Abstract
The pontomedullary reticular formation (PMRF) of the monkey produces motor outputs to both upper limbs. EMG effects evoked from stimulus-triggered averaging (StimulusTA) were compared with effects from stimulus trains to determine whether both stimulation methods produced comparable results. Flexor and extensor muscles of scapulothoracic, shoulder, elbow, and wrist joints were studied bilaterally in two male M. fascicularis monkeys trained to perform a bilateral reaching task. The frequency of facilitation versus suppression responses evoked in the muscles was compared between methods. Stimulus trains were more efficient (94% of PMRF sites) in producing responses than StimulusTA (55%), and stimulus trains evoked responses from more muscles per site than from StimulusTA. Facilitation (72%) was more common from stimulus trains than StimulusTA (39%). In the overall results, a bilateral reciprocal activation pattern of ipsilateral flexor and contralateral extensor facilitation was evident for StimulusTA and stimulus trains. When the comparison was restricted to cases where both methods produced a response in a given muscle from the same site, agreement was very high, at 80%. For the remaining 20%, discrepancies were accounted for mainly by facilitation from stimulus trains when StimulusTA produced suppression, which was in agreement with the under-representation of suppression in the stimulus train data as a whole. To the extent that the stimulus train method may favor transmission through polysynaptic pathways, these results suggest that polysynaptic pathways from the PMRF more often produce facilitation in muscles that would typically demonstrate suppression with StimulusTA.
Keywords: Reticulospinal, Electromyography, Electrical stimulation, Reaching, Macaque
Introduction
The pontomedullary reticular formation (PMRF) is the primary source of the reticulospinal system, a major motor system (Kuypers 1975, 1981; Peterson et al. 1974; Peterson 1979). Reticulospinal neurons have widespread monosynaptic and polysynaptic axonal projections to multiple motor pools on both sides of the spinal cord, with single axons often affecting cervical, thoracic, and lumbar circuits (Jankowska et al. 2003; Matsuyama and Drew 1997; Peterson et al. 1974, 1975). Reticulospinal neurons terminate primarily in lamina VII and VIII, but also reach motoneurons in lamina IX (Matsuyama et al. 1997; Peterson et al. 1975). Reticulospinal axons usually descend ipsilaterally, but affect contralateral motoneurons through axon collaterals that decussate or via commissural interneurons (Bannatyne et al. 2003; Jankowska et al. 2003). Segmental interneurons and motoneurons for axial and proximal limb muscles appear to be the main target of reticulospinal neurons (Jankowska et al. 2003; Matsuyama and Drew 1997; Peterson 1979; Peterson et al. 1979). Reticulospinal outputs may even affect distal motoneurons controlling the hand (Riddle et al. 2009).
Microstimulation studies in the cat using stimulus trains at rest and during locomotion have shown that the reticulospinal system recruits axial and proximal limb muscles (Drew and Rossignol 1984, 1990a, b; Peterson et al. 1975). Previous work in the monkey using stimulus-triggered averaging (StimulusTA) has also shown the capacity of the reticulospinal system to recruit upper limb muscles during voluntary reaching (Davidson and Buford 2004, 2006). PMRF stimulation evokes a prevalence of ipsilateral limb flexor and contralateral limb extensor muscle excitation, often coupled with reciprocal suppression effects in the antagonist muscles (Davidson and Buford 2004, 2006; Drew and Rossignol 1990a, b; Sprague and Chambers 1954).
Motor outputs revealed by StimulusTA are thought to primarily reveal monosynaptic and disynaptic connections onto motoneurons (Cheney and Fetz 1985). The results of spike-triggered averaging (SpikeTA) are also thought to be heavily biased towards monosynaptic and disynaptic effects (Baker and Lemon 1998; Davidson et al. 2007b). A high level of agreement for SpikeTA effects and StimulusTA effects was found in the motor cortex (Cheney and Fetz 1985). This was expected because adjacent cells in motor cortex have similar output fields (Asanuma and Rosen 1972). In comparing SpikeTA and StimulusTA effects in arm muscles based on PMRF recordings in the monkey, we found strong agreement in results from these two methods at sites from which SpikeTA effects were found (Davidson et al. 2007a). This supports the view that StimulusTA reveals the output of reticulospinal neurons through relatively direct pathways, and suggests that local groups of PMRF cells may have similar outputs.
The purpose of this study was to compare the results from StimulusTA and stimulus trains applied at the same PMRF stimulus sites in the monkey to determine whether these two stimulation methods produce similar responses in arm and shoulder muscles. Since StimulusTA effects from the monkey (Davidson and Buford 2006) were similar to the movements evoked with stimulus trains in the cat (Drew and Rossignol 1990a, b; Sprague and Chambers 1954), we hypothesized that effects evoked by stimulus trains and StimulusTA should usually be similar in the monkey. Nevertheless, stimulus trains could engage polysynaptic pathways (Patton 1982); thus, responses evoked with stimulus trains might show characteristics different from those observed with StimulusTA. Preliminary results of these studies have been reported in abstracts (Buford et al. 2007; Herbert et al. 2007).
Methods
Subjects and task
Subjects were two male monkeys (M. fascicularis) trained to perform an instructed-delay, bilateral reaching task controlled by Tempo software (Reflective Computing, Olympia, WA, USA). The details of the task have been described in a previous report (Davidson and Buford 2006). The task involved a control period, including an instructed-delay interval, when the monkey waited with both hands on start switches at waist level. During the subsequent movement period, he responded to the instruction by reaching with the left or right arm to a target presented on a touch-screen computer monitor. After the trial, he was free to use either hand to retrieve a food reward, and then he started the next trial.
Animal care
Subject care complied with the NIH Guide for the Care and Use of Laboratory Animals, and the institutionally approved animal care protocol for our laboratory. Surgeries were performed under veterinary supervision in aseptic conditions. As previously reported (Davidson and Buford 2004, 2006), animals were pre-treated with antibiotics and were pre-anesthetized with Ketamine HCL (13 mg/kg im) followed by isoflurane gas (1–2%). Antibiotics and non-steroidal anti-inflammatory analgesics were administered postoperatively. A stainless-steel recording chamber was mounted to the skull over a craniotomy of the left parietal bone, allowing bilateral access to the PMRF. The EMG electrodes were pairs of multi-stranded, teflon-coated stainless-steel wires chronically implanted in twelve arm and shoulder muscle pairs per arm, 24 total. The muscles tested were extensor carpi ulnaris (ECU), flexor carpi radialis (FCR), biceps (BIC), brachialis (BRAC), triceps (long head: TRLO; lateral head; TRLA), anterior deltoid (ADLT), middle deltoid (MDLT), posterior deltoid (PDLT), upper trapezius (UTR), middle trapezius (MTR), pectoralis major (PMJ), and latissimus dorsi (LAT). EMG activity was recorded from all implanted muscles during stimulation for both StimulusTA and stimulus train methods using Spike2 software and a Power 1401 data acquisition unit (CED, Cambridge, England). Muscles that showed cross-talk in an off-line analysis or eventual implant failure were removed from the analysis, as described in Davidson and Buford (2006).
