause of associated neurological co-morbidities that persist into adulthood.
Ischemia in the adult brain is associated with an increase in hippocampal neurogenesis. The data in the immature brain is controversial; some data show an increase in the survival of hippocampal precursor neurons and others show a decrease in the survival of this cell population [8–10]. We have recently shown that post-stroke the rate of new-cell proliferation and survival in the sub-granular zone (SGZ) of the hippocampus of CD1 mice subject to unilateral carotid ligation at P12 is negatively modulated [10,11]. However a trend towards recovery in proliferation rates of SGZ neural stem cells was detected 3 weeks following the injury [11]. Little is known about circuit integration of post-injury neurogenesis into functional networks and subsequently the associated neurobehavioral co-morbidities in animal models. Clinically, it is not clear whether neonates make a better recovery from strokes than adults because of the fact that the immature brain has a better potential for neurogenesis compared to the mature brain; a feature that is also known to diminish with age [12,13]. Therefore better information on long-term clinical outcomes in children who suffered neonatal strokes and criteria for identification of high-risk groups for severe neurologic morbidity remain a topic of interest [3,14].
Ramirez-Amaya et al., have successfully used exploratory behavioral tests that require spatial information processing to identify new neurons that have integrated into networks based on their ability to induce expression of IEG gene Arc [15,16]. Studies in which hippocampal afferents arising in the entorhinal cortex were lesioned demonstrated profound depression of experience dependent Arc-induction at early post-lesion intervals [17]. Recovery was seen in the time course required for reinnervation of the DG due to synaptic reorganization [18]. Neonatal stroke injury causes widespread neuronal death followed by gliosis most notable in the watershed zones of the major cerebral arteries [19,20]. The present study aimed to determine the effects of injury and subsequent synaptic reorganization on the ability of newly formed neurons to incorporate into functional neuronal networks within the hippocampus. In addition, we assessed the long-term consequences of neonatal stroke including the severity of epilepsy and cognitive impairments; our analysis measured correlations between the severity of the epilepsy and post-stroke neurogenesis, hippocampal functional circuits and behavioral co-morbidities.
Methods
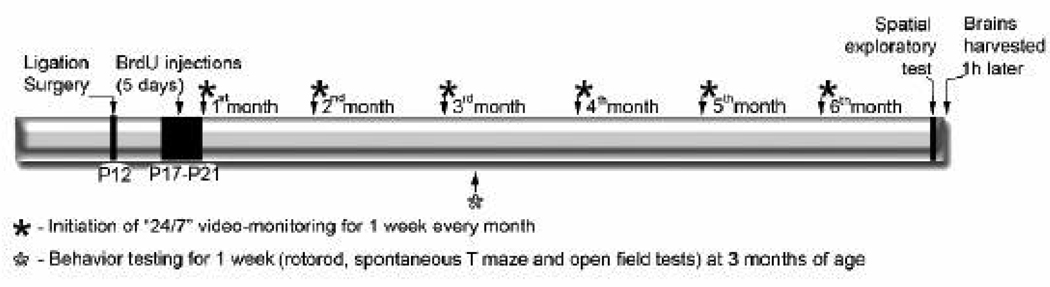
All research was conducted according to a protocol approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee (IACUC). Previous studies measuring hippocampal injury after carotid ligation in mice ligated at P12 found a strong correlation for the severity of brain injury and acute seizure scores for the model [21]. Using this correlation, equal numbers of ligated male and female mice with acute seizure scores following ligation (n=12 total) were introduced into the 7-month study (see schematics of the experimental protocol in Figure 1).
Figure 1.
Schematics of experimental protocol for the 7 month study.
Surgical procedure for ischemic model
All litters of CD1 mice were purchased from Charles River Laboratories Inc (Wilmington, MA). Newly born litters of pups arrived at postnatal five days old (P5) and were allowed to acclimate for seven days. Animals were housed in polycarbonate cages on a 12 hour light:dark cycle and food provided ad libitum. On P12, animals underwent permanent unilateral ligation of the carotid artery as previously described [10].
Acute seizure scoring
Acute post-ligation seizure activity was scored according to a seizure rating scale as previously reported [22] and previously described in this model [21]. Seizures were scored at 5 minute intervals during the four hour period immediately following surgery and the score corresponding to the highest level of behavioral seizure activity observed during that time period was recorded. After four hours, the mice were returned to the dam and each of their seizure scores was individually summed (i.e., highest behavioral seizure scores assigned to every 5 min block of the 4h monitoring period were added) to produce a total acute seizure score. The mean acute seizure score for ligated mice introduced into the chronic study (n=12) 6 males and 6 females was 14±2.8 (range from 3 to 35).
BrdU labeling of post-ischemia neurogenesis
Each animal [n=11 ligated (5 males and 6 females;1 male with high seizure score died 2 weeks after ligation) and n=10 sham-control mice] received daily intraperitoneal injections of 50mg/kg BrdU (Sigma, St. Louis, MO) over a period of 5 days starting on postnatal day 17 through 21. Afterward, animals were kept undisturbed in their home cages (2 same-sex mice per cage) for 6 months during which a sub-set of mice from each group (i.e., ligates and sham-controls) underwent behavioral monitoring with video-cameras to investigate occurrence of spontaneous recurrent motor seizures.
Behavioral monitoring for epilepsy
Video of mouse behavior in the home cage setting (4 available units, therefore the mice were same-sex housed, 2 mice per cage, n=8, n/n=8/11 ligated, 4 males and 4 females) was acquired and analyzed. The ligation-injured mice (n=8) and sham-controls (n=6) were videotaped 24 h per day continuously for 1 week every month (i.e. 25% monitoring time; Figure 1) starting at 1 month of age for 6 consecutive months using a digital color bullet camera connected to an EverPlex4CQ color quad processor (i.e., all 4 cages on one screen). These files were burned on DVDs for further analysis. The mice were housed in a 12-h light /dark cycle from 6 am to 6 pm (i.e., tube lights on for 12 hours and a darkroom safe light on for 12 hours controlled by a timer) in clean standard housing cages, complete with bedding, food, and water, located inside a sound attenuating chamber (Coulbourn Habitat Isolation Cubicles Model# H10–24). Behavior was recorded through a video camera positioned inside the sound attenuating chamber, but outside the housing cages. Spontaneous recurrent behavioral seizures were scored using the Racine scale (class 1–5) to determine the severity of motor seizure activity [23] as follows: Class 1 – Frozen (immobility with open eyes) and or “wet dog shakes” associated with facial convulsions (vibrissae twitching, jaw clonus, blepharospasms); Class 2 – Same behaviors as class 1 with head bobbing; Class 3 -Forelimb clonus with a lordotic posture; Class 4 - Forelimb clonus continued along with rearing; Class 5 – Same behaviors as class 3 and 4 seizure, but the mice also fall over. The video analysis was conducted initially in fast-forward mode to detect behavioral postures (i.e., freezing, lordosis, straight tail, forelimb clonus, and/or rearing). Once a behavioral posture was observed, the video was re-examined at real-time speed, the seizure was scored on the scale and time of day and duration of seizure was noted. With the 1-week-per-month protocol, the protocol amounted to 42 days (i.e. 42 DVDs) of 24-h video-monitoring.
Behavioral tests
All behavior tests were conducted at 3 months of age in the week following the 3rd month of video-monitoring for all the mice in the 7 month study (n=21; Figure 1).
Rotorod
The Rotorod (Accuscan, Columbus, OH) test was conducted as previously described [21]. To begin a trial the mouse was placed on top of the beam facing away from the experimenter, in the orientation opposite to that of its rotation, so that forward locomotion was necessary to avoid fall. The Rotorod accelerated gradually without jerks from 0 to 35 rpm over a 2 minute trial. Latencies for the mice to fall from the rod were recorded automatically by computer. Each mouse was given 4 trials with a 10-min inter-trial interval.
T-maze spontaneous alternation
This procedure was carried out in an enclosed “T” shaped maze (Med Associated, St. Albans, VT) in which the long arm of the T (47 cm × 10 cm) serves as a start arm and the short arms of the T (35 cm × 10 cm) serve as the goal arms. The mice were tested as previously described [21].When a mouse had fully entered the choice arm (tail tip all the way in) the arm was closed and the mouse was confined to the choice arm for 30 seconds. The mouse was then removed, the guillotine door lifted and the next trial initiated. This was repeated for a total of 15 trials. If the mouse did not make a choice within 2 minutes the trial was ended and advanced to the next. At the conclusion of each trial the maze was cleaned with 70% ethanol to get rid of odors.
Open-field habituation
All procedures were carried out in a square open-field chamber (40.6 × 40.6 cm, Accuscan, Columbus, OH) mounted within sound attenuating shells. Behavior was monitored via a grid of invisible infrared light beams mounted on the sides of the walls of the arena. Data was collected and analyzed via VersaMax Analyzer software (Accuscan, Columbus, OH). To examine activity levels and habituation, mice were exposed to the test chambers for 30 minutes on each of two consecutive days at 3 months after the ligation. To begin a session, each mouse was placed in the center of the chamber and allowed to move about freely. The arena was cleaned with 70% ethanol after each mouse completed a session.
Novel spatial exploration for Arc-induction at 7months of age
Seven months after the last BrdU injection, stroke-injured mice (n=11) and sham-controls (n=10) were habituated to single cage housing for 3 weeks. All animals were handled once daily for 7 days and then allowed to explore a novel environment as previously described by Ramirez-Amaya [15,16]. Animals were allowed to explore a square open box; 25 × 25 inches with 16-inch-high walls made of ABS plastic material that contained four novel objects (small animal pet toys) and guidance cues on the walls of the box. The box was placed in a dimly lit room. Each mouse [i.e., 8 ligated mice; n/n=8/11(total of 4 males and 4 females of which 3 males and 3 females underwent novel-exploration from the video-monitored group and 1 male and 1 female underwent novel-exploration the non-video-monitored group) and 6 sham-controls, n/n=6/10; (total of 3 males and 3 females of which 2 males and 2 females from the video-monitored group and 1 male and 1 female from the non-video-monitored group underwent novel-exploration)] was tested individually. Immediately after the exploration session, the mouse was placed back in its home cage and kept undisturbed for 1h before being euthanized for brain harvesting. The remaining 3 ligated mice [1 male and 2 females (2 from the chronically video-monitored group and 1 from the non-video monitored group)] and 4 sham-controls [2 males and 2 females (2 of each sex from the chronically video-monitored and non-video-monitored groups)] were kept in their home- cages (cage-controls) and euthanized for tissue harvest at the same time as the ligated and sham-control mice that had the novel-exploration treatment. The 3 ligation-injured mice assigned to the cage-control group comprised of two chronically video-monitored mice with post-stroke epilepsy and hyperactivity co-morbidities (1 each) and one non-video monitored mouse. All mice (11 ligates and 10 sham-controls) were video-monitored in their home-cages for 24h prior to being tested, in-order rule out behavioral seizures modulating the Arc-induction in the study.
