Abstract
Background
Dual tasking can interfere with activity after stroke.
Objective
The authors examined the interactions between 3 different cognitive tasks and the swing and double-limb support (DLS) components of the gait cycle in community-dwelling individuals poststroke.
Methods
Acquisition of cognitive and gait data were synchronized to study the cognitive–motor interference effects during the different phases of the gait cycle. Participants performed 3 different cognitive tasks in isolation and in combination with walking as well as a single walking task. Tasks were performed continuously for 3 minutes, generating 131 ± 39 gait cycles per person for analysis for each walking trial. Data were analyzed for 8 participants 7.6 ± 4.2 months poststroke.
Results
A significant increase was found in the proportion of the gait cycle spent in DLS in dual-task walking because of an increased duration of the DLS phase associated with paretic weight acceptance. There was a significant dual-task effect on nonparetic swing duration: participants reduced the amount of time in paretic single-limb stance in the 3 dual-task conditions. Temporal asymmetry of gait did not increase significantly under dual-task conditions. Reaction times were not affected by whether the stimuli were present during the swing or DLS phase of the gait cycle.
Conclusions
The findings from this pilot study provide evidence that cognitive–motor interference during gait may be influenced by the phase of the gait cycle, especially DLS involving paretic weight acceptance, which may affect community ambulators with hemiparetic stroke.
Keywords: dual task, gait, stroke
Introduction
Despite the increasing frequency of dual-task studies in clinical populations, a relatively limited number of studies have investigated gait-related cognitive–motor interference effects in people after stroke.1–10 Many have focused primarily on the dual-task effects on gait speed,1,4,7–10 stride duration,2,3,6,8 and cadence.5,7,8,10 Although average parameters such as gait speed are important determinants for functional walking,11 other temporal parameters of gait can reveal more about gait stability.12 For instance, the amount of time spent in double-limb support (DLS) and measures of temporal asymmetry may be useful indicators of balance control during walking.13,14
Dual-task effects on DLS duration in people after stroke have been explored in only 1 study.1 Bowen et al1 found an increase in DLS when individuals at 120 ± 48 days post-stroke were required to perform a cognitive task simultaneously. The concurrent changes in swing duration and dual-task effects on the cognitive task performance were not reported. A further limitation was that gait was measured over only 8 m, and only 1 type of cognitive task was examined. To expand on this research, we investigate the interactions between DLS and swing duration, and 3 different cognitive tasks during continuous overground walking (3 minutes). Ascertaining how DLS and swing durations interact with additional cognitive demands may help quantify gait instability during dual-task walking in people after stroke as well as help clinicians identify specific limitations to be targeted in therapy.
Regnaux et al6 reported longer reaction times (RTs) to tactile stimuli applied during DLS than swing in people poststroke. Longer RTs for stimuli presented during DLS would imply that this phase of the gait cycle demands more attentional resources than the swing phase. This seems counterintuitive at first because DLS is the most stable part of the gait cycle and could logically be presumed to require fewer attentional resources to maintain stability than the relatively unstable swing phase. One possible explanation is that paretic preswing is more attention demanding than paretic swing execution for persons with hemiparesis. This account seems plausible from a motor planning perspective. The previous study did not examine RTs for each DLS phase independently, so it is not known if the dual-task effect on RT was the same for both DLS phases. The current study examines the effects of each DLS phase on cognitive task performance as well as the effects of cognition on the duration of each DLS phase.
The degree of asymmetry between the paretic and non-paretic limbs for temporal parameters of gait may provide insight into the control of balance during walking.14 Indeed, temporal asymmetry is highly prevalent in independent ambulators following stroke15 and correlates strongly with motor recovery and gait speed.16,17 Although a growing body of literature provides evidence that attention-demanding cognitive tasks have destabilizing effects on control of posture,18 voluntary step execution,19 and continuous walking8 after stroke, to our knowledge, dual-task effects on temporal asymmetry of gait after stroke have not been measured.
The purpose of this study was to examine the interactions between cognition and each phase of the gait cycle during dual-task walking using 3 distinct cognitive tasks. The specific aims were the following: (1) to investigate cognitive–motor interference effects on DLS and swing duration, (2) to assess dual-task effects on temporal asymmetry of gait, and (3) to examine the effects of DLS and swing phases on RT to auditory stimuli requiring mental processing and a verbal response.