Stimulus-triggered averaging
StimulusTA procedures are provided in a previously published report (Davidson and Buford 2006). Stimulation was applied following a neural recording without changing the electrode position, and also at 0.5 mm intervals as the electrode was withdrawn from the bottom of the recording track. At each stimulation site, 4,000 biphasic pulses were delivered at a rate of 10 Hz by a digital stimulus controller (Master-8, AMPI, Israel) connected to an analog stimulus isolator (Model 2200, AM-Systems, Carlsborg, WA, USA). Stimulation for StimulusTA was delivered throughout all phases of the task (pre-trial, instructed-delay, reaching, reward retrieval, etc.). The subjects appeared to be unaware of this background stimulation. The stimulus current used was 30 μA in most cases because previous studies in PMRF output in the macaque indicate this is an effective stimulus intensity for StimulusTA (Davidson and Buford 2004, 2006). This current level has also been proven effective for studies in PMRF output in the cat (Cowie and Robinson 1994; Davidson and Buford 2004; Drew and Rossignol 1990a). In 18 cases when single pulses evoked visible muscle twitches at a given site, the current was reduced until twitches were not visible. The lowest current used was 10 μA.
Procedures for compiling StimulusTAs and the acceptance criteria for StimulusTA effects are described in detail in a previous manuscript (Davidson and Buford 2006); only a brief description is presented here. A custom written Spike2 software program was used to compile Stimulus-TAs and to identify potential EMG responses for analysis. EMG data were averaged over an 80-ms peristimulus window, consisting of a 20-ms pre-trigger and a 60-ms post-trigger period. EMG responses that exceeded ±2 standard deviations of a 15 ms pre-trigger (−20 to −5 ms) baseline EMG mean during the period between 3.5 and 15 ms following the trigger were considered StimulusTA effects. When StimulusTA revealed a sequence of responses in a given muscle, such as suppression followed by facilitation; only the first response was analyzed because this was considered most likely to reflect the direct output of the PMRF.
Stimulus trains
In some cases, stimulus trains were applied following StimulusTA when the StimulusTA was conducted immediately following a neuronal recording. However, the perceptible muscle twitches evoked by stimulus trains distracted the subject and sometimes stopped behavior. To avoid this, stimulus trains were usually applied as the electrode was withdrawn, immediately following StimulusTA from that site. A train of 12 biphasic pulses was applied at 333 Hz (pulse duration 0.2 ms/phase, amplitude 30 μA). Stimulation was manually triggered during the control period (pre-trial and instructed-delay) while the monkey sat with both hands on start switches awaiting the go cue before reaching. This behavioral state was chosen as the most reproducible because it was impracticable to apply a sufficient number of stimulus trains to create representative averages for all possible phases of the behavior. At some sites, stimulation trains were applied when the subject was not performing the task, but while the arms were at rest on or near the switches. Ten stimulus trains were usually applied at each stimulation site (mean 10.1 ± 1.34 SD, range 5–16).
EMG responses to stimulus trains were averaged and then smoothed using an 80 Hz low pass zero-lag Butterworth filter (Fig. 1, black traces) to capture the main features of the responses. A 36-ms period beginning 47 ms before stimulus train onset (ending before the train actually began) was used to calculate the mean baseline level of this smoothed EMG activity. Having the baseline period end before the train began prevented any effects of stimulus artifact from entering the baseline. Potential responses to stimulus trains were automatically detected if the smoothed EMG departed from the baseline mean by +2 SD for facilitation or −1.65 SD for suppression. The −1.65 SD threshold was required to detect suppression because there was little background EMG to begin with, making suppression relatively hard to detect. Onset and offset times determined from the smoothed data were manually adjusted to match the time when the raw EMG data (Fig. 1, gray traces) crossed the mean and SD thresholds calculated from the unsmoothed averages (e.g., Fig. 2). Only responses with onsets that began during the stimulus train or within 3 ms after the last stimulus pulse were accepted for further analysis. There was often a sequence of responses in a given muscle in the stimulus train data. To match the approach used to analyze the StimulusTA data, only the first response was analyzed in these cases. A response duration of at least 10-ms was required to avoid spurious responses from entering the dataset for responses from stimulus trains, since a relatively small number of trains were averaged in these awake subjects. With a 33-ms stimulus train duration, it was reasonable to expect at least a 10-ms response.
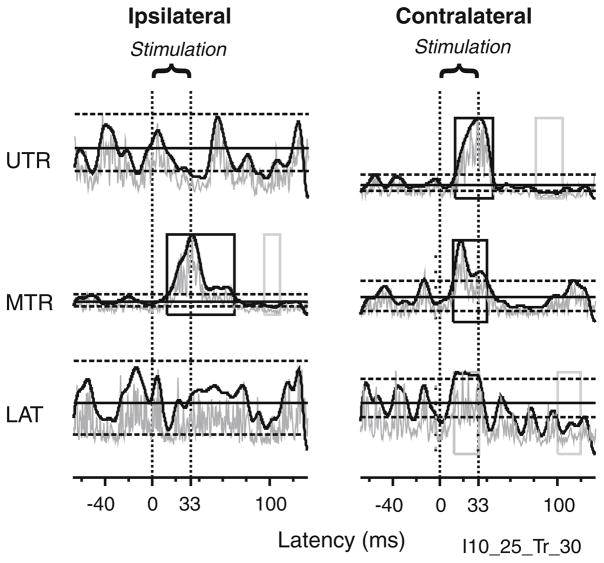
Fig. 1.
Example of potential and accepted responses for stimulus trains. The two vertical lines for each panel represent the 33-ms stimulation period. The gray traces show the raw averages and the black traces show the smoothed responses. The solid horizontal lines indicate the mean EMG levels for the baseline period; dashed horizontal lines above and below the mean represent +2 SD and −1.65 SD thresholds used for response detection. Gray boxes outline potential responses, black boxes outline accepted responses
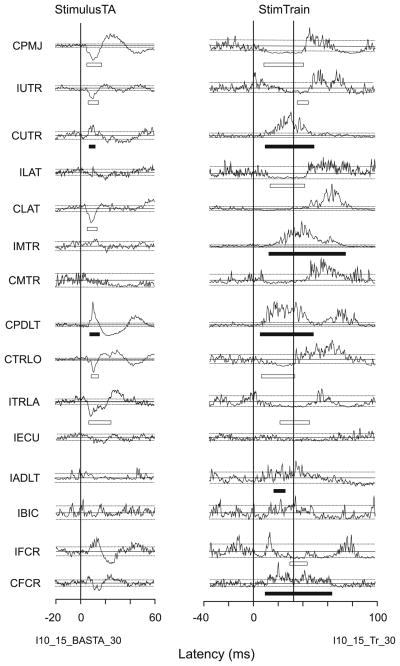
Fig. 2.
Example of responses for StimulusTA and stimulus trains from a PMRF site. Facilitation is indicated by a filled bar below the trace, suppression by an open bar. The solid horizontal lines indicate the mean EMG levels for the baseline period; dashed horizontal lines above and below the mean represent +2 SD and −1.65 SD thresholds used for response detection
With these objective criteria, visual inspection of the data was still required in order to eliminate responses that were clearly noise. In Fig. 1, for example, the automatic response detection indicated a possible facilitation in contralateral LAT. Visual inspection, however, showed this response was due to stimulus artifact superimposed on noise, so this was omitted. The automatic response detection also indicated a facilitation response in contralateral MTR and contralateral UTR; these facilitation events were accepted.