Histology
All animals were anesthetized with chloral hydrate (90 mg/ml; intraperitoneal) before the whole brain was removed, rapidly fresh frozen in a slush of isopropanol and dry ice, and placed in −80°C storage. Coronal brain sections 40µm thick were cut on a cryostat in serial order to create 4 series of adjacent sections and mounted on super frost plus glass slides.
Immunohistochemistry with triple labeling
Slides mounted with fresh frozen sections were fixed in formalin. Before the detection of Arc the tissue was permeabilized with acetone/methanol (50:50, v/v; Sigma) at 4°C for 8 min. After blocking nonspecific reactions the tissues were sequentially incubated overnight in 4°C with primary antisera which included polyclonal rabbit anti-Arc antibody (1:500; a gift from Dr. P.F. Worley’s laboratory). For the detection of BrdU incorporation, the DNA was denatured with 2N HCL for 30 min at 37°C and followed by incubation with mouse anti-BrdU monoclonal antibody (1:200, Roche, Germany). Secondary antibodies used sequentially were Alexa 594 (Arc) and Alexa 488 (BrdU) for 1 hour at room temp. Sections were then counter-stained with Hoechst (33342, Invitrogen, Oregon, USA) and slides were cover slipped with Pro-long anti-fade reagent (Invitrogen, Oregon, USA).
Confocal microscopy
Confocal images of immunostained sections were taken on a FV 1000 confocal system that is based on an Olympus IX81 inverted microscope stand. Stacked images (1µm thick - Z axis) were taken of bilateral hippocampi from 40µm thick coronal sections to confirm co-labeling. All Arc labeled cells constituted the granule cells involved in the exploration- test activated circuits in the DG and all the BrdU labeled cells represented the new cells born between P17 and P21 following the neonatal stroke. Total counts of BrdU and Arc-positive cells were made in the granule cell layer (GCL) of the dorsal hippocampus in ipsi- and contralateral GCLs at bregma coordinates −1.46 to −2.30 mm for each injured and uninjured brain [24] such that the 1st section quantified had both upper and lower blades of the GCLs clearly defined. The ischemic injury in the CD1mouse model lies in the middle and posterior cerebral artery perfusion territories and the ipsilateral ventral hippocampus is lost to the stroke-infarct (Figure 2). Cell counts were restricted to the dorsal hippocampus where direct comparisons between counts and GCL areas in ligation-injured and sham-controls could be made. Counts of BrdU/Arc co-labeled cells were determined for each DG and represented the new neurons that were activated during the exploration test. Unbiased stereology using a modified method described previously [10] was used to estimate total counts of Arc-positive, BrdU-positive and Arc/BrdU co-labeled cells in 4 consecutive coronal sections and mean counts were determined for each left and right GCL. Additionally to account for injury related atrophy of hippocampi, total counts in each section were normalized to infarct related atrophy in the GCL (i.e., cells/mm2 area of corresponding GCLs) in the same 4 coronal sections and means densities were reported.
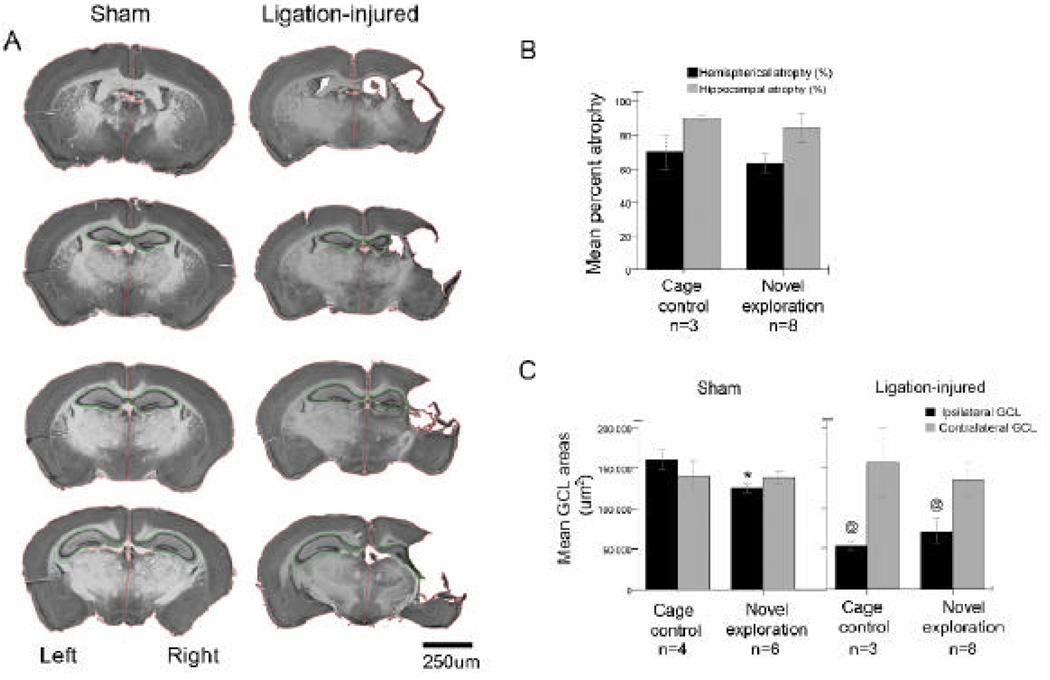
Figure 2.
Unilateral neonatal stroke injury in the ligation-injured group of mice that had acute seizure scores (A; right panel) compared to control (A; left panel) at 7 months post-ligation (quantified in B and C). Scale bar = 250um. B and C. Stroke-injury related atrophy B. Hippocampal and hemispheric atrophy was not significantly different between the two groups of novel-exploration and cage-control mice within the ligation-injured group of mice. C. In order to control for stroke-injury related atrophy of ipsilateral hippocampi, mean areas of the corresponding GCLs were quantified by high magnification tracings in neuro-investigator (MicroBrightfield Inc.). As expected ipsilateral hippocampi in the ligation-injured group of both novel-exploration and cage-control mice had GCL areas that were significantly smaller (@) than their respective sham-controls, however not significantly different from each other.
Timm stain for mossy fiber sprouting
A complete series of adjacent fresh frozen coronal sections (40µm thickness) from brains of ligation-injured and sham-control mice were processed for Timm stain. Slides mounted with sections were dipped 6–7 times in freshly prepared sodium sulphide solution (0.37%), allowed to air dry briefly after draining off excess solution, and then fixed in 10% formalin overnight. After drying at room temperature the slides were stored in a slide box in preparation for Timm stain. Timm stain was developed according to a modified protocol described by Babb et al. [25]. Briefly, slide mounted sections were placed in a solution consisting of 180 ml gum Arabic (50% w/v), 30 ml 2.25 M citrate buffer, 45 ml 0.5 M hydroquinone, and 50 ml 0.04% silver lactate. Sections were developed for approximately 70 min in the dark at room temperature, rinsed later with water and lightly counterstained with cresyl violet.
Histology and computerized measurement of atrophy and GCL areas
Brain atrophy measurements were done as previously described [10]. Using MCID 7.0 Elite (InterFocus Imaging Ltd., Cambridge UK), hemispheric areas of 40 µm-thick, Nissl-stained coronal sections equally spaced and spanning rostral striatum to caudal hippocampus were measured (n=10–12 sections per animal). Hippocampal and hemispheric atrophy was calculated for each section as follows:
[1-(ipsilateral area/contralateral area)] × 100 = percent ipsilateral hippocampal or hemispheric atrophy. The values from each section were then averaged to calculate the hippocampal and hemispheric brain atrophy for each brain. Areas of GCLs were measured by tracing closed contours using Neurolucida software (MicroBrightField Inc. VT, USA) and acquiring closed contour measurements for every dentate gyrus in which total Arc and BrdU-labeled cell counts were done.
Methods of analysis
Statistical analyses were run in SPSS for Windows (SPSS Inc., Chicago, Illinois, USA) and SYSTAT 12 (SYSTAT software, Inc., San Jose, CA). ANOVAs were carried out to analyze rotorod, open-field, and the area and count data for the ipsi- and contralateral sides in the ligation-injured group compared to controls. With the exception of the rotorod data, there were no performance differences between male and females on any of the behavioral tests; therefore, analysis was carried out collapsed across gender. Correlations were reported whenever statistical significance was noted. A probability below 0.05 was considered significant.
