Abstract
Recent studies have revealed the critical role of programmed death-1 (PD-1) in exhaustion of HIV- and SIV-specific CD8+ T cells. In this study, we show that high expression of PD-1 correlates with increased ex vivo spontaneous and CD95/Fas-induced apoptosis, particularly in the “effector-memory” CD8+ T cell population from HIV+ donors. High expression of PD-1 was linked to a proapoptotic phenotype characterized by low expression of Bcl-2 and IL7-Rα, high expression of CD95/Fas and high mitochondrial mass. Expression of PD-1 and CD57 was differentially associated with the maturation status of CD8+ T cells in HIV infection. CD57 was linked to higher apoptosis resistance, with cells expressing a PD-1LCD57H phenotype exhibiting lower levels of cell death. The majority of HIV-specific CD8+ T cells were found to express a PD-1HCD57L or PD-1HCD57H phenotype. No correlation was found between PD-1 expression and ex vivo polyfunctionality of either HIV- or CMV-specific CD8+ T cells. Contrary to CD57, high expression of PD-1 was characterized by translocation of PD-1 into the area of CD95/Fas-capping, an early necessary step of CD95/Fas-induced apoptosis. Thus, our data further support the role of PD-1 as a preapoptotic factor for CD8+ T cells in HIV infection.
Despite a broad HIV-specific CD8+ T cell response, the immune system ultimately fails to control the virus. It is now well recognized that some functions of virus-specific immunity are defective in HIV infection (1). In particular, HIV-specific CD8+ T cells exhibit an exhausted phenotype characterized by reduced ex vivo survival (2) and impaired cytokine production (3). However, the molecular mechanism(s) leading to this exhaustion remain undefined.
Our previous studies have shown that HIV-specific CD8+ T cells are characterized by 1) reduced Bcl-2 related anti-apoptotic potential (4); 2) high binding of the mitochondrial-specific dye Mitotracker Green FM (an index of mitochondrial mass) (5); and 3) increased expression of programmed death-1 (PD-1)3 (6), a negative regulator of T cells (7). In terms of their ability to produce cytokines directly ex vivo, an inverse correlation was found between viral load and the polyfunctionality of HIV-specific CD8+ T cells in HIV progressors (3). Furthermore, the preservation of polyfunctional HIV-specific CD4+ and CD8+ T cell responses could be at least partially responsible for the good clinical outcome observed in HIV-2, as compared with HIV-1, infection (8).
PD-1 is a negative regulator of T cells, originally identified as a surface receptor involved in apoptosis (9). An increasing body of evidence has revealed the critical role of PD-1 in regulating virus-specific T cell responses both in vivo (10–12) and ex vivo (13–15). Although this role is well established at the cellular level, the molecular pathways linking PD-1 to exhaustion of virus-specific T cells in chronic infection are poorly understood. Some studies have linked PD-1 expression to a reduced ability of T cells to produce cytokines (10, 13, 16). More recently, a correlation was found between PD-1 expression on effector CD8+ T cells and the frequency of IL-10+ suppressor CD8+ T cells in advanced HIV infection (17). We, like others, have emphasized the predominant role of this receptor in regulating the survival of T cells (6, 11, 18–21). PD-1-induced signaling suppresses PI3K/Akt activation and reduces the expression of Bcl-xL, a potent anti-apoptotic factor (22), in human T cells (23, 24). Activation of the PI3K/Akt axis is a critical mediator that transduces a plethora of extracellular signaling events into multiple functional outcomes affecting metabolism, survival, and proliferation of T cells (25, 26).
The surface molecule CD57 has been used as a marker of replicative senescence in HIV infection (27, 28). Expression of CD57 was associated with an inability of HIV-specific CD8+ T cells to divide, and total CD57+CD8+ T cells were prone to ex vivo death upon stimulation with PMA for 48 h (27). Furthermore, high expression of adhesion molecules, cytotoxic potential, and low expression of cell-cycle related genes was recently described for CD8+CD57+ T cells from both HIV infected and uninfected subjects, suggesting that such cells migrate to nonlymphoid tissues without further cycling (29). A recent study, however, described that CD8+CD57+ T cells from healthy donors do have a capacity for rapid expansion and production of IL-5, an anti-apoptotic cytokine (30, 31), upon TCR stimulation (32), thereby challenging the use of CD57 as a marker of terminal differentiation.
In this study, we further investigated the role of PD-1 in regulating HIV-specific CD8+ T cell survival. Our data show that PD-1 and CD57 reach maximal expression on CD8+ T cells at opposing ends of the memory maturation spectrum. HIV-specific CD8+ T cells predominantly express a PD-1H/DCD57L phenotype. CD8+ T cells expressing only CD57 are resistant to spontaneous and CD95/Fas-induced apoptosis. PD-1HCD8+ T cells express a proapoptotic phenotype, characterized by reduced levels of Bcl-2, increased mitochondrial mass and high levels of CD95/Fas. Finally, copolarization of PD-1 and CD95/Fas was observed during experimentally induced CD95/Fas capping.
Materials and Methods
Donors
Peripheral blood was collected from HIV+ individuals (n = 32) and HIV− donors (n = 5). Signed informed consent was obtained in accordance with the Declaration of Helsinki and approved by the relevant Institutional Review Board. All HIV+ individuals were infected for at least 1 year (range, 1–24 years), the median CD4 count was 395 cells/μl (range, 4–1401 cells/μl), and the median viral load was 460 RNA copies/ml plasma (range, <50 to 1325850 RNA copies/ml blood); 10 individuals were asymptomatic and 21 were on antiretroviral treatment. The vast majority of experiments were conducted using freshly isolated PBMC; in other cases, cells were cryo-preserved until use. RPMI 1640 (Life Technologies) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin-sulfate was used for culturing PBMC or Jurkat cells. Fresh myeloid dendritic cells (mDC) were prepared, cultured, and stimulated with a TLR-7/8 agonist as previously described (6).
Abs-fluorescent reagents
The following directly conjugated mAbs were used: 1) CD3-Cy7 allophycocyanin, CD8-allophycocyanin, IFN-γ-FITC, TNF-α-Cy7PE, PD-1-FITC, active capsase 3-PE, Bcl-2-FITC, CD95/Fas-PE, CD95/Fas-allophycocyanin, CD57-FITC, CD70-FITC, CD11c-allophycocyanin (BD Biosciences) and 2) CD45RO-TexasRedPE, CD127-PE (Beckman Coulter). The following mAbs were conjugated in our laboratory (http://drmr.com/abcon/index.html): IL-2–allophycocyanin, CD14–Pacific blue, CD19–Pacific blue, CD8-Qdot 705, CD8-Qdot 585, CD57-Qdot 545, CD57-Qdot 705, CD27-Cy5PE, CCR7-Cascade blue. The unconjugated mAbs were obtained from BD Biosciences. Cascade blue was obtained from Molecular Probes and Cy5 from BD Biosciences. Quantum dots were obtained from Invitrogen. The violet (vivid), aqua and green (gravid) amine reactive viability dyes were obtained from Invitrogen. Annexin V-FITC and annexin V-allophycocyanin were obtained from BD Bioscience. Biotinylated anti-PD-1 Ab was obtained from R&D Systems (BAF 1086) and streptavidin (Cy7PE or Qdot 655) was obtained from Molecular Probes. The mitochondria specific dye MitoTracker Green FM was obtained from Molecular Probes.
Flow cytometry
Cells were analyzed with a modified LSRII flow cytometer (BD Immunocytometry Systems) as previously described (6). Between 200,000 and 1 × 106 events were collected and electronic compensation was conducted with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. Data were analyzed using FlowJo version 8.0 (TreeStar). Forward scatter area vs forward scatter height was used to gate out cell aggregates. CD14+, CD19+, and dead cells were removed from the analysis to reduce background staining. For mitochondrial mass evaluation, PBMC were stained for surface markers using CD3-Cy7 allophycocyanin, CD8-allophycocyanin, PD-1-SA-655, CD27-Cy5PE, CD45RO-TRPE, and vivid (or aqua in combination with annexinV-Cascade blue) then incubated with 100 nM of MitoTracker Green FM for 45 min at 37°C, 5% CO2. Cells were fixed with 1% paraformaldehyde and events were collected immediately after the assay was completed. The combination Grivid/CD3-Cy7 allophycocyanin/CD8-Qd705/CD27-Cy5PE/CD45RO-TRPE/PD-1-SA-Cy7PE/CD127-PE/CCR7-Cascade blue was used for further characterization of cells located in the CD27H CD45ROH compartment. CD70 expression was analyzed in αCD3/αCD28 stimulated PBMC from HIV− donors and sorted, live mDC (CD14low CD11chigh) stimulated through TLR 7 and 8.