Data analysis
Because of the differences in stimulation methods, which would be expected to result in latency and amplitude differences, and due to the differences in detection criteria required for the two methods, detailed comparisons of response amplitude and onset latencies were not conducted. The sign of the response detected, facilitation or suppression, was considered the most reliable finding, and this was compared in detail for results from the two stimulation methods. The StimulusTA dataset from previously published StimulusTA results (Davidson and Buford 2006), referred to here as the main StimulusTA dataset, was one source of data for comparison. However, because that data-set was constructed from stimuli applied during reaching as well as during rest, it was not the most comparable data. Hence, the results from these studies were reanalyzed using only stimuli applied while both hands were resting on the start switches to create the averages. This control period before reaching provided a behavioral task epoch in which the monkey’s behavior was relatively consistent across trials and most comparable to the conditions under which stimulus trains were applied. Stimulus triggered averages constructed from stimulation during this period are referred to as the control-period StimulusTA dataset.
In our previously published report (Davidson and Buford 2006) a minimum of 500 triggers were required for a StimulusTA response to be included in the analysis. Application of this criterion for control-period StimulusTA data was too stringent because with only 4,000 stimuli to begin with and much of the time spent reaching, there were often not enough triggers to test. McKiernan et al. (1998) found that spike triggered averages of EMG for cortical recordings based on a minimum of 200 triggers were not substantially different from those with 1,000 or more. Davidson et al. (2007b) presented spike triggered averages of EMG for cortical recordings from primary motor cortex that contain visible spike triggered effects with only 100 triggers. Based on these findings, we required at least 200 triggers for a StimulusTA response during the control period to be included in the analysis for comparison with stimulus trains. To avoid introduction of spurious results from this lower number of triggers, StimulusTA responses found in this data subset were used for comparison only if a corresponding response was found in the main StimulusTA data.
SPSS 17.0 and Microsoft Office Excel 2003 were used for all data analyses. Descriptive statistics were used to describe characteristics of evoked responses. Chi-squared tests were used to investigate significant differences in proportions of stimulus-evoked effects (facilitation vs. suppression responses, extensor vs flexor responses, etc.). The Wilcoxon signed rank test was used to test for differences in stimulation effectiveness between StimulusTA and stimulus trains. A criterion level of P < 0.05 was considered statistically significant in each of these statistical tests.
Results
Comparison of main stimulusTA and control-period stimulusTA datasets
As described in “Methods”, our previous results from StimulusTA (Davidson and Buford 2006) were obtained from single pulse stimulation applied during all phases of performance of a bilateral reaching task. The StimulusTA data presented here are based on a reanalysis including StimulusTA data compiled only from the control period between trials, while the hands rested on start switches. This is the only behavioral state during which stimulus trains were applied. Before comparing these StimulusTA results with responses to stimulus trains, we first performed a comparison of the control-period StimulusTA dataset to the main (previously published) StimulusTA dataset to determine whether the responses obtained during the control-period data were similar. Of the 1611 responses from the previously published, main StimulusTA dataset, 1,257 potential responses were found for stimuli applied during the control period; 864 of these were also represented in the main StimulusTA dataset. The remaining 393 control-period responses that had no corresponding event in the main data-set were not significantly different from 864 comparable control-period responses in terms of distribution of suppression versus facilitation or ipsilateral versus contralateral effects. Likewise, the 747 unmatchable responses in the main dataset were not significantly different from the 864 that could be compared.
The 864 comparable control-period StimulusTA responses were elicited from 293 of the 535 PMRF sites (55%). In the main dataset, the full complement of 1,611 responses came from 435 sites (81%). Hence fewer PMRF sites are represented in the control-period dataset. However, when the 864 responses were compared for the control-period versus main StimulusTA datasets, agreement was 97%, with agreement defined as the same type of response (facilitation or suppression) in a given muscle from a given stimulation site. For the 3% that differed, half were facilitation in main dataset and suppression in the control-period data, and the other half were the opposite. As would be expected with 97% agreement, the proportions of facilitation and suppression by muscle and laterality in these 864 responses were not significantly different for the control-period and main StimulusTA datasets. For the remainder of this report, only these 864 control-period StimulusTA responses are used for comparison with responses to stimulus trains.
Overall comparison of stimulusTA and stimulus train evoked effects
Stimulus trains were applied at 473 PMRF sites; 444 of these sites (94%) were effective for activating one or more muscles. A total of 2582 EMG responses were included in the database from stimulus trains. Compared to StimulusTA, stimulus trains were more effective for evoking EMG responses (Wilcoxon signed rank test, P < 0.0001). In sum, nearly twice as many sites were effective for stimulus trains as for StimulusTA, and about three times as many responses were found for stimulus trains as for StimulusTA.
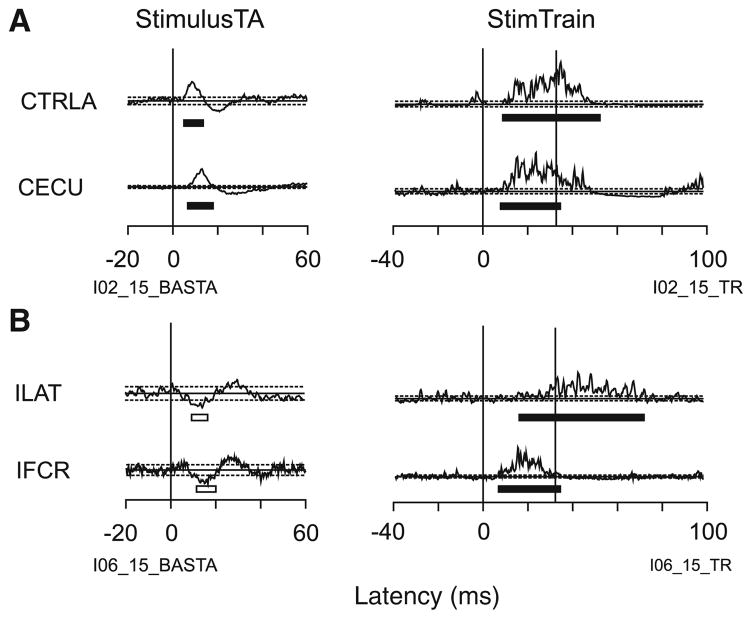
Figure 2 presents a comparison of StimulusTA and stimulus train responses evoked from one of the most effective sites stimulated. In this example, seven muscles showed StimulusTA effects, eleven muscles showed responses to stimulus trains, and both methods produced bilateral responses. Of the six StimulusTA responses in Fig. 2 for which there was a corresponding stimulus train response, all of the responses had matching signs (CPMJ, IUTR, CUTR, CPDLT, CTRLO, ITRLA), and the analyses below will indicate this was a common finding. Likewise, this example shows that stimulus trains were more effective—there are five stimulus train responses (ILAT, IMTR, IADLT, IFCR, CFCR) for which the same muscle had no response detected in the StimulusTA record. This was expected as stimulus trains are more likely to excite neurons via polysynaptic pathways. Conversely, there was one StimulusTA response (CLAT) for which the same muscle did not show a response to stimulus trains. This can be expected when the signal-to-noise ratio is better for StimulusTA.
The acceptance criteria resulted in some plausible responses not being accepted in both StimulusTA and stimulus train records. For example, in the StimulusTA record (Fig. 2), because the traces for IFCR and CFCR did not have corresponding responses in the main StimulusTA dataset they were not accepted. In the stimulus train record (Fig. 2), because the trace for CMTR never went below threshold, this was not accepted as a suppression response. One could also question why a facilitation response was not found in IBIC even though there was one in IADLT. Again, the response detection in the smoothed data (see “Methods”, Fig. 1), did not identify this BIC response as a viable candidate. Hence, some apparent facilitation and suppression responses were hard to detect for both stimulation methods. Within the limits of experimental error, the acceptance criteria were designed to ensure that those responses which were ultimately accepted into the dataset for comparison were valid.