Methods
This article presents a further analysis of data that were partially described previously.8 The focus of the current report is the analysis of the synchronously recorded cognitive and gait data and analysis of dual-task effects on distinct phases of the gait cycle. Previously, we reported the cognitive–motor interference effects on gait speed, stride duration (mean and SD), stride length, and cadence of 3 different cognitive functions (working memory, visuospatial cognition, and production of spontaneous narrative). Subsequently, the synchronization of the auditory stimuli and verbal responses with the footfall data in the RT tasks allowed us to identify the phase of the gait cycle during which each stimulus onset occurred. Therefore, we were able to examine differences in RTs to stimuli delivered during DLS and swing. The current report presents the results from this analysis. We also describe the dual-task effects on swing and DLS duration of the gait cycle under the 3 dual-task conditions relative to walking without an added task.
Participants
Thirteen community-dwelling individuals who had suffered a stroke participated in this study. To be included, the participants had to be able walk at least 10 m without physical assistance, follow a 3-step command, and be able to perform the cognitive tasks. Participants were ineligible to participate if they had preexisting neurological disorders, orthopedic conditions affecting walking, severe aphasia, or uncorrected visual or hearing impairment. The analyses presented in this article required footfall data from both paretic and nonparetic limbs, and thus, the sample reported here is a subset (n = 8) of the earlier group, including only those for whom synchronized data were available for both feet. The 8 participants (7 men) in this analysis were 60.3 ± 18.2 years old and 7.6 ± 4.2 months after stroke onset, with self-selected gait speed of 0.82 ± 0.34 m/s. Except for 1 participant whose self-selected gait speed was 0.15 m/s, all other participants walked 0.67 to 1.19 m/s. Thus, the sample mostly reflects individuals who are independent community ambulators. The participants had moderate to marked lower-extremity motor impairment, as assessed using the lower-extremity portion of the Fugl-Meyer Motor Assessment Scale20: mean score 25.9, range 18 to 30 (maximum possible score is 34). Two of the participants wore an ankle-foot-orthosis on the hemiparetic leg; one also used a quad cane. The stroke was ischemic in all participants, with right side hemiparesis in 5 of the 8 cases. The performance of the 8 participants on a battery of cognitive tasks is summarized in Table 1. All participants provided informed consent. The study was approved by the institutional review board at the University of Florida.
Table 1.
Cognitive Characteristics of the Sample
| Cognitive Assessment | Mean | SD |
|---|---|---|
| MMSE (maximum 30) | 26.9 | 3.3 |
| Digit symbol substitution (number correct in 90 s) | 32.6 | 8.9 |
| Digit symbol copy (time in seconds to complete) | 146.5 | 48.5 |
| Backward digit span (maximum 14) | 4.9 | 1.6 |
| Digit ordering (maximum 24) | 12.6 | 4.0 |
| WAIS vocabulary (maximum 70) | 53.6 | 13.6 |
| Stroop test (total correct; baseline minus interference) | 22.1 | 9.5 |
Abbreviations: SD, standard deviation; MMSE, Mini Mental State Examination; WAIS, Wechsler Abbreviated Scale of Intelligence.
Procedures
The procedures and tasks have been described in detail previously.8 Briefly, the participants performed 3 cognitive tasks (auditory 1-back, auditory visuospatial clock task, and spontaneous speech) while sitting and in combination with walking. The walking task was also performed in isolation (single walk). No instructions regarding gait speed or task prioritization were given. The participants self-selected their walking speed in each task. The order of the 3 dual tasks was randomized. Auditory stimuli were delivered through wireless headphones, and verbal responses (yes/no) were recorded via a wireless microphone. The footfall data were collected using footswitch technology (B&L Engineering, Tustin, CA).
