Abstract
Objective
The protein deacetylase SirT1 inhibits apoptosis in a variety of cell systems by distinct mechanisms, yet its role in chondrocyte death has not been explored. Here we assess the role of SirT1 in the survival of osteoarthritic human chondrocytes.
Methods
SirT1, PTP1B, PTP1Bmutant expression plasmids and SirT1siRNA and PTP1BsiRNA were transfected into primary human chondrocytes. Levels of apoptosis were determined by flow cytometry and activation of components of the IGFR/Akt pathway was assessed by immunoblotting. Immunohistochemistry was performed on osteoarthritic (OA) and normal knee cartilage samples.
Results
Expression of SirT1 in chondrocytes led to increased chondrocyte survival in either the presence or absence of TNFα/Actinomycin D, while a reduction of SirT1 by siRNA led to increased in chondrocyte apoptosis. Expression of SirT1 in chondrocytes led to activation of the IGF receptor (IGFR) and the downstream kinases PI3K, PDK1, mTOR and Akt which in turn led to phosphorylation of MDM2, inhibition of p53 and a block to apoptosis. Activation of the IGFR occurs at least in part via SirT1-mediated repression of the protein tyrosine phosphatase PTP1B. Expression of PTP1B in chondrocytes increased apoptosis and reduced IGFR phosphorylation, while downregulation of PTP1B by siRNA significantly decreased apoptosis. Examination of cartilage from normal donors and osteoarthritic patients revealed that PTP1B levels are elevated in OA cartilage where SirT1 levels are decreased.
Conclusion
This is the first demonstration that SirT1 is a mediator of human chondrocyte survival via downregulation of PTP1B a potent chondrocyte proapoptotic protein that is elevated in OA cartilage.
Introduction
Maintenance of healthy adult articular cartilage is dependent on the synthetic capabilities of the chondrocytes residing within this tissue. Chondrocytes produce the unique extracellular matrix (ECM) proteins that provide this tissue with the mechanical characteristics required for resistance to cyclic loading. Osteoarthritis (OA), the predominant age-related arthritic disease of the west, primarily affects this hyaline cartilage of the load bearing joints. One key feature of this disease is the elevation of chondrocyte death in cartilage, which leads to a reduction in the number of cells able to produce the specialized ECM (1). It has been demonstrated that a correlation exists between the level of chondrocyte death and both the age of individual and the severity of disease (2). Additional studies have revealed that cell death is elevated in OA cartilage and that this death has features attributed to apoptosis (3-6). In total these data indicate that apoptosis of chondrocytes plays a role in the pathology of OA (1).
Since OA is an age-associated disease it is relevant to explore the gene products that prolong aging in mammals, with regard to the mechanisms underlying chondrocyte apoptosis. An important mediator of aging in mammals is SirT1 an NAD-dependent histone deacetylase. SirT1 has been shown to be responsible for lifespan extension during caloric restriction in a variety of organisms (7-8). Additionally, SirT1 can enhance cell survival and affect differentiation and proliferation (7-8). While little is known of the function of SirT1 in human chondrocytes, it has been recently demonstrated that SirT1 can positively regulate expression of a number of cartilage specific extracellular matrix genes, such as collagen type II(α1), type IX(α1), aggrecan and COMP (9). It appears that SirT1 proteins levels are reduced in chondrocytes derived from OA cartilage compared to normal cartilage (9). Additionally it has been demonstrated that resveratrol, a natural product known to activate a number of cellular proteins including SirT1, can enhance chondrocyte survival (10,11). In other cell systems, SirT1 has been demonstrated to block apoptosis and that it accomplishes this function through a variety of mechanisms (7). For example, SirT1 can directly deacetylate and inactivate the p53 and p73 tumor suppressors (12-15), it can deacetylate the FOXO transcription factors (16-19) and it can deacetylate Ku70 thereby inhibiting Bax-induced apoptosis (20). Given that SirT1 appears to be downregulated in OA chondrocytes (9), it suggests that the increased chondrocyte death in OA cartilage is due in part to a reduction in SirT1 levels.
Here we explored the role of SirT1 in the survival of chondrocytes derived from knee joints of osteoarthritic patients. The results show that SirT1 is a potent prosurvival protein and that it accomplishes this function by activating the IGF-receptor/Akt pathway via suppression of a potent proapoptotic factor, namely protein tyrosine phosphatase 1B (PTP1B). Consistent with the increase in chondrocyte death in OA cartilage, PTP1B protein levels are enhanced in this diseased cartilage compared to normal cartilage.
Materials and Methods
Cell culture, transfections and flow cytometry
Human chondrocytes were isolated from patients undergoing total knee arthroplasty (provided by NDRI, Philadelphia, PA). The average age was 62 years old (range 54-70). Chondrocyte isolation and culture was as previously described (9). Passage 0, 1 and 2 (P0, P1, P2) human articular chondrocytes were used for all experiments since cartilage marker genes are expressed optimally in these cells. Monolayer cultures were maintained in DMEM (4.5 g/l glucose with L-glutamine) supplemented with 10% FBS, 50 U/ml penicillin and 50 ug/ml streptomycin. All transfection experiments were initiated on 50 % confluent monolayer cultures. Plasmids (4 ug) were transfected by Amaxa nucleofector technology for human chondrocytes according to the manufacturers protocol (9). The murine SirT1 expression plasmid was obtained from Upstate/Millipore (Boston, MA). The PTP1B and PTP1BMutant plasmids were as previously described (38,39). SiRNAs were purchased from Ambion (Applied Biosystems).
Actinomycin D was purchased from Sigma-Aldrich Chemical Co (St Louis, MO) and TNFα was purchased from R&D Systems, Inc (Mpls, MN).