Effectiveness of stimulation by muscle
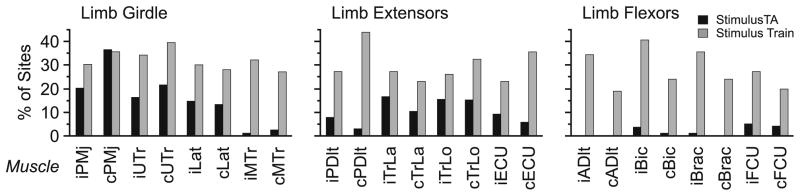
Figure 3 compares the effectiveness of stimulation for each muscle for stimulus trains versus StimulusTA. Effectiveness was calculated as the number of sites from which a stimulus-evoked response could be observed in a muscle divided by the total number of sites where EMG was recorded for that muscle during a stimulation attempt. A response was counted as one or more muscles being facilitated or suppressed from a given site. For StimulusTA, effectiveness varied among muscles from 0 to 35%, with the highest degree of effectiveness found in limb girdle muscles (15%), moderate effectiveness found in the limb extensors (10%), and the lowest effectiveness found in the limb flexors (2%) (χ2 = 324.2, P < 0.0001). For stimulus trains, effectiveness varied from 18 to 42%, and there was barely a significant difference by muscle group. Overall stimulus train effectiveness was 31% for the limb girdle muscles, 29% for limb extensors, and 27% for limb flexors (χ2 = 8.4, P = 0.014). The tendency for more limb girdle and fewer flexor muscles to respond, with intermediate responsiveness for extensors, was significantly more disparate for StimulusTA than for stimulus trains (χ2 = 142.1, P < 0.0001).
Fig. 3.
Comparison of the effectiveness of stimulation for StimulusTA vs stimulus trains. The effectiveness of stimulation was determined as a percentage for each muscle by counting the number of sites at which a stimulus-evoked response was observed and dividing this number by the total number of sites where EMG was recorded for that muscle during a stimulation attempt
For each effective stimulation site, the number of muscles responding for stimulus trains ranged from one to fourteen, with a median of six and a mean of 6 ± 3 (SD). On average, half as many muscles responded per site for StimulusTA, with a range of one to eleven muscles per site, a median of two and a mean of 3 ± 2 (SD).
Effects from StimulusTA versus stimulus trains
For stimulus trains, ipsilateral and contralateral responses were equally prevalent, with 51% of the effects observed in ipsilateral muscles and 49% in contralateral muscles. Bilateral responses were evoked by stimulus trains from 83% of all effective sites; 9% of sites evoked only ipsilateral responses and 8% of sites evoked only contralateral responses. Most StimulusTA sites (57%) also evoked bilateral outputs, 18% of sites evoked only ipsilateral responses and 25% of sites evoked only contralateral responses. Hence, there was a greater tendency for bilateral outputs to be revealed from stimulus trains, but neither method favored ipsilateral or contralateral effects.
Facilitation was the prevailing response to stimulus trains for both upper limbs, accounting for 71% of ipsilateral responses and 73% of contralateral responses. Suppression was more common (61%) than facilitation (39%) in the results for StimulusTA. This was true for both upper limbs, with suppression accounting for 67% of ipsilateral responses and 54% of contralateral responses.
In summary, stimulus trains and StimulusTA had a similar capacity to affect ipsilateral and contralateral muscles, with bilateral responses being most common, especially for stimulus trains. Facilitation was more common in response to stimulus trains, but suppression was more common in the results for StimulusTA (χ2 = 294.3, P < 0.0001).
Comparison of effects by muscle
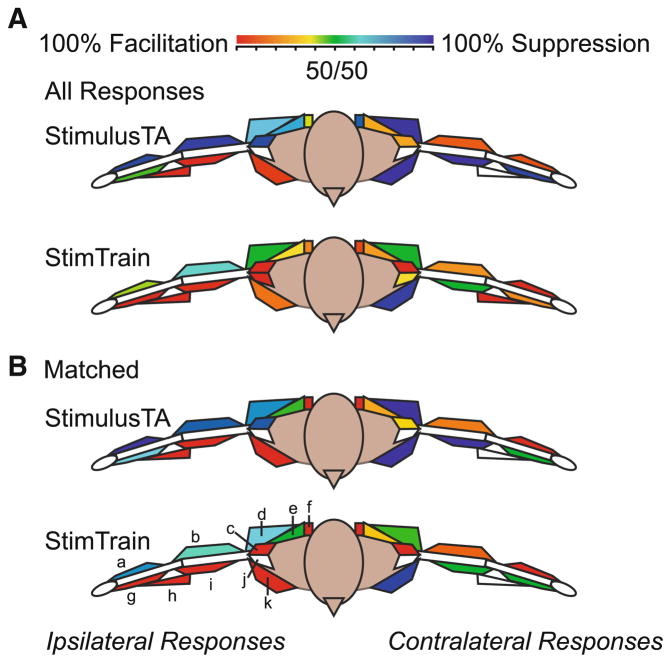
Figure 4 provides a schematic diagram of the proportion of stimulus-evoked facilitation and suppression responses in upper limb muscles for each method. The top two diagrams (Fig. 4a) illustrate ipsilateral and contralateral muscles color coded for all 864 control-period StimulusTA and all 2,582 stimulus train responses. The colors represent the overall percentage of facilitation effects evoked for each muscle, with red indicating a preponderance of facilitation effects, blue indicating a preponderance of suppression effects, and green representing equal proportions of facilitation and suppression. The white color indicates that no StimulusTA responses in ipsilateral ADLT, and contralateral ADLT and BRAC were available for comparison. In agreement with the results from the main StimulusTA data-set (Davidson and Buford 2006), the overall pattern for the control-period StimulusTA dataset was a double reciprocal pattern between the upper limbs, with ipsilateral flexors and contralateral extensors facilitated and ipsilateral extensors and contralateral flexors suppressed.
Fig. 4.
This schematic representation color codes muscles according to the proportion of facilitation versus suppression for each stimulation method. As shown in the key, shades of red indicate muscles that were mostly facilitated, shades of blue indicate muscles that were mostly suppressed, and colors in between represent a mixture of responses; white no response. Responses are referred to as ipsilateral or contralateral to the stimulation site. The top two diagrams a illustrate the response patterns for all control-period StimulusTA and stimulus train results. The bottom two b illustrate the response patterns for all control-period StimulusTA and stimulus train results constrained to sites where both methods evoked responses for a given muscle. In order to portray the triceps muscle, the proportion of responses for TRLA and TRLO were averaged. Muscle key: a ECR, b TRI, c PDLT, d LATS, e UTR, f MDLT, g FCR, h BRACH, i BIC, j ADLT, k PMJ
For ipsilateral flexors and contralateral extensors, StimulusTA and stimulus trains both produced a preponderance of facilitation. As shown in Table 1, there was more facilitation from stimulus trains than for StimulusTA for many of these muscles. However, in two ipsilateral extensors, UTR and PDLT, where suppression was the typical response for StimulusTA, facilitation was the prevalent response for stimulus trains (Fig 4a; Table 1). In two contralateral flexors (FCR and MTR), stimulus trains also resulted in a prevalence of facilitation even though suppression was typically the response for StimulusTA (Fig 4a; Table 1). In sum, stimulus trains favored facilitation of ipsilateral flexors and contralateral extensors even more strongly than StimulusTA. Conversely, the tendency for stimulus trains to suppress ipsilateral extensors and contralateral flexors was less pronounced, and in some cases stimulus trains were most likely to facilitate muscles that were typically suppressed for StimulusTA. This prevalence of facilitation from stimulus trains was found throughout the muscles studied, regardless of the muscle’s location (proximal vs. distal) or function (flexor vs. extensor).