Results
Long-term effects of neonatal strokes on behaviors and physical development
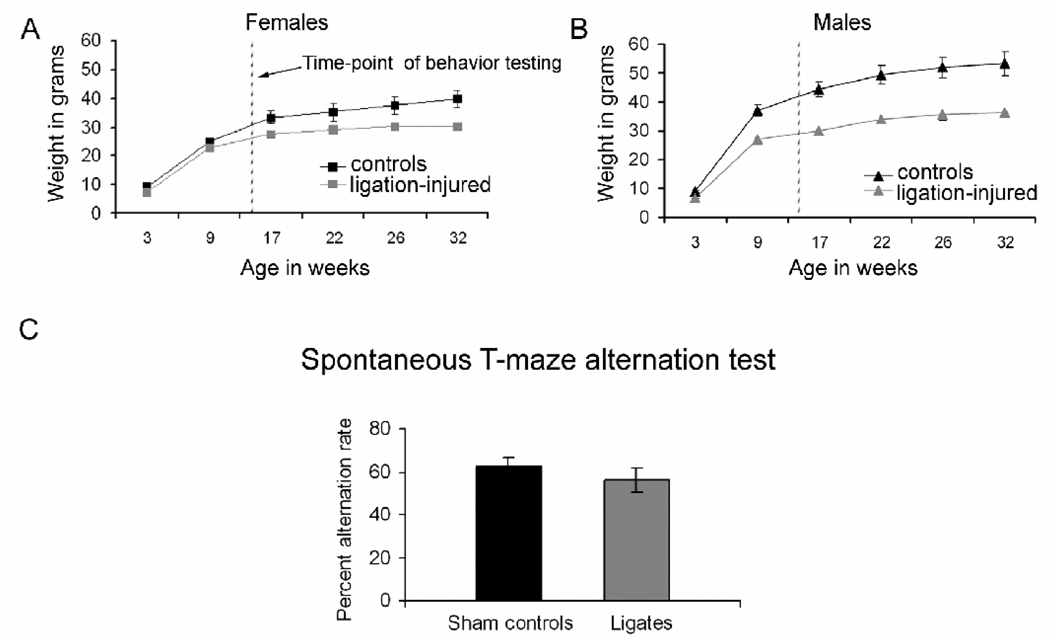
We have previously reported behavioral results from post-stroke CD1 mice at P40 (i.e., 4 weeks following the P12 stroke injury). The assays in the previous report were limited to rotorod, open-field, and T-maze spontaneous alternation [21]. However in the present study, chronic video-monitoring of the 8 ligated mice (n/n=8/11), 1 week per month over a six month period following the stroke was also included, and revealed temporally progressive hyperactivity behaviors that were not noted in the P40 study. Lateralized “mad running” behaviors became apparent in 4 out of the 8 ligation-injured monitored mice (i.e., 50%, 3 clockwise, 1 counterclockwise; 2 males and 2 females) beginning at 2 to 3 months of age. The mice continuously circled the perimeter of their cages for extended periods of time with a running gait. This behavior progressively worsened as evidenced by an increase in episodes and the amount of time spent running around the cage. In 3 of those mice (i.e., 38%, 2 females, 1 male) the activity progressed from running around the periphery of the cage to tighter clockwise rotations at 4 to 5 months of age. This behavior was sometimes associated with “tail chasing” for extended periods of time that was not associated with behavioral features of post-ictal depression like malaise and inactivity. On the contrary there was an overall locomotor hyperactivity. The shams that were monitored for the same periods of time did not show similar behaviors with advancing age. Concomitant weight monitoring over the period of the study, detected considerable lag in weight gain in all the ligation-injured mice (males more than females, Figure 3A and B) that was also temporally progressive akin to the increase in hyperactive behaviors. Since the lag in weight gain was not limited to the hyperactive circling mice, the finding cannot be attributed to hyperactivity alone. The differences in weight between ligated and sham-control mice were significant in males (p=0.002) compared to females (p=0.053) at the time when behavioral testing was conducted at 3 months of age (Figure 3; compare A to B, dotted lines).
Figure 3.
Animal weights as a function of time and performance on the Rotarod and spontaneous T-maze alternation tests. A and B. Compared to controls both female (A) and male (B) ligation-injured mice showed impaired weight gain as a function of time over the 7 month duration of the study. The male ligation-injured however showed significantly lower weights than their control litter mates as opposed to females at the time of behavioral testing (dashed line). C. Although ligation-injured mice showed a lower rate of alternation on the T maze, this was not significant compared to their control littermates.
Motor and cognitive testing
Behavior testing was done on the all the mice in the study (i.e., 11 ligates and 10 sham-controls) at 3 months of age.
Rotorod test
Rotorod data was analyzed by a 2-way repeated measures ANOVA with Treatment (sham vs. ligated) and Gender as the between factors and Trial as the repeated measure factor. There was no main effect of Treatment or Trial nor were there any interactions. Only the main effect of Gender was significant (F1,18 = 5.011, p = 0.038). Since the effect of Gender did not impact any other factor it is likely that this main effect was due to the overall shorter fall latencies for all male mice (trial 1: 56.997 ± 7.32; trial 2: 46.1 ± 6.17; trial 3: 53.66 ± 7.79) relative to all female mice (trial 1: 73.89 ± 6.42; trial 2: 62.49 ± 4.99; trial 3: 65.87 ± 7.67). Nevertheless, the rotorod test detected no motor learning deficits for the ligation-injured mice at 3 months after the ligation and thus was similar to previously reported performances at P40 [21].
T-maze spontaneous alternation
To measure exploratory behavior as well as memory, we assessed performance on a T-maze spontaneous alternation task. Results from the T-maze spontaneous alternation task can be seen in figure 3D which shows that the ligation-injured mice alternated at a lower mean rate than sham-control mice that was not significant (p=0.3). There was no difference in the number of trials completed by each groups since. Ligation-injured mice completed on average 12.3±0.9 of the 15 trials compared to the 12.7±0.7 trials by the control group of mice. We also examined the percent of right turns made and found no significant differences between the two groups (43.6±2.8% for sham-control mice and 51.6±4.5% for ligation-injured mice; p=0.15). This indicated that there was no significant lateralized preference for making right turns (i.e., towards the side of the unilateral stroke injury) in the ligation-injured group compared to controls.
Open-field activity and habituation
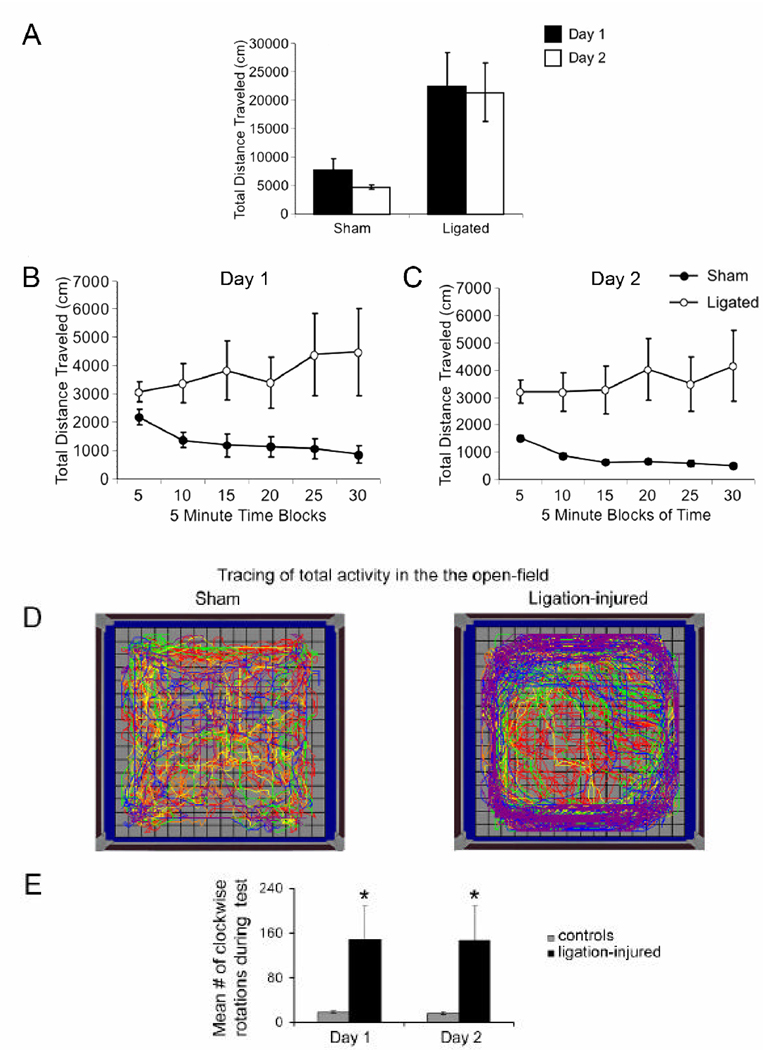
To examine overall locomotor activity and habituation, spatial distribution and rotational bias, mice were observed in an open-field over 2 consecutive daily sessions. We first examined locomotor activity across the 2 days (Figure 4A) and analysis revealed only a main effect of Treatment (F1,20 = 8.322, p = 0.009) reflecting the overall higher levels of activity in the ligates compared to the controls. There was no main effect of Day or a Day × Treatment interaction. Thus, although there was a trend toward less activity in the controls on day 2 relative to day 1 (across session habituation), this difference was not significant. To examine habituation within a session, the total distance traveled during the 30 minute session was analyzed in 5 minute blocks. Analysis of the Day 1 session (Figure 4B) revealed significant main effects of Treatment (F1,20 = 7.519, p = 0.013) due to the overall longer distance traveled by the ligated mice, but no effect of Time Block nor a Time Block × Treatment interaction. This was surprising giving the apparent decrement in activity over the course of the session in the sham-control mice, therefore, we performed one-way Anova’s for each treatment group and found a significant effect of Time Block for the sham-controls (F5,20 = 47.317, p < 0.001), indicating that this group habituated, or decreased locomotion over the length of the session. However no such effect was observed in the ligated group. Analysis of the day 2 session (Figure 4C) also yielded a significant main effect of Treatment (F1,20 = 10.371, p = 0.004), but in contrast to the day 1 session, the day 2 session analysis also revealed a significant Time Block × Treatment interaction (F5,100 = 2.423, p = 0.041). Posthoc analysis revealed this interaction to be due to a decrease in locomotion over the course of the session in the sham-controls (p<0.001), but not in the ligated mice (p = 0.502). Thus, the ligated mice had higher overall levels of activity, and the only habituation observed was in the sham-control mice within each session.
Figure 4.
Open-field test: total open-field activity and habituation. A. Ligation-injured mice showed significantly higher levels of locomotor activity compared to controls in the open-field. B and C. Ligation-injured mice exhibited a complete lack of habituation within sessions associated with hyperactivity, while control mice showed classic within session habituation both on day 1 (B) and day 2 (C). Neither group showed significant between session habituation. D and E. A clockwise revolution (i.e., lateralized circling) was evident in ligate-injured mice compared to controls by their open-field activity traces (D) that was confirmed with video- monitoring. Quantification of the activity showed significant differences (E, p=0.042)
In addition to habituation, we determined if the spatial distribution of activity differed between the two groups. Therefore, the time mice spent in the four quadrants of the open-field (left front, left rear, right front, and right rear) as well as the time mice spent along the peripheral and the center zones of the field were examined. Analysis of the quadrant data from day 1 yielded no significant main effects or an interaction. In contrast, analysis of the quadrant data from day 2 yielded a significant main effect of Quadrant (F3,60 = 3.095, p = 0.033) due to all mice spending more time in the left rear quadrant overall, and there was a significant Quadrant × Treatment interaction (F3,60 = 3.146, p = 0.032). Follow up analysis revealed that this interaction was most likely due to the ligated mice spending more time in the left rear quadrant relative to the sham mice although the difference was statistically marginal (p = 0.057; data not shown). There were no other significant main effects or interactions. Next we examined the time mice spent in the peripheral and central zones of the field during the two daily sessions and analysis yielded only a significant effect of Zone (Day 1: F1,20 = 401.647, p < 0.001 and Day 2: F1,20 = 415.824, p < 0.001) due to all mice (i.e., ligation-injured and shams) spending more time along the walls of the field than in the center of the field (data not shown).