Apoptosis studies
PBMC (1–1.5 × 106) were cultured in 24-well plates (BD Biosciences) in the absence or presence of plate-bound anti-human CD95/Fas (IgM, CH11; Upstate Biotechnology; 5 μg/ml) for 12–14 h at 37°C. Cells were harvested, washed, and surface stained with annexin V, CD3, CD8, CD27, CD45RO, PD-1, CD57, CD14, CD19 and violet amine reactive viability dye. In some experiments surface staining was followed by fixation/permeabilization (Cytofix/CytoPerm kit; BD Biosciences) and intracellular staining with anti-active caspase 3. When MitoTracker Green FM was used, cells were incubated for 45 min at 37°C in the presence of 100 nM MitoTracker followed by surface staining. In all staining steps 2.5 mM CaCl2 was included.
Cytokine production
PBMC (2 × 106) were diluted to 1 ml with medium containing the co-stimulatory mAbs (αCD28 and αCD49d) (1 μg/ml each; BD Biosciences), monensin (0.7 μg/ml; BD Biosciences), and brefeldin A (10 μg/ml; Sigma-Aldrich), in the absence or presence of peptides (15mers overlapping by 11 residues) corresponding to full-length HIV-1 Gag (2 μg/ml each peptide, 5 μl/ml; National Institutes of Health AIDS Research and Reference Reagent Program) or CMV pp65 Ag (2 μg/ml, 5 μl/ml; Microbix Biosystems) for 6 h. After washing, cells were surface stained for PD-1, CD57, CD8, CD27, CD45RO, CD14/CD19, and violet amine reactive viability dye. Following permeabilization (Cytofix/Cytoperm kit; BD Biosciences), cells were stained for CD3, IFN-γ, IL-2, and TNF-α.
Plasmids and transfection
PD-1 was amplified from human-activated T cell cDNA using Phusion DNA polymerase (New England Biolabs). The 5′ primer included a Kozak sequence for higher expression in eukaryotic cells (33). The amplified open reading frame was cloned into the mammalian expression vector pCI (Pro-mega). Insert integrity was confirmed by sequencing. PD-1 was fused to the enhanced GFP mutant FRED143 (34) cloned into the pCMVkan (35) that provided the CMV promoter and growth hormone polyadenylation signal. Cultured Jurkat cells (American Type Culture Collection) were transfected with PD-1-GFP or empty parental vector using the Amaxa system (kit V, program X-001, Amaxa). Following overnight culture, live (vivid-) cells were sorted and subjected to further analysis.
Imaging experiments: confocal analysis
CD95/Fas capping was induced in live-sorted transfected Jurkat cells as previously described (5, 36). In brief, 1–2 × 106 cells were incubated with anti-CD95/Fas Ab (clone CH-11; final concentration 10 μg/ml) for 30 min on ice, transferred to 37°C for 2–3 min and transferred again on ice. After incubation with a Rhodamine Red-X-conjugated goat anti-mouse IgM Ab (Jackson Immunoresearch Laboratories), cells were transferred onto poly-L-lysine-coated slides (Sigma-Aldrich), mounted with mounting medium (Gold anti-FADE with 4′, 6-diamidine-2′-phenylindole dihydrochloride, Invitrogen), and visualized using a confocal microscope (Leica TCS SP2; Leica Microsystems) equipped with a 63×/1.4 oil-immersion objective lens (Leica). The Z-stack feature of the software was used to obtain a library of images of various sections of cells; three-dimensional images were acquired using Leica Confocal Software (Leica Microsystems), and Adobe Photoshop 6.0 software (Adobe Systems) was used to process them. Multitracking mode was used to eliminate spillover between fluorescence channels.
Imaging experiments: ImageStream analysis
Untransfected Jurkat cells were stained with anti-CD95/Fas-PE, phalloidin-TRPE (Molecular Probes), and Draq5 (for nuclear localization, Biostatus) or anti-CD3ε-PE, anti-TCRαβ-FITC, and Draq5. Alternatively, Vivid− PD-1-GFP transfected Jurkat cells were stained with an anti-CD95/Fas-PE Ab and Draq5 following CD95/Fas capping and fixed with 1% paraformaldehyde. Primary Vivid−CD3+CD8+CD27− T cells were sorted from HIV infected donors under sterile conditions by using a FACS Aria system in a BSL-3 facility and incubated for 1 to 3 h at 37°C with CH-11 Ab. Cells were washed and stained with anti-PD-1-FITC (or anti-CD57-FITC), anti-CD95/Fas-PE, and Draq5. Primary or Jurkat cells were analyzed on an ImageStream Imaging Flow Cytometer (Amnis Corporation) using 488 nm laser excitation. Classifiers were used to eliminate collection of debris based on low area in the brightfield imagery while camera saturating events, based on the presence of peak intensities greater than 1022, were also excluded. Typical files contained imagery for 10,000 to 60,000 cells with each cell imaged with side scatter, brightfield, and three channels of fluorescence. Images of fixed cells were analyzed using ImageStream Data Exploration and Analysis Software. Spectral compensation was digitally performed on a pixel-by-pixel basis before data analysis. Following compensation, similarity analysis was conducted on in-focus single cell images (supplementary Fig. 2B, lower panel)4 (37).
Statistical analysis
Experimental variables were analyzed using the nonparametric Mann-Whitney U test. Spearman rank correlation analysis was used when clinical/demographic data and experimental variables were analyzed. The effect of antiretroviral treatment on measured parameters was analyzed by applying the Mann-Whitney U test. Bars depict median values. When “boxes and whiskers” graphs are used, the box size represents the limits of the data for the second and third quartiles. p values <0.05 were considered significant. The GraphPad Prism statistical analysis program (GraphPad Software) was used throughout. Analysis and graphical representation of cytokine production in relation to PD-1 expression was conducted by using the data analysis program Simplified Presentation of Incredibly Complex Evaluations (SPICE version 2.9; provided by M. Roederer, National Institutes of Health, Bethesda, MD) (38).