Table 1.
Frequency of responses for StimulusTA and stimulus trains
| Muscle | StimulusTA |
Stimulus Train |
||||
|---|---|---|---|---|---|---|
| FAC | SPR | % FAC | FAC | SPR | % FAC | |
| CECU | 21 (15) | 4 (2) | 85 (88) | 148 (16) | 8 (1) | 95 (94) |
| CFCR | 3 (1) | 20 (1) | 13 (50) | 91 (1) | 25 (1) | 78 (50) |
| CBIC | 0 (0) | 3 (2) | 0 (0) | 38 (1) | 36 (1) | 51 (50) |
| CBRAC | 0 (0) | 0 (0) | −(−) | 64 (0) | 0 (0) | 100 (−) |
| CTRLA | 43 (18) | 3 (1) | 93 (95) | 92 (18) | 8 (1) | 92 (95) |
| CTRLO | 47 (19) | 14 (7) | 77 (73) | 84 (20) | 45 (6) | 65 (77) |
| CADLT | 0 (0) | 0 (0) | − (−) | 22 (0) | 13 (0) | 63 (−) |
| CPMJ | 3 (1) | 93 (47) | 3 (2) | 8 (6) | 85 (42) | 9 (13) |
| CPDLT | 3 (2) | 1 (1) | 75 (67) | 62 (3) | 0 (0) | 100 (100) |
| CLAT | 4 (1) | 61 (25) | 7 (4) | 63 (14) | 67 (12) | 48 (54) |
| CMTR | 2 (1) | 9 (0) | 18 (100) | 106 (1) | 17 (0) | 86 (100) |
| CUTR | 72 (41) | 25 (16) | 74 (72) | 136 (38) | 43 (19) | 76 (67) |
| IECU | 5 (1) | 32 (18) | 14 (5) | 53 (4) | 39 (15) | 58 (21) |
| IFCR | 13 (4) | 16 (8) | 45 (33) | 152 (11) | 8 (1) | 95 (92) |
| IBIC | 10 (3) | 0 (0) | 100 (100) | 101 (3) | 11 (0) | 90 (100) |
| IBRAC | 3 (1) | 0 (0) | 100 (100) | 94 (1) | 0 (0) | 100 (100) |
| ITRLA | 4 (1) | 62 (20) | 6 (5) | 50 (6) | 60 (15) | 45 (29) |
| ITRLO | 9 (6) | 59 (16) | 13 (27) | 37 (13) | 81 (9) | 31 (59) |
| IADLT | 0 (0) | 0 (0) | − (−) | 46 (0) | 2 (0) | 96 (−) |
| IPMJ | 56 (35) | 8 (1) | 88 (97) | 79 (33) | 18 (3) | 81 (92) |
| IPDLT | 2 (1) | 12 (5) | 14 (17) | 48 (6) | 1 (0) | 98 (100) |
| ILAT | 19 (6) | 47 (23) | 29 (21) | 66 (10) | 70 (19) | 48 (34) |
| IMTR | 3 (1) | 2 (0) | 60 (100) | 118 (1) | 31 (0) | 79 (100) |
| IUTR | 19 (16) | 56 (22) | 25 (42) | 98 (17) | 58 (21) | 63 (45) |
Facilitation and suppression evoked responses during the control phase of the task in each muscle for both methods are expressed as counts. The numbers in parentheses are control data constrained to sites where both methods evoked a response in a given muscle. The prevalence of facilitation responses evoked in each muscle is expressed as a percentage. Each percentage of facilitation is calculated by dividing the number of facilitation responses by the total number of evoked responses for that muscle
I ipsilateral, C contralateral
Site-by-site comparison of effects from stimulus trains and stimulusTA
Directly comparable responses
To follow up on the finding that stimulus trains were more likely to produce facilitation, a more specific comparison was conducted with the analysis constrained to directly comparable responses where both methods evoked a response in a given muscle from the same site, the best chance for agreement. Of 356 directly comparable responses from 164 sites, facilitation accounted for 58% of the responses for stimulus trains, a higher proportion than the 45% found for StimulusTA (χ2 = 11.9, P = 0.001).
The bottom two diagrams in Fig. 4b illustrate ipsilateral and contralateral muscles color coded for these corresponding StimulusTA and stimulus train responses. The white color indicates that no responses in ipsilateral ADLT and contralateral ADLT and BRAC were available for direct comparison between methods. For StimulusTA, the bilateral reciprocal response pattern was still evident in this subset of the data. Stimulus trains also usually facilitated ipsilateral flexors and contralateral extensors, consistent with the typical response for StimulusTA. However, stimulus trains reduced the tendency for reciprocal suppression, especially for ipsilateral extensors.
Matching StimulusTA and stimulus train responses
Overall, 80% of the 356 comparable responses matched, with a match being defined as the same type of response (facilitation or suppression) evoked in a given muscle for a given stimulus site for both methods. Matches were equally common for facilitation and suppression, and for ipsilateral and contralateral effects. Figure 5a is representative of matching StimulusTA and stimulus train responses from a single stimulation site. StimulusTA and stimulus trains both evoked facilitation of CECU and CTRLA. These were typical StimulusTA responses for these muscles. Examples of responses that matched for StimulusTA and stimulus trains were also presented in Fig. 2.
Fig. 5.
Selected examples of matching and mismatching effects for StimulusTA and stimulus trains. a Illustrates a matching responses, and b illustrates mismatches. Formatted like Fig. 2
Mismatched StimulusTA and stimulus train responses
At sites with comparable stimulus train and StimulusTA responses, 20% (72/356) of the responses were mismatches, with a mismatch being defined as a case where facilitation was evoked by one method and suppression was evoked by the other. Most (82%) mismatches were cases where facilitation was evoked by the stimulus trains but suppression was evident in the record for StimulusTA (χ2 = 29.4, P < 0.0001). Mismatches were also more common ipsilaterally (63%) than contralaterally (38%) (χ2 = 4.5, P = 0.03), and more common in extensors (81%) than flexors (19%) (χ2 = 26.9, P < 0.0001). The most common type of mismatch (40%) was when ipsilateral extensors were suppressed by StimulusTA, but this typical effect was replaced by facilitation for stimulus trains. Many of the mismatches (35%) were also cases where the response for StimulusTA was suppression in a muscle that was typically facilitated, and the stimulus train produced the more typical facilitation response; this was most common in contralateral extensors.
Figure 5b illustrates an example of a mismatch in responses from StimulusTA and stimulus train for a single stimulation site. In this example, suppression responses were evoked in IFCR and ILAT by StimulusTA, stimulus trains produced facilitation for these two muscles. The suppression response in IFCR was atypical from StimulusTA, but the suppression response in ILAT was typical.
