Abstract
Insufficient availability of n-3 polyunsaturated fatty acids (PUFA) during pre- and neonatal development decreases accretion of docosahexaenoic acid (DHA, 22:6n-3) in the developing brain. Low tissue levels of DHA are associated with neurodevelopmental disorders including attention deficit hyperactivity disorder (ADHD). In this study, 1st-and 2nd-litter male Long-Evans rats were raised from conception on a Control diet containing α-linolenic acid (4.20 g/kg diet), the dietarily essential fatty acid precursor of DHA, or a diet Deficient in α-linolenic acid (0.38 g/kg diet). The Deficient diet resulted in a decrease in brain phospholipid DHA of 48% in 1st-litter pups and 65% in 2nd-litter pups. Activity, habituation, and response to spatial change in a familiar environment were assessed in a single-session behavioral paradigm at postnatal days 28 and 70, inclusive. Activity and habituation varied by age with younger rats exhibiting higher activity, less habituation, and less stimulation of activity induced by spatial novelty. During the first and second exposures to the test chamber, 2nd-litter Deficient pups exhibited higher levels of activity than Control rats or 1st-litter Deficient pups and less habituation during the first exposure, but were not more active after introduction of a novel spatial stimulus. The higher level of activity in a familiar environment, but not after introduction of a novel stimulus is consistent with clinical observations in ADHD. The observation of this effect only in 2nd-litter rats fed the Deficient diet suggests that brain DHA content, rather than dietary n-3 PUFA content, likely underlies these effects.
Keywords: polyunsaturated fatty acid, omega-3, docosahexaenoic acid, rat, brain, locomotor activity, novelty, habituation, force-plate actometer
1. Introduction
Neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) involve alterations in attention, response to novelty, habituation, and other processes. Such disorders typically have onset during childhood. Of note, ADHD is reported to affect as many as 5% of school-aged children [1]. Accordingly, it is desirable to study mechanisms underlying activity and attention-related processes in animal models at various points in development, particularly at ages corresponding to childhood in humans. Rodents are typically used in these studies; however, the rate of maturation in rodents is sufficiently rapid that the training required for many of the well established tests of attentional function, such as the sustained attention task [2, 3], which is analogous to the continuous performance task used in humans [4, 5], can span several critical developmental periods in rodents (e.g., pre-adolescence, peri-adolescence, adolescence, etc.), potentially confounding the interpretation of the results. Overt symptoms of ADHD include restlessness, fidgeting, and generally unnecessary gross body movements [6, 7] which are more pronounced in familiar environments than in novel environments [8, 9]. Previously, we developed a behavioral paradigm for rats that enabled inferences about habituation and attention to a novel stimulus in a familiar environment implemented in a single test session without prior training of the animals [10]. In this study, this paradigm was used to assess the behavior of rats at various points in development from peri-adolescence to young adulthood, demonstrating its utility for studying the behavior of developing rats.
In addition, this study assessed the effects of low dietary and brain n-3 (omega-3) polyunsaturated fatty acid (PUFA) content during development. Epidemiologic and clinical findings indicate that low dietary and tissue levels of n-3 PUFAs are associated with ADHD and may thus contribute to its etiology. Of note, patients with ADHD exhibited lower plasma or erythrocyte levels of docosahexaenoic acid (DHA; 22:6n-3) and other PUFAs than normal controls [11–15], and lower n-3 PUFA status correlated with higher scores of hyperactivity [15]. In addition, children with ADHD had higher levels of ethane in exhalant than normal controls suggesting that the rate of oxidative metabolism of n-3 PUFAs may be higher in these individuals and that these individuals may consequently have a higher dietary requirement for these fatty acids [16]. ADHD is also associated with a polymorphism in a fatty acid desaturase (FADS2), further supporting a role for altered fatty acid metabolism in affected individuals [17]. Clinical and preclinical studies suggest that the prenatal and early postnatal periods represent critical intervals when inadequate accumulation of n-3 PUFAs may be particularly detrimental (for review see: [18]). Clinical trials with DHA and other n-3 PUFA supplements, however, have yielded mixed results (for review see: [19–21]). To further assess the potential role of n-3 PUFAs in ADHD, the effects of diet-induced manipulation of the percentage of DHA in brain phospholipids on ADHD-related behaviors were also determined in rats across post-weaning development.
2. Methods
All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Kansas Medical Center Animal Care and Use Committee.
2.1. Animals and husbandry
Male, Long-Evans rats were raised from conception on diets containing (Control) or deficient in α-linolenic acid (18:3n-3), the precursor of DHA (22:6n-3). Each rat in a group was from a different litter. Sample sizes for ages 28, 35, 42, 49, 56, and 70 days, respectively, were 1st-litter Control: 13, 9, 9, 16, 17, and 14; 2nd litter Control: 11, 13, 14, 11, 15 and 11; 1st-litter Deficient: 15, 17, 19, 20, 14, and 19; 2nd-litter Deficient: 12, 9, 9, 9, 13, and 12. Rats were housed in a temperature- and humidity-controlled facility with a 12-hour light-dark cycle and given food and water ad libitum. Breeding stock (females 80–89 days; male proven breeders; Harlan, Indianapolis, IN) were obtained 5 days prior to the beginning of the treatment and were handled regularly. During the acclimation period, rats were fed Teklad laboratory Rodent Diet (W) #8604. Dams were individually housed and randomly placed on the experimental diets at the time of initial mating as previously described in detail [22]. Litters were culled to 8 on postnatal day 1 (P1) with preference for males. Pups were weaned on postnatal day 20, group-housed, and fed the maternal diet until the completion of the study. Dams were re-mated 7 days after weaning of the 1st-litter to produce 2nd-litter pups. Rats were weighed regularly and were accustomed to handling prior to the behavioral testing.
2.2. Experimental diets
The Control diet was prepared from a purified basal mix (TD00235; Teklad, Indianapolis, IN) with pure unhydrogenated soybean oil (70 g/kg diet), and was identical in composition to Teklad AIN-93G, which meets all current nutrient standards for rat pregnancy and growth [23]. Accordingly, the Control diet contained 4.20 g/kg diet α-linolenic acid (18:3n-3) and 33.81 g/kg diet linoleic acid (18:2n-6). The Deficient diet was the same as the Control diet, except it was prepared with safflower oil (66.5 g/kg diet) and soybean oil (3.5 g/kg diet), and thus contained 0.38 g/kg diet α-linolenic acid (18:3n-3) and 45.96 g/kg diet linoleic acid (18:2n-6).