Results
PD-1HCD27LCD45ROH/DCD8+ T cells from HIV-infected donors have the greatest sensitivity to ex vivo spontaneous and CD95/Fas-induced apoptosis
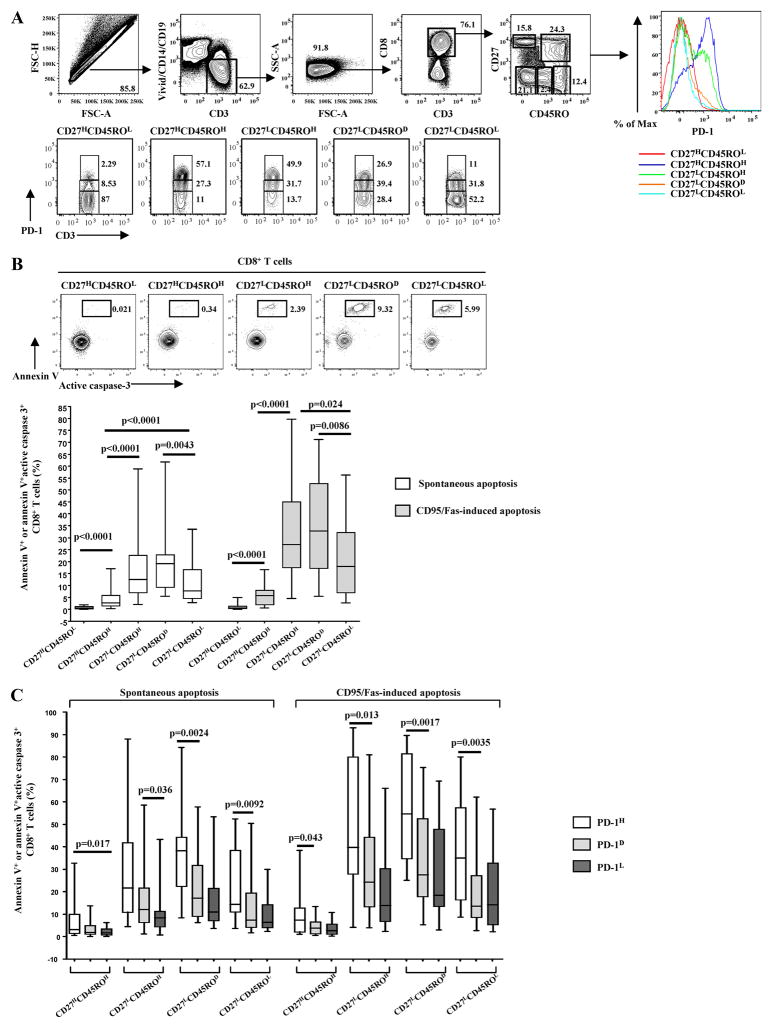
Our previous studies have shown that PD-1 expression is associated with increased susceptibility to ex vivo apoptosis of total and virus-specific CD8+ T cells from HIV-infected donors, irrespective of Ag specificity (6). Furthermore, the absolute level of PD-1 expression was the primary indicator of apoptosis sensitivity of virus-specific CD8+ T cells (6). In this study, we sought to further investigate the role of PD-1 in the ex vivo apoptosis of CD8+ T cells from HIV positive donors. Polychromatic flow cytometry was used to quantify the expression level of PD-1 (high/dim/low) within multiple memory and naive CD8+ T cell compartments (Fig. 1A). Naive (CD27HCD45ROL) CD8+ T cells were used to set the gates for PD-1 expression on memory CD8+ T cell populations (Fig. 1A, lower panel). Apoptosis sensitivity was measured either using annexin V or an Ab against activated caspase 3 or both (Fig. 1B). Positivity for annexin V was consistently accompanied by activation of caspase 3 (Fig. 1B, upper panel), indicating that the apoptosis is caspase-mediated, in agreement with previous studies (4, 39 – 42). There was increased spontaneous and CD95/Fas-induced apoptosis with progressive maturation of CD8+ T cells (Fig. 1B, lower panel). Specifically, CD27LCD45ROH and CD27LCD45ROD were the populations expressing the highest apoptosis sensitivity (Fig. 1B). CD27HCD45ROH cells expressed the lowest sensitivity among memory populations, although this sensitivity was still significantly higher (p < 0.0001, for both spontaneous and CD95/Fas-induced apoptosis) than of naive CD8+ T cells (Fig. 1B). When apoptosis sensitivity was analyzed in relation to PD-1 expression, ex vivo survival was found to be significantly reduced in cells expressing a PD-1high phenotype in all memory populations tested (Fig. 1C). A similar trend, although not statistically significant, was observed between PD-1D and PD-1L cells (Fig. 1C). In addition, CD8+ T cells from HIV uninfected individuals showed similar characteristics. PD-1HCD27LCD45ROH was the population exhibiting the highest sensitivity to both spontaneous (5.4 ± 4.4 vs 0.5 ± 0.2 (%)Vivid−AnnexinV+CD8+ T cells for PD-1H and PD-1L populations, respectively) and CD95/Fas-induced apoptosis (5 ± 1.9 vs 0.84 ± 0.2 for PD-1H and PD-1L populations, respectively). No correlation was found between age, CD4 T cell counts, and the percentage of CD8+ T cells expressing a PD-1H phenotype in memory populations. A negative correlation, however, was found between CD8 T cell counts and this percentage in the CD27LCD45ROD population (p = 0.0023). In contrast, a strong positive correlation was found between viral load and the percentage of PD-1HCD8+ T cells in the CD27HCD45ROH (p = 0.0007), CD27LCD45ROH (p = 0.0001) and CD27LCD45ROD (p = 0.0029) populations. The percentage of PD-1HCD27LCD45ROHCD8+ T cells was significantly lower in Highly Active Antiretroviral Therapy (HAART)-treated donors (7.1 ± 9) compared with untreated ones (31.9 ± 13, p = 0.006). No correlation was found between age, CD4 T cell counts, CD8 T cell counts, viral load, and ex vivo spontaneous or CD95/Fas-induced apoptosis within total CD8+ T cell memory populations. PD-1H cells, however, in the CD27LCD45ROH compartment from treated donors were more sensitive to CD95/Fas-induced apoptosis compared with treatment naive individuals (p = 0.0231). In agreement with our previous data, these data show that high expression of PD-1 is a strong indicator of poor ex vivo survival of memory CD8+ T cells from HIV-infected donors (6). This is most evident in highly differentiated memory populations.
FIGURE 1.
The absolute expression of PD-1 is a primary indicator of ex vivo apoptosis of CD8+ T cells in HIV infection. A, The polychromatic flow cytometry gating scheme for identification of CD8+ T cell populations expressing low, dim, and high levels of PD-1 is shown. Histograms depict the PD-1 expression in naive and memory populations of CD8+ T cells from the same sample. Memory subsets identified by CD27 and CD45RO staining of total CD8+ T cells are also presented. B, Representative flow cytometry plots showing simultaneous measurement of annexin V binding and active caspase 3 levels in naive and memory CD8+ T cell from an HIV+ donor cultured for 12–14 h at 37°C (upper panel). Pooled data showing the percentage (%) of apoptotic naive and memory CD8+ T cells from HIV+ donors (n = 26) cultured in the absence or presence of anti-CD95/Fas Ab for 12–14 h (lower panel). C, Pooled data showing the percentage (%) of spontaneous and CD95/Fas-induced apoptosis in naive and memory CD8+ T cell compartments from HIV+ donors (n = 26) and in relation to expression of PD-1. Apoptosis sensitivity was evaluated based on annexin V binding or the simultaneous measurement of annexin V binding and active caspase 3 expression. Bars depict median values. p values were calculated using Mann-Whitney U test.
High expression of PD-1 in CD8+ T cells from HIV-infected donors is accompanied by a proapoptotic phenotype
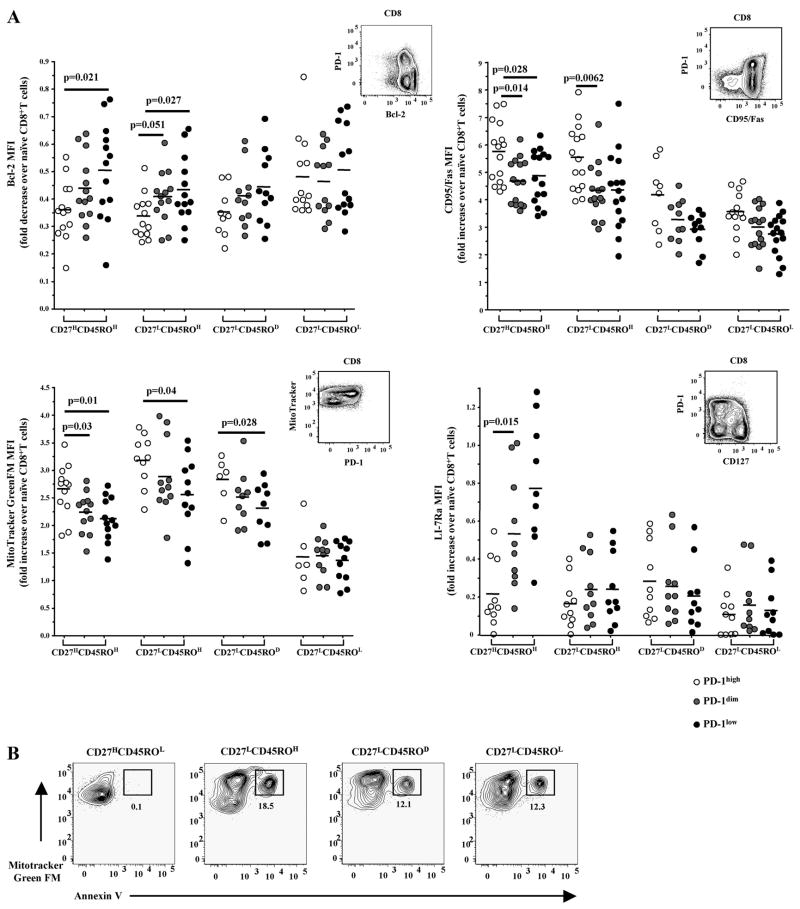
The molecular mechanism(s) governing the increased ex vivo apoptosis sensitivity of CD8+ T cells in HIV-infected donors is not well understood. Our previous studies have revealed a potential role for Bcl-2 family molecules in regulating this phenotype (4). In addition, we have also shown that “mitochondrial mass” is increased, especially in HIV-specific CD8+ T cells, suggesting a role for mitochondria in this phenotype (5). Therefore, the relation between PD-1 and apoptosis-related factors was analyzed in CD8+ T cells from HIV-infected donors. To minimize variations due to comparison of mean fluorescence intensity (MFI) values obtained from different experiments all parameters were expressed as fold change over values in naive CD8+ T cells. Similar data, however, were obtained when raw MFI values were analyzed (data not shown). Bcl-2 levels were found to be reduced in PD-1H as compared with PD-1D CD8+ T cells in all memory compartments, with the exception of CD27LCD45ROL cells (Fig. 2A, upper left panel). The difference in Bcl-2 expression was most evident in the CD27LCD45ROH population (p = 0.05 and p = 0.027 for comparison between PD-1H/PD-1D and PD-1H/PD-1L, respectively) (Fig. 2A, upper left panel). Similarly, PD-1H cells were found to express higher levels of CD95/Fas surface receptor than PD-1D cells, reaching statistical significance in CD27HCD45ROH (p = 0.014 for PD-1H vs PD-1D) and CD27LCD45ROH cells (p = 0.0062 for PD-1H vs PD-1D) (Fig. 2A, upper right panel). Previously, MitoTrackerGreen FM has been used as a marker of “mitochondrial mass” (5, 43, 44). PD-1H cells showed increased binding of MitoTracker in all memory CD8+ T cell compartments with the exception of CD27LCD45ROL cells (Fig. 2A, lower left panel). This binding was significantly higher compared with 1) PD-1D (p = 0.03) and PD-1L (p = 0.01) cells in the CD27HCD45ROH compartment; 2) PD-1L (p = 0.04) cells in the CD27LCD45ROH compartment; and 3) PD-1L (p = 0.03) cells in CD27LCD45ROD memory populations. In parallel, apoptosis sensitivity was measured in relation to Mitotracker binding in memory populations. In agreement with our previous data (5) annexin V positivity was almost exclusively detected in the MitotrackerH compartment (Fig. 2B). Next, the expression of IL-7Rα (CD127), a major survival factor for memory CD8+ T cells (45), was examined in relation to PD-1 expression in all memory compartments. CD127 was found to be predominantly expressed in the CD27HCD45ROH compartment (Fig. 2A, lower right panel). This expression was significantly reduced in PD-1H cells (p = 0.015 for PD-1H vs PD-1D and 0.002 for PD-1H vs PD-1L, respectively).