Finally, we examined the number of clockwise and counter clockwise revolutions made by the mice in the open-field during each of the two daily sessions. Paired comparisons of the data from day 1 did not reveal any significant differences in the numbers of clockwise and counterclockwise rotations for either the shams (19.0 ± 2.08 vs. 40.8 ± 16.69, respectively) or the ligated mice (148.45 ± 61.82 vs. 78.55 ± 53.02 respectively). Similarly comparisons of the data from day 2 did not reveal any significant differences in the numbers of clockwise and counterclockwise rotations for either the shams (17.18 ± 2.37 vs. 18.1 ± 2.52, respectively) or the ligated mice (147.45 ± 62.63 vs. 59.64 ± 34.38 respectively). Analysis of the clockwise rotations across day yielded only a main effect of Treatment (F1,20 = 4.722, p = 0.042; Figure 4D and E) due to the higher number of clockwise rotations by the ligates on both days. However analysis of the counter clockwise revolutions across both days did not reveal any significant differences, indicating that the number of counter-clockwise revolutions were similar in the two groups of mice across both days. This open-field test finding was consistent with the clockwise rotational hyperactivity detected in 3 out of 4 ligation-injured mice with lateralized circling hyperactivity of the ligation-injured and video-monitored mice that showed temporal exacerbation.
Unilateral stroke-injury related atrophy in the ipsilateral hemisphere
All the ligated mice introduced in the long-term study (i.e. 100%) showed significant stroke injury in the ipsilateral hemisphere as predicted by their acute seizure scores (Figure 2A, right column compared to the left). Stroke severity was quantified and ranged between 60–80% hemispheric atrophy and 80–90% hippocampal atrophy. Injury-severity in mice randomly assigned to the cage-control and novel-exploration group of ligated mice did not show significant differences (Figure 2B). GCL areas in which counts of BrdU and Arc-positive cells were done were measured. Ipsilateral GCLs in ligation-injured mice were significantly smaller than controls (71602±15749 µm2 and 125127±4775 µm2 respectively for the novel-exploration group, p=0.006 and 53282±4771 µm2 and 160461±12191 µm2 respectively for the cage-control group of mice, p=0.001). Contralateral GCL areas in the ligation-injured mice were not significantly different from sham-controls (p=0.7). Stroke related hippocampal atrophy was severe and not uniform. The dorsal hippocampus was atrophied but relatively spared compared to the ventral hippocampus which in most ligation-injured mice was completely lost to the stroke-injury (Figure 2A, left column) accounting for the overall high percent of atrophy in the ligation-injured mice. As previously reported in the model, CA3 and CA1 neurons are more susceptible to cell death than the DG in the hippocampus [10]. Therefore, inspite of significant overall hippocampal atrophy, corresponding DGs in the dorsal hippocampi were relatively spared (i.e., GCL areas in ligation- injured mice were 56% and 33% of control in novel-exploration tested and cage-control mice respectively).
Post-stroke neurogenesis
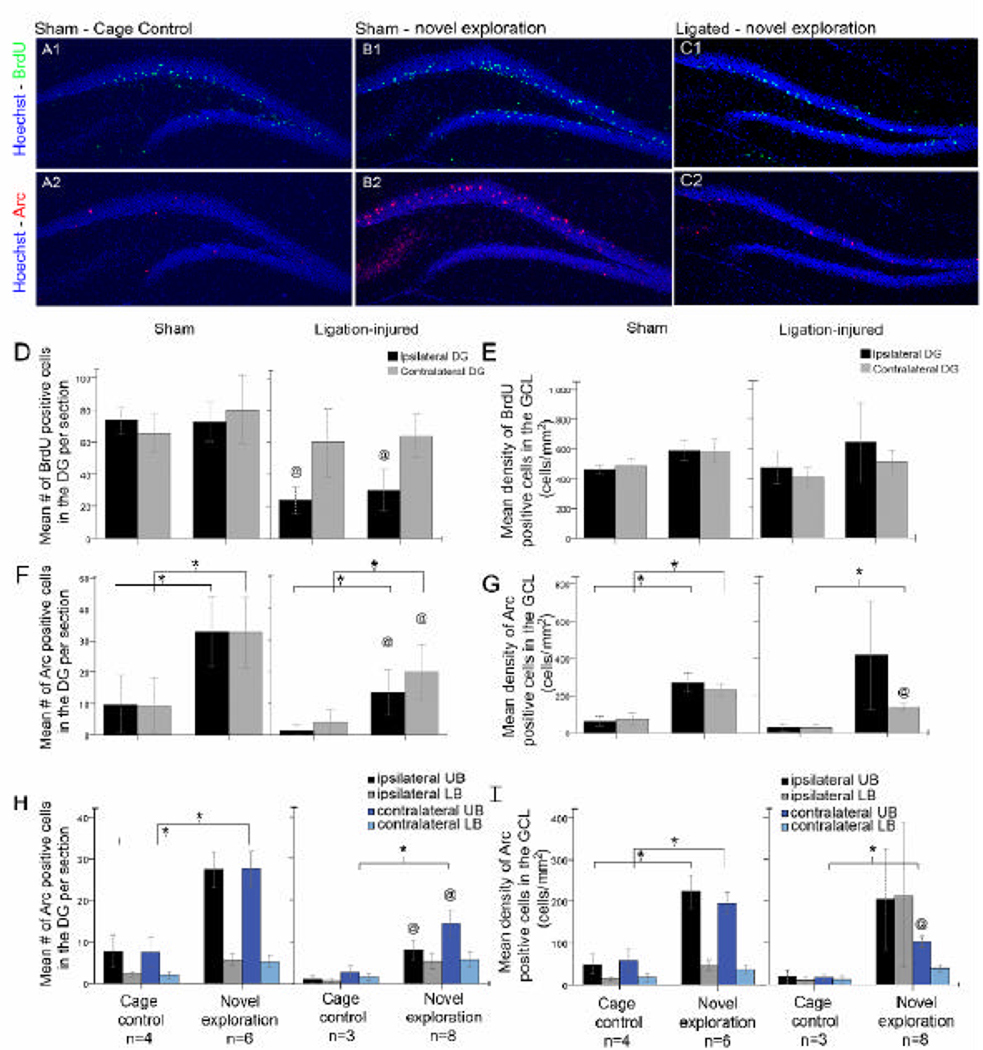
At the age of 7 months, BrdU incorporated into dividing cells at P17–21 and detected by green fluorescence (Alexa 488, Chemicon) was distinct in the nuclei of cells in the granule cell layer at low magnifications (10×, Figure 5A1,B1 and C1). Total counts of BrdU-positive-cells in the GCL revealed significant differences between ligation-injured and sham-control brains (Figure 5D) in the ipsilateral DG. Mean counts of BrdU-positive cells in novel-exploration tested ligation-injured brains were significantly lower compared to the novel-exploration tested shams in the ipsilateral GCLs (30.4±6.4 vs. 72.7±6.3, Figure 5D, black bars, p<0.0001). Total counts of BrdU-positive-cells in the cage-control group of mice also showed a similar trend as would be expected (ligation-injured mice 24.4±4.2 compared to 73.4±4 cells in sham-controls, p <0.0001). In mice that underwent novel-exploration, counts of BrdU-positive-cells in uninjured contralateral hippocampi of ligation- injured brains were 64.6±6.2 compared to 80.3±10.6 cells in sham-controls and therefore marginally but not significantly lower (Figure 5D, gray bars p<0.6). The ipsilateral reduction of endogenous post-stroke neurogenesis had a negative correlation with the corresponding percent atrophy of the injured hippocampi (Figure 2) that did not reach significance (r=−0.55, p=0.1). Counts of BrdU-positive-cells when normalized to their corresponding GCL areas (i.e., cells/mm2 of GCL), were found to be similar to the sham-controls (Figure 5E, compare black to gray bars both for the novel-exploration and cage-control groups of mice), indicating the lower counts of BrdU-positive-cells in the ipsilateral injured hippocampi resulted from the severity of the GCL atrophy associated with the ischemic stroke.
Figure 5.
BrdU-positive-cells and Arc-induction in the GCL following novel spatial exploration test conducted 7 months after neonatal-stroke. A1 and 2 show BrdU labeled cells (A1) and basal Arc expression levels (A2) from the same coronal section in a cage-control mouse from the sham-group. B1 and 2 show BrdU labeled cells (B1) and Arc-induction 1h after exposure to a 5 min novel spatial exploration test from the sham-group (B2). Note predominantly upper blade induction of Arc in the dorsal hippocampus of the novel-exploration mouse. C1 and 2 show BrdU-labeled cells (C1) and Arc-induction 1h after exposure to a 5 min novel spatial exploration test from the ligation-injured group (C2). D. Mean number of BrdU-positive-cells in the ipsilateral injured hippocampi of ligation-injured mice was significantly lower (@) than controls (black bars; p =0.001) however contralaterally they were similar (gray bars). E. Mean counts of BrdU-positive cells normalized to their respective GCL areas showed mean density counts to be similar between all groups and ipsi- and contralateral DGs. F. Arc-induction following exposure to a novel environment was significantly robust both in the sham and ligation-injured groups of mice as compared to their respective cage-control littermates (*). Mean counts of Arc-positive cells in the ligation-injured group that underwent novel-exploration however was significantly lower (@) than the sham-controls that underwent novel-exploration, both ipsi- and contralaterally. G. Mean Arc-positive cell count densities normalized to their respective GCL areas in ligation-injured mice retained the significance of robust-induction in the contralateral upper blade that was also significantly lower than the sham-control counterparts (@). Ipsilaterally however, densities of Arc-positive cells showed a large variance and on average a higher density of Arc cells /mm2 when normalized to their respective GCL areas in the stroke-injured ipsilateral hippocampi. H. Mean counts of Arc-positive cells in the upper vs. lower blades of novel-exploration and cage-control littermates from the sham and ligation-injured mice show higher counts of Arc-positive cells in the upper blades for both groups. However induction related increase in numbers of Arc-positive cells in the novel-exploration group of shams was significantly higher due to activation in upper blade neurons vs. lower blade neurons. I. Mean Arc-positive cell count densities normalized to their respective GCL areas in sham and ligation-injured mice from the upper vs. lower blades of novel-exploration and cage-control littermates also showed similar trends as in H.