2.3. Apparatus
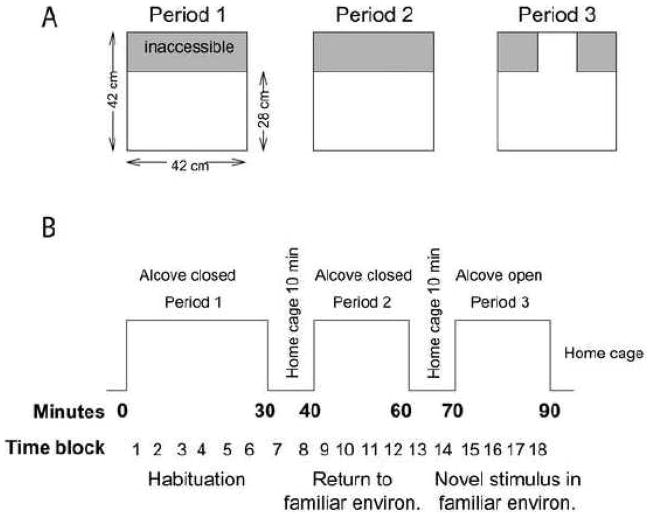
Modified force-plate actometers [24] were used to assess response to environmental spatial change. The modified plexiglass actometer chamber consisted of a “main” compartment (28 × 42 cm) and a small “alcove” (14 × 14 cm) separated from the main compartment area by a removable guillotine-type door (Fig. 1). Spatial resolution of the actometer was less than 2 mm and temporal resolution was 0.02 sec.
Fig. 1. Schematic of the experimental apparatus (A) and test protocol (B).
2.4. Behavioral Procedure
All testing occurred between 10:00 h and 13:00 h and was conducted under dim, indirect lighting conditions. Rats were tested on P28, 35, 42, 49, 56, and 70, with different rats used at each time point. On the test day, rats were transported in their home cages to the testing room and weighed 1 hr prior to testing. Activity and response to spatial change in the test environment were measured during a 3-stage, 90-min session in modified force-plate actometer chambers (Fig. 1) as previously described [10]. Initially, each rat was placed in the main compartment (Period 1 - Habituation) with the door to the alcove closed. After 30 min, the rat was removed from the main compartment, placed in the home cage for 10 min, and then returned to the unchanged main compartment (Period 2 – Return to a familiar environment) for 20 min. After Period 2, the rat was again removed from the main compartment to the home cage for 10 min, and then returned to the main compartment with the door to the alcove open (Period 3 - Novel stimulus in familiar environment) for 20 min. Movements of the rat in the main compartment and alcove were automatically quantified by the x-y coordinates of the center of force derived from the force plate software [24]. At the end of the test session, rats were euthanized by decapitation. Brains were rapidly removed, frozen on dry ice, and stored at −80° C.
2.5. Brain total phospholipid fatty acid composition
Whole brain total phospholipid fatty acid composition was determined as previously described [25] in one hemisphere from 5–6 brains randomly selected from each group. Phospholipids were isolated by thin layer chromatography. The band containing total phospholipids was removed and transmethylated with boron trifluoride methanol (Sigma, St. Louis, MO) to yield fatty acid methyl esters. Individual fatty acid methyl esters were separated on a Varian 3400 gas chromatograph with an SP-2330 capillary column (30 m, Supelco, Inc., Belfonte, PA), with helium used as the carrier gas. The resulting peaks were identified by comparison to authentic standards (PUFA 1 and 2; Supelco, Inc. and 22:5n-6, Nu-Chek Prep, Elysian, MN) and expressed as percent of total fatty acids on the basis of peak area.
2.6. Data analysis
Data are presented as the group means ± SEM. The dependent variables for the test procedure were activity, assessed as distance traveled (m), and time spent in the alcove (expressed as the percentage of the observation period). Data were analyzed in 5-min time blocks. Between-time block comparisons of interest were within-period habituation, recovery of activity upon replacement in the test chamber in Period 2, and stimulation of activity by the novel spatial stimulus in Period 3. In order to control for differences in baseline activity [26], these between-time block parameters were calculated as the relative activity in the first and last time blocks of the relevant behavior period(s) for each rat. Accordingly, habituation for Period 1 was defined as ratio of the distances traveled in time blocks 6 and 1. Period 2 recovery was the ratio of distances traveled in time blocks 9 and 6. Period 2 habituation was the ratio of distances traveled in time blocks 12 and 9. Period 3 stimulation was the ratio of distances traveled in time blocks 15 and 12. Period 3 habituation was the ratio of distances traveled in time blocks 18 and 15.
Data were analyzed for statistically significant effects by various ANOVA procedures, followed by Tukey’s or Dunnett’s tests (SYSTAT v.12; Systat Software, Inc, Chicago, IL). Outliers in the biochemical data identified by Systat were removed. Significance was assumed at P<0.05. Behavioral data were initially analyzed for all four experimental groups (1st-litter Control, 2nd-litter Control, 1st-litter Deficient, and 2nd-litter Deficient). Because behavior, as well as brain fatty acid composition and weight were highly similar for 1st-and 2nd-litter Control diet groups, these groups were combined into a single “Control” group (n = 24–33 at various ages) for the final analysis of the behavioral data.