FIGURE 2.
CD8+PD-1H T cells are characterized by a proapoptotic phenotype. A, Pooled data showing the ex vivo expression of Bcl-2 (upper left), CD95/Fas (upper right), mitochondrial mass (lower left), and IL-7Rα (CD127) (lower right) in memory CD8+ T cell populations from HIV+ individuals in relation to PD-1 expression. MFI values for the parameters tested were expressed as fold increase (decrease) over those of naive cells. Representative flow cytometry plots are also shown. Horizontal lines depict median values. p values were calculated using Mann-Whitney U test. B, Representative flow cytometry plots showing annexin V binding in relation to mitochondrial mass (binding of Mitotracker Green FM) of memory CD8+ T cells from HIV+ donor cultured for 12–14 h at 37°C.
Surface expression of PD-1, CD127, and CCR7 was then assessed in the memory CD8+ T cell compartments (supplementary Fig. 1). A significant proportion of CD27HCD45ROH cells from HIV-infected donors were found to express a CCR7H phenotype, although this expression was lower compared with CCR7 in naive cells (supplementary Fig. 1). Our analysis revealed that this memory compartment also contains a population expressing a PD-1LCD127HCCR7H phenotype (supplementary Fig. 1). This population exhibited a Bcl-2HCD95LMitotrackerL phenotype and minimum levels of ex vivo spontaneous as well as CD95/Fas-induced apoptosis (Fig. 1C, annexin V positivity for PD-1L cells).
Differential association of PD-1 and CD57 with survival ability of CD8+ T cells from HIV-infected donors
CD57 expression is increased on T cells from HIV-infected donors and under conditions associated with immune activation and increasing age (46, 47). HIV-specific CD8+ T cells expressing CD57 were found to exhibit replicative senescence and are characterized by increased sensitivity to ex vivo death upon stimulation with PMA (27). We therefore investigated the relative expression of PD-1 and CD57 on total and virus-specific CD8+ T cells from HIV-infected donors as well as their association with ex vivo spontaneous and CD95/Fas-induced apoptosis.
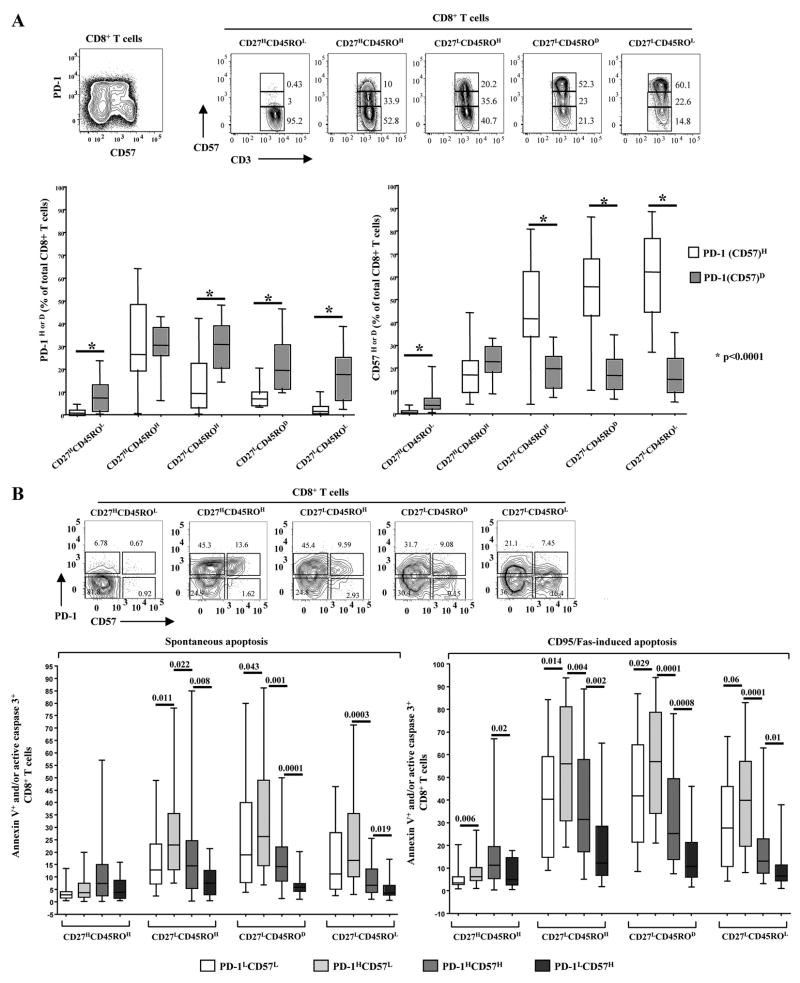
CD57 levels gradually increased during maturation of CD8+ T cells with the CD27LCD45ROL memory compartment expressing the highest levels (Fig. 3A). Analyzing both PD-1 and CD57, we observed that the two surface receptors reach maximal expression levels at opposing ends of the maturation spectrum (Fig. 3A, lower panel). The ex vivo sensitivity to spontaneous and CD95/Fas-induced apoptosis was examined in memory populations expressing all possible combinations of PD-1 and CD57 receptors (Fig. 3B, lower panel). The PD-1HCD57L population exhibited the highest sensitivity to apoptosis for both types of treatments in all but the CD27H CD45ROH memory populations (Fig. 3B, lower panel). In contrast, cells expressing CD57 in the absence of PD-1 were the most resistant to ex vivo death (Fig. 3B, lower panel). Interestingly, the apoptosis sensitivity of CD8+PD-1LCD57H cells was significantly lower than that of CD8+PD-1HCD57H cells. Furthermore, spontaneous and CD95/Fas-induced apoptosis was comparable within the CD8+PD-1LCD57H compartment across all memory populations (Fig. 3B, lower panel).
FIGURE 3.