The laptop computer that delivered and recorded the stimuli and responses for the RT tasks (1-back and clock tasks) had a synchronized start-up with Vicon Workstation v4.6 (Vicon, Los Angeles, CA) on a separate computer, to which the footfall signals were transmitted wirelessly. One data file was created from each computer—one for the stimulus and RT sequences and one for the footfall events. Because each event was time stamped with synchronized start-up, the 2 files could be merged to create a master file with each event in order: stimulus onset, onset of verbal response, and gait events (heel strike and toe-off for each foot). A synchronized file was created for each participant for each dual-task condition in which RTs were collected (ie,1-back and clock tasks only). In other words, analysis of the effects of DLS and swing on cognition, which required synchronization of the gait and cognitive data, was possible only for the RT tasks. Spontaneous speech was recorded with a digital recorder. The speech recordings were not time labeled; thus, we were not able to analyze the effects of DLS and swing phases on speech performance. In contrast, we were able to examine the effects of each of the 3 cognitive tasks on DLS and swing duration because these analyses involved only the gait data in each condition. The overall dual-task effects on spontaneous speech have been described elsewhere.8
Each task lasted approximately 3 minutes, resulting in the acquisition of 131 (±39) continuous strides for each walking trial. This is a considerably larger number of uninterrupted strides than that in most previous gait-related dual-task studies in stroke, which typically analyzed dual-task walking over 4 to 8 meters.1,4,7
Outcome Measures
The dependent variable for examining cognitive–motor interference effects on cognition was RT (time in milliseconds from stimulus onset to onset of verbal response). The participants made too few errors in either the 1-back or the clock task8 to warrant an investigation of errors for each gait cycle phase. The dependent variables for examining cognitive–motor interference effects on gait were DLS duration and swing duration, expressed as a percentage of the gait cycle duration. We also analyzed dual-task effects on swing duration variability (coefficient of variation, CV) and temporal asymmetry. The temporal asymmetry was quantified by calculating the swing–stance ratio for each limb, then dividing the larger ratio by the smaller ratio.14 Thus, perfect symmetry is represented by a ratio of 1.0, and values greater than 1.0 indicate asymmetry.
Statistical Analysis
A repeated-measures analysis of variance (ANOVA) was used to examine the dual-task effects for each of the gait variables, with Tukey’s least-squared differences post hoc procedures applied to examine where the significance occurred. For violations of sphericity, Greenhouse-Geisser corrections to the degrees of freedom were applied. For variables in which the data were not normally distributed, nonparametric tests were used for statistical analysis. For each repeated-measures ANOVA, we present the partial eta squared (η2p) as a measure of effect size; values may range between 0 and 1, with higher values representing higher proportions of variance explained by the independent variable. Paired t tests were used to compare the RTs for each phase of the gait cycle for the 1-back and clock tasks, with measures of effect size reported as Cohen’s d.
Results
Cognitive–Motor Interference Effects on Double-Limb Support
Table 2 displays the temporal gait data as a function of task condition. The dual-task effects on gait speed were reported previously8; however, to facilitate interpretation of the current findings, the mean gait speeds of the 8 participants in this analysis are shown in Table 2. There was a significant dual-task effect on DLS duration [Friedman test, ; P < .05; Figure 1]. Multiple comparisons using the Wilcoxon signed-ranks test with Bonferroni corrected levels of observed significance showed that compared with single walking, DLS was significantly greater in the 1-back (Z = 2.521; n = 8; P = .012) and speech tasks (Z = 2.380; n = 8; P = .017). The difference in DLS duration between single walking and the clock task was not statistically significant (Z = 1.820; n = 8; P = .069).
Table 2.
Mean Values for Gait Variables Under Single- and Dual-Task Conditions (n = 8)
| Gait Parameter | Single Task |
Dual Task |
P | ||
|---|---|---|---|---|---|
| Walk (SD) | 1-Back (SD) | Clock (SD) | Speech (SD) | ||
| Gait speed (m/s) | 0.82 (0.34) | 0.77 (0.32) | 0.72 (0.35) | 0.69 (0.31) | .009 |
| DLS (% gait cycle) | 31.92 (12.68) | 34.69 (12.11) | 34.94 (9.97) | 35.71 (12.33) | .012 |
| Paretic preswing (% gait cycle) | 18.51 (13.43) | 20.08 (13.46) | 19.57 (10.70) | 20.45 (13.50) | .080 |
| Paretic weight acceptance (% gait cycle) | 13.08 (3.17) | 14.63 (3.07) | 15.23 (2.46) | 15.32 (2.86) | <.001 |
| Paretic swing (% gait cycle) | 38.13 (7.72) | 36.77 (7.55) | 37.16 (6.56) | 36.29 (8.05) | .319 |
| Nonparetic swing (% gait cycle) | 29.74 (8.68) | 28.04 (8.64) | 27.76 (8.91) | 27.68 (8.41) | <.001 |
| Paretic swing CV | 7.01 (1.80) | 7.82 (2.63) | 8.10 (3.97) | 8.50 (2.57) | .478 |
| Nonparetic swing CV | 8.12 (3.03) | 8.67 (2.79) | 8.49 (3.78) | 8.89 (3.22) | .907 |
| Temporal symmetrya | 1.74 (0.77) | 1.82 (0.92) | 2.07 (1.52) | 1.85 (0.98) | .326 |
Abbreviations: SD, standard deviation; DLS, double-limb support; CV, coefficient of variation (%).