Additional control analyses for mismatches
As noted earlier in the results, 3% of effects from the control-period StimulusTA were opposite in sign, altered from the effect from the corresponding site in the main StimulusTA dataset. We checked to see whether these altered effects showed up with a disproportionately high incidence in the mismatches between StimulusTA and stimulus train responses described above, but this was not the case. Only three of these altered responses entered into the data. Two of these were atypical facilitation from StimulusTA replaced by suppression from stimulus trains and one was atypical suppression from StimulusTA replaced by facilitation from stimulus trains. Therefore, responses in the control-period StimulusTA dataset that were altered from the response in the main StimulusTA data could not account for the finding that suppression from StimulusTA was often replaced by facilitation from stimulus trains.
Next, we examined the extent to which measurement error could account for different responses observed between methods. To test for this possibility, the control-period StimulusTA dataset the data file was split in two and the StimulusTAs compiled from these two datasets were compared. Each response found in the split data had to come from at least 100 triggers, half the overall acceptance criteria of at least 200 triggers for a StimulusTA response. In this comparison, 496 StimulusTA responses were found. There was 96% agreement in response type (facilitation or suppression) for each response found in the split StimulusTA datasets.
Referring back to the actual comparison of StimulusTA and stimulus train responses, for the 72 mismatches found, only one came from a site that produced different responses for the two halves of the StimulusTA data. Hence, sites from which StimulusTA responses could be described as unstable were not the source of differences between the StimulusTA and stimulus train responses. There was a significantly higher proportion of disagreement in the comparison between StimulusTA and stimulus train responses than there was between the two halves of the StimulusTA dataset (χ2 = 19.11, P < 0.001). Consequently, the 20% difference found between the stimulus train and StimulusTA results exceeds the expected measurement error.
Unmatched responses
For 86% (n = 2,222) of the stimulus train responses, there was no corresponding StimulusTA response in that muscle for that site; these were called unmatched responses, and were present in one or more muscles from 243 sites. A smaller proportion (56%) of the StimulusTA responses (n = 464) were unmatched; these were evoked from 78 sites. For the unmatched subset of stimulus train responses, facilitation was even more prevalent (74%) than in the matched subset (57%). In these unmatched train responses, 52% of the effects were ipsilateral, 48% were contralateral, and the bilateral reciprocal pattern of ipsilateral flexor and contralateral extensor facilitation was evident in the data. For the unmatched StimulusTA responses there was 65% suppression, more than for matched StimulusTA responses (55%). Again, 52% of the effects were ipsilateral and 48% were contralateral, and the bilateral reciprocal pattern was evident.
In sum, data that entered into the site-by-site comparison for directly comparable responses appear the most similar overall as subsets of the data from the two stimulation methods. In fact, the StimulusTA responses available for direct comparison with stimulus train results had more, not less facilitation, than could be found in the overall control-period dataset. Hence, a lack of facilitation in the StimulusTA results cannot account for the excess facilitation from stimulus trains.
Anatomical organization of effective PMRF stimulus-evoked responses
The locations of sites evoking responses for StimulusTA were published in Davidson and Buford (2006). As stated in “Methods”, stimulus trains were applied at most StimulusTA sites. Sites evoking facilitation and suppression responses for given muscles with stimulus trains were interspersed. There was no special concentration of stimulus train sites producing the most effects, nor were there special concentrations of sites producing ipsilateral, contralateral, or bilateral effects. There were not certain locations from which facilitation versus suppression responses were elicited. PMRF sites evoking matched and mismatched StimulusTA and stimulus train responses were also evenly dispersed throughout the PMRF.
Discussion
Double reciprocal pattern of PMRF output
In the aggregate, results from StimulusTA and stimulus trains revealed a common pattern of bilateral motor output to the upper limbs: ipsilateral flexor and contralateral extensor muscle facilitation, with suppression (or at least less facilitation) of the reciprocal muscles. One explanation for this consistent finding could be that most reticulospinal neurons distribute their outputs in a way that contributes to this overall pattern. Matsuyama et al. (1997) traced individual reticulospinal axons and found that many terminated ipsilaterally, but some terminated bilaterally. These axons terminated in laminae VII and VIII, where they could contact interneurons or the dendrites of motoneurons. Classical studies indicate that there are monosynaptic pathways from the PMRF to motoneurons in the cat (Grillner and Lund 1966; Peterson et al. 1974, 1975; Peterson 1979) and in the monkey (Shapovalov 1972). Jankowska’s physiological data indicate that bilateral PMRF effects are mediated by pathways comprised of monosynaptic synapses on the motoneurons or disynaptic connections onto excitatory, inhibitory and commissural interneurons (Bannatyne et al. 2003; Jankowska 2007; Jankowska et al. 2003). Thus, there is ample evidence for mono and disynaptic pathways from the PMRF to motoneurons that are bilaterally distributed. However, most of these studies are in the lumbar cord of the cat and there is a need to define this in the cervical cord of the macaque.
A pattern combining extension of one limb with flexion of the other has its roots in the control of locomotion. Activation of the PMRF directly, or indirectly from mesencephalic locomotor region stimulation, is sufficient for initiation of locomotion in reduced preparations (Deliagina et al. 2008; Drew et al. 2004; Shik et al. 1969). Hence, the tendency for PMRF output to produce this double reciprocal pattern is well established (Drew and Rossignol 1990a, b; Sprague and Chambers 1954). Data from our previous work and from the cat also shows that PMRF neurons are strongly modulated during voluntary reaching (Buford and Davidson 2004; Schepens et al. 2008; Schepens and Drew 2004, 2006). As Drew et al. (2004) noted, these findings indicate the pathways used for initiation and regulation of locomotion by the PMRF may overlap with pathways engaged by the PMRF during reaching.
The double reciprocal pattern observed with PMRF stimulation is also consistent with the upper limb movements associated with the asymmetric tonic neck reflex (ATNR), which is thought to reflect brainstem output after cortical injury (Denny-Brown 1966). Humans often present with movement patterns that mimic the ATNR after a cortical stroke. Dewald et al. (1995) demonstrated a predominance of stroke-related muscle synergies in the hemiparetic upper limb during reaching that are consistent with the ATNR and the historical descriptions of stroke synergies from Brunnstrom (1970). Subjects in Dewald et al. (1995) study typically flexed the elbow when elevating the shoulder, and had the greatest difficulty recruiting elbow extensors coupled with shoulder flexors. Our results from both stimulation methods are consistent with Dewald’s argument that these stroke synergies may be explained by increased reliance on the reticulospinal system for voluntary control of reaching.
Differences in results for stimulus trains versus stimulusTA
Although both methods produced results that largely agreed and reflected the double reciprocal pattern, there were two important differences. First, there was excess facilitation from stimulus trains. Second, stimulus trains were more effective for recruiting limb muscles, whereas StimulusTA results were more heavily represented in the limb girdle muscles.
Despite the excess facilitation from stimulus trains, only 20% of responses were different in the directly comparable individual responses. However, in the larger datasets, the difference was more pronounced. Facilitation accounted for 72% of the responses to stimulus trains, but only 39% of the responses to StimulusTA. For an investigator forced to choose between one method or the other, this could present somewhat different conclusions from a study. One factor that probably contributed to this difference in results for the two methods was the design of our study. Suppression of EMG when movement is not underway is relatively hard to detect. The improvement of signal-to-noise with the relatively large number of triggers in StimulusTA would be expected to make that the more sensitive technique for subtle responses, since a relatively small number of stimulus trains were applied. Perhaps if stimulus trains were applied during reaching movements, more suppression could have been detected. For practical reasons explained in the methods, however, this was not feasible in the present study. Even considering this limitation, however, the analysis of the directly comparable responses along with the control analyses described at the end of the results show that there was still a clear tendency for stimulus trains to evoke facilitation where the response for StimulusTA was suppression. This was especially true for extensor muscles. Our conclusion is that at least some of this over-representation of facilitation from stimulus trains represents a true physiological difference between the pathways for muscle recruitment for the two methods.