Differential association of PD-1 and CD57 with ex vivo spontaneous and CD95/Fas-induced apoptosis of CD8+ T cells from HIV infected donors. A, Representative flow cytometry plots showing the expression of PD-1 vs CD57 (upper left) as well as the expression of CD57 in memory CD8+ T cell populations (upper right). Pooled data showing the relative expression of PD-1 and CD57 in relation to maturation status of CD8+ T cells from HIV+ donors (n = 26) are shown in the lower panels. Bars depict median values. p values were calculated using the Mann-Whitney U test. B, Representative plots showing the expression of PD-1 in relation to CD57 one in naive and memory CD8+ T cell populations (upper panel). Pooled data showing the percentage (%) of apoptotic memory CD8+ T cells from HIV+ donors (n = 28) with respect to PD-1 and CD57 expression are shown in the lower panels. Bars depict median values. p values were calculated using Mann-Whitney U test.
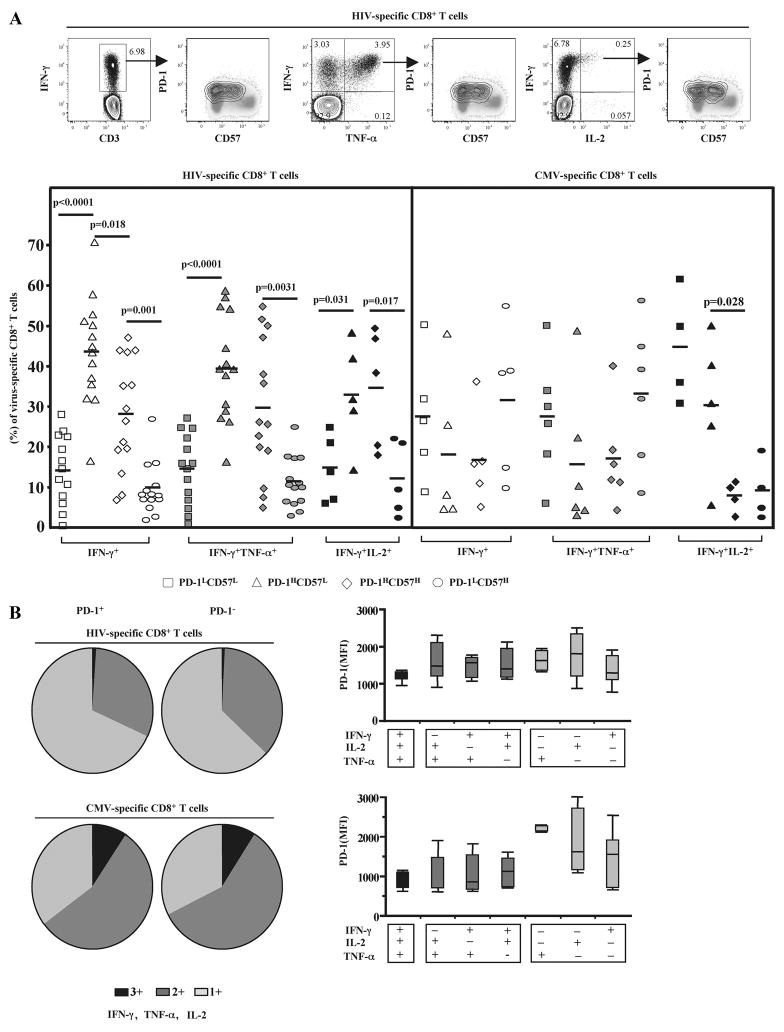
Next, the expression of PD-1 and CD57 on virus-specific CD8+ T cells from HIV infected donors was investigated. Virus-specific CD8+ T cells were identified by intracellular staining for IFN-γ, TNF-α and IL-2 production upon ex vivo stimulation with appropriate peptide pools (Fig. 4A, upper panel). Our gating strategy enables detection of CD8+ T cells exhibiting each and every combination of these three cytokines. No correlation between PD-1 expression and ex vivo cytokine production was found for either HIV- or CMV-specific CD8+ T cells (Fig. 4B); this was true for all of the cytokine combinations. The majority of HIV-specific CD8+ T cells were found to be either PD-1HCD57L or PD-1HCD57H while PD-1LCD57H was the least frequent population in all groups tested (Fig. 4A, lower panel). In contrast, CMV-specific CD8+ T cells were found to exhibit a PD-1LCD57L or PD-1LCD57H phenotype in IFN-γ and IFN-γ/TNF-α groups (Fig. 4A, lower panel). Interestingly, CMV-specific CD8+ T cells producing both IFN-γ and IL-2 expressed mostly a PD-1LCD57L or PD-1HCD57L phenotype (Fig. 4A, lower panel). Overall, our data show opposing expression of PD-1 and CD57 during CD8+ T cell maturation with PD-1 expression having the greatest impact upon ex vivo sensitivity to apoptosis.
FIGURE 4.
HIV-specific CD8+ T cells express predominantly a PD-1HCD57L phenotype that is not associated with their ex vivo ability to produce cytokines. A, Representative flow cytometry plots depicting the expression of PD-1 and CD57 in Gag-specific CD8+ T cells identified by IFN-γ+, IFN-γ+TNF-α+, or IFN-γ+IL-2+ cytokine production (upper panel). Pooled data showing the phenotype of HIV- and CMV-specific CD8+ T cells with respect to expression of PD-1 and CD57 (lower panel). Bars depict median values. p values were calculated using Mann-Whitney U test. B, Functional composition of the HIV- (n = 8) and CMV-specific (n = 8) CD8+ T cell responses in relation to PD-1 expression. Each slice of the pie represents the fraction of the total response that consists of CD8+ T cells positive for a given number of functions (left panel). MFI values of PD-1 for every possible combination of responses are shown on the x-axis (right panel). Boxes represent interquartile ranges; mean and SD lines are shown.
PD-1 but not CD57 copolarizes with CD95/Fas upon ex vivo induction of CD95/Fas capping
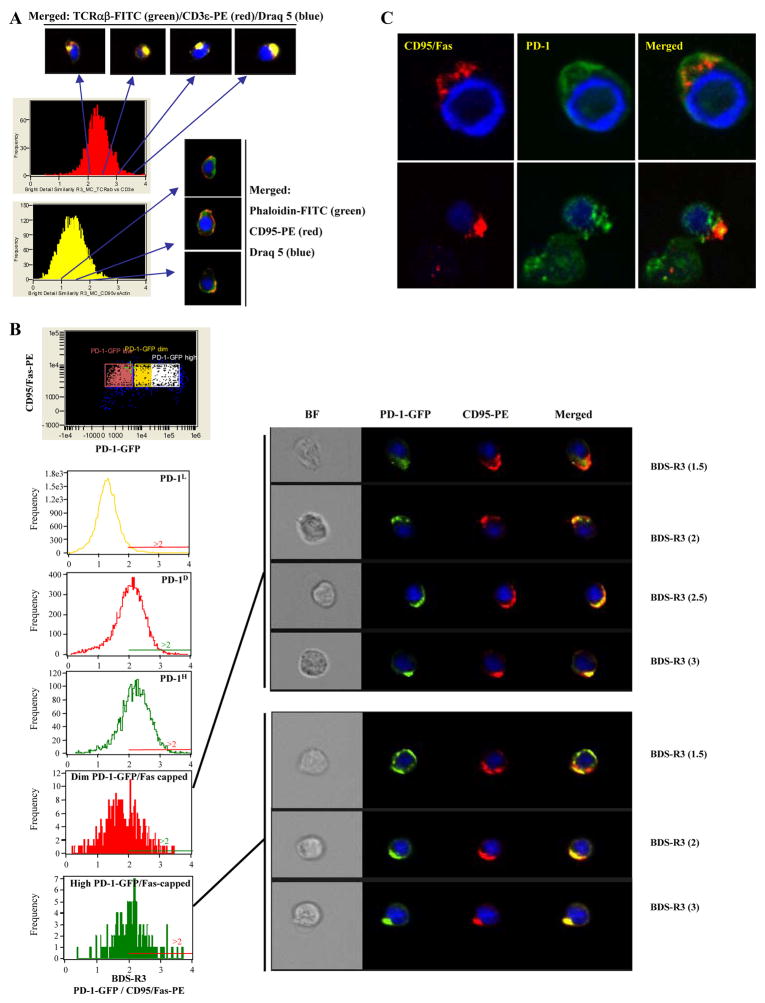
CD95/Fas capping is an essential early molecular event upon ex vivo cross-linking of the receptor (36, 48, 49). Recent studies, however, have shown that recruitment of other surface receptors in the proximal area of CD95/Fas capping can modulate CD95/Fas-induced apoptosis (50–52), possibly by affecting events at the level of the cytoplasmic membrane (50, 51). Therefore, we sought to examine the localization of PD-1 under ex vivo conditions promoting CD95/Fas capping. We started by using Jurkat cells, a cell line that does not express PD-1 (data not shown) and have been extensively used in CD95/Fas capping experiments (36, 49). Cells were transiently transfected with an expression vector coding for human PD-1 tagged with GFP. Primary CD8+ T cells transfected with this vector revealed that an anti-PD-1-PE Ab recognized PD-1-GFP, thereby confirming the proper surface expression of PD-1 by the transgene (supplementary Fig. 2A). The relative localization of PD-1-GFP and CD95/Fas under capping conditions was analyzed in live transfected Jurkat cells by using the Image Stream System. The parameter bright detailed similarity R3 (BDS-R3) was used to estimate the proximity of these two surface receptors in cells induced to cap CD95/Fas (Fig. 5, A and B). Jurkat cells were also stained either for TCRα β and CD3 or CD95/Fas and actin (Fig. 5A). BDS-R3 values >2 indicate proximal localization of the two surface receptors under investigation (Fig. 5A). PD-1 was localized in the proximal area of CD95/Fas capping in 51 ± 7.6% (n = 3 experiments) of cells expressing a high PD-1-GFP phenotype (Fig. 5B). Similar localization was found in 41 ± 1.5% and 6 ± 1% of the cells with dim and low PD-1-GFP expression, respectively (Fig. 5B). Control experiments using the parental-empty vector show a diffused distribution of GFP even in CD95/Fas-capped cells (supplementary Fig. 2C). We further analyzed colocalization using confocal microscopy. Similar to the previous analysis, capped cells were characterized by copolarization of PD-1 and Fas (Fig. 5C).