Larger swing–stance ratio/smaller swing–stance ratio.
Figure 1.

Double-limb support duration (as a percentage of gait cycle duration) shown as a function of task. Mean double-limb support duration was significantly longer in the 1-back and speech dual-task conditions, relative to single walk. The upper and lower margins of the box indicate the interquartile range. The horizontal line through the box indicates the median and the solid diamond represents the mean. The error bars indicate the range of values, with dots representing outliers (>1.5 times the interquartile range)
Dual-task effects on the 2 DLS phases (paretic preswing and nonparetic preswing) were also examined individually. There was a significant dual-task effect for duration of non-paretic preswing, which is the period of weight acceptance on the paretic limb [F(3, 21) = 8.598; P < .001; ; Figure 2], such that it increased significantly in the 3 dual-task conditions relative to single walking (P < .05). There was no dual-task effect on paretic preswing [Friedman test, ; P > .05]. These findings suggest that the increase in overall DLS as a percentage of gait cycle duration is primarily a result of an increase in the DLS phase associated with paretic limb weight acceptance.
Figure 2.
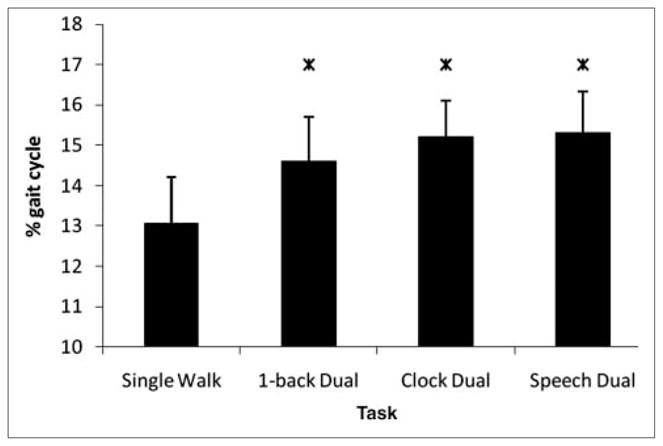
Duration of the double-limb support phase associated with paretic weight acceptance shown as a function of task: error bars indicate standard error of the mean. There was a significant increase in duration of paretic weight acceptance in each dual-task condition relative to single walk. Star denotes significant difference from single walk (P < .001)
Cognitive–Motor Interference Effects on Swing
There was a significant dual-task effect on swing duration of the nonparetic limb [F(3, 21) = 8.702; P < .001; ; Figure 3]. Post hoc tests revealed that this effect was a result of significantly shorter swing duration in the 3 dual-task conditions compared with the single walking condition (P < .05). The difference between the 3 dual tasks was not significant. Paretic limb swing was not significantly affected by the dual tasks [F(1.65, 11.55) = 1.230; P > .05; , with Greenhouse-Geisser adjustment; ε = 0.55; Table 3].
Figure 3.
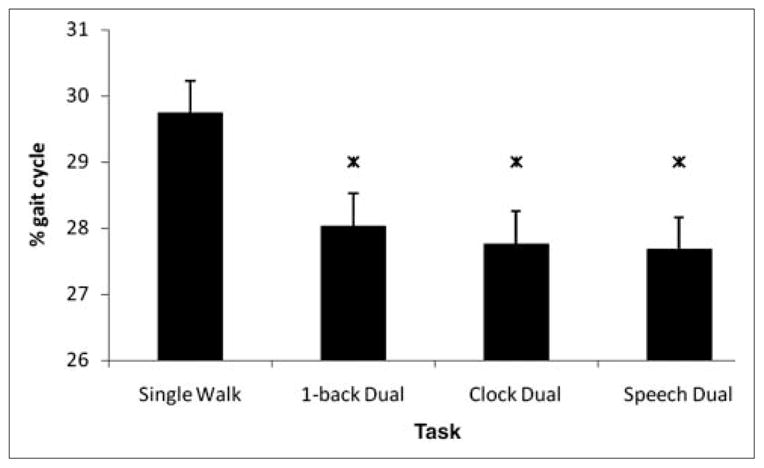
Nonparetic swing duration (as a percentage of gait cycle duration) shown as a function of task: error bars indicate standard error of the mean. There was a significant reduction in nonparetic swing duration in all dual-task conditions relative to single walk. Star denotes significant difference from single walk (P < .001)
Table 3.