Percent apoptotic cells were determined by flow cytometry as in Gagarina et al., 2008 (21) on a Coulter Profile 2, Flow Cytometer. Apoptosis is evident as a population of cells with less than 2N (diploid) DNA content (21). A TUNEL assay was used to determine the percent apoptotic cells in cartilage tissue. SirT1 activity assays were performed as previously described (9).
RNA isolation and RT/PCR analysis
Total RNA was isolated from cells by the Trizol method (Gibco/BRL). Oligo dT was used as the primer in the reverse transcription reaction. Real-time PCR reactions were carried out using 10ng of cDNA and Syber Green mix (BioRad Laboratories, Hercules, CA), as previously described by (22). Quantitative analyses were performed using the BioRad iCycler software. Additionally, where indicated, semi-quantitative RT/PCR reactions were performed with the appropriate primers. For the RT/PCR reactions, one microgram of total RNA from the cells was used in each reaction. All RNA samples were DNase I treated prior to the PCR reactions. Additionally, as a control, PCR done in the absence of RT was negative for any ethidium bromide stained bands (data not shown).
Protein analysis and immunoblotting
Cell lysis and the generation of protein extracts were carried out as previously described (9,21). The protein extracts were resolved by SDS-PAGE (5-10 ug protein/lane) and transferred onto nitrocellulose membranes for immunoblotting. The blots were processed as previously described (9,21) and then probed with the antibodies indicated below. The blots were developed by using secondary antibodies to either alkaline phosphatase (BCIP/NBT) as a color developer or horseradish peroxidase (Chemiluminescence) and exposed to X-ray film.
Immunohistochemistry
For immunohistochemistry, cartilage samples were fixed in 4% paraformaldehyde for 24 hours, dehydrated in a graded series of ethanol baths, embedded in parrafin and sectioned (4 uM). The sections on slides were rehydrated and incubated with anti-SirT1, anti-PTP1B or anti-MMP13 specific antibodies and visualized using a broad-spectrum immunohistochemistry kit (DAB) (Zymed, Invitrogen, Carlsbad, CA).
Statistical analyses
Statistical analysis was obtained using one-way ANOVA, assuming confidence levels of 95% or less (p≤0.05) to be statistically significant. Error bars indicate the standard deviation around the mean value of each data point. An asterisk (*) was used to express a statistically significant difference between controls and the experimental condition. The immunoblots and co-immunoprecipitation assays represent three repetitions from two distinct cell sources (n=6). The SirT1 activity assays were carried out in triplicates from two different transfections (n=6). The qPCR were generated from 3 runs of two separate experiments (n=6)).
Results
Expression of SirT1 in human chondrocytes reduces apoptosis
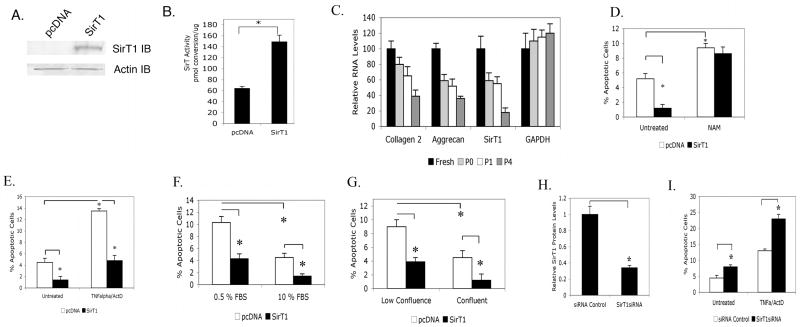
To determine if SirT1 has a prosurvival effect on human chondrocytes, it was transiently overexpressed in these cells by Amaxa nucleofector technology resulting in 90-95 % transfection efficiency using the solutions and electroporation program recommended by the manufacturer. As shown in Figure 1, SirT1 was efficiently overexpressed by day two posttransfection, as assessed by both western blot (Figure 1A) and SirT1 activity assay (Figure 1B). The ectopically expressed SirT1 was also found to be targeted to the nucleus (data not shown). As shown in Figure 1C, the P1 cells used display relatively high levels of collagen 2(α1) and aggrecan gene expression, compared to that of freshly isolated cells. The transfected cells were then monitored for levels of apoptosis by flow cytometry. Figure 1D shows that the level of apoptosis drops by 5.5-fold when SirT1 was expressed. Adding the SirT1 inhibitor nicotinamide (NAM) to the culture at the time of transfection abolished SirT1's protective effect against apoptosis (Figure 1D). When an enzymatically inactive SirT1 mutant (SirT1H355Y) was expressed in chondrocytes, it had no effect on chondrocyte survival (data not shown).
Figure 1. SirT1 is a positive regulator of survival of osteoarthritic human chondrocyte.
OA human chondrocytes (P1) were Amaxa transfected with a SirT1 expression plasmid or pcDNA plasmid control (empty vector). Extracts generated at 24 hours posttransfection were used in immunoblot analysis (A) or SirT activity assays (B). C. Real-time PCR analysis of the indicated genes from freshly isolated, P0, P1 and P4 chondrocytes. D-E. Chondrocytes transfected as in (A) were left untreated or treated with nicotinamide (NAM, 1 mM) (D) for 24 hours or treated with TNFα (10 ng/ml) and Actinomcyin D (ActD, 0.2 ug/ml) (E) for 24 hours and the percent apoptotic cells measured. F-G. Chondrocytes transfected as in (A) were either cultured in low vs high serum (0.5 % vs 10% FBS) (F) or were plated at subconfluence vs confluence (20% vs 100%) (G). Apoptosis was assessed at 24 hours. H-I. P1 chondrocytes were transiently transfected with a control or SirT1 siRNA. The level of SirT1 protein was determined by immunoblot and quantitated by scanning densitometry (H). I. Chondrocytes transfected as in (E) were treated with or without TNFα/ActD and the percent apoptotic cells measured. The error bars in all graphs indicate the standard deviation and the statistical significance is indicated by an asterisk (*) (p<0.05).