FIGURE 5.
Cotranslocation of PD-1 and CD95/Fas under ex vivo induction of CD95/Fas-capping. A, Jurkat cells were stained with either TCRab/CD3 or CD95/actin and the relative distribution of these molecules was analyzed using the ImageStream Imaging Flow Cytometer. Histograms depict the values of Bright Detailed Similarity R3 (an index of relative colocalization) for the pairs of molecules tested (left panel). Representative images showing the relative distribution of these molecules in Jurkat cells for different values of BDS-R3 (right panel). B, PD-1-GFP transfected Jurkat cells were analyzed under induction of CD95/Fas-capping. Histograms depict the BDS-R3 values for PD-1 and CD95/Fas localization in PD-1L, PD-1D, and PD-1H populations of total cells or cells in which CD95/Fas is capped (left panel). Representative images for different BDS-R3 values are shown (right panel). Draq 5 (a nuclear staining) is shown in blue, PD-1-GFP in green and CD95/Fas-PE in red. C, Confocal images of Jurkat cells showing the localization of PD-1-GFP and CD95/Fas under experimental conditions inducing CD95/Fas-capping.
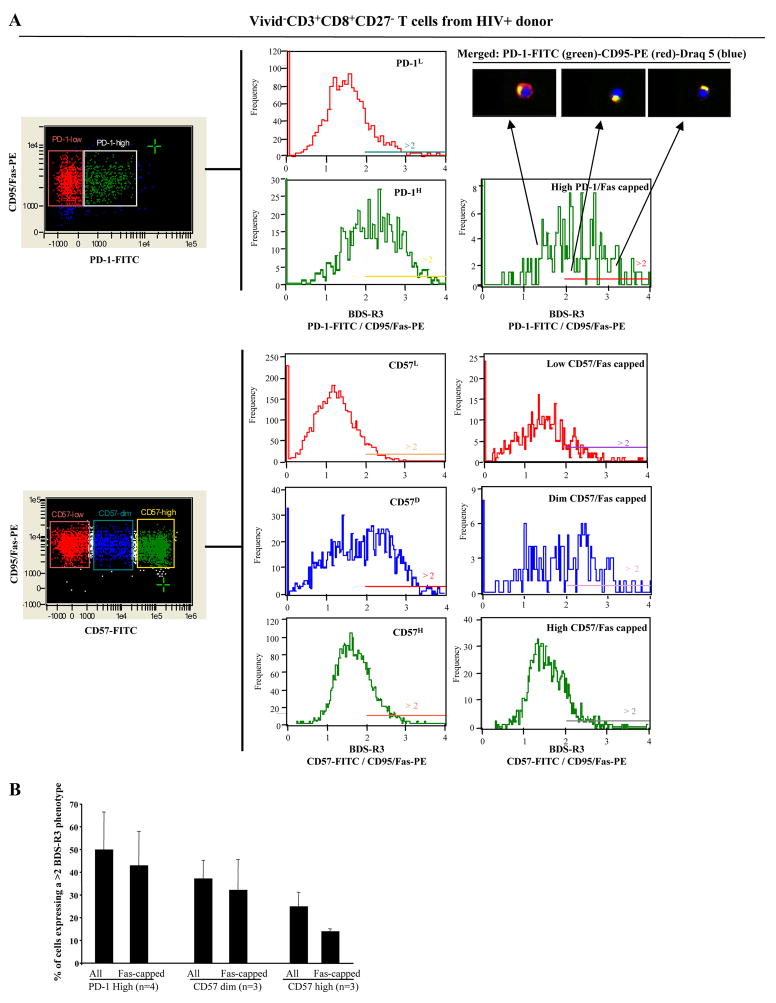
The relative distribution of PD-1 (or CD57) and CD95/Fas was next examined in primary sorted CD27−CD8+ T cells (the memory compartment that shows maximum sensitivity to ex vivo apoptosis) from HIV-infected donors under ex vivo conditions inducing CD95/Fas-capping. Again, endogenous PD-1 was found to cotranslocate to the area of capping (Fig. 6A). Similar data were obtained when sorted CD3+CD8+CD27LPD-1L cells were transfected with PD-1-GFP vector (supplementary Fig. 2D). In sharp contrast, CD57 was “excluded” from this area in the majority of capped cells, especially in cells expressing a CD57H phenotype (Fig. 6B). Taken together, our data reveal an orchestrated movement of PD-1 and CD95/Fas during the early steps of CD95/Fas-induced apoptosis, thereby further supporting an active role of PD-1 in this process.
FIGURE 6.
In contrast to PD-1, highly expressed CD57 is excluded from the area of CD95/Fas-capping in primary CD8+ T cells from HIV-infected donors. A, Flow cytometry plots showing the expression of PD-1 (CD57) and CD95/Fas in “focused” sorted Vivid−CD3+CD8+CD27H T cells from an HIV+ donor analyzed by the “ImageStream Data Exploration and Analysis Software” (left panel). Histograms depict the BDS-R3 values for PD-1 (CD57) vs CD95/Fas in total or CD95/Fas-capped cells and in relation to PD-1 (CD57) levels (right panel). Representative images for different BDS-R3 values are also shown. B, Pooled data showing the percentages (%) of primary sorted Vivid−CD3+CD8+CD27L T cells expressing a phenotype characterized by BDR-S3 values >2. Group of cells expressing high PD-1, dim CD57, and high CD57 levels are shown. Bars depict means ± SD.
Discussion
In this study, we report on the differential association of PD-1 and CD57 with ex vivo sensitivity to spontaneous and CD95/Fas-induced apoptosis in CD8+ T cells from HIV-infected donors. Furthermore, we provide data showing that PD-1 expression is linked to a proapoptotic phenotype of CD8+ T cells characterized by low expression of Bcl-2 and IL-7Rα, increased levels of CD95/Fas, and high mitochondrial mass. In agreement with our previous data (6), no correlation was found between PD-1 levels and ex vivo cytokine production analyzing either single or combinations of multiple cytokines (IFN-γ, TNF-α, and IL-2). Prior studies have revealed an increased sensitivity of CD45ROHCD8+ T cells from HIV-infected donors to ex vivo CD95/Fas-induced apoptosis (53, 54), a phenomenon associated predominantly with “effector memory” cells (2). In line with our previous data (6), ex vivo apoptosis was primarily observed in the CD27LCD45ROH and CD27L CD45ROD compartments followed by lower levels in the highly differentiated CD27LCD45ROL population. Although apoptosis in the CD27HCD45ROH memory compartment was significantly higher compared with the naive compartment (CD27HCD45ROL), this population still exhibits relatively high resistance to both spontaneous and CD95/Fas-induced apoptosis compared with other memory groups. Still, the majority of apoptotic cells are characterized by high expression of PD-1 even in this memory compartment. A hierarchy was found when apoptosis was analyzed in relation to PD-1 levels, indicating that the absolute level of PD-1 is a primary determinant of apoptosis sensitivity in memory CD8+ T cells (6), especially CD27LCD45ROH and CD27LCD45ROD cells. This is further supported by the finding of a similar trend in CD8+ T cells from HIV uninfected donors. The lower ex vivo apoptosis sensitivity of CD8+ T cells from HIV uninfected donors compared with HIV donors, even within the PD-1H compartment, indicates that additional mechanism(s) contribute to the high ex vivo apoptosis of CD8+ T cells in HIV infection. A strong correlation between viral load and PD-1 expression in memory CD8+ T cells was found. We have previously described the lack of such correlation when HIV-specific CD8+ T cells were analyzed (6). We hypothesize that chronic Ag-specific TCR stimulation, a major mechanism leading to high sustained PD-1 levels in virus-specific CD8+ T cells (20, 55), probably overrides non-TCR stimuli that potentially affect the expression of PD-1 in total CD8+ T cell populations in a non-Ag specific manner (56). Furthermore, the ex vivo lower percentage of PD-1H CD27LCD45ROH in HAART-treated donors is associated with a higher sensitivity to CD95/Fas-induced apoptosis compared with cells from untreated donors. Whether this is a reflection of higher in vivo turnover of PD-1H “effector memory” cells upon HAART treatment needs further investigation.