Novel exploration-dependant Arc-induction in sham and ligation-injured groups of mice at 7 months of age
Every mouse in sham and ligation-injured groups explored all novel objects (i.e. pet toys) spread around the four corners of the novel-exploration field. Neither “mad running” behaviors nor Racine class 1–5 behavioral seizures were noted in the 5 minutes of the novel enriched environment exposure period. Continuous video-monitoring done in the hour following the novel exploration, when the mice were returned to their home cages, did not reveal any behavioral seizures, although “mad running” behaviors were seen in the mice that had shown the behavior in the 6 months preceding testing. Hippocampal circuits activated by exploration have routinely been studied in the dorsal hippocampus. Following novel exploration robust Arc-induction was seen in the control (Figure 5B2 and F) and ligation-injured mice (Figure 5C2 and F) after novel-exploration compared to their respective cage-controls (i.e., sham and ligation-injured mice not exposed to the novel-exploration; Figure 5A2 and F) in the dorsal hippocampus. Counts of Arc-positive cells in the ligation-injured mice however were significantly lower than control mice after induction (Figure 5F). This finding indicated that, inspite of significant unilateral stroke-related loss of brain volume, neuronal networks linked to learning and memory, and identified by behavior-dependent induction of the immediate early gene- Arc in GCL neurons, remained functional but were significantly impaired. Total counts of Arc-positive cells in the DG revealed significant differences between ligation-injured and sham-control brains (Figure 5F) in both the ipsilateral and contralateral DG. After novel-exploration there were significantly fewer Arc-positive neurons in the ipsilateral DG of ligation-injured brains as compared to the sham-controls (13.4±3.6 vs. 32.8±5.5, p=0.02; Figure 5F, black bars, @). More importantly, counts of Arc-positive cells in uninjured contralateral hippocampi of ligation-injured brains after novel-exploration were also significantly lower than sham-controls (17.2±3 vs. to 32.6±5.6; p=0.02; Figure 5F, gray bars, @). In the cage-control group, counts of Arc-positive cells in ligation-injured mice were lower than the shams, however not significantly (1.5±0.9, vs. 9.8± 4.6, p=0.19 ipsilaterally and 4.1±2 vs. 9.3±4.4, p=0.4 contralaterally). Arc-induction following the novel-exploration test, however was significantly higher in the novel-exploration versus cage-control groups of mice, both in the sham-control (p= 0.01, both ipsi- and contralaterally) and ligation-injured mice (p=0.03 ipsilaterally and p=0.005 contralaterally). The results show the efficacy of the 5 min exposure to the novel environment for robust IEG activation and protein expression when examined 1 h later in the CD1 mouse strain. The impaired induction detected in ligation-injured mice ipsilaterally, is likely directly stroke-injury related. However the contralateral impairment of Arc induction indicated that inter-hemispheric commissural input is essential for bilateral functional hippocampal circuit activation.
Similar to the BrdU-cell counts, Arc-positive cell counts were also normalized to their corresponding GCL areas (Figure 5G) and Arc-positive cell densities still maintained significance of induction in the novel-exploration group over the cage-control group in the shams (269.6±50.6 and 62.2±27.7 cells/mm2 respectively ipsilaterally, p=0.015; and 232.6±33.5 and 75.1±34.5 cells/mm2 respectively contralaterally; p= 0.014). In ligation-injured mice the significance between the novel-exploration and cage-control groups was maintained contralaterally (123.7±12.8 and 26.8±14 cells/mm2 respectively; p=0.002) but lost ipsilaterally (417±292.7 and 28.15±17.6 cells/mm2 respectively; p=0.3) due to the large variability associated with stroke-injury related atrophy of the ipsilateral GCLs. After normalization to injury (i.e., corresponding GCL areas in which counts were done) in the group of mice that underwent novel-exploration, Arc-induction remained bilaterally significant in sham-controls and contralaterally in ligation-injured mice, however ipsilaterally this significance was lost (p=0.6). Ipsilaterally, Arc-induction was present but variable and no significant correlation to their corresponding GCL areas was evident (r2=−0.2, p=0.7). This finding may indicate that the variable Arc-induction in the ipsilateral GCLs of ligation-injured mice is dependent on factors other than GCL atrophy, which may include variability of impaired inputs coming in from the injured somatosensory, parietal and visual cortex. In contrast, the density of Arc-positive neurons in the contralateral hippocampus of ligation-injured mice after novel-exploration remained significantly impaired compared to controls after novel exploration. They however showed a significant positive correlation to their corresponding GCL areas (r=0.829, p=0.011) likely due to lack of any ischemic injury related atrophy and thus remained similar to controls. In the cage-control group of mice, baseline Arc activity normalized to injury related GCL atrophy in ligation-injured mice compared to sham-controls, was lower bilaterally, however not significantly [28.2±17.6 vs. 62.2±27.7 ipsilaterally (p=0.4) and 26.8±14 vs. 75.1±34.5 contralaterally (p=0.3); Figure 5G].
As previously described there was distinct predominance of Arc-positive cells in the upper or dorsal blade of the DG (Figure 5B2 and H) as compared to the corresponding lower or ventral blade [16,17]. This was significantly true for sham-controls that underwent novel-exploration [i.e., 27.3±4.4 and 27.4±4.4 in the upper blade, 5.6±1.5 and 5.1±1.4 in the lower blade, ipsi- (p=0.002) and contralaterally (p=0.001)] respectively] and not statistically significant for sham cage-controls [i.e.7.6±3.9 and 7.4±3.6 in the upper blade, 2.2±0.6 and 1.8±0.9 in the lower blade, ipsi- (p=0.19) and contralaterally (p=0.16) respectively]. Similar observations were made in the ligation-injured group of mice that underwent novel-exploration [i.e., 8.1±2.3 and 12.5±2 in the upper blade, 5.3±1.8 and 4.7±1.2 in the lower blade, ipsi- (p=0.2) and contralaterally (0.002) respectively]. The cage-control group of ligation-injured mice had non-significant trend for a lower baseline of constitutive Arc expression than sham-controls (Fig. 5H and I), and failed to show upper blade predominance of Arc expression due to the lower counts [i.e., 0.9±0.8 and 2.7±1.4 in the upper blade, 0.6±0.4 and 1.4±0.8 in the lower blade of ligation-injured mice, ipsi- (p=0.7) and contralaterally (p=0.4) respectively]. Arc-induction following novel-exploration in the sham-group of mice, when compared to their cage-control littermates, revealed that the significant number of induction related positive cells were in the upper blade bilaterally (* Fig. 5H and I) as opposed to the lower blade. In the lower blade, the numbers of Arc-positive-cells following novel-exploration in sham-controls were marginally higher however not significantly different from the counts of Arc-positive-cells in the lower blade of their cage-control littermates (p = 0.12 ipsi- and contralaterally). In ligation-injured mice, Arc-induction in the novel-exploration tested mice was also found to be significant in the upper blades of the contralateral DG [p=0.02 for counts, p=0.002 for density (cells/mm2)] but not in the severely atrophied upper blades of the ipsilateral DGs (p=0.06 for counts and p=0.2 for density; * Fig. 5H and I). Arc-positive cell densities in ipsilateral upper blades of GCLs were variable relative to the severity of injury in their respective hippocampi and did not show significant correlation to the associated atrophied GCLs (i.e., corresponding GCL areas, r2 = −0.3, p=0.6). Contralateral upper-blade Arc-positive cell densities remained significantly lower than the sham-group that underwent novel exploration (Fig. 5I, @). In summary, the present, but reduced, Arc-induction detected the in contralateral uninjured hippocampi was a novel finding for this study. Its persistence at >6 months after neonatal stroke, indicate opportunities for enhancement strategies.
Integration of post-stroke born granule cells into functional circuits
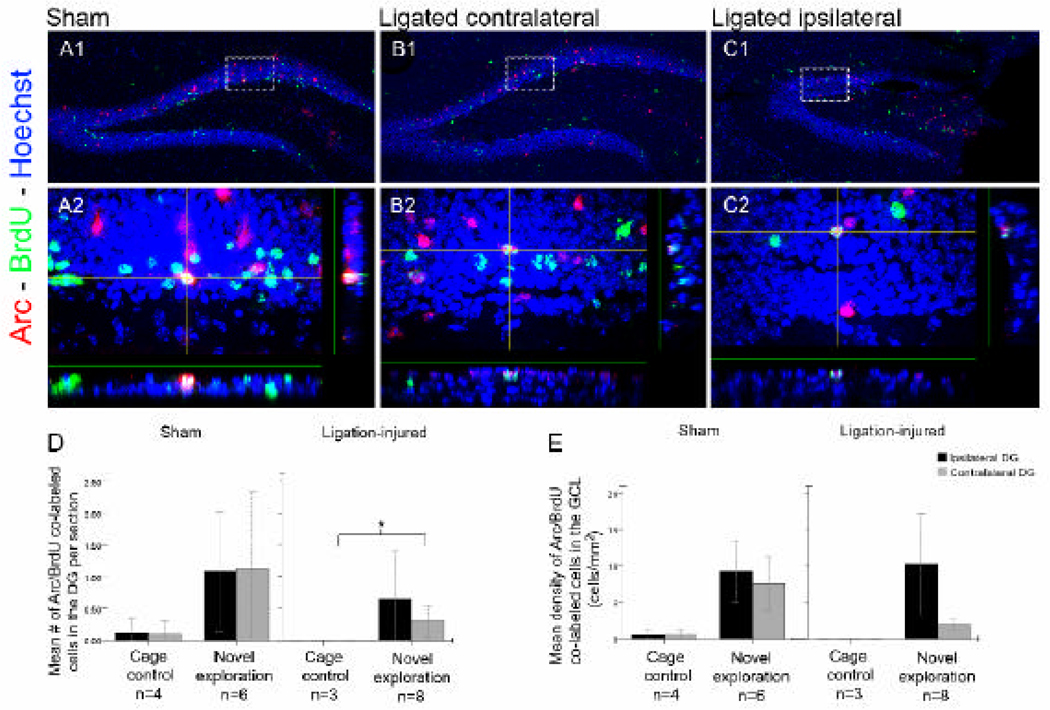
Sub-granular zone neural stem cells proliferating in the 5–9 days after stroke were BrdU-labeled during the S phase of cell division. New-cells that survived the process of differentiation and maturation were then tested after novel-exploration, for their functional integration into hippocampal networks. The number of neurons that were co-labeled with BrdU and Arc represented the newborn cells labeled in the post-stroke period that had integrated into the functional circuits during routine spatial tasks in the cage-control mice and acquisition of novel spatial information in the novel-exploration mice (Figure 6A, B and C). As expected, the counts of co-labeled neurons were higher after novel-exploration in the sham-group of mice. Surprisingly, the counts of co-labeled neurons were also higher in the ligation-injured mice (Figure 6D and E) in whom robust Arc-induction had occurred following novel-exploration (Figure 5F and H) compared to their cage-control littermates that was significant in the contralateral DGs (i.e., in the uninjured dorsal hippocampi; p=0.03; Figure 6D). Following novel exploration, counts of co-labeled cells in the ligation-injured group of mice were 0.65± 0.4 ipsilaterally and 0.25 ± 0.1 contralaterally, therefore were marginally but not significantly lower (p=0.5 ipsilaterally and p=0.1 contralaterally) compared to the shams (1.1±0.5 ipsilaterally and 1.1 ± 0.6 contralaterally). No baseline activity related co-labeled cells were detected in the DG of the cage-control group of ligation-injured mice (i.e., zero both ipsi- and contralaterally). Comparatively in the sham-control mice, a few co-labeled cells were detected (0.12±0.12 ipsilaterally and 0.1 ± 0.1 contralaterally), however the difference was not statistically significant (p=0.4 bilaterally). When normalized to the injury- related atrophy of corresponding GCL areas in which the counts were done (Figure 6E) comparisons described above, remained similar. Percentage of new neurons in which Arc-induction occurred in ligation-injured mice compared to sham-controls following novel exploration was 2.4±1.7% vs. 1.5±0.7% ipsi- (p=0.6) and 0.5±0.2% vs. 1.3±0.6% (p=0.2) contralaterally, which again was not significantly different. In the cage-control group of mice, percentage of new neurons in which Arc- induction occurred was also not significantly different between the ligation-injured and sham-group of mice [ 0% vs. 0.16±0.16% ipsi- (p=0.4) and 0% vs. 0.15±0.15% contralaterally (p=0.4) respectively]. These percentages were similar to the percentages of co-labeled new neurons detected when a similar pilot protocol was run on ligation-injured and sham-control CD1 mice at P60 following the P12 unilateral stroke insult. No significant differences were noted between sham-control and ligation-injured mice for new-neuron integration. A pilot study was also conducted at P40 to investigate the earliest post-stroke period at which the new-neurons (i.e. labeled in the week following ligation) could functionally integrate into hippocampal networks involved in novel exploration and no new neurons were found to co-label with Arc (unpublished observations). Therefore new neurons born in early post-stroke period while not yet functionally integrated at P40 were able to integrate into hippocampal functional circuits to a similar degree as new born neurons in the sham-controls at P60 (i.e., 6 weeks after dividing cells were labeled with BrdU) and the trend remained similar in the current study at 6 months after the neonatal stroke.