Studies in the cat where stimulus trains were used also report a preponderance of facilitation (Drew and Rossignol 1990b). Previously, we attributed the difference between the findings from the cat and our findings with StimulusTA (Davidson and Buford 2006) to the lack of EMG activity in the resting state for the cat making suppression harder to detect. In the present analysis, we controlled for that and still found facilitation in response to stimulus trains more often than facilitative StimulusTA effects. The present findings suggest that responses to stimulus trains are similar in the cat and monkey. Further, we suspect a StimulusTA study in the cat would yield results similar to those found in the monkey, where suppression was more common than facilitation. Indeed, when SpikeTA has been conducted from PMRF neurons in the cat (Schepens and Drew 2006), suppression was more common than facilitation, and SpikeTA and StimulusTA results tend to agree (Cheney and Fetz 1985; Davidson et al. 2007a, b).
The second difference was the relatively equal distribution of effects among proximal and more distally located muscles for responses to stimulus trains. For StimulusTA, there was clearly a preponderance of effects in the limb girdle muscles, moderate responsiveness in limb extensors, and very few responses in limb flexors. But with stimulus trains, all three muscle groups were quite responsive and there was only a small difference among groups. Again, this is consistent with what was described in the cat with stimulus trains, where flexor responses in limb muscles were common (Drew and Rossignol 1990b). Recent data from Baker’s lab indicates that stimulation of reticulospinal and other axons in the medial longitudinal fasciculus (MLF) of the rhesus monkey can evoke monosynaptic EPSPs in forearm motoneurons and even in intrinsic hand motoneurons (Riddle et al. 2009). The tendency for stimulus trains to have more effects distally suggests that these connections may be mostly polysynaptic.
The unmatched results in the StimulusTA and stimulus train datasets may be explained by differing sensitivities and specificities of the two methods. StimulusTA by its nature is able to extract very small effects as long as they are consistently present. Stimulus trains, in comparison would be expected to reveal a larger number of effects overall by engaging polysynaptic pathways (Patton 1982), but might not succeed at revealing real but very weak effects. A stimulus train that succeeded as well as possible at eliciting a very weak effect would still potentially be hard to recognize because there are very few stimulus trains to average and the weak effect might be hard to discern from the background EMG activation that was present in these awake subjects.
Implications for reticulospinal output pathways
The physiological difference between stimulus trains versus StimulusTA is thought to be that through temporal summation, stimulus trains are better able to reveal polysynaptic pathways (Patton 1982), whereas StimulusTA results tend to reflect monosynaptic and disynaptic connections to motoneurons (Baker and Lemon 1998; Cheney and Fetz 1985; Fetz et al. 1976). Accepting for the moment that stimulus train and StimulusTA results were different in the ways described above, this suggests that a slightly different set of neural pathways was being studied for the two methods. Similarities could be attributed to the most direct pathways activated well by both methods, while differences could be attributed to more polysynaptic routes better revealed by stimulus trains. The tendency to facilitate ipsilateral flexors and inhibit ipsilateral extensors may be explained by monosynaptic reticulospinal projections to motoneurons in combination with disynaptic connections through excitatory and inhibitory interneurons. The tendency to facilitate contralateral extensors while simultaneously inhibiting contralateral flexors may be explained by known populations of excitatory and inhibitory commissural interneurons (Bannatyne et al. 2003; Jankowska et al. 2003). This double reciprocal pattern is present with both StimulusTA and stimulus trains. Results from previous SpikeTA and StimulusTA studies in the monkey have also revealed the typical, double reciprocal pattern (Davidson et al. 2007a; Davidson and Buford 2004, 2006), which further indicates that the double reciprocal pattern with a preponderance of suppression revealed by StimulusTA is more likely to reflect relatively direct pathways. However, facilitation of ipsilateral extensors and contralateral flexors also exists and may become more prevalent with stimulus trains. The finding that suppression responses from StimulusTA could be replaced by facilitation from stimulus trains suggests that there is an alternative route, where the more direct pathway promotes suppression and a more polysynaptic pathway allows for an alternative response of facilitation, especially in extensors.
This is not the first evidence that reticulospinal motor outputs may take alternative pathways with opposite effects for a given motor pool. In the cat, reciprocal activation patterns between flexors and extensors in the forelimbs can vary depending on the behavioral state (rest or movement) of the animal. Drew and Rossignol (1990b) reported that stimulus trains applied at rest facilitated ipsilateral forelimb flexors and contralateral forelimb extensors, but rarely suppressed EMG activity in any limb muscles. Stimulation applied during the swing phase of locomotion for the ipsilateral forelimb also facilitated ipsilateral flexors and contralateral extensors, but stimulation applied during swing for the contralateral forelimb facilitated contralateral flexors, opposite to the pattern observed at rest and during ipsilateral swing (Drew 1991; Drew and Rossignol 1984; Perreault et al. 1994). Drew and colleagues have interpreted this result to suggest that reticulospinal outputs can facilitate or suppress flexors and extensors bilaterally, but the pathways capable of conducting responses to stimulation will depend on behaviorally appropriate gating of spinal circuits through which the reticulospinal effects are conveyed (Schepens and Drew 2003, 2004, 2006).
Drew et al. (2004) suggested that the direct and alternative pathways explaining the nature of reticulospinal outputs exist at the spinal level. We concur that there is ample evidence that well established spinal circuits associated with locomotion can support this double reciprocal pattern and that circuits for coactivation of flexors and extensors can also be demonstrated at the spinal level, so this proposal has the advantage of parsimony and plausibility. However, there is another possibility worth considering: polysynaptic pathways revealed by stimulus trains may also involve circuitry intrinsic to the PMRF. In primary motor cortex, intracortical microstimulation activates cells in the immediate vicinity of corticomotoneuronal cells indirectly (Baker et al. 1998; Bennett et al. 1989) and activates corticospinal cells trans-synaptically (Bennett et al. 1989; Jankowska et al. 1975). Stimulus trains applied to the MLF activate some reticulospinal neurons directly and some trans-synaptically via antidromic activation of reticulospinal collaterals (Edgley et al. 2004; Jankowska et al. 2003). Therefore, it seems likely that stimulus trains delivered in the PMRF would recruit polysynaptic pathways within the reticular formation. In contrast to findings in motor cortex of adjacent cells showing similar motor outputs (Asanuma and Rosen 1972), our findings showed no anatomical organization within PMRF producing facilitation and suppression effects. Hence, engagement of polysynaptic circuits to spread the activation within the PMRF might have mixed effects, whereas polysynaptic engagement within a local region of the motor cortex might not produce as much difference.
Another interesting polysynaptic route with known projections to the more distal muscles in cat and in monkey is the C3–4 propriospinal system (Alstermark et al. 1987, 1999). C3–4 propriospinal neurons are also a strong target of reticulospinal projections in the cat, but may be a weaker target in the monkey (Lemon 2008; Maier et al. 1998). This system has been implicated even in control of skilled digit movements in monkeys via direct cortical projections to the propriospinal neurons (Alstermark et al. 1999) and with corticoreticular influences on reticulospinal cells that project to the propriospinal neurons (Nishimura et al. 2007; Pettersson et al. 2007).