The survival ability of a mammalian cell is determined by numerous extracellular factors, which can induce death signals as well as the intracellular pathways that control the transduction of such signals. We analyzed several parameters that have been associated with the survival of CD8+ T cells from HIV+ donors. PD-1 was found to be consistently associated with a preapoptotic phenotype; specifically, that of high mitochondrial mass and low Bcl-2 expression. These data indicate that these cells are potentially more susceptible to mitochondria-mediated cell death. Whether PD-1 induces a direct signal affecting mitochondria or if this process could be mediated by other cellular pathways such as Akt-mediated signals (24) is not known and warrants further investigation.
A somewhat unexpected finding was that although CD27H CD45ROHCD8+ T cells express the highest levels of both PD-1 and CD95/Fas, they are relatively resistant to ex vivo apoptosis. These cells, however, express high levels of CD27, a receptor that delivers positive signals in vivo for CD8+ T cells which we recently reported can rescue virus-specific CD8+ T cells from CD95/Fas-induced death during the development of an anti-viral response (57). Our data show that ex vivo stimulated CD4+ and CD8+ T cells as well as mDC treated with a TLR7/8 agonist express high levels of CD70, the ligand for CD27 (supplementary Fig. 3), indicating that an extended in vivo cellular network could potentially provide survival signals to CD27H cells. Furthermore, mitochondrial mass was higher in CD27LCD45ROH and CD27HCD45ROD cells compared with CD27HCD45ROH cells while IL-7Rα was significantly higher on CD27HCD45ROH cells, particularly the ones expressing low levels of PD-1. The presence of CCR7 on many of them could also indicate a “central memory” phenotype that is associated with high resistance to apoptosis. Although the balance of surface receptors inducing pre- or proapoptotic signals may be important, execution of apoptosis is largely dependent on the relative expression/function of intracellular mediators of CD95/Fas-induced signaling. The relative expression of cellular caspase-8-like inhibitory protein (anti-apoptotic) and Fas-associated death domain (preapoptotic) could critically affect the CD95/Fas-induced apoptosis of T cells. More recently, the critical role of Rac-mediated signaling in this process was described (58). Investigation of the relative expression of such factors would be very informative regarding the sensitivity of memory CD8+ T cell populations to ex vivo apoptosis.
For CD95/Fas-induced apoptosis, two types of cell lines have been described based on apoptosis signaling pathways (59): 1) type I cells, in which apoptosis is dependent on the integrity of the actin network and high levels of CD95/Fas results in massive death-inducing signaling complex (DISC) formation and mitochondria-independent death; and 2) type II cells that express lower levels of CD95/Fas and DISC-induced signaling, in which apoptosis requires a mitochondria-dependent amplification process. This latter pathway of cell death is independent of actin. All of the CD8+ T cells we tested were positive for CD95/Fas, in agreement with our previous data (2). Our previous data have shown that Bcl-2-related molecules and mitochondria may play a critical role in the survival of HIV-specific CD8+ T cells (4, 5) while destruction of actin abolishes the CD95/Fas-induced apoptosis (60). Overall, our data indicate that primary PD-1H effector memory CD8+ T cells from HIV infected patients exhibit a mixed phenotype where the integrity of actin is necessary but the intracellular signal induced by the lower expression of CD95/Fas expression (compared with CD27HCD45ROH cells) can be amplified through an extended mitochondria network. This hypothesis is in agreement with recent reports showing that both CD95/Fas-induced and mitochondria-mediated pathways cooperate to shut down antiviral immune responses during chronic infection (61, 62).
The percentage of CD8+ T cells expressing CD57, a marker of replicative senescence in CD8+ T cells from HIV+ donors (27), was found to increase with differentiation reaching maximum levels in the CD27LCD45ROL population. We have previously shown that CD57HCD8+ T cells from HIV+ donors are susceptible to activation-induced cell death upon ex vivo treatment for 48 h (27). In this study, we report on the lack of association between CD57 and spontaneous or CD95/Fas-induced apoptosis. Previous studies(63, 64) have shown that TCR-induced apoptosis in HIV infection is CD95/Fas-independent. Furthermore, upon TCR-stimulation, both CD4+ and CD8+ T cells up-regulate PD-1 (65). Therefore de novo synthesized PD-1 could also contribute to activation-induced cell death. Our data also revealed that CD8+ T cells can express both PD-1 and CD57 and these cells are sensitive to apoptosis. The low sensitivity to ex vivo apoptosis of CD57HCD8+ T cells is in agreement with recent reports of in vivo accumulation of this population in HIV-infected donors (66). We have previously shown that HIV-specific CD8+ T cells are characterized by increased sensitivity to CD95/Fas-induced death even compared with other virus-specific CD8+ T cells, i.e., CMV-specific cells from the same HIV-infected donors (2). The PD-1/CD57 profile of HIV- and CMV-specific CD8+ T cells described in this study, according to which the majority of HIV-specific CD8+ T cells are characterized by a PD-1H and or PD-1HCD57H phenotype, is consistent with apoptosis sensitivity being predominantly mediated by PD-1.
CD95/Fas-capping is a very early and necessary molecular event during the formation of DISC and the initiation of death signaling (67). Recent studies have focused on the recruitment of other surface receptors to the area of CD95/Fas-capping (50–52). Such comobilization could affect the sequestration of CD95/Fas and DISC formation through physical interactions or by changing the dynamics of interactions between CD95/Fas and other signaling molecules (68). Our data show a copolarization between PD-1 and CD95/Fas upon ex vivo Fas capping in a large proportion of cells. In contrast, no such copolarization of CD95/Fas and CD57 was observed. How the cotranslocation of PD-1 and CD95/Fas receptors could affect the initiation of death signal(s) is currently under investigation.
In this study, we describe a population of CD8+ T cells exhibiting a CD27HCD45ROH IL7RαH CCR7H PD-1L CD95L Bcl-2H Mitochondrial MassL phenotype. This population is characterized by increased resistance to both spontaneous and CD95/Fas-induced cell death. Central memory cells are long-lasting cells presumably resistant to cell death. We propose that the use of combination of both differentiation and survival-related parameters could potentially help to further identify and characterize central memory cells. However, whether the above combination characterizes “central memory” CD8+ T cells or an early activated, less matured population is under investigation.
We have recently proposed a model where HIV-specific CD8+ T cells could be eliminated upon repetitive encounter with FasL expressed on HIV-infected cells (69). HIV-infected cells up-regulate FasL by a Nef-dependent mechanism (70, 71). HIV infection could also potentially induce the expression of PD-L1. We hypothesize that the orchestrated expression of both ligands in HIV-infected cells could further contribute to the elimination of HIV-specific CD8+ T cells.
Our data point to a complex regulation of CD8+ T cell survival that is linked to their differentiation level and the ability of intra-cellular pathways to transduce apoptotic signals. The low apoptotic potential of less mature CD27HCD45ROH cells is consistent with the hypothesis that expression of both PD-1 and CD95 are necessary but not sufficient for apoptosis of CD8+ T cells from HIV-infected donors. Furthermore, the collaboration of two surface receptors (PD-1 and CD95/Fas) could be crucial in the process of CD8+ T cell exhaustion in chronic viral infections.