3. Results
3.1. Effects of experimental treatments on brain fatty acid composition and growth
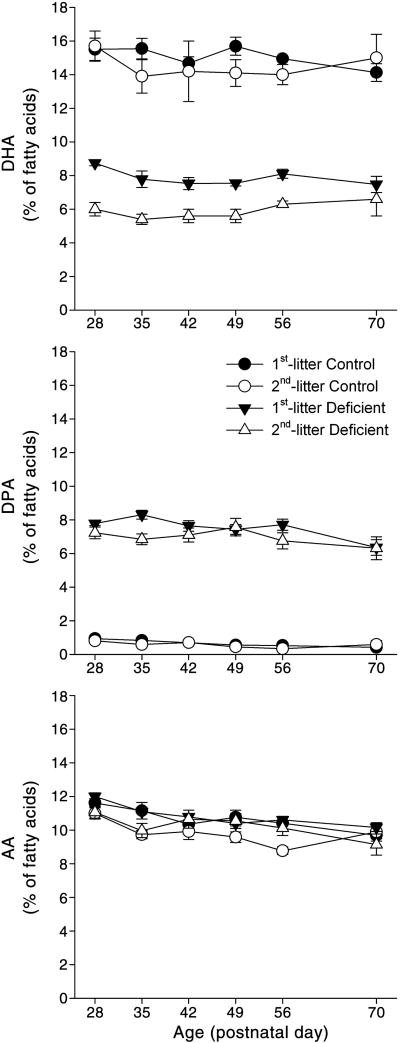
In agreement with previous findings [22], three-way ANOVA with factors of diet, litter, and age indicated significant main effects on brain DHA content of diet (F(1,103)=688.29, P<0.0001), litter (F(1,103)=18.63, P<0.0001), and an interaction of diet with litter (F(1,103)=4.92, P<0.05) (Fig. 2). Post hoc analysis of marginal means indicated that the percentage of DHA in brain phospholipids was not different between 1st- and 2nd-litter Control pups. Brain DHA of 1st-litter Deficient pups was 52% of 1st-litter Controls (P<0.001). Brain DHA of 2nd-litter Deficient pups was 35% of 2nd-litter Controls (P<0.001) and was significantly lower than in 1st-litter Deficient pups (P<0.001).
Fig. 2. Effects of the experimental treatments on brain phospholipid fatty acid composition.
Data for docosahexaenoic acid (DHA, 22:6n-3), docosapentaenoic acid (DPA, 22:5n-6), and arachidonic acid (AA, 20:4n-6) are presented as the group means ± SEM (n = 5–6 per group selected at random from the total sample). The percentages of DHA and DPA in brain phospholipids were not different between 1st- and 2nd-litter Control pups. For all ages, brain DHA of 1st- and 2nd-litter Deficient pups was decreased compared to 1st- and 2nd-litter Controls (P<0.001). Brain DHA of 2nd-litter Deficient pups was also lower than 1st-litter Deficient pups (P<0.001). Brain DPA of 1st- and 2nd-litter Deficient pups was higher than their respective control groups at all ages (P<0.001). There was a main effect of litter on brain AA such that 1st-litter pups had 8% higher AA than 2nd-litter pups (P<0.001), but there was no effect of diet or age.
In agreement with previous studies [27], the decrease in brain DHA in pups raised on the Deficient diet was accompanied by a compensatory increase in the percentage of docosapentaenoic acid (DPA, 22:5n-6), which exhibited significant main effects of diet (F(1,105)=1843.32, P<0.001), litter (F(1,105)=7.06, P<0.001), and age (F(5,105)=3.86, P<0.01), and interactions of diet with litter (F(1,105)=4.92, P<0.05) and diet with age (F(1,105)=2.76, P<0.05). Post hoc analysis of marginal means indicated that the percentage of DPA in brain phospholipids was not different between 1st-and 2nd-litter Control pups. Brain DPA of 1st- and 2nd-litter Deficient pups was 11-times that of their respective control groups (P<0.001), and was 10% higher in 1st-litter Deficient pups than 2nd-litter (P<0.01). The main effect of age on brain DPA was such that the percentage of this fatty acid was 22% lower at P70 than at P28 (P<0.01).
The percentage of arachidonic acid (AA) in brain phospholipids exhibited significant main effects of litter (F(1,105)=20.36, P<0.001) and age (F(5,105)=5.67, P<0.001). Brain AA was 8% higher in 1st-litter pups than in 2nd-litter pups (P<0.001), and decreased with age such that this fatty acid was 13% lower at P70 than at P28 (P<0.001).
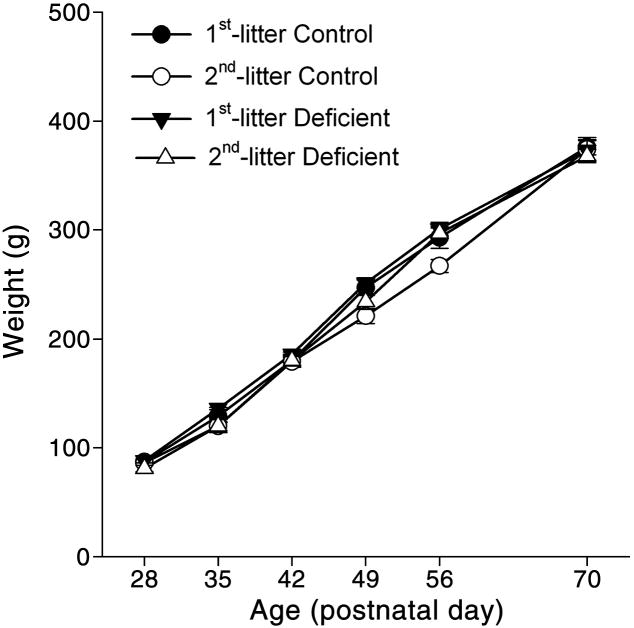
Body weight exhibited significant main effects of age (F(5,323=1124.66, P<0.0001) and litter (F(1,323=14.28, P<0.0001) by three-way ANOVA with factors of diet, litter, and age, such that 2nd-litter pups weighed 4% less than 1st-litter pups (P<0.001). There was no main effect of diet or any significant interaction of factors (Fig. 3).
Fig. 3. Effects of experimental treatments on body weight.
Data are presented as the group means ± SEM. Sample sizes for ages 28, 35, 42, 49, 56, and 70 days, respectively, were 1st-litter Control: 13, 9, 9, 16, 17, and 14; 2nd-litter Control: 11, 13, 14, 11, 15 and 11; 1st-litter Deficient: 15, 17, 19, 20, 14, and 19; 2nd-litter Deficient: 12, 9, 9, 9, 13, and 12. There was a significant effect of litter on weight, with 2nd-litter pups weighing 4% less than 1st-litter pups (P<0.001), but no main effect of treatment or interaction of litter and treatment.
3.2. Effects of age on behavior in rats raised on the Control diet
Analysis of the data indicated that behavior of 1st-and 2nd-litter Controls was highly similar. Because brain fatty acid composition and body weight were also highly similar in these groups, data from 1st-and 2nd-litter Controls were combined into a single Control group for all subsequent analyses.