Mean Reaction Times for Stimuli Presented in Different Phases of the Gait Cycle
| Gait Cycle Phase | 1-Back (SD) | Clock (SD) |
|---|---|---|
| Double-limb support | 1657.05 (115.05) | 2235.02 (615.97) |
| Swing | 1638.03 (145.92) | 2148.81 (661.24) |
| Paretic swing | 1646.24 (157.08) | 2187.95 (723.27) |
| Nonparetic swing | 1610.79 (167.71) | 2130.79 (682.62) |
| Paretic preswing | —a | 2183.52 (463.74) |
| Nonparetic preswing | — | 2008.14 (299.56) |
Dash indicates that insufficient data were available to calculate the mean.
Variability in swing duration expressed as CV did not change across conditions for either the paretic [F(3, 21) = 0.858; P > .05; ] or nonparetic limbs [F(3, 21) = 0.183; P > .05; ; Table 3].
Cognitive–Motor Interference Effects on Gait Symmetry
Table 2 reveals considerable temporal asymmetry, even in single-task walking. Six of the 8 participants had asymmetry with longer stance duration on the nonparetic limb, whereas 1 person had asymmetry with longer paretic stance duration. One participant had no temporal asymmetry. On average, the temporal asymmetry observed in single-task walking increased slightly under dual-task conditions, but the effect was not statistically significant [F(1.08, 7.59) = 1.136; P > .05; , with Greenhouse-Geisser adjustment; ε = 0.36].
Effects of Gait Cycle Phase on Reaction Time in Cognitive Tasks
Table 3 displays RT as a function of task and gait cycle phase. To examine whether there was a difference in RT for the auditory stimuli delivered during DLS or swing, we compared the mean RT for stimuli occurring in each phase of the gait cycle. In the clock task, the mean RT for stimuli delivered during DLS was slightly longer than for stimulus onsets in the swing phase (Table 2). The mean difference of 86.21 ms was not statistically significant [t(7) = 1.433; P > .05; d = 0.14]. Similarly, in the 1-back task, there was no difference between the RTs for stimuli occurring in the swing and DLS phases [t(7) = 0.0376; P > .05; d = 0.15].
To determine whether response latencies differed according to whether stimuli occurred in the swing phase of the paretic or the nonparetic limb, we compared the mean RTs for stimuli presented during the 2 swing phases (Table 3). There was no significant difference in mean RT for stimuli occurring during the paretic or nonparetic swing phase in either the clock task [t(6) = 1.077; P > .05; d = 0.09] or the 1-back task [t(6) = 1.125; P > .05; d = 0.24].
We also compared mean RTs for the 2 DLS phases: paretic preswing and nonparetic preswing (Table 3). There were 6 participants with a sufficient number of trials in each DLS phase to be able to perform this analysis for the clock task. RTs were slightly longer for stimuli occurring during the DLS phase preceding nonparetic swing compared with the DLS phase preceding paretic swing. We found that 4 of the 6 participants tended to be slower to respond during paretic weight acceptance. However, with a small number of participants and large between-participant variability, the difference (175.38 ms) was not statistically significant [t(5) = 1.214; P > .05; d = 0.49].
For the 1-back task, there were too few trials delivered in each DLS phase to be able to compare RTs to stimuli delivered during the DLS associated with paretic preswing with that of nonparetic preswing. In the future, it may be necessary to deliberately manipulate the onset of the stimuli to ensure that an adequate number of stimuli occur during each phase of the gait cycle.
Discussion
This is the first investigation of cognitive–motor interference related to different phases of the gait cycle during continuous overground walking after stroke. Our unique analysis was possible because we synchronized the acquisition of the cognitive and gait data. Knowledge gained from the 2 previous studies examining cognitive–motor interference associated with DLS and swing has been limited because the studies have focused only on gait (DLS)1 or cognition (RT).6 Here, we have reported the cognitive–motor interference effects associated with distinct phases of the gait cycle for both gait and cognitive performance in a group of community-dwelling individuals poststroke.