Acknowledgments
We thank Dr. Jeffrey I. Cohen (National Institute of Allergy and Infectious Diseases) for providing access to the Image-Stream flow cytometer (Amnis).
Footnotes
The studies were supported in part by R01 AI46719 (to P.D.K.); D.A.P. is a Medical Research Council (U.K.) Senior Clinical Fellow.
Abbreviations used in this paper: PD-1, programmed death-1; mDC, myeloid dendritic cell; MFI, mean fluorescence intensity; BDS-R3, bright detailed similarity R3; DISC, death-inducing signaling complex; H, high; D, dim; L, low; HAART, Highly Active Antiretroviral Therapy.
The online version of this article contains supplementary material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 2.Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD, Katsikis PD. Increased CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Immunity. 2001;15:871–882. doi: 10.1016/s1074-7613(01)00246-1. [DOI] [PubMed] [Google Scholar]
- 3.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrovas C, Mueller YM, Dimitriou ID, Bojczuk PM, Mounzer KC, Witek J, Altman JD, Katsikis PD. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J Immunol. 2004;172:4444–4453. doi: 10.4049/jimmunol.172.7.4444. [DOI] [PubMed] [Google Scholar]
- 5.Petrovas C, Mueller YM, Dimitriou ID, Altork SR, Banerjee A, Sklar P, Mounzer KC, Altman JD, Katsikis PD. Increased mitochondrial mass characterizes the survival defect of HIV-specific CD8+ T cells. Blood. 2007;109:2505–2513. doi: 10.1182/blood-2006-05-021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Poly-functional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 11.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7–H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875–4884. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 14.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 17.Elrefaei M, Baker CA, Jones NG, Bangsberg DR, Cao H. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol. 2008;180:7757–7763. doi: 10.4049/jimmunol.180.11.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 19.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -γ and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–1949. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 23.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 24.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 26.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 27.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 28.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C, Combadiere B. High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV-infected and uninfected individuals. J Immunol. 2006;177:5145–5154. doi: 10.4049/jimmunol.177.8.5145. [DOI] [PubMed] [Google Scholar]
- 30.Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001;98:2239–2247. doi: 10.1182/blood.v98.7.2239. [DOI] [PubMed] [Google Scholar]
- 31.Uehara T, Miyawaki T, Ohta K, Tamaru Y, Yokoi T, Nakamura S, Taniguchi N. Apoptotic cell death of primed CD45RO+ T lymphocytes in Epstein-Barr virus-induced infectious mononucleosis. Blood. 1992;80:452–458. [PubMed] [Google Scholar]
- 32.Chong LK, Aicheler RJ, Llewellyn-Lacey S, Tomasec P, Brennan P, Wang EC. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur J Immunol. 2008;38:995–1000. doi: 10.1002/eji.200737687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 34.Stauber RH, Horie K, Carney P, Hudson EA, Tarasova NI, Gaitanaris GA, Pavlakis GN. Development and applications of enhanced green fluorescent protein mutants. BioTechniques. 1998;24:462–466. 468–471. doi: 10.2144/98243rr01. [DOI] [PubMed] [Google Scholar]
- 35.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori DC, Markham P, Felber BK, Pavlakis GN. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol. 2005;79:8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 37.George TC, Fanning SL, Fitzgeral-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, et al. Quantitative measurement of nuclear translocation events using similarity analysis of multi-spectral cellular images obtained in flow. J Immunol Methods. 2006;311:117–129. doi: 10.1016/j.jim.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnoult D, Petit F, Lelievre JD, Lecossier D, Hance A, Monceaux V, Hurtrel B, Ho Tsong Fang R, Ameisen JC, Estaquier J. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ. 2003;10:1240–1252. doi: 10.1038/sj.cdd.4401289. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira Pinto LM, Garcia S, Lecoeur H, Rapp C, Gougeon ML. Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1)- and TNFR2-mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood. 2002;99:1666–1675. doi: 10.1182/blood.v99.5.1666. [DOI] [PubMed] [Google Scholar]
- 41.Genesca M, Rourke T, Li J, Bost K, Chohan B, McChesney MB, Miller CJ. Live attenuated lentivirus infection elicits polyfunctional simian immunodeficiency virus Gag-specific CD8+ T cells with reduced apoptotic susceptibility in rhesus macaques that control virus replication after challenge with pathogenic SIVmac239. J Immunol. 2007;179:4732–4740. doi: 10.4049/jimmunol.179.7.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsikis PD, Garcia-Ojeda ME, Torres-Roca JF, Tijoe IM, Smith CA, Herzenberg LA, Herzenberg LA. Interleukin-1 β converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu X, Wan S, Lyu YL, Liu LF, Qi H. Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation. PLoS ONE. 2008;3:e2009. doi: 10.1371/journal.pone.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pendergrass W, Wolf N, Poot M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A. 2004;61:162–169. doi: 10.1002/cyto.a.20033. [DOI] [PubMed] [Google Scholar]
- 45.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 46.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 47.Merino J, Martinez-Gonzalez MA, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira ML. Progressive decrease of CD8high+ CD28+ CD57+ cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME. Molecular ordering of the initial signaling events of CD95. Mol Cell Biol. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gajate C, Mollinedo F. The antitumor ether lipid ET-18-OCH(3) induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860–3863. doi: 10.1182/blood.v98.13.3860. [DOI] [PubMed] [Google Scholar]
- 50.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111:2290–2299. doi: 10.1182/blood-2007-06-096529. [DOI] [PubMed] [Google Scholar]
- 51.Mielgo A, Brondani V, Landmann L, Glaser-Ruhm A, Erb P, Stupack D, Gunthert U. The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis. 2007;12:2051–2061. doi: 10.1007/s10495-007-0115-3. [DOI] [PubMed] [Google Scholar]
- 52.Giraud S, Lautrette C, Bessette B, Decourt C, Mathonnet M, Jauberteau MO. Modulation of Fas-induced apoptosis by p75 neurotrophin receptor in a human neuroblastoma cell line. Apoptosis. 2005;10:1271–1283. doi: 10.1007/s10495-005-2649-6. [DOI] [PubMed] [Google Scholar]
- 53.Gougeon ML, Lecoeur H, Dulioust A, Enouf MG, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 54.McCloskey TW, Bakshi S, Than S, Arman P, Pahwa S. Immunophenotypic analysis of peripheral blood mononuclear cells undergoing in vitro apoptosis after isolation from human immunodeficiency virus-infected children. Blood. 1998;92:4230–4237. [PubMed] [Google Scholar]
- 55.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 57.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramaswamy M, Dumont C, Cruz AC, Muppidi JR, Gomez TS, Billadeau DD, Tybulewicz VL, Siegel RM. Cutting edge: Rac GTPases sensitize activated T cells to die via Fas. J Immunol. 2007;179:6384–6388. doi: 10.4049/jimmunol.179.10.6384. [DOI] [PubMed] [Google Scholar]
- 59.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrovas C, Mueller YM, Yang G, Altork SR, Jacobson JM, Pitsakis PG, Mounzer KC, Altman JD, Katsikis PD. Actin integrity is indispensable for CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Apoptosis. 2007;12:2175–2186. doi: 10.1007/s10495-007-0128-y. [DOI] [PubMed] [Google Scholar]
- 61.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 63.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen JC. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–4966. [PubMed] [Google Scholar]
- 64.Katsikis PD, Garcia-Ojeda ME, Wunderlich ES, Smith CA, Yagita H, Okumura K, Kayagaki N, Alderson M, Herzenberg LA, Herzenberg LA. Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int Immunol. 1996;8:1311–1317. doi: 10.1093/intimm/8.8.1311. [DOI] [PubMed] [Google Scholar]
- 65.Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell Immunol. 2004;230:89–98. doi: 10.1016/j.cellimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Ladell K, Hellerstein MK, Cesar D, Busch R, Boban D, McCune JM. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J Immunol. 2008;180:7907–7918. doi: 10.4049/jimmunol.180.12.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter ME, Chan AC. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koncz G, Kerekes K, Chakrabandhu K, Hueber AO. Regulating Vav1 phosphorylation by the SHP-1 tyrosine phosphatase is a fine-tuning mechanism for the negative regulation of DISC formation and Fas-mediated cell death signaling. Cell Death Differ. 2008;15:494–503. doi: 10.1038/sj.cdd.4402282. [DOI] [PubMed] [Google Scholar]
- 69.Petrovas