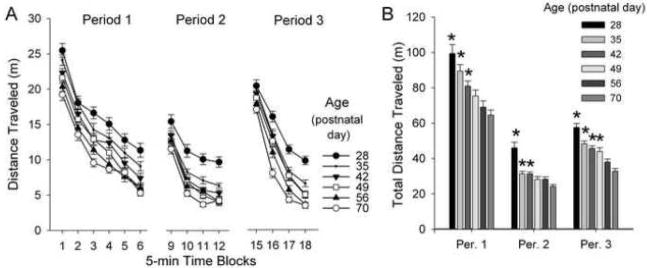
At all ages, Control rats exhibited habituation to the environment, recovery of activity when replaced in a familiar environment, and stimulation of activity by the novel spatial stimulus, in agreement with our previous study [10]. For distance traveled (Fig. 4A), repeated-measures ANOVA with factors of age and time block indicated significant main effects of age (F(5,334)=25.07, 40.828, and 39.231 for Periods 1–3, respectively; all P<0.01), time block (F(5,1720)=1457.56 for Period 1, F(3,1032)=2093.04 for Period 2, and F(3,1032)=749.93 for Period 3; all P<0.01) and interactions of age with time block (F(25,1720)=2.35 for Period 1, F(15,1032)=6.01 for Period 2, and F(15,1032)=3.67 for Period 3; all P<0.01). In all test periods, distance traveled decreased across time blocks. Distance traveled also decreased with increasing age in a graded manner. Accordingly, the total distance traveled for the entire test period was significantly greater (P<0.05) at P28, P35, and P42 than at P70 during Periods 1, 2, and 3; and also at P49 during Period 3 (Fig. 4B).
Fig. 4. Activity of Control rats in the test paradigm across postnatal development.
Activity was assessed on the basis of distance traveled. Data are presented across time blocks (A) and as the main effect for each test period (B). Data were collected in 5-min time blocks, were analyzed by repeated-measures ANOVA with factors of age and time block, and are presented as the group means ± SEM. Sample sizes for ages 28, 35, 42, 49, 56, and 70 days, respectively, were 24, 29, 33, 29, 32, and 31 reflecting the combined 1st and 2nd Control litters into a single Control group. In Panel A, there were significant main effects of age and time block, and interactions of age with time block during each of the test Periods (P<0.01). For clarity, differences between ages in each time block are not indicated. *P<0.05 v. P70 by ANOVA and Dunnett’s test.
Time spent in the alcove during Period 3 varied by time block by repeated-measures ANOVA (F(3,1032)=332.73, P<0.01), such that all rats spent increasingly more time in the alcove during Period 3, in agreement with previous findings [10]. However, there was no effect of age or interaction of age with time block (not shown).
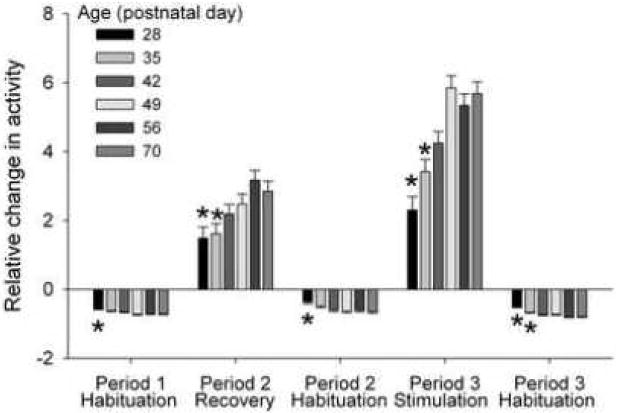
Habituation, recovery of activity in a familiar environment, and stimulation of activity induced by spatial change also varied by age (Fig. 5). Younger rats exhibited less habituation such that habituation was significantly less at P28 than at P70 during all three test periods and also at P35 during Period 3 (P<0.01). Younger rats also exhibited less recovery of behavior when replaced in the familiar environment in Period 2, which was significantly less at P28 and P35 than at P70 (P<0.05). The stimulation of activity induced by the introduction of a spatial change in Period 3 was also smaller in younger rats, and was significantly less at P28 and P35 than at P70 (P<0.01).
Fig. 5. Habituation, recovery of activity, and novelty-induced stimulation of activity across postnatal development in Control rats.
Data are presented as group means ± SEM for each of the three test Periods. Sample sizes for ages 28, 35, 42, 49, 56, and 70 days, respectively, were 24, 29, 33, 29, 32, and 31 reflecting the combined 1st and 2nd Control litters into a single Control group. Period 1 habituation was calculated as ratio of the distances traveled in time blocks 6 and 1. Period 2 recovery was the ratio of distances traveled in time blocks 9 and 6. Period 2 habituation was the ratio of distances traveled in time blocks 12 and 9. Period 3 stimulation was the ratio of distances traveled in time blocks 15 and 12. Period 3 habituation was the ratio of distances traveled in time blocks 18 and 15. *P<0.05 v. P70 by ANOVA and Dunnett’s test.
3.3. Effects of diet and litter on behavior across development
Distance traveled, habituation, recovery of activity in a familiar environment, stimulation of activity induced by spatial change, and alcove time, were analyzed for effects of treatment (i.e., Control, 1st-litter Deficient, and 2nd-litter Deficient) and interactions with age.