Effects of Cognition on Double-Limb Support
The significant dual-task effect on DLS, expressed as a percentage of gait cycle duration, is consistent with the only other study examining this question1 and suggests that balance during walking may be disrupted under dual-task conditions in independent community ambulators after stroke. Of note, Bowen et al1 reported that despite their group effect, DLS did not increase under dual-task conditions for all the participants: 2 of 11 showed no change, and 1 participant showed a slight reduction in DLS duration. A visual inspection of individual data for our participants showed that we also had 2 participants who did not behave according to the group effect. These 2 participants were at the opposite ends of the spectrum in terms of percentage of the gait cycle spent in DLS; one (left hemiplegia) had the lowest percentage DLS for the group, whereas the other (right hemiplegia) had the highest percentage of DLS. Despite these differences, both participants had marked lower-extremity motor impairment (22/34 and 18/34 Fugl-Meyer scores, respectively), and both demonstrated considerable variability in DLS duration in all 4 walking tasks relative to the rest of the group. Neither of the participants differed remarkably from the rest of the group with respect to performance on the neuropsychological battery. Future research with larger samples is needed to investigate differences in patterns of cognitive–motor interference based on characteristics such as severity of motor impairment.
The overall increase in DLS duration as a percentage of the gait cycle duration was a result of a significant increase in duration of the DLS phase associated with paretic weight acceptance (ie, preswing of the nonparetic limb). In contrast, the DLS phase associated with preswing of the paretic limb did not increase under dual-task conditions (Table 2). Therefore, in addition to the moderate effect for reduced cognitive performance during paretic weight acceptance (discussed in more detail later), this phase of the gait cycle was also associated with interference with respect to motor control. In other words, both motor and, to a lesser extent, cognitive performances were affected during paretic weight acceptance. Together, these findings suggest that loading the paretic limb may be the most attention-demanding phase of the gait cycle in persons with hemiparesis. This has important clinical implications. Therapists may need to focus on single-task part-practice of this phase of the gait cycle to improve automaticity of paretic limb loading. Dual-task part-practice may be an important step in progression to dual-task whole-practice of gait.
Effects of Cognition on Swing
Although the duration of paretic swing did not change under dual-task conditions, there was a significant dual-task effect on nonparetic swing duration, such that participants reduced the duration of nonparetic swing relative to single-task walking. Together with the findings for DLS, this indicates that under dual-task conditions, the participants increased the amount of time (as a percentage of total gait cycle duration) spent loading weight onto the paretic limb during initial DLS and then reduced the amount of time spent in paretic single-limb support. No previous studies of the dual-task effects on swing duration after stroke are available for comparison. Nonetheless, the increase in DLS with parallel decrease in paretic single-limb support implies that balance control during dual-task walking may be significantly compromised in independent community ambulators who have suffered a stroke. The destabilizing effect of cognitive–motor interference on gait in this population has the potential to have a substantial impact on community mobility. An important area for future research will be to quantify the impact of gait-related cognitive–motor interference on participation.
We reported previously that the 1-back task may not have been cognitively demanding enough to produce noteworthy gait interference; it had the smallest effect on gait speed, and its effect on stride duration was not statistically significant (unlike the clock and speech tasks).8 The current findings indicate however, that the 1-back task had a significant effect on the timing of the phases of the gait cycle, even if the total gait cycle duration was not affected. Our previous report involved a slightly larger sample (n = 13). It is likely that the current analysis was underpowered to detect significant differences between the 3 dual tasks (1-back, clock, speech), although the trend in the means for duration of paretic weight acceptance and paretic single-limb stance (nonparetic swing) support our earlier theory that generating spontaneous narrative produced the most interference with gait, whereas the 1-back had the smallest impact.