Figure 6.
BrdU-positive-cells (green) expressing Arc protein (red) after exposure to novel spatial environment in ipsilateral DGs of sham and ligation-injured mice contra- and ipsilaterally. A1, B1 and C1 show DGs (10× magnification) for the three groups. Bottom panels (A2, B2 and C2) show high magnification view (60×) of co-labeled cells in the three groups with orthogonal views of the stacked z axis. D. On average, counts of BrdU-positive cells that co-labeled with Arc expression were higher in the tested group of sham and ligation-injured mice compared to their cage-control litter-mates both ipsi- and contralaterally. However, in injured mice they were significantly higher (*) only in the contralateral DG of ligation-injured mice (gray bar). E. Total mean density of BrdU/Arc co-labeled cells mirrored total mean counts in the DG. Contralateral density of co-labeled cells in the ligation-injured mice after novel exploration was lower compared to the novel-exploration sham-group but not significantly.
Spontaneous behavioral seizure activity
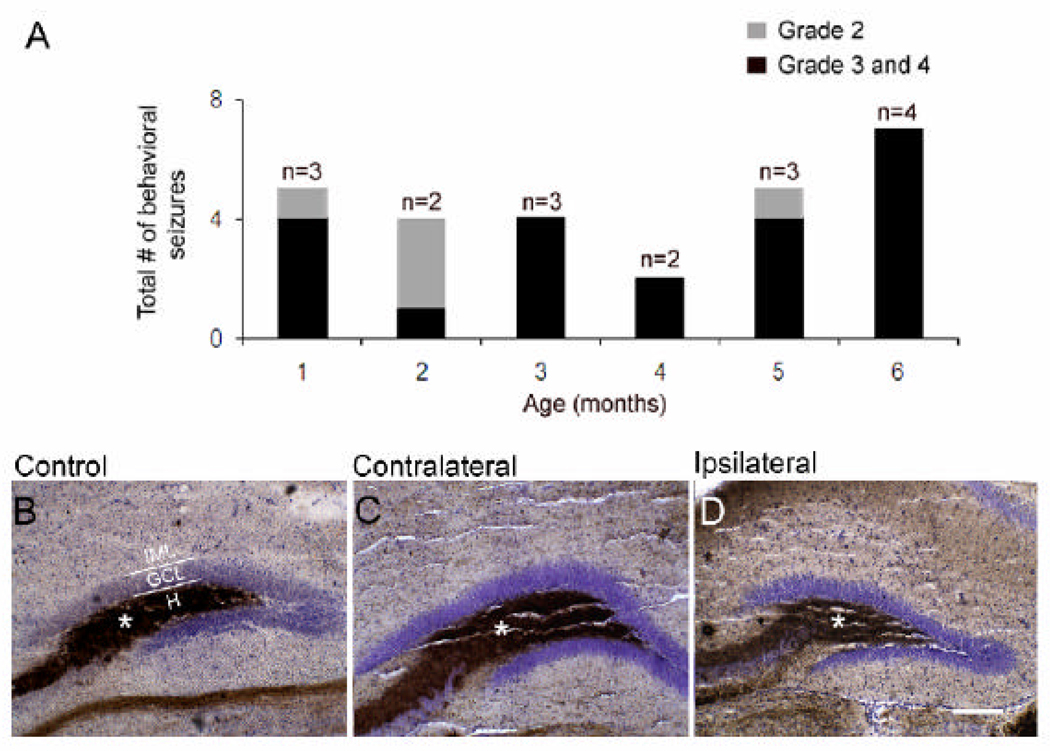
Understanding how post-stroke plasticity can lead to chronic recurrent seizures after a latent period is an important question in epilepsy research. Video-monitoring was done for 25% of the time in the long-term study (i.e., 1 week per month for 6 months). Behavioral seizures graded on the Racine scale [26] revealed the occurrence of recurrent spontaneous class 2, 3 and 4 behavioral seizures in 5 out of the 8 video-monitored mice (63%; 3 females, 2 males). Only one of the female mice with spontaneous seizures also showed the consistent lateralized circling related hyperactivity. Therefore, while there was overlap between groups, circling behaviors and occurrence of seizures did not occur precisely in the same group of ligation-injured mice. Low frequency seizures (i.e., seizures/day were 0.24 at 1 month, 0.29 at 2 months, 0.19 at 3 months, 0.14 at 4 months, 0.24 at 5 months and 0.25 at 6 months of age) were detected throughout the six-month period in which the mice were behaviorally monitored (Figure 7A). Mean seizure rates were 0.22±0.02 seizures/day for the period of monitoring (i.e., 6 months; min 0.14, max 0.28). No class 5 seizures were detected. Behavioral manifestations of class 2 to 4 seizures ranged from ~10 to 60 seconds in duration. A total of 27 seizures were detected of which 22 were class 3 and 4 seizures and the remaining 5 were class 2 seizures. Of the 27 events, 19 (70%) occurred during the light-cycle, similar to previously reported in rodent models of temporal lobe epilepsy [27]
Figure 7.
Low frequency spontaneous behavioral seizures (Racine scale, Racine, 1971) in the months following neonatal stroke and absence of mossy fiber sprouting. A. In addition to the temporally progressive increase in overall activity associated with clockwise running (see Figure 3) progressing to tail-chasing behaviors and a failure to gain weight (see Figure 2A and B) stroke-injured mice were detected to have spontaneous behavioral seizures. These low frequency seizures ranged from grade 2 to 4 on the Racine scale with durations ranging from 5–40 seconds in duration. These low frequency behavioral seizures were not associated with detectable aberrant mossy fiber sprouting (normal mossy fiber shown as brown precipitate (*) in the hilus where the mossy fibers extend to innervate the CA3 neurons) in the inner molecular layer of the DG neither ipsi- nor contralaterally and were therefore similar to controls (compare C and D to B). Scale bar in D = 200 µm applies to B, C and D. H-hilus, GCL-granule cell layer, IML-inner molecular layer
Experiments exposing neonatal rats to insults that result in infarcts have demonstrated induction of atypical mossy fiber sprouting in the inner molecular layer of the dentate gyrus [28]. The CD1 neonatal stroke mouse model shows a qualitatively similar type of neuronal cell death in the hippocampus with an affinity for CA1 and CA3 pyramidal neurons [10]. However, Timm staining done on adjacent series of coronal sections did not show presence of stain product in the inner molecular layer (Figure 7B–D). Although mossy fiber sprouting has been proposed to be a prerequisite for chronic seizure activity in experimental temporal lobe epilepsy, non-progressive epilepsy is generally not associated with mossy fiber sprouting [29,30]. The seizure rates of the post-stroke epilepsy in the CD1 neonatal mouse model remained low for the period monitored in the study and rates did not show a progression as a function of time over the period of monitoring. Therefore, the absence of mossy fiber sprouting was consistent with the absence of clear progressive seizure activity at 7 months following the ischemic insult.
Correlations: neonatal stroke-injury, post-stroke neurogenesis and co-morbidities
In this study, the acute post-stroke seizure scores (i.e., behavioral seizures monitored in the 4h following ligation surgery) did not predict the severity of the post-stroke epilepsy (i.e., counts of spontaneous behavioral seizures video-monitored in the 6 months thereafter; r2=0.22 p=0.3; one tailed). There was a positive correlation between the severity of post-stroke epilepsy (i.e., counts of the spontaneous chronic seizures) in the epileptic mice to the severity of those seizures (i.e., sum of the seizures = grade 3 in severity on the Racine scale; r2=0.97 p=0.0001).
Although no correlation was found between the severity of the post-stroke epilepsy and endogenous post-stroke neurogenesis evaluated by new-neurons labeled by BrdU in the week following the stroke (r2=0.1, p=0.4) in the ipsilateral injured GCL; contralaterally a significant positive correlation [31] was found both for mean counts and densities (r2=0.6, p=0.02 and r2=0.6, p=0.03 respectively; one tailed; n=11). In contrast, there were negative correlations between the counts of the post-stroke spontaneous behavioral seizures and counts of Arc-positive cells following novel-exploration (n=8) both ipsi- and contralaterally that did not reach statistical significance (r2=−0.53, p=0.09 ipsi- and r2=−0.08, p=0.4 contralaterally; one tailed). However, there was a significant negative correlation (r2=−0.7, p=0.03; one tailed) between the severities of the post-stroke epilepsy and the counts of network integrated new neurons (i.e., BrdU labeled cells that co-labeled with Arc) in the ipsilateral GCL. No similar correlations were noted between acute seizure scores and post-stroke neurogenesis or network activity in new neurons.