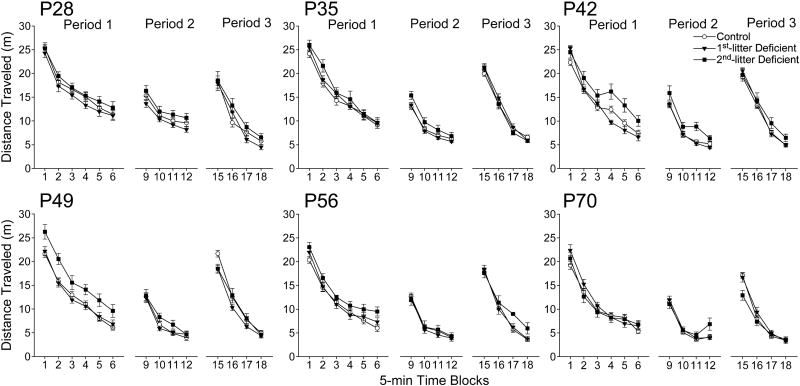
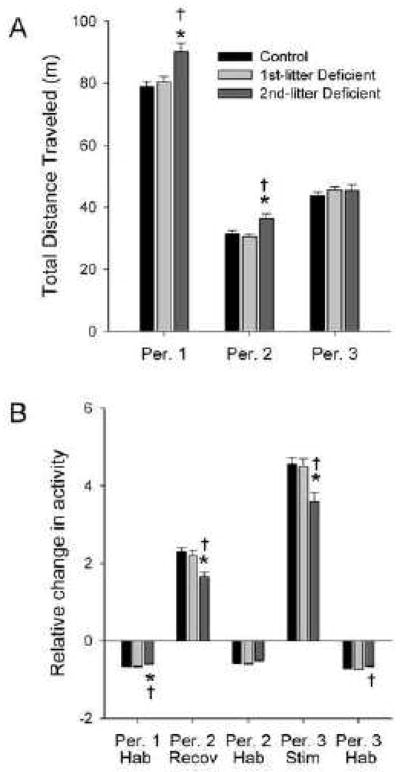
In Period 1, distance traveled exhibited significant main effects of age (F(5,332)=23.21, P<0.001), treatment (F(2,332)=10.21, P<0.001), and time block (F(5,1660)=1278.28, P<0.001), and interactions of age with time block (F(25,1660)=2.68, P<0.001), and treatment with time block (F(10,1660)=3.43, P<0.001) by repeated-measures ANOVA with factors of age, treatment, and time block (Fig. 6). The interaction of age, treatment, and time block was not quite significant (F(50,1660)=1.35, P=0.052). Post-hoc analysis of the marginal means indicated that 2nd-litter Deficient rats were 14% more active than Controls (P<0.01), and 12% more active than 1st-litter Deficient rats (P<0.01) overall. Although the interaction of age, treatment, and time block was not quite significant (P=0.052), this effect appeared to be most pronounced at P42 and P49. Habituation in Period 1 exhibited significant main effects of age (F(5,332)=6.78, P<0.001) and treatment (F(2,332)=4.09, P<0.05) by two-way ANOVA with factors of age and treatment. Post hoc analysis indicated that 2nd-litter Deficient rats exhibited less habituation during Period 1 than Controls or 1st-litter Deficient rats (P<0.05) (Fig. 7).
Fig. 6. Effects of dietary n-3 fatty acid content and litter on activity in the test paradigm across development.
Activity was assessed on the basis of distance traveled. Data were collected in 5-min time blocks, were analyzed by repeated-measures ANOVA with factors of treatment, age and time block, and are presented as group means ± SEM. Sample sizes for ages 28, 35, 42, 49, 56, and 70 days, respectively, for Control rats were 24, 29, 33, 29, 32, and 31 reflecting the combined 1st and 2nd Control litters. Sample sizes for 1st litter Deficient rats were 15, 17, 20, 19, 16, and 19; and 2nd litter Deficient rats were 12, 12, 10, 9, 13, and 12. There was no significant interaction of treatment and age. The main effect of treatment on distance traveled is presented in Fig. 7.
Fig. 7. Main effects of dietary n-3 fatty acid content and litter on total distance traveled (A) and habituation, recovery of activity, and novelty-induced stimulation of activity (B).
Data are presented as group means ± SEM for each of the three test Periods. Sample sizes were 178 for Control, 106 for 1st-litter Deficient, and 68 for 2nd-litter Deficient. Habituation, recovery, and stimulation were calculated as described for Fig. 5. *P<0.05 v. Control, †P<0.05 v. 1st-litter Deficient by ANOVA and Tukey’s test.
In Period 2, distance traveled exhibited significant main effects of age (F(5,332)=36.61, P<0.001), treatment (F(2,332)=9.35, P<0.001), and time block (F(3,996)=619.73, P<0.001), and an interaction of age with time block (F(15,996)=3.60, P<0.001) by repeated-measures ANOVA with factors of age, treatment, and time block (Fig. 6). Post-hoc analysis of the marginal means indicated that 2nd-litter Deficient rats were 15% and 18% more active than Controls and 1st-litter Deficient rats, respectively (P<0.01). Period 2 recovery of activity exhibited significant main effects of age (F(5,330)=4.68, P<0.01) and treatment (F(2,330)=4.33, P<0.01) by two-way ANOVA with factors of age and treatment. Post hoc analysis indicated that 2nd-litter Deficient rats exhibited less recovery of activity in Period 2 than Controls or 1st-litter Deficient rats (P<0.05) (Fig. 7) Habituation in Period 2 exhibited only a main effect of age (F(2,330)=7.685, P<0.001).
In Period 3, distance traveled exhibited significant main effects of age (F(5,332)=37.39), P<0.001) and time block (F(3,996)=1773.65, P<0.001), and interactions of age with time block (F(15,996)=5.83, P<0.001) and treatment with time block (F(6,996)=3.10, P<0.01) by repeated-measures ANOVA with factors of age, treatment, and time block (Fig. 6). However, post hoc analysis of the marginal means indicated that distance traveled by the 1st- or 2nd-litter Deficient groups was not different from Controls during any time block. Stimulation of activity by the alcove in Period 3 exhibited significant main effects of age (F(5,331)=18.36, P<0.001) and treatment (F(2,331)=5.83, P<0.01) (Fig. 7), as well as an interaction of age with treatment (F(10,331)=1.91, P<0.05). Post hoc analysis indicated that 2nd-litter Deficient rats exhibited less stimulation of activity in Period 3 than Controls or 1st-litter Deficient rats (P<0.05) overall, and that this effect was most pronounced at P42 and P70 (not shown). Habituation in Period 3 also exhibited significant main effects of age (F(5,331)=24.14, P<0.001) and treatment (F(2,331)=5.03, P<0.01), but was different only between 2nd-litter and 1st-litter Deficient rats (P<0.01). Alcove time indicated a significant effect only of time block (F(3,996)=256.59, P<0.001) (not shown).