Effects of Cognition on Temporal Asymmetry
Temporal asymmetry may be a useful indicator of gait dys-control following stroke.14 Although the participants in this study showed signs of reduced balance during dual-task walking (eg, increased DLS duration), they did not exhibit a significant increase in temporal asymmetry. On average, participants had severe15 temporal asymmetry even at baseline (index > 1.5). With regard to dual-task effects on temporal asymmetry, 2 participants who were extremely asymmetrical (index > 2.5) influenced the group data. Only these 2 showed a noteworthy increase in asymmetry under dual-task conditions. Interestingly, these were the same individuals who did not behave according to the group effect for DLS. The remaining participants (baseline asymmetry index range: 1.03–1.62) did not show an increase in asymmetry across tasks. It is possible that dual-task effects on gait symmetry are related to motor impairment severity or gait variability. The degree of variation in temporal asymmetry observed in our small sample is not unusual for community-dwelling ambulators poststroke.15 Future research should explore dual-task effects on temporal asymmetry in different subgroups of ambulators poststroke.
Effects of Gait Cycle Phase on Cognitive Task Performance
The current finding that the cognitive–motor interference effects of DLS and swing on RT were minimal is consistent with our previous conclusion that the cognitive tasks were prioritized over gait.8 The only exception was a moderate effect size for longer RTs in the auditory clock task during the DLS phase associated with paretic weight acceptance relative to the DLS phase associated with paretic preswing. When auditory stimuli were delivered during paretic weight acceptance (initial DLS for the paretic limb), the RTs tended to be longer than when stimuli occurred during terminal DLS for the paretic limb (ie, paretic preswing). Despite the moderate effect size (d = 0.49), the effect was not statistically significant. It is likely that the large variance and the small sample size resulted in insufficient statistical power to detect a significant difference. Indeed, post hoc power analysis (G*Power 3, Faul et al21) reveals inadequate protection against type II error (power = 0.2). Thus, the current results should be viewed as hypothesis generating and should be explored further in a larger group.
Although previous research has reported significantly longer RTs during DLS compared with swing in a subgroup of patients poststroke,6 we did not find a significant difference between DLS and swing. These conflicting results may be due to differences in the walking task (overground vs treadmill) and/or the nature and difficulty of the cognitive task. It is also possible that differences in patient characteristics contributed to the discrepancy between our study and the previous study. Indeed, Regnaux et al6 only found a statistically significant effect between DLS and swing for the group of patients who performed more poorly at baseline on the RT task than healthy controls. The previous study did not distinguish between initial and terminal DLS, so it is not known if the effect was equal for paretic preswing and paretic weight acceptance. The current findings shed new light on cognitive–motor interference during walking after stroke and suggest that increases in RT during DLS may be associated with one particular DLS phase. Thus, a hypothesis that emerges from the current data is that paretic weight acceptance may be more attention demanding than paretic preswing and may contribute to slower cognitive processing (longer RTs) during DLS compared with swing. Future research should discriminate between the 2 DLS phases of the gait cycle to address this question.
Limitations
A limitation of this study was the absence of a control group. This was a novel analysis of the interactions between cognitive performance and specific components of the gait cycle. Future investigations will need to determine whether these findings are a result of age-related changes in the control of walking or the effect of the stroke. A more important limitation was the small sample size. Complexities in the technological setup to conduct this innovative analysis led to occasional missing data; thus, some participants from the primary cohort8 could not be included in this report. Collecting data on an instrumented walkway might overcome some of the difficulties encountered, but the collection of a sufficiently large sample of uninterrupted strides would be severely compromised by the length of the walkway. Another alternative would be to use an instrumented treadmill; however, we remain predominantly interested in studying how people voluntarily modulate their gait during dual-task situations in an overground environment that closely mimics real life. Advances in technology (and/or collaboration with engineers) could streamline synchronized data acquisition. Unfortunately, lesion location information was not available for all participants, so we could not examine for relationships between lesion location and cognitive–motor interference and different cognitive tasks.
Conclusion
Research in cognitive–motor interference during gait in people who have suffered a stroke is still in its infancy. A consistent finding from the emerging body of research is that gait speed is significantly reduced under dual-task conditions.1,4,7–10 The findings from the current study provide new information to suggest that an increase in DLS, especially paretic weight acceptance, underlies this compromise in gait velocity. Furthermore, we demonstrated that paretic weight acceptance may also be associated with deterioration in simultaneous cognitive task performance, although this needs to be verified in a larger sample. Nonetheless, the current results provide the first evidence that interference between cognition and gait in people poststroke may be influenced by different phases of the gait cycle. Establishing the interactions between components of the gait cycle and different cognitive processes may provide insight into the cognitive factors involved in control of the step cycle.