With regards to the temporally progressive behavioral co-morbidities detected in the open-field testing, there was a strong positive correlation between total distances covered by the ligation-injured mice (n=11, i.e., with and without novel-exploration) to the counts of total revolutions (r2=0.96, p=0.0001). This finding supported the observation of that the overall temporally progressive hyperactivity was associated lateralized circling behaviors detected with the chronic video-monitoring. Also important for the grouping variability between ligation-injured mice assigned to novel-exploration and cage-control groups was that open-field hyperactivity between the two groups were not different from each other with regards to total activity and lateralized revolutions (p=0.5 and 0.7 respectively). Correlations between the behavioral co-morbidities of hyperactivity and lateralized circling and post-stroke neurogenesis or Arc-induction were consistently negative however none reached significance. Therefore although post-stroke plasticity likely induced the long-latency to onset, progressive hyperactivity behaviors, they did not correlate significantly with the impaired post-stroke neurogenesis or the impaired Arc induction in the injured hippocampi.
Severity of stroke-injury quantified as both hemispheric and hippocampal atrophy had positive correlations with hyperactivity and lateralized circling behaviors quantified with the open-field testing at 3 months of age in the ligation-injured group of mice that only reached significance between hemispheric atrophy and hyperactivity (n=11; p=0.05 and 0.1 for hemispheric atrophy and p= 0.2 and 0.3 for hippocampal atrophy respectively for hyperactivity and counts of lateralized circling behaviors; one tailed). Significant negative correlations between severity of stroke-injury as quantified by hippocampal atrophies (n=11) and counts of BrdU-positive cells in the ipsilateral injured GCLs were noted (r2=−0.73, p=0.005) as has been reported in prior studies from our group [10]. Similar negative correlations between hemispheric atrophies and contralateral BrdU-positive cell counts did not reach significance (p=0.1) in this study. Stroke-injury also showed significant negative correlations between severity of hemispheric and hippocampal atrophies and counts of Arc-positive cells in the ipsilateral injured hippocampi (r2=−0.76, p=0.015 and r2=−0.73, p=0.021 respectively) that was not significant contralaterally. Therefore severity of the stroke injury predicted the severity of impairment of both post-stroke neurogenesis and exploration induced activation of hippocampal circuits in the injured hippocampi.
Discussion
In this study the IEG Arc was induced in the GCL of the hippocampus using a novel behavioral experience that strongly upregulates the IEG in a cell-type specific manner [32,33] and new neurons born in the post-stroke period were labeled with BrdU. Arc-induction was network specific and detected in both sham and ligation-injured mice following exploration responses in both the general pool of neurons and in the neurons born after the neonatal stroke. However, Arc-induction was a magnitude lower and negatively correlated to the severity of the stroke-injury in ligation-injured hippocampi and was found to be impaired even in the uninjured contralateral hippocampi compared to controls. The severe stroke related porencephaly and hippocampal atrophy would indicate significant disruptions of the polysynaptic circuits that are normally are involved in experience dependent learning. Therefore, the preservation of some Arc-induction following novel-exploration in this study represents plasticity (i.e., adaptive plasticity;[34]) of young brains [35]) and represents the potential for positively modulating hippocampal functional recovery especially in the uninjured contralateral hippocampi. There may be a role for rehabilitative interventions to restore post-stroke recovery by enhancing new neuron integration into these relatively intact circuits. On the other hand, the temporally progressive hyperactivity disorder [36] and recurrent spontaneous seizures also detected in the ligation-injured mice in this study may represent disorders associated with “gain of function” plasticity that is excessive or maladaptive in the long-term [34,37]. Therefore, future work addressing post-stroke neurogenesis and regeneration will need to continue to take into account which regenerative changes are adaptive and which are maladaptive.
Behavior in ligation-injured and sham-control mice
The current study found no differences in the two groups on the rotorod indicating that the ligation procedure and the late-onset lateralized circling behaviors [38] did not overtly impact motor coordination or power. No significant deficits in the ligation-injured mice were noted on the T-maze task in the current study, whereas clear deficits were seen at P40 (previous study) [21]. At P40 the mice are still developing, whereas at 3 months (current study) the mice are adults and developmental changes have slowed dramatically. This could potentially produce a different behavioral profile since age has been known to impact behavioral outcomes [39–41]. At 3-months of age ligation-injured mice may have developed a non-hippocampal strategy that allowed them to perform the T-maze that was not present at P40.
In the previous study the sham mice showed clear habituation between sessions, and both ligates and sham-controls exhibited within session habituation. In the current study, although the shams exhibited within session habituation the ligated-injured mice did not. Instead, the activity levels of the ligated mice appeared to increase over the course of each session (see Figures 3B and 3C). In addition, the overall level of activity of the ligated mice was significantly greater than that of the shams during both sessions. Again this was not observed in the previous study and may be due to chronically worsening brain asymmetries related to nigro-striatal pathway functions compensating for the loss of dopaminergic neurons [38,42] in the ipsilateral substantia-nigra of ligation-injured mice (unpublished observations). The unilateral loss of dopaminergic neurons may also explain the increase in the frequency of lateralized circular running around the cage periphery in 50% of the ligated mice. First noticeable at 3 months of age, this “mad running” behavior progressed to “tail chasing” at 6 months of age as documented in the video-monitoring conducted over the 7 month period. These new findings in the model may clinically relevant to the late-onset neurobehavioral impairments, such as hyperactivity disorders described in children with a history of neonatal stroke [3].
Arc-induction, epilepsy, and hippocampal-dependent cognition
Significant negative effects of epileptic seizures were detected both upon Arc-induction and upon the ability of new neurons to integrate into the ipsilateral GCL networks activated by exploration. Therefore the severity of post-stroke epilepsy was associated with long-term deficits in functional hippocampal circuit learning and recruitment of new neurons. This observation suggests that prevention of recurrent post-stroke seizures with anticonvulsants may have a role in optimizing cognitive recovery after neonatal stroke. More studies are needed to address this hypothesis.
This study also noted a significant positive correlation of spontaneous epileptic seizures with contralateral post-stroke neurogenesis that was not detected ipsilaterally. This suggests that either not all of the post-stroke contralateral neurogenesis is adaptive and may in fact contribute to the generation of epilepsy, or alternatively that post-stroke epilepsy stimulates neurogenesis contralaterally whether adaptive or maladaptive. Further work is needed to assess these hypotheses. Seizure discharges have been shown to similarly stimulate dentate granule cell neurogenesis after pilocarpine-induced status epilepticus [31] and in this context the neurogenesis is thought to be maladaptive.
When initially discovered, Arc was shown to be rapidly induced in neurons in models of adult plasticity using maximal electric shock (MES) stimuli commonly used in the kindling model of epilepsy [43]. Arc-induction occurs in groups of neurons that have recently participated in synaptic activation sufficient enough to trigger synaptic plasticity. NMDA receptor activation is a critical step both for synaptic plasticity and Arc activation [43]. Although Arc activation in the GCL is considered neuronal specific [44], presence of Arc has been reported in reactive astrocytes in the molecular layer of the DG [45]. Counts related to Arc-induction in this study, however, were restricted to the GCL layer. Hypersynchronous neuronal firing activity associated with hippocampal seizures in the stroke model would also be expected to induce Arc in the networks through which the seizure may have propagated. In another novel observation in this study, the post-neonatal stroke model in CD1 mice showed occurrence of spontaneous behavioral seizures. Therefore the possibility of the spontaneous behavioral seizures modulating hippocampal Arc-induction was addressed by analyzing behavioral data recorded by continuous video-monitoring in the 24h preceding novel-exploration based Arc-induction. Although no behavioral seizure activity was detected, modulation by electrographic non-convulsive seizure activity in these mice could not be ruled out. However, none of the mice that demonstrated behavioral seizures in the prior 7 months showed Arc activation in excess of that shown by their group mates. These observations indicate that it is unlikely that hippocampal ongoing seizure activity induced modulation perturbed the exploration induced Arc-induction reported here. On the contrary, the presence of epilepsy in the ligation-injured mice was found to negatively correlate with Arc-induction and new-cell integration in the injured hippocampi. This finding indicated that post-lesion chronic seizure activity hindered recovery of Arc expression plasticity, as noted in hippocampal networks in the GCL in other brain injury models [17] that did either did not recover or further deteriorated in the 6 months following stroke in the mice with post-stroke epilepsy.
The “mad running” behaviors were pervasive and continued off and on in the time preceding the time of sacrifice. Inspite of this aberrant activity that was independent of the detected episodes of behavioral seizures, there was persistence of low basal Arc activity levels in the cage-control ligation-injured mice. This observation also indicated that the hyper-activity and tail-chasing behaviors were not associated with synchronous neuronal hyperactivity in the hippocampus and therefore likely not “seizure activity”[46,47]. The new observation of co-morbidities like the long-term hyperactivity disorders in this study may represent an ADHD -like syndrome that may further negatively modulate cognition [48].
Arc protein has been proposed to be involved in the development of spatial working memory that is regulated by both ERK1/2 signaling and BDNF [49,50]. Baseline counts of Arc-positive cells in the DG have also been proposed to be "constitutive" IEG expression during rest that is not random; representing memory consolidation mechanisms [51,52]. In this study the constitutive Arc expression in ligation-injured mice was marginally but not significantly lower than the sham-group of mice (Figure 5 F and G). Arc mRNA is rapidly induced and distributed to dendritic processes following synaptic stimulation and also translated locally to facilitate synapse-specific modifications [43]. Additionally, levels of Arc were reported to be significantly higher in aged animals supplemented with flavinoids that also showed improved performance on spatial memory tasks relative to control aged animals that were on a standard diet [49]. Thus, Arc-induction may be a useful tool to test functional integrity of hippocampal circuits required for learning and cognition.