4. Discussion
The behavioral paradigm used in this study assessed exploratory behavior, habituation, and response to a spatial change in a familiar environment in a single test session and required no prior training of the animals. Thus, the procedure enabled the evaluation of behavior at discrete points during development. The behavioral changes occurring during habituation of the exploratory response reflect an elementary form of behavioral plasticity involving learning, memory, arousal level, and attention [26, 28], processes that are highly relevant to developmental disorders such as ADHD. This test assessed within-period “habituation”, which involves gaining familiarity with a novel environment; adaptation in the form of between-period habituation, which involves memory of the prior period [26]; and response to spatial change. As in previous studies [29–31], the present data showed that activity of rats in this paradigm decreased with age, and older rats exhibited more habituation during each test period, and exhibited greater novelty-induced stimulation of activity. Mature patterns of behavior (i.e., not different from behavior at P70) began to emerge by P42 (Figs. 4 and 5). While this change in behavior likely reflects neurodevelopmental maturation of the animals, it must be noted that the size of the rats also increased as they matured. This raises the possibility that the changing size relationship of the rat and the apparatus may contribute to the changes in activity. However, the rats’ weight increased linearly throughout the study whereas activity plateaued around P56-P70, indicating that weight was not the critical factor. Even so, the length and volume of the rats were not measured; thus, it is not possible to eliminate the potential contribution change in size may have made to the changes in activity. Nevertheless, the present characterization of rats’ behavior with this procedure enables the assessment of the effects of other manipulations on those parameters in the developing rat.
Several lines of evidence suggest that low dietary and/or tissue levels of DHA and other n-3 PUFAs are associated with ADHD [11–21]. The present findings indicate that 2nd-litter rats raised on the n-3 PUFA-deficient diet (65% decrease in brain DHA relative to Controls), exhibited higher levels of activity during Periods 1 and 2 in the test chamber, less habituation during Periods 1 and 3, less recovery of activity when replaced in the now-familiar chamber in Period 2, and less stimulation of activity induced by the novel spatial stimulus in Period 3 regardless of age (Figs. 6 and 7). Although the 2nd-litter rats fed either diet were slightly smaller than the 1st-litter, there was no main effect of diet on weight gain across development. Furthermore, at P56 when a difference in weights was apparent between treatment groups (P<0.05 by ANOVA and Tukey’s test), the heavier 2nd-litter Deficient animals were more active than the lighter Control rats, contrary to what would be predicted from the developmental profile of rats in this test paradigm (Fig. 4). Thus, the differences in behavior at P56 cannot be attributed to differences in weight. Accordingly, this pattern of behavior suggests that rats with substantially decreased brain DHA exhibit a more immature pattern of behavior. Furthermore, that 2nd-litter Deficient rats exhibited less habituation during Period 1, and were more active when replaced in the now-familiar environment in Period 2 but not after the introduction of a novel spatial stimulus (which produced a powerful stimulatory effect) in Period 3, is consistent with clinical observations in ADHD [8, 9]. The higher levels of activity of the 2nd-litter Deficient rats was most notable at P42 and P49, suggesting that the effects of decreased brain DHA content may be most pronounced in the periadolescent period. However, as this study did not examine rats older than P70, future studies must determine whether higher levels of activity persists throughout out life or represents a delay in maturation.
Altered motor function in animals with inadequate accumulation of brain DHA during development has been reported in several previous studies, though the specific effects varied. We have previously shown increased locomotor activity in rats raised on an n-3 PUFA deficient diet; however, this effect was observed in adult [32, 33], and not in immature rats [33]. Additionally, the rats in those studies had a smaller decrease in brain DHA than those studied here (−20% and −33%). This discrepancy may also have been due to differences in the methods and apparatus used (manually-counted line crosses or photocell cages v. the force-plate actometer) and the length of the observation period (20–30 min. v. 2 hrs). In other studies, the DHA content of rats’ frontal cortex was inversely related to levels of nocturnal locomotor activity, though these findings differed from the present results in that rats with low DHA exhibited increased locomotor response to novelty [34]. On the other hand, adult rats with a 70% decrease in brain DHA demonstrated less exploratory behavior in a novel environment [35]. Finally, rhesus monkeys with long-term deficiency of n-3 PUFAs exhibited more stereotyped behavior and locomotor activity than control animals [36]. Taken together, these studies support a role inadequate DHA accretion in the modulation of systems affecting motor function, though the relationship between DHA level and specific behavioral effects appears complex.
The underlying pathology of ADHD involves dysregulation of the CNS dopamine systems, most notably frontolimbic dysfunction [37]. Dopamine also plays a role in the neurochemistry underlying the behavioral processes studied here [26]. A number of alterations in the CNS dopamine systems have been noted in adult rats with decreased brain DHA content from conception. Rats with multi-generational n-3 PUFA deficiency, which produces about a 75% decrease in brain DHA, exhibited decreases in the densities of dopamine-immunoreactive vesicles, D2 receptor mRNA, and the vesicular monoamine transporter (VMAT2) in the frontal cortex [38–42]. The density of dopamine vesicular transporter (VMAT2) and amphetamine-stimulated dopamine release also were decreased in the nucleus accumbens; however, basal dopamine release and the density of D2 dopamine receptors were increased in this brain region [42, 43]. Increased expression of D1 and D2 dopamine receptors in cortex, limbic regions, and striatum; decreased expression of tyrosine hydroxylase in the substantia nigra and ventral tegmental area; and decreased expression of the monoamine vesicular transporter (VMAT2) in striatum and hippocampus have also been reported in rats raised on an n-3 PUFA-deficient diet that contained even less α-linoleic acid than the diet used in this study [44]. Rats prepared identically to the 2nd-litter Deficient pups used in this study had one-third fewer dopamine neurons in both the substantia nigra pars compact and the ventral tegmental area compared to controls [45]; however, no differences in the densities or affinities of D1 or D2 dopamine receptors and the dopamine transporter in the striatum, nucleus accumbens, or striatal and nigral dopamine content or turnover were detected between 1st- or 2nd-litter Deficient rats and Controls that could explain differences in behavior observed in this study (unpublished observation). Accordingly, the observed alterations in behavior may be the result of changes in other dopaminergic parameters that remain to be determined. Furthermore, in addition to affecting the dopamine systems, inadequate accretion of DHA affects visual function at both the retinal and cortical levels [46]. The decreases in tissue DHA accretion in our animals would thus be anticipated to result in poorer than normal vision. However, normal vision in rats is notably poor even with adequate dietary n-3 PUFAs. Under the low light testing conditions used in this study, exploratory behaviors in rats rely heavily on olfactory and tactile function, rather than vision [47]. Even so, effects on the visual system may also contribute to the altered behavior observed in these studies.