Further implications
Finally, on a methodological note, a common neurophysiological technique in cerebral cortex is to use stimulus trains to map motor outputs, recording movements evoked without EMG analysis. Can simple observation of movement or even EMG recording with stimulus trains in the PMRF suffice, or is the extra time associated with StimulusTA required for an accurate representation of motor outputs from this part of the brain? The large agreement between StimulusTA and stimulus trains in these results support the use of stimulus trains for a general description of motor outputs. However, stimulus trains may under estimate the amount of suppression produced by the PMRF. If a comparison was planned as the result of a treatment that could change the effects of the more direct PMRF output pathways or to specifically focus on changes in suppression, then the StimulusTA method might be required to reveal those changes. If the intent was to study effects that were mediated by polysynaptic routes, stimulus trains would be a better approach. Future investigations should choose methods carefully with these differences in mind.
Acknowledgments
The authors thank Stephanie Moran and Jacob Banks for excellent technical assistance. Supported in part by NIH R01 NS037822 to JA Buford and by PODS I and PODS II awards to WJ Herbert from the Foundation for Physical Therapy, Inc.
Contributor Information
Wendy J. Herbert, Division of Physical Therapy, School of Allied Medical Professions, The Ohio State University, Columbus, OH 43210, USA
Adam G. Davidson, Neuroscience Graduate Studies Program, The Ohio State University, Columbus, OH 43210, USA
John A. Buford, Email: Buford.5@osu.edu, Division of Physical Therapy, School of Allied Medical Professions, The Ohio State University, Columbus, OH 43210, USA. Neuroscience Graduate Studies Program, The Ohio State University, Columbus, OH 43210, USA. Center for Brain and Spinal Cord Repair, The Ohio State University, Columbus, OH 43210, USA
References
- Alstermark B, Kummel H, Pinter MJ, Tantisira B. Branching and termination of C3–C4 propriospinal neurones in the cervical spinal cord of the cat. Neurosci Lett. 1987;74:291–296. doi: 10.1016/0304-3940(87)90312-0. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Ohki Y, Saito Y. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C(3)–C(4) propriospinal neurons in the Macaca fuscata. J Neurophysiol. 1999;82:3580–3585. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Rosen I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14:243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Baker SN, Lemon RN. Computer simulation of post-spike facilitation in spike-triggered averages of rectified EMG. J Neurophysiol. 1998;80:1391–1406. doi: 10.1152/jn.1998.80.3.1391. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. An investigation of the intrinsic circuitry of the motor cortex of the monkey using intra-cortical microstimulation. Exp Brain Res. 1998;123:397–411. doi: 10.1007/s002210050585. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KMB, Lemon RN, Werner W. Indirect excitation of corticospinal neurones by intracortical stimulation in the conscious monkey. J Physiol. 1989;418:103P. [Google Scholar]
- Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. Harper and Row; New York: 1970. [Google Scholar]
- Buford JA, Davidson AG. Program No. 918.4.2004. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2004. [Accessed 01 November 2004]. Movement-related and preparatory activity in the reticular formation during a bilateral reaching task. Online. [Google Scholar]
- Buford JA, Herbert WJ, Davidson AG. Program No 191 6 2007 Abstract Viewer/itinerary Planner. Society for Neuroscience; Washington, DC: 2007. Stimulus trains and stimulus triggered averaging of bilateral arm and shoulder muscles from the pontomedullary reticular formation in the monkey. Online. [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol. 1994;72:2648–2664. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus triggered averaging. J Neurophysiol. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007a;27:8053–8058. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, O’Dell R, Chan V, Schieber MH. Comparing effects in spike-triggered averages of rectified EMG across different behaviors. J Neurosci Methods. 2007b;163:283–294. doi: 10.1016/j.jneumeth.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev. 2008;57:212–221. doi: 10.1016/j.brainresrev.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D. The cerebral control of movement. Charles C Thomas; Springfield: 1966. [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Drew T. Functional organization within the medullary reticular formation of the intact unanesthetized cat. III. Microstimulation during locomotion. J Neurophysiol. 1991;66:919–938. doi: 10.1152/jn.1991.66.3.919. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in thalamic cats. J Neurophysiol. 1984;52:653–675. doi: 10.1152/jn.1984.52.4.653. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol. 1990a;64:767–781. doi: 10.1152/jn.1990.64.3.767. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J Neurophysiol. 1990b;64:782–795. doi: 10.1152/jn.1990.64.3.782. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD, German DC. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res. 1976;114:505–510. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. A descending pathway with monosynaptic action on flexor motoneurones. Experientia. 1966;22:390. doi: 10.1007/BF01901155. [DOI] [PubMed] [Google Scholar]
- Herbert WJ, Buford JA, Davidson AG. Program No 191 5 2007 Abstract Viewer/itinerary Planner. Society for Neuroscience; Washington, DC: 2007. Stimulus trains of bilateral upper limb muscles from the pontomedullary reticular formation in the monkey. Online. [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2007;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. The mode of activation of pyramidal tract cells by intracortical stimuli. J Physiol. 1975;249:617–636. doi: 10.1113/jphysiol.1975.sp011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HG. Handbook of physiology. Sect. I. The nervous system, vol II. Motor control, pt 1, vol II. Motor control, pt. 1. The American Physiological Society; Bethesda, MD: 1981. Anatomy of descending pathways; pp. 597–666. [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Maier MA, Illert M, Kirkwood PA, Nielsen J, Lemon RN. Does a C3–C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey. J Physiol. 1998;511(Pt 1):191–212. doi: 10.1111/j.1469-7793.1998.191bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;389:617–641. doi: 10.1002/(sici)1096-9861(19971229)389:4<617::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervicothoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol. 1997;377:234–250. [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol. 1998;80:1961–1980. doi: 10.1152/jn.1998.80.4.1961. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- Patton HD. Excitable tissues and reflex control. Vol. 4. W.B Saunders; Philadelphia, PA: 1982. Spinal reflexes and synaptic transmission. [Google Scholar]
- Perreault MC, Rossignol S, Drew T. Microstimulation of the medullary reticular formation during fictive locomotion. J Neurophysiol. 1994;71:229–245. doi: 10.1152/jn.1994.71.1.229. [DOI] [PubMed] [Google Scholar]
- Peterson BW. Reticulospinal projections to spinal motor nuclei. Annu Rev Physiol. 1979;41:127–140. doi: 10.1146/annurev.ph.41.030179.001015. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Anderson ME, Filion M. Responses of pontomedullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res. 1974;21:19–44. doi: 10.1007/BF00234256. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and branching of reticulospinal neurons. Exp Brain Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate—recovery after selective spinal cord lesions. Acta Physiol (Oxf) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Strategies for the integration of posture and movement during reaching in the cat. J Neurophysiol. 2003;90:3066–3086. doi: 10.1152/jn.00339.2003. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol. 2006;96:2229–2252. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol. 2008;100:2235–2253. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- Shapovalov AL. Extrapyramidal monosynaptic and disynaptic control of mammalian alpha-motoneurons. Brain Res. 1972;40:105–115. doi: 10.1016/0006-8993(72)90114-x. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr Clin Neurophysiol. 1969;26:549. [PubMed] [Google Scholar]
- Sprague JM, Chambers WW. Control of posture by reticular formation and cerebellum in the intact, anesthetized and unanesthetized and in the decerebrated cat. Am J Physiol. 1954;176:52–64. doi: 10.1152/ajplegacy.1953.176.1.52. [DOI] [PubMed] [Google Scholar]