Apoptosis was induced in chondrocytes by the addition of TNFα and Actinomycin D, which elevated the level of cell death by over 3-fold (Figure 1E). Overexpression of SirT1 in these cells significantly reduced this TNFα/ActD-mediated apoptosis (Figure 1E). Apoptosis was also induced by culture in low serum (0.5% FBS) (Figure 1F) or at low confluence (20%) (Figure 1G). Under both physiological apoptotic conditions, expression of SirT1 led to decreased apoptosis. A SirT1 specific siRNA was transfected into chondrocytes, which lowered the levels of endogenous SirT1 in untransfected human chondrocytes by 3-fold (Figure 1H). This reduction in SirT1 resulted in a corresponding increase in the level of apoptosis in either the presence or absence of TNFα/ActD (Figure 1I). In total, these data indicate that SirT1 is an anti-apoptotic protein in human chondrocytes and that this function requires its enzymatic activity.
Expression of SirT1 in chondrocytes leads to activation of the IGF receptor/Akt pathway
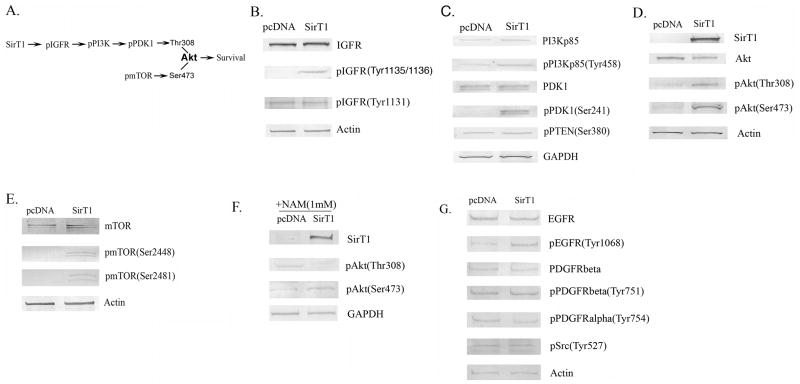
To address the mechanism by which SirT1 protects chondrocytes against apoptosis, the IGF receptor (IGFR) pathway was examined (Figure 2A), since IGF1 is a well-known survival factor for chondrocytes (23,24) and SirT1 has been shown to affect components of this pathway (13, 25,26). Chondrocytes were transfected with the SirT1 expression plasmid and IGFR levels were assessed. As shown in Figure 2B, total IGFR levels were unchanged by SirT1, however levels of the activated/tyrosine phosphorylated form of the receptor, pIGFR(Tyr1135/1136), were significantly elevated in the presence of elevated SirT1 (Figure 2B). Activation of the IGFR leads to phosphorylation of PI3kinase which in turn leads to phosphorylation of PDK1 (27), as outlined in Figure 2A. As shown in Figure 2C the levels of the phosphorylated forms of both PI3kinase and PDK1 were significantly increased by SirT1, while there was no change in the total levels of PI3Kinase, PDK1 or GAPDH. Additionally SirT1 did not change the phosphorylation status of the phosphatase PTEN (Figure 2C) a negative regulator of this pathway. pPDK1 is known to phosphorylate the prosurvival kinase Akt on Thr308, resulting in Akt activation (27). As shown in Figure 2D, pAkt(Thr308) levels were significantly increased by SirT1.
Figure 2. Activation of the IGF receptor and Akt in human chondrocytes expressing SirT1.
A. Pathway outlining phosphorylation of Akt by IGF-activated protein kinases and mTOR. pAkt(Thr308) is a target of pPI3Kinase while pAkt(Ser473) is a target of pmTOR. B-E. P1 human chondrocytes were transiently Amaxa transfected with a SirT1 expression plasmid or a pcDNA control (empty vector). Extracts were generated at 24 hours posttransfection and used in immunoblot analysis with the indicated antibodies. F. Chondrocytes transfected as in (B) were treated with NAM (1 mM) where indicated, extracts were generated and immunoblotted with the indicated antibodies. G. Chondrocytes were transfected as in (B), extracts were generated and immunoblotted with the indicated antibodies.
In order to achieve optimal activation of Akt, phosphorylation on Ser473 is also required (Figure 2A). As shown in Figure 2D, pAkt(Ser473) levels were significantly elevated by SirT1. The protein kinase responsible for phosphorylation of Ser(473) is mTOR, which in turn needs to be phosphorylated in order to be activated. Assessment of the phosphorylation status of mTOR, on residues Ser2448 and Ser2481 show increased phosphorylation in the presence of SirT1 (Figure 2E), indicating mTOR was activated.
To confirm that SirT1 mediates the activation of Akt by phosphorylation on Thr308 and Ser473, the SirT1 inhibitor NAM was added to the cultures following the SirT1transfection. In the presence of NAM, phosphorylation of Akt on Ser473 and Thr308 was not elevated by SirT1 (Figure 2F). In additional control experiments the IGFR antagonist AG1024 or the PI3Kinase inhibitor LY294002 was added to the cells following the SirT1 transfection. These inhibitors significantly blocked the phosphorylation of Akt on Thr308 in the presence of elevated SirT1 (data not shown), indicating that activation of Akt by SirT1 occurs via the IGFR and PI3Kinases. Taken together, these data show that elevated expression of SirT1 in human chondrocytes leads to the activation of the IGFR, which initiates a phosphorylation cascade culminating in phosphorylation of Akt on the two amino-acid residues needed for activation.