That rats raised on the α-linolenic acid-deficient diet, but with differing brain DHA contents should exhibit differing behavioral profiles suggests that brain phospholipid fatty acid composition, rather than dietary α-linoleic acid content, may be the critical factor underlying the behavioral alterations. Most of the DHA in brain accumulates during later prenatal and early neonatal life [48]. Although litter order does not affect offspring brain fatty acid composition if the dam is fed a diet containing adequate α-linoleic acid, the essential fatty acid precursor of DHA, litter order does affect brain DHA content in rat pups raised from conception on diets containing inadequate n-3 PUFAs [22]. As such, 2nd-litter pups exhibit a greater decrease in brain DHA than 1st-litter pups raised on the identical n-3 PUFA-deficient diet, most likely due to the depletion of maternal DHA stores during gestation and nursing of the first litter [49, 50]. The behavioral alterations observed in this study were observed in 2nd-litter Deficient pups, but not in 1st-litter Deficient pups, which were raised in the same diet but have a smaller decrease in brain DHA. This suggests that the resulting brain fatty acid composition, rather than diet n-3 PUFA content, is the critical factor in producing these effect. However, consumption of an n-3 PUFA-deficient diet during pregnancy and lactation is known to also affect maternal brain fatty acid composition and neurochemistry [51, 52]. Despite the extensive literature on the effects of dietary PUFA manipulations in pregnant and nursing dams, there are no reports on the effects of n-3 PUFA-deficient diets on specific maternal behaviors, such as time spent in various nursing postures. Although attempts have been made to differentiate between the contributions of maternal behavior from the effects of variation in brain fatty acid composition using methods such as artificial rearing [53], such methods introduce additional potential confounds in the offspring. Furthermore, because fetal and neonatal brain DHA accumulation is dependent on maternal nutritional and reproductive status, cross fostering cannot be used to control for maternal variables. Thus, while it would be desirable to definitively rule out maternal factors in the behavioral effects presented here, there are currently no satisfactory methods for doing so. Nevertheless, dams fed the Deficient diet exhibited no gross alterations in maternal behavior with either their 1st or 2nd litters (unpublished observation), and pups reared by dams fed an n-3 PUFA-deficient diet have been reproducibly shown to exhibit normal weight gain, which indicates that maternal care was sufficiently normal to result in adequate nutrition (e.g., Fig. 3, [35, 45]).
In summary, in this single-session behavioral paradigm, activity, habituation, and response to spatial novelty varied by age. Second litter pups raised from conception on an n-3 PUFA-deficient diet, which had a 65% decrease in brain DHA, exhibited higher levels of activity that were consistent with a more immature pattern of behavior in the test. That 2nd-litter Deficient rats exhibited less habituation in Period 1, and higher activity in the familiar environment in Period 2 but not after the introduction of the novel spatial stimulus in Period 3, is consistent with clinical observations in ADHD. This effect was observed in 2nd-litter, but not 1st-litter pups raised on the same n-3 PUFA-deficient diet, suggesting the behavioral effects are likely due to differences in brain DHA accumulation during development rather than the n-3 PUFA content of the diet.
Acknowledgments
The authors thank Marlies K. Ozias, Paul F. Davis, and Guillermo Lona for expert technical assistance, and Ann Manzardo, Ph.D. for statistical consultation. Supported by NIH MH071599 (BL), P30 HD02528 (BL, SCF), and P20 RR016475 from the INBRE Program of the National Center for Research Resources (BL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lavigne JV, Gibbons RD, Christoffel KK, Arend R, Rosenbaum D, Binns H, Dawson N, Sobel H, Isaacs C. Prevalence rates and correlates of psychiatric disorders among preschool children. J Am Acad Child Adolesc Psychiatry. 1996;35:204–214. doi: 10.1097/00004583-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Skjoldager P, Fowler SC. Scopolamine attenuates the motor disruptions but not the attentional disturbances induced by haloperidol in a sustained attention task in the rat. Psychopharmacology (Berl) 1991;105:93–100. doi: 10.1007/BF02316869. [DOI] [PubMed] [Google Scholar]
- 3.Brockel B, Fowler S. Effects of chronic haloperidol on reaction time and errors in a sustained attention task: Partial reversal by anticholinergics and by amphetamine. The J Pharmacol Exp Ther. 1995;275:1090–1098. [PubMed] [Google Scholar]
- 4.Kornetsky C. The use of a simple test of attention as a measure of drug effects in schizophrenic patients. Psychopharmacologia. 1972;24:99–106. doi: 10.1007/BF00402907. [DOI] [PubMed] [Google Scholar]
- 5.Chee P, Gordon L, Schachar R, Lindsay P, Wachsmuth R. Effects of event rate and display time on sustained attention in hyperactive, normal, and control children. J Abnorm Child Psychol. 1989;17:371–391. doi: 10.1007/BF00915033. [DOI] [PubMed] [Google Scholar]
- 6.Taylor E. Clinical foundations of hyperactivity research. Behav Brain Res. 1998;94:11–24. doi: 10.1016/s0166-4328(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 7.Porrino LJ, Rapoport JL, Behar D, Ismond DR, Bunney WE., Jr A naturalistic assessment of the motor activity of hyperactive boys. II. Stimulant drug effects. Arch Gen Psychiatry. 1983;40:688–693. doi: 10.1001/archpsyc.1983.04390010098013. [DOI] [PubMed] [Google Scholar]
- 8.Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder--from brain dysfunctions to behaviour. Behav Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- 9.Sleator EK, Ullmann RK. Can the physician diagnose hyperactivity in the office? Pediatrics. 1981;67:13–17. [PubMed] [Google Scholar]
- 10.Fowler SC, Zarone TJ, Levant B. Methylphenidate attenuates rats’ preference of a novel spatial stimulus introduced into a familiar environment: assessment using a force plate actometer. J Neurosci Meth. 2010 doi: 10.1016/j.jneumeth.2010.03.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell EA, Aman MG, Turbott SH, Manku MS. Clinical characteristics and serum fatty acid levels in hyperactive children. Clin Pediatrics. 1987;26:406–411. doi: 10.1177/000992288702600805. [DOI] [PubMed] [Google Scholar]
- 12.Burgess JR, Stevens L, Zhang W, Peck L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr. 2000;71:327–330. doi: 10.1093/ajcn/71.1.327S. [DOI] [PubMed] [Google Scholar]