Acknowledgments
The authors are sincerely grateful to Dawn Saracino and Emily Fox for assistance with data collection. We also acknowledge the support of staff and volunteers at the Brooks Center for Rehabilitation Studies and Ryan Knight of the Brain Rehabilitation Research Center at the Malcom Randal VA Medical Center in Gainesville, Florida who provided technical and engineering support.
Funding
The authors received no financial support for the research and/or authorship of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Bowen A, Wenman R, Mickelborough J, Foster J, Hill E, Tallis R. Dual-task effects of talking while walking on velocity and balance following a stroke. Age Ageing. 2001;30:319–323. doi: 10.1093/ageing/30.4.319. [DOI] [PubMed] [Google Scholar]
- 2.Cockburn J, Haggard P, Cock J, Fordham C. Changing patterns of cognitive-motor interference (CMI) over time during recovery from stroke. Clin Rehabil. 2003;17:167–173. doi: 10.1191/0269215503cr597oa. [DOI] [PubMed] [Google Scholar]
- 3.Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry. 2000;69:479–486. doi: 10.1136/jnnp.69.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyndman D, Pickering R, Ashburn A. Reduced sway during dual task balance performance among people with stroke at 6 and 12 months after discharge from hospital. Neurorehabil Neural Repair. 2009;23:847–854. doi: 10.1177/1545968309338192. [DOI] [PubMed] [Google Scholar]
- 5.Kemper S, McDowd J, Pohl P, Herman R, Jackson S. Revealing language deficits following stroke: the cost of doing two things at once. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:115–139. doi: 10.1080/13825580500501496. [DOI] [PubMed] [Google Scholar]
- 6.Regnaux JP, David D, Daniel O, Smail DB, Combeaud M, Bussel B. Evidence for cognitive processes involved in the control of steady state of walking in healthy subjects and after cerebral damage. Neurorehabil Neural Repair. 2005;19:125–132. doi: 10.1177/1545968305275612. [DOI] [PubMed] [Google Scholar]
- 7.Canning CG, Ada L, Paul SS. Is automaticity of walking regained after stroke? Disabil Rehabil. 2006;28:97–102. doi: 10.1080/09638280500167712. [DOI] [PubMed] [Google Scholar]
- 8.Plummer-D’Amato P, Altmann LJP, Saracino D, Fox E, Behrman AL, Marsiske M. Interactions between cognitive tasks and gait after stroke: a dual task study. Gait Posture. 2008;27:683–688. doi: 10.1016/j.gaitpost.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis A, Dawes H, Elsworth C, et al. Fast walking under cognitive-motor interference conditions in chronic stroke. Brain Res. 2009;1287:104–110. doi: 10.1016/j.brainres.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Lord S, Rochester L, Weatherall M, McPherson KM, McNaughton HK. The effect of environment and task on gait parameters after stroke: a randomized comparison of measurement conditions. Arch Phys Med Rehabil. 2006;87:967–973. doi: 10.1016/j.apmr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26:555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 14.Sibley K, Tang A, Patterson K, Brooks D, McIlroy WE. Changes in spatiotemporal gait variables over time during a test of functional capacity after stroke. J Neuroeng Rehabil. 2009;6:27. doi: 10.1186/1743-0003-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89:304–310. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 16.Brandstater M, de Bruin H, Gowland C, et al. Hemiplegic gait: analysis of temporal variables. Arch Phys Med Rehabil. 1983;64:583–587. [PubMed] [Google Scholar]
- 17.Titianova E, Tarkka I. Asymmetry in walking performance and postural sway in patients with chronic unilateral cerebral infarction. J Rehabil Res Dev. 1995;32:236–244. [PubMed] [Google Scholar]
- 18.Bensoussan L, Viton J-M, Schieppati M, et al. Changes in postural control in hemiplegic patients after stroke performing a dual task. Arch Phys Med Rehabil. 2007;88:1009–1015. doi: 10.1016/j.apmr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Melzer I, Tzedek I, Or M, et al. Speed of voluntary stepping in chronic stroke survivors under single- and dual-task conditions: a case-control study. Arch Phys Med Rehabil. 2009;90:927–933. doi: 10.1016/j.apmr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Hsueh IP, Hsu MJ, Sheu CF, Lee S, Hsieh CL, Lin JH. Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale and 2 versions of the Stroke Rehabilitation Assessment of Movement. Neurorehabil Neural Repair. 2008;22:737–744. doi: 10.1177/1545968308315999. [DOI] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]