As controls we examined the status of other tyrosine kinases potentially affected by SirT1. Figure 2G shows a modest increase in EGF receptor tyrosine phosphorylation in the presence of SirT1, however no increases were detected in tyrosine phosphorylation of the PDGFalpha receptor, PDGFbeta receptor or Src.
Activation of Akt by SirT1 leads to phosphorylation of MDM2 and inhibition of the proapoptotic protein p53
Activated Akt has multiple cellular targets that participate in cell survival, which includes MDM2, a protein that functions in part by binding and blocking the pro-apoptotic protein p53. It is known that activated Akt phosphorylates human MDM2 on Ser186 (Figure 3A), leading to increased affinity of MDM2 for p53 (28-30). As shown in Figure 3B, chondrocytes overexpressing SirT1 show a significantly increased level of pMDM2Ser186 compared to the control transfected cells while the total levels of MDM2 did not vary. These data are consistent with fact that SirT1 activates Akt, which in turns leads to phosphorylation of MDM2.
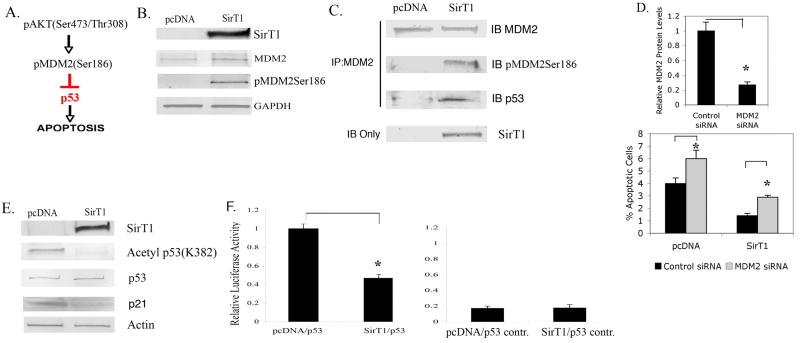
Figure 3. Phosphorylation of MDM2 and inactivation of p53 in chondrocytes expressing SirT1.
A. Pathway outlining MDM2 phosphorylation by Akt. B. P1 human chondrocytes were transiently Amaxa transfected with a SirT1 expression plasmid or pcDNA control (empty vector). Extracts generated at 24 hours posttransfection were used in immunoblot analysis with the indicated antibodies. C. Extracts from (B) were immunoprecipitated with an MDM2 specific antibody were then immunoblotted and probed with the indicated antibodies. D. Chondrocytes were transfected with a control siRNA or an MDM2 siRNA. The level of MDM2 protein was determined by immunoblot and quantitated by scanning densitometry (upper panel). Lower Panel; Chondrocytes were transfected with a pcDNA control, the SirT1 plasmid, a control siRNA and an MDM2 siRNA where indicated. The percent apoptotic cells was assessed by flow cytometry. E. Chondrocytes were transfected with a pcDNA control or the SirT1 plasmid. Extracts were generated and immunoblotted with the indicated antibodies. F. Chondrocytes transfected with a pcDNA control, a SirT1 expressing plasmid, a p53 responsive promoter/luciferase construct or a control promoter/luciferase construct were assayed for luciferase activity. The error bars in all graphs indicate the standard deviation and the statistical significance is indicated by an asterisk (*) (p<0.05).
It has been demonstrated that phosphorylated MDM2 binds p53 more efficiently than unphosphorylated MDM2 (28). To determine if this was the case in our chondrocyte extracts, MDM2 was immunoprecipitated (IP) from extracts of the control and SirT1 transfected cells and these IPs were then immunoblotted for MDM2, pMDM2 and p53 (Figure 3C). It is clear from the figure that in cells expressing SirT1, there is a significant increase in the amount of pMDM2 in the MDM2 IP while the total amount of MDM2 does not change between the two conditions. Consistent with this increase in phosphorylated MDM2 there is a large increase in the amount of p53 in the IP from the SirT1 expressing extracts which contain elevated pMDM2. These data indicate that p53 associates more efficiently with phosphorylated MDM2 as has been reported (28).
If MDM2 mediates the survival effect of SirT1 in chondrocytes, then downregulation of MDM2 by siRNA transfection should abolish the anti-apoptotic effect of SirT1. Chondrocytes were transfected with either a control siRNA or MDM2 siRNA and the levels of MDM2 protein assessed. From Figure 3D, upper panel, it is clear that the MDM2 siRNA reduced the level of MDM2 protein by about 4-fold. When cells were cotransfected with the MDM2 siRNA and the SirT1 expression plasmid (Figure 3D, lower panel), SirT1 was not nearly as effective at reducing the level of apoptosis in the presence of the MDM2 siRNA, compared to the control siRNA (the number of apoptotic cells increased 2-fold in the presence of the MDM2 siRNA). Thus, reducing MDM2 levels reduces the ability of SirT1 to block apoptosis.
While p53 can be blocked by its association with pMDM2, it also has been demonstrated that p53 can be inactivated by SirT1-mediated deacetylation (12,14). To determine if p53 was affected by SirT1 in human chondrocytes, the acetylation status of p53 was assessed. As shown in Figure 3E, SirT1 did not affect total levels of p53 but dramatically reduced the level of acetylated p53. Since p53 activity can be blocked by both pMDM2 binding and by deacetylation it was expected that p53 target gene expression should be reduced by SirT1. One such gene is p21 and as shown in Figure 3E, p21 protein levels were down regulated by SirT1. Additionally, cells were transfected with a p53-responsive promoter/luciferase in the presence or absence of SirT1. As shown in Figure 3F, left panel, this p53 responsive promoter was significantly repressed in cells expressing SirT1, while a control promoter was not affected (Figure 3F right panel). In total these data indicate that activation of Akt leads to phosphorylation of MDM2 and inactivation of the pro-apoptotic protein p53.
It is recognized that active Akt serves an anti-apoptotic function by phosphorylating a number of proteins in addition to MDM2, which includes the FOXO family of proteins (40,41) and Bad (42,43). In our experiments we detected no increased phosphorylation of Bad, FOXO1, FOXO3a or FOXO4 following overexpression of SirT1 in chondrocytes (data not shown), indicating that at least in chondrocytes Akt activation by SirT1 may not lead to efficient phosphorylation of these proteins.
SirT1 represses expression of protein tyrosine phosphatase 1B (PTP1B), a pro-apoptotic protein that targets the IGF receptor
The data above show that SirT1 is able to activate the IGFR pathway. To determine if the IGFR was activated by autocrine production of IGF1 and IGF2, the levels of these growth factors were assessed in chondrocytes expressing SirT1. The IGFs (IGF1 in particular), are known to activate Akt (23), are a well-known component of cartilage and can be secreted by chondrocytes (34). SirT1 had no effect on IGF1 at either the RNA or protein level (data not shown). SirT1 was able to induce IGF2 in chondrocytes at both the RNA and protein level, however, pure IGF2 had no effect on reducing the level of chondrocyte apoptosis (data not shown) while IGF1 had an anti-apoptotic effect consistent with previous reports (23,24). These data suggested that activation of the IGFR by SirT1 may be due to factors in addition to IGF1. One factor that has been demonstrated to block IGFR activity is the protein tyrosine phosphatase PTP1B, which dephosphorylates the IGFR thereby inactivating it (31,32). Importantly, SirT1 can repress expression of PTP1B, thereby enhancing insulin signaling (33). It was therefore thought that SirT1 activates the IGFR pathway in chondrocytes by repression of PTP1B. As shown in Figures 4A and 4B, SirT1 had a significant effect on repression of PTP1B at both the RNA and protein levels. When additional PTPs were assessed (Figure 4B), PTPalpha levels were not changed in cells expressing elevated Sirt1, indicating that SirT1 does not affect it. We could not detect PTPgamma, PTPkappa or LAR by immunoblot in these chondrocyte extracts (data not shown).
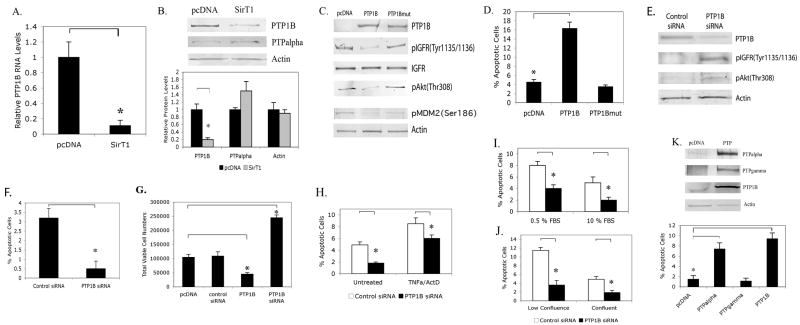
Figure 4. PTP1B is a potent pro-apoptotic protein in human osteoarthritic chondrocytes, is repressed by SirT1 and reduces the level of activated IGF Receptor.
A-B. P1 human chondrocytes were transfected with a SirT1 expression plasmid or pcDNA control. RNA and protein extracts were used in quantitative RT/PCR (A) or immunoblot analysis (B) with the indicated antibodies. The blot in B (upper panel) was scanned and relative protein levels determined (lower panel). C & D. Chondrocytes were transfected with a PTP1B or PTP1Bmutant expression plasmid or a pcDNA control. Protein extracts were used in immunoblot analysis with the indicated antibodies (C) or the cells were processed for flow cytometry and the percent apoptotic cells determined (D). E & F. Chondrocytes were transfected with a control siRNA or a PTP1BsiRNA. Protein extracts were used in immunoblot analysis with the indicated antibodies (E) or the cells were processed for flow cytometry and the percent apoptotic cells determined (F). G. Chondrocytes transfected with the PTP1B expression plasmid and the PTP1BsiRNA as in (C & E) were assayed for viable adherent cell numbers at 24 hours posttransfection. The error bars in all graphs indicate the standard deviation and the statistical significance is indicated by an asterisk (*) (p<0.05). H-J. Chondrocytes were transfected with a control siRNA or a PTP1BsiRNA. H. The cells were left untreated or treated with TNFα (10 ng/ml) and Actinomcyin D (ActD, 0.2 ug/ml) for 24 hours and the percent apoptotic cells measured. I, J. The cells were either cultured in low vs high serum (0.5 % vs 10% FBS) (F) or were plated at subconfluence vs confluence (20% vs 100%) (G). Apoptosis was assessed at 24 hours. K. Chondrocytes were transfected with the PTPalpha, PTPgamma and PTP1B expression plasmids. Upper panel; immunoblot of extracts. Lower Panel; assessment of percent apoptosis. The error bars in all graphs indicate the standard deviation and the statistical significance is indicated by an asterisk (*) (p<0.025).
Since repression of PTP1B by SirT1 was associated with a decrease in apoptosis it would be expected that increased expression of PTP1B would lead to increased apoptosis. PTP1B was therefore overexpressed in chondrocytes (Figure 4C). When percent apoptosis was assayed (Figure 4D), it was clear that PTP1B was a potent pro-apoptotic protein, leading to a significant increase in chondrocyte cell death. This proapoptotic effect of PTP1B was dependent upon its enzyme activity since expression of an enzymatically inactive mutant PTP1B in chondrocytes (Figure 4C) had no effect on apoptosis (Figure 4D).
When IGFR was assessed in cells expressing PTP1B there was a clear down regulation in pIGFR(Tyr1135/1136) levels indicating that the phosphatase was effective in dephosphorylating the receptor. In addition, pAkt and pMDM2 levels were significantly decreased in cells expressing PTP1B (Figure 4C), indicating that tyrosine dephosphorylation markedly impaired the activation of Akt and MDM2. As controls the inactive PTP1B mutant had no effect on the levels of pIGFR, pAkt or pMDM2 (Figure 4C).
To further assess the role of PTP1B in the IGFR pathway, cells were transfected with a PTP1B siRNA. From Figure 4E, it was clear that PTP1B levels dropped significantly in cells expressing PTP1B siRNA. Correspondingly, phosphorylation of the IGFR and Akt increased significantly in cells transfected with the PTP1B siRNA (Figure 4E). Measurement of the percent apoptotic cells and total viable cell number following PTP1B siRNA transfection indicated that cell death dramatically declined while viable cell numbers increased when PTP1B levels were reduced (Figures 4F & G). Additionally, survival was assessed after PTP1B siRNA transfection and following induction by TNFα/ActD (Figure 4H) treatment or culture in low serum (0.5%) (Figure 4I) and at low confluence (20%) (Figure 4J). The data show a reduction in apoptosis under all conditions following PTP1BsiRNA transfection. These data indicate that PTP1B is a potent negative regulator of chondrocyte survival.
As controls we also determined whether other PTP's were able to induce apoptosis. As shown in Figure 4K, PTPalpha and PTPgamma were efficiently overexpressed (upper panel) in chondrocytes, however only PTPalpha was able to induce apoptosis. This data indicates that only some PTPs induce chondrocyte apoptosis and that of those tested, only PTP1B is regulated by SirT1.
SirT1 and PTP1B show inverse expression patterns in OA and normal cartilage samples
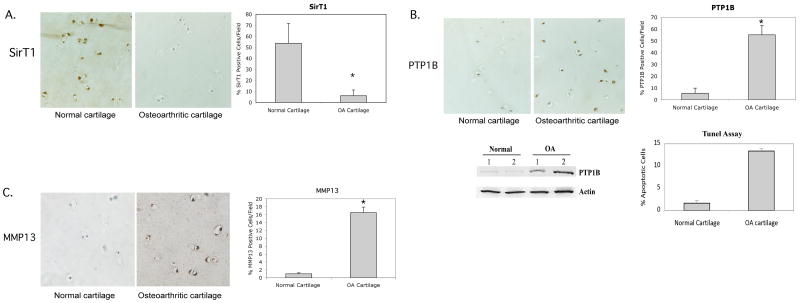
Recent data indicates that SirT1 protein levels are downregulated in the chondrocytes from OA knee cartilage compared to normal knee cartilage as assessed by immunoblot (ref 9, Supplemental Data). Assessment of cartilage sections by immunohistochemistry confirmed this finding. The staining intensity and number of stained cells of SirT1 is reduced in the OA cartilage sections compared to similar sections from normal cartilage (Figure 5A (left panel). The percent of SirT1-stained cells was quantitated in Figure 5A (right panel), and shows a significant decrease in the percent of SirT1-postive cells in the OA cartilage sections.
Figure 5. SirT1 levels are reduced in OA cartilage where PTP1B levels are elevated.
Normal and OA human cartilage samples were processed for immunohistochemistry for either SirT1 (A), PTP1B (B) or MMP13 (C) (left panels). The percent positive stained cells per field was determined and is shown in the graphs (right panels). Stained cells are reflective of greater than 10-fold intensity above the background (as determined by scanning densitometry). An average of 10 fields from three sections of six separate OA and Normal cartilage samples were assessed. Each field was blindly read by two different individuals. The images in A, B and C represent the intermediate layer of cartilage. B. Immunoblot of extracts from freshly isolated chondrocyte extracts from two normal and two OA cartilage samples probed with the indicated antibodies. Percent apoptotic cells in the OA and Normal cartilage samples were assessed by TUNEL assay (B). The error bars in all graphs indicate the standard deviation and the (*) indicates a statistically significant difference (p<0.05).
Since SirT1 is a repressor of PTP1B, a decrease in SirT1 levels in OA cartilage should lead to an upregulation of PTP1B. This appears to be the case (Figure 5B (left panel). The staining intensity and number of stained PTP1B-positive cells was significantly elevated in the OA cartilage samples compared to normal cartilage. This elevation in PTP1B levels was confirmed by the western blots in Figure 5B, demonstrating elevated PTP1B protein in freshly isolated chondrocyte extracts from OA patients. The percent of PTP1B-stained cells in the cartilage sections was quantitated in Figure 5B (right panel) and revealed a significant elevation in the percent of SirT1-postive cells in the OA cartilage sections. When percent apoptotic cells were assessed in these sections, a relationship was evident between PTP1B levels and percent apoptosis (Figure 1B). As a control, assessment of MMP13 showed significant expression within the extracellular matrix in the OA samples (Figure 5C, left & right panels), while very little is detected in the sections from normal cartilage samples, which consistent with its reported expression pattern in OA cartilage (36,37). Together these data indicate that when OA and normal knee articular cartilage was compared, there exists an inverse relationship in expression pattern between SirT1 and PTP1B; SirT1 levels are high in normal cartilage where PTP1B levels are lower, while the reverse appears to be the case in OA cartilage where SirT1 levels are low and PTP1B levels are high.
Discussion
It has been previously demonstrated that SirT1 blocks cell death in different cell systems and that it does so through distinct mechanisms (12,14-20). In light of these findings, it was hypothesized that SirT1 would play a positive role in human chondrocyte survival. When apoptosis was assayed in primary human chondrocytes overexpressing SirT1 (either transiently or by retroviral expression) it was clear that SirT1 reduced both the background level of cell death and the apoptosis mediated by TNFα/ActD (Figure 1). In contrast, reduction of endogenous SirT1 levels by siRNA or inactivation of SirT1 by treatment of cells with nicotinamide led to an increase in apoptosis. Thus, SirT1 appears to be a modulator of human chondrocyte survival.
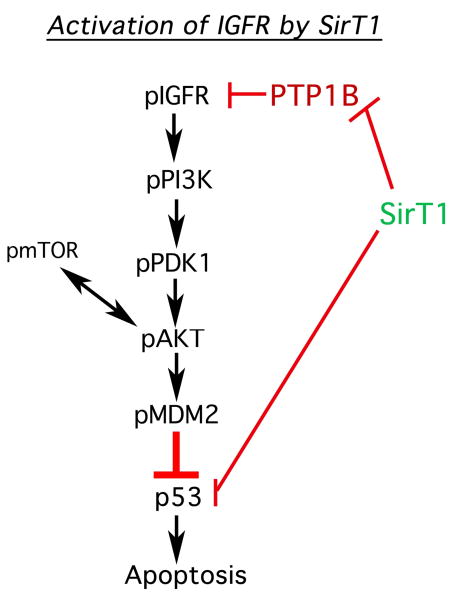
The mechanism by which SirT1 mediates chondrocyte survival appears to involve, at least in part, the activation of the IGF receptor (IGFR) pathway (outlined in Figure 6). Activation of the IGFR leads to activation via phosphorylation of a number of proteins known to participate in cell survival. These proteins include PI3Kinase, PDK1, mTOR, Akt and MDM2 (Figure 6). Phosphorylation of MDM2 culminates in the binding of pMDM2 to p53 and inhibition of p53 activity. Elevation of SirT1 therefore leads to activation of a well-defined prosurvival pathway. The data presented here are consistent with those of other groups showing that treating chondrocytes with resveratrol enhances chondrocyte survival (10,11). However it should be noted that while resveratrol is known to activate SirT1, it also has multiple additional protein targets within the cell so it cannot be concluded that its sole effect is on SirT1.
Figure 6. SirT1-mediated activation of the IGFR pathway and suppression of apoptosis in human chondrocytes.
The pathway outlines that the proteins are affected by increased expression of Sirt1 in chondrocytes. A reduction in PTP1B levels directly by SirT1 led to activation of the IGFR along with the attendant kinases PI3K, PDK1 and Akt. Activation of mTOR is thought to occur via receptor tyrosine kinases through unknown intermediates. pAkt also functions to activate mTOR by phosphorylation, leading to increased phosphorylation of pAkt (on residue Ser473). Sirt1 also functions to directly block p53 by deacetylation.
Of critical interest is the mechanism by which SirT1 activates the IGFR. This appears independent of any effect on IGF1 since its levels are not affected by SirT1 (data not shown). While IGF2 levels were increased by SirT1 this growth factor does not appear to affect chondrocyte survival in our system (data not shown). However, a powerful mediator of IGFR activity is PTP1B (protein tyrosine phosphatase 1b). This phosphatase can inactivate both the insulin and IGF receptor tyrosine kinases through tyrosine dephosphorylation (31,32). It has been recently demonstrated that SirT1 can elevate insulin sensitivity in mice via repression of PTP1B, thereby increasing the activity of the insulin receptor (33). In chondrocytes when SirT1 is overexpressed, we find significantly reduced levels of PTP1B at both the protein and RNA levels. Correspondingly, in cells overexpressing PTP1B, the levels of active/phosphorylated IGFR were dramatically down regulated. Additionally, when PTP1B levels were reduced by siRNA, pIGFR levels increased and the number of apoptotic cells declined. These data strongly point to PTP1B as an intermediate in SirT1-mediated chondrocyte survival. It would then be predicted that PTP1B would be pro-apoptotic when overexpressed in these chondrocytes and in fact was found to be a very potent inducer of apoptosis. This is the first demonstration that PTP1B is a pro-apoptotic protein for human chondrocytes.
Recent data has indicated that SirT1 levels are reduced in the knee articular cartilage of OA patients relative to normal cartilage (9). A reduction in SirT1 levels in OA cartilage appears to coincide with a derepression of PTP1B (Figure 5) resulting in an inverse relationship between SirT1 and PTP1B levels in normal and OA cartilage samples. Given that chondrocyte death is elevated in OA cartilage and that PTP1B is a potent inducer of chondrocyte death, the data presented here would suggest that the elevation of PTP1B in OA in part plays an important role in chondrocyte death and could be a contributing factor in the pathology of this disease. In addition, since IGF signaling can affect expression of both cartilage ECM proteins and matrix degrading enzymes (24, 35) it is possible that PTP1B plays additional roles in regulating these other important aspects of chondrocyte biology. SirT1 could aid in maintaining chondrocyte phenotype by directly regulating such factors as Sox9 (9) and by modulating growth factor receptor activity through PTP1B.
In conclusion, the studies here show a link between SirT1 and chondrocyte survival. PTP1B appears to be an intermediate in SirT1-mediated survival via regulation of the IGFR pathway. Finally, the inverse relationship between SirT1 and PTP1B levels in the samples of OA and normal articular cartilage suggest that PTP1B is a critical SirT1 target gene, playing a role in OA pathology.
Acknowledgments
We thank NDRI, Philadelphia, PA, for providing the human cartilage tissue samples. We would like to thank Dr. James Simone (NIAMS) for assistance with flow cytometry. This work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Center for Complementary and Alternative Medicine (NCCAM) within the National Institutes of Health (NIH), Bethesda, MD.
Contributor Information
Viktoria Gagarina, Cartilage Molecular Genetics Group, Cartilage Biology and Orthopedics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
Odile Gabay, Cartilage Molecular Genetics Group, Cartilage Biology and Orthopedics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
Mona Dvir-Ginzberg, Cartilage Molecular Genetics Group, Cartilage Biology and Orthopedics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
Eun-Jin Lee, Cartilage Molecular Genetics Group, Cartilage Biology and Orthopedics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
Jillian K. Brady, Cartilage Molecular Genetics Group, Cartilage Biology and Orthopedics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
Michael J. Quon, Diabetes Unit, Laboratory of Clinical Investigation, National Center for Complementary and Alternative Medicine, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
David J. Hall, Cartilage Molecular Genetics Group, Cartilage Biology and Orthopedics Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, 50 South Drive, Rm 1524, Bethesda, MD 20892.
References
- 1.Kuhn K, D'Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarth & Cart. 2004;12:1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27:455–62. [PubMed] [Google Scholar]
- 3.Heraud F, Heraud A, Harmand MF. Apoptosis in normal and osteoarthritic human articular cartilage. Ann Rheum Dis. 2000;59:959–65. doi: 10.1136/ard.59.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yatsugi N, Tsukazaki T, Osaki M, Koji T, Tamashita S, Shindo H. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. J Orthop Sci. 2000;5:150–6. doi: 10.1007/s007760050142. [DOI] [PubMed] [Google Scholar]
- 5.Kouri JB, Aguilera JB, Reyes J, Lozoya KA, Gonzalez S. Apoptotic chondrocytes from osteo-arthritic human articular cartilage and abnormal calcification of subchondroal bone. J Rheumatol. 2000;27:1005–9. [PubMed] [Google Scholar]
- 6.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondroyctes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–9. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Blander G, Guarente L. The sir2 family of protein deacetylases. Ann Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 8.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Ann Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 9.Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribisyltransferase. J Biol Chem. 2008;283:36300–10. doi: 10.1074/jbc.M803196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arth & Rheum. 2008;58:2786–97. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 11.Csaki C, Keshishzadeh N, Fischer K, Shakibaei M. Regulation of inflammation signaling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol. 2008;75:677–87. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Nikolaev AY, Imai SI, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 13.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 15.Dai JM, Wang ZY, Sun DC, Wang SQ. Sirt1 interacts with p73 and suppresses p73-dependent transcriptional activity. J Cell Phys. 2007;210:161–6. doi: 10.1002/jcp.20831. [DOI] [PubMed] [Google Scholar]
- 16.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 17.Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004;101(27):10042–7. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 19.van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–9. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 20.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 21.Gagarina V, Carlberg AL, Pereira-Mouries L, Hall DJ. Cartilage oligomeric matrix protein protects cells against death by elevating members of the IAP family of survival proteins. J Biol Chem. 2008;283:648–59. doi: 10.1074/jbc.M704035200. [DOI] [PubMed] [Google Scholar]
- 22.Derfoul A, Miyoshi AD, Freeman DE, Tuan RS. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007;15:646–55. doi: 10.1016/j.joca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Kurmasheva RT, Houghton PJ. IGF1 mediated survival pathways in normal and malignant cells. Biochem Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. J Biol Chem. 2003;278:36563–71. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-1/IRS-2/RAS/ERK1/2 signaling and protects neurons. Cell. 2008:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemieux ME, Yang X, Jardine K, He X, Jacobsen KX, Staines WA, Harper ME, McBurney MW. The SirT1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals. Mech of Ageing Develop. 2005;126:1097–1105. doi: 10.1016/j.mad.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;29:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. Her-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–81. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 29.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances MDM2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–50. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 30.Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol. 2006;26:8840–56. doi: 10.1128/MCB.01846-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley DA, Cheng A, Kiely PA, Tremblay ML, O'Connor R. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22:1998–2010. doi: 10.1128/MCB.22.7.1998-2010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenner KA, Anyanwu E, Olefsky J, Kusari J. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J Biol Chem. 1998;271:19810–16. doi: 10.1074/jbc.271.33.19810. [DOI] [PubMed] [Google Scholar]
- 33.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SirT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Mol Cell. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Trippel SB. Growth factor actions on articular cartilage. J Rheumatol Suppl. 1995;43:129–32. [PubMed] [Google Scholar]
- 35.Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD, Tang D, Sheu TJ, Li TF, Shi Q, Wang YJ. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarth Cart. 2009;17:100–106. doi: 10.1016/j.joca.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15:805–829. doi: 10.1053/berh.2001.0195. [DOI] [PubMed] [Google Scholar]
- 37.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Frontiers In Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Wertheimer SJ, Lin CH, Katz SL, Amrein KE, Burn P, Quon MJ. Protein-tyrosine phosphatases PTP1B and Syp are modulators of insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. J Biol Chem. 1997;272:8026–8031. doi: 10.1074/jbc.272.12.8026. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Cong LN, Li Y, Yao ZJ, Wu L, Zhang ZY, Burke TR, Quon MJ. A phosphotyrosyl mimetic peptide reverses impairment of insulin-stimulated translocation of GLUT4 caused by overexpression of PTP1B in rat adipose cells. Biochemistry. 1999;38:384–389. doi: 10.1021/bi9816103. [DOI] [PubMed] [Google Scholar]
- 40.Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27:2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- 41.Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol. 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 42.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Chong ZZ, Li F, Maiese K. Activating AKT and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]