- 13.Stevens L, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, Burgess JR. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 14.Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2006;75:299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case-control study. Nutr J. 2008;7:8. doi: 10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross BM, McKenzie I, Glen I, Bennett CP. Increased levels of ethane, a non-invasive marker of n-3 fatty acid oxidation, in breath of children with attention deficit hyperactivity disorder. Nutr Neurosci. 2003;6:277–281. doi: 10.1080/10284150310001612203. [DOI] [PubMed] [Google Scholar]
- 17.Brookes KJ, Chen W, Xu X, Taylor E, Asherson P. Association of fatty acid desaturase genes with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60:1053–1061. doi: 10.1016/j.biopsych.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 18.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Chalon S. The role of fatty acids in the treatment of ADHD. Neuropharmacology. 2009;57:636–639. doi: 10.1016/j.neuropharm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr. 2009 doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- 21.Raz R, Gabis L. Essential fatty acids and attention-deficit-hyperactivity disorder: a systematic review. Dev Med Child Neurol. 2009;51:580–592. doi: 10.1111/j.1469-8749.2009.03351.x. [DOI] [PubMed] [Google Scholar]
- 22.Ozias MK, Carlson SE, Levant B. Maternal parity and diet (n-3) polyunsaturated fatty acid concentration influence accretion of brain phospholipid docosahexaenoic acid in developing rats. J Nutr. 2007;137:125–129. doi: 10.1093/jn/137.1.125. [DOI] [PubMed] [Google Scholar]
- 23.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 24.Fowler SC, Birkestrand BR, Chen R, Moss SJ, Voronstova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Meth. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- 25.Levant B, Ozias MK, Jones KA, Carlson SE. Differential effects of modulation of docosahexaenoic acid content during development in specific regions of rat brain. Lipids. 2006;41:407–414. doi: 10.1007/s11745-006-5114-6. [DOI] [PubMed] [Google Scholar]
- 26.Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Galli C, Trzeciak HI, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochim Biophys Acta. 1971;248:449–454. [Google Scholar]
- 28.Berlyne DE. Arousal, reward and learning. Ann N Y Acad Sci. 1969;159:1059–1070. doi: 10.1111/j.1749-6632.1969.tb12997.x. [DOI] [PubMed] [Google Scholar]
- 29.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 30.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- 31.Frantz KJ, Van Hartesveldt C. The locomotor effects of quinpirole in rats depend on age and gender. Pharmacol Biochem Behav. 1999;64:821–826. doi: 10.1016/s0091-3057(99)00162-8. [DOI] [PubMed] [Google Scholar]
- 32.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Levant B, Ozias MK, Carlson SE. Sex-specific effects of brain LC-PUFA composition on locomotor activity in rats. Physiol Behav. 2006;89:196–204. doi: 10.1016/j.physbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Vancassel S, Blondeau C, Lallemand S, Cador M, Linard A, Lavialle M, Dellu-Hagedorn F. Hyperactivity in the rat is associated with spontaneous low level of n-3 polyunsaturated fatty acids in the frontal cortex. Behav Brain Res. 2007;180:119–126. doi: 10.1016/j.bbr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 35.Enslen M, Milon H, Malnoe A. Effect of low intake of n-3 fatty acids during development on brain phospholipid fatty acid composition and exploratory behavior in rats. Lipids. 1991;26:203–208. doi: 10.1007/BF02543972. [DOI] [PubMed] [Google Scholar]
- 36.Reisbick S, Neuringer M, Hasnain R, Connor WE. Home cage behavior of rhesus monkeys with long-term deficiency of omega-3 fatty acids. Physiol Behav. 1994;55:231–239. doi: 10.1016/0031-9384(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 37.Bush G. Attention-Deficit/Hyperactivity Disorder and Attention Networks. Neuropsychopharmacology. 2009 [Google Scholar]
- 38.Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 39.Zimmer L, Delpal S, Guilloteau D, Aioun J, Durand G, Chalon S. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284:25–28. doi: 10.1016/s0304-3940(00)00950-2. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer L, Durand G, Guilloteau D, Chalon S. n-3 polyunsaturated fatty acid deficiency and dopamine metabolism in the rat frontal cortex. Lipids. 1999;34 (Suppl):S251. doi: 10.1007/BF02562309. [DOI] [PubMed] [Google Scholar]
- 41.Zimmer L, Hembert S, Durand G, Breton P, Guilloteau D, Besnard JC, Chalon S. Chronic n-3 polyunsaturated fatty acid diet-deficiency acts on dopamine metabolism in the rat frontal cortex: a microdialysis study. Neurosci Lett. 1998;240:177–181. doi: 10.1016/s0304-3940(97)00938-5. [DOI] [PubMed] [Google Scholar]
- 42.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
- 43.Zimmer L, Delion S, Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, Chalon S. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J Lipid Res. 2000;41:32–40. [PubMed] [Google Scholar]
- 44.Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J Neurochem. 2008;106:662–671. doi: 10.1111/j.1471-4159.2008.05418.x. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad SO, Park JH, Radel JD, Levant B. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci Lett. 2008;438:303–307. doi: 10.1016/j.neulet.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeffrey BG, Weisinger HS, Neuringer M, Mitchell DC. The role of docosahexaenoic acid in retinal function. Lipids. 2001;36:859–871. doi: 10.1007/s11745-001-0796-3. [DOI] [PubMed] [Google Scholar]
- 47.Prusky GT, Douglas RM. Vision. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat. New York: Oxford University Press; 2005. pp. 49–59. [Google Scholar]
- 48.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22:474–480. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 49.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J Nutr. 2006;136:2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- 50.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. J Nutr. 2007;137:2425–2430. doi: 10.1093/jn/137.11.2425. [DOI] [PubMed] [Google Scholar]
- 51.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis PF, Ozias MK, Carlson SE, Reed GA, Winter MK, McCarson KE, Levant B. Dopamine receptor alterations in females rats with diet-induced decreased brain docosahexaenoic acid (DHA): Interactions with reproductive status. Nutr Neurosci. 2010 doi: 10.1179/147683010X12611460764282. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stark KD, Lim SY, Salem N., Jr Artificial rearing with docosahexaenoic acid and n-6 docosapentaenoic acid alters rat tissue fatty acid composition. J Lipid Res. 2007;48:2471–2477. doi: 10.1194/jlr.M700317-JLR200. [DOI] [PubMed] [Google Scholar]