Conclusion
Exploration induced IEG activation of Arc in a model of neonatal stroke in CD1 mice showed impaired but significant induction both in the injured and uninjured hippocampi of stroke-injured mice with unilateral lesions. This induction, along with the suppressed but present post-stroke neurogenesis, represents the adaptive plasticity of young brains [53]. Understanding both the positive and negative aspects of this plasticity will be key to developing future novel strategies to enhance post-stroke recovery. Significant impairment in the long-term functional plasticity detected in the hippocampi of ligation-injured brains may represent opportunities for enhanced rehabilitation by therapeutic manipulations. Behavioral impairments showed severe deficits in the ability to habituate within an open-field testing session. Associated behaviors showed a temporal progression in “mad running” behaviors likely originating from unilateral impairments of the dopaminergic pathway followed by maladaptive plasticity. Post-stroke epilepsy was low-frequency in the time monitored and not associated with aberrant mossy fiber sprouting. However the severity of the post-stroke epilepsy modulated the post-stroke functional hippocampal circuit activity in the uninjured contralateral hippocampus and new-cell circuit-integration in the injured ipsilateral hippocampus. The hyperactivity disorder and the post-stroke epilepsy are important clinically relevant aspects detected in this model that are common co-morbidities of neonatal stroke [3] and warrant further characterization. The Arc-induction protocol, impaired habituation and hyperactivity behaviors may provide convenient measures to test the long-term effects of acute or sub-acute interventional therapies aimed at improving post-stroke recovery in the model. Recent findings in our laboratory with a pilot study using similar testing protocols at P60 indicate new neurons integrate into functional hippocampal circuits in numbers similar to those found at 7 months of age in CD1 mice [54]. Ongoing studies will use the protocol from this study at the P60 time-point to evaluate enhanced rehabilitation by therapeutic interventions starting soon after the neonatal stroke.
Acknowledgements
This study was supported by NS52166-01A1 (awarded to AMC) and by NCRR grant 1PO40 RR017688 (awarded to the Neurogenetics and Behavior Center).
List of abbreviations
- GCL
granule cell layer
- IEG
immediate early gene
- Arc
Activity regulated cytoskeleton associated protein
- BrdU
Bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke Workshop on Perinatal and Childhood Stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 3.Hartel C, Schilling S, Sperner J, Thyen U. The clinical outcomes of neonatal and childhood stroke: review of the literature and implications for future research. Eur J Neurol. 2004;11:431–438. doi: 10.1111/j.1468-1331.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson V, Jacobs R, Spencer-Smith M, Coleman L, Anderson P, Williams J, et al. Does Early Age at Brain Insult Predict Worse Outcome? Neuropsychological Implications. J Pediatr Psychol. 2009 doi: 10.1093/jpepsy/jsp100. [DOI] [PubMed] [Google Scholar]
- 5.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Bebin M, Morawetz R. Congenital porencephaly: MR features and relationship to hippocampal sclerosis. AJNR Am J Neuroradiol. 1998;19:135–141. [PMC free article] [PubMed] [Google Scholar]
- 6.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Bebin M, Morawetz R. Congenital porencephaly and hippocampal sclerosis. Clinical features and epileptic spectrum. Neurology. 1997;49:1382–1388. doi: 10.1212/wnl.49.5.1382. [DOI] [PubMed] [Google Scholar]
- 7.Burneo JG, Faught E, Knowlton RC, Martin RC, Bebin M, Morawetz R, et al. Temporal lobectomy in congenital porencephaly associated with hippocampal sclerosis. Arch Neurol. 2003;60:830–834. doi: 10.1001/archneur.60.6.830. [DOI] [PubMed] [Google Scholar]
- 8.Bartley J, Soltau T, Wimborne H, Kim S, Martin-Studdard A, Hess D, et al. BrdU-positive cells in the neonatal mouse hippocampus following hypoxic-ischemic brain injury. BMC Neurosci. 2005;6:15. doi: 10.1186/1471-2202-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YC, Tzeng SF, Yu L, Huang AM, Lee HT, Huang CC, et al. Early-life fluoxetine exposure reduced functional deficits after hypoxic-ischemia brain injury in rat pups. Neurobiol Dis. 2006;24:101–113. doi: 10.1016/j.nbd.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Kadam SD, Mulholland JD, McDonald JW, Comi AM. Neurogenesis and neuronal commitment following ischemia in a new mouse model for neonatal stroke. Brain Res. 2008;1208:35–45. doi: 10.1016/j.brainres.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadam SD, Mulholland JD, McDonald JW, Comi AM. Poststroke subgranular and rostral subventricular zone proliferation in a mouse model of neonatal stroke. J Neurosci Res. 2009 doi: 10.1002/jnr.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- 13.Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–F192. doi: 10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson V, Spencer-Smith M, Leventer R, Coleman L, Anderson P, Williams J, et al. Childhood brain insult: can age at insult help us predict outcome? Brain. 2009;132:45–56. doi: 10.1093/brain/awn293. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temple MD, Worley PF, Steward O. Visualizing changes in circuit activity resulting from denervation and reinnervation using immediate early gene expression. J Neurosci. 2003;23:2779–2788. doi: 10.1523/JNEUROSCI.23-07-02779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steward O. Reorganization of neuronal connections following CNS trauma: principles and experimental paradigms. J Neurotrauma. 1989;6:99–152. doi: 10.1089/neu.1989.6.99. [DOI] [PubMed] [Google Scholar]
- 19.Volpe JJ, Pasternak JF. Parasagittal cerebral injury in neonatal hypoxic-ischemic encephalopathy: clinical and neuroradiologic features. J Pediatr. 1977;91:472–476. doi: 10.1016/s0022-3476(77)81328-0. [DOI] [PubMed] [Google Scholar]
- 20.Volpe JJ. Neurology of the newborn. Major Probl Clin Pediatr. 2001;22:1–648. [PubMed] [Google Scholar]
- 21.Kadam SD, Mulholland JD, Smith DR, Johnston MV, Comi AM. Chronic brain injury and behavioral impairments in a mouse model of term neonatal strokes. Behav Brain Res. 2009;197:77–83. doi: 10.1016/j.bbr.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Franklin KM. The mouse brain in stereotaxic coordinates. 2nd Edition. Academic Press; 2001. [Google Scholar]
- 25.Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- 26.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 27.Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- 28.Kadam SD, Dudek FE. Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;505:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorter JA, van Vliet EA, Aronica E, da Silva FHL. Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. European Journal of Neuroscience. 2001;13:657–669. doi: 10.1046/j.1460-9568.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, et al. Relations between Brain Pathology and Temporal Lobe Epilepsy. J Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinaud R, Penner MR, Robertson HA, Currie RW. Upregulation of the immediate early gene arc in the brains of rats exposed to environmental enrichment: implications for molecular plasticity. Molecular Brain Research. 2001;91:50–56. doi: 10.1016/s0169-328x(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 34.Johnston MV. Clinical disorders of brain plasticity. Brain Dev. 2004;26:73–80. doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 35.Sadato N, Yamada H, Okada T, Yoshida M, Hasegawa T, Matsuki K, et al. Age-dependent plasticity in the superior temporal sulcus in deaf humans: a functional MRI study. BMC Neurosci. 2004;5:56. doi: 10.1186/1471-2202-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ten VS, Wu EX, Tang H, Bradley-Moore M, Fedarau MV, Ratner VI, et al. Late Measures of Brain Injury After Neonatal Hypoxia-Ischemia in Mice. Stroke. 2004;35:2183–2188. doi: 10.1161/01.STR.0000137768.25203.df. [DOI] [PubMed] [Google Scholar]
- 37.Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Dev. 2009;31:1–10. doi: 10.1016/j.braindev.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loscher W. Abnormal circling behavior in rat mutants and its relevance to model specific brain dysfunctions. Neuroscience & Biobehavioral Reviews. 2010;34:31–49. doi: 10.1016/j.neubiorev.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Anderson MJ, Barnes GW, Briggs JF, Ashton KM, Moody EW, Joynes RL, et al. Effects of ontogeny on performance of rats in a novel object-recognition task. Psychol Rep. 2004;94:437–443. doi: 10.2466/pr0.94.2.437-443. [DOI] [PubMed] [Google Scholar]
- 40.Chapillon P, Roullet P. Use of proximal and distal cues in place navigation by mice changes during ontogeny. Dev Psychobiol. 1996;29:529–545. doi: 10.1002/(SICI)1098-2302(199609)29:6<529::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Rossier J, Schenk F. Olfactory and/or visual cues for spatial navigation through ontogeny: olfactory cues enable the use of visual cues. Behav Neurosci. 2003;117:412–425. doi: 10.1037/0735-7044.117.3.412. [DOI] [PubMed] [Google Scholar]
- 42.Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res. 2005;58:297–301. doi: 10.1203/01.PDR.0000169971.64558.5A. [DOI] [PubMed] [Google Scholar]
- 43.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 44.Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci U S A. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez JJ, Davies HA, Errington ML, Verkhratsky A, Bliss TV, Stewart MG. ARG3.1/ARC expression in hippocampal dentate gyrus astrocytes: ultrastructural evidence and co-localization with glial fibrillary acidic protein. J Cell Mol Med. 2008;12:671–678. doi: 10.1111/j.1582-4934.2007.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindemann S, Lessenich A, Ebert U, Loscher W. Spontaneous paroxysmal circling behavior in the ci2 rat mutant: epilepsy with rotational seizures or hyperkinetic movement disorder? Exp Neurol. 2001;172:437–445. doi: 10.1006/exnr.2001.7802. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama K, Ishikawa M, Saito A. mRNA expression of activity-regulated cytoskeleton-associated protein (arc) in the amygdala-kindled rats. Brain Research. 2008;1189:236–246. doi: 10.1016/j.brainres.2007.10.102. [DOI] [PubMed] [Google Scholar]
- 48.Max JE, Mathews K, Manes FF, Robertson B, Fox PT, Lancaster JL, et al. Attention deficit hyperactivity disorder and neurocognitive correlates after childhood stroke. Journal of the International Neuropsychological Society. 2003;9:815–829. doi: 10.1017/S1355617703960012. [DOI] [PubMed] [Google Scholar]
- 49.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radical Biology and Medicine. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Huang F, Chotiner JK, Steward O. Actin Polymerization and ERK Phosphorylation Are Required for Arc/Arg3.1 mRNA Targeting to Activated Synaptic Sites on Dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marrone DF, Schaner MJ, McNaughton BL, Worley PF, Barnes CA. Immediate-early gene expression at rest recapitulates recent experience. J Neurosci. 2008;28:1030–1033. doi: 10.1523/JNEUROSCI.4235-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89:269–284. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- 54.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, et al. Adult-Born Hippocampal Neurons Are More Numerous, Faster Maturing, and More Involved in Behavior in Rats than in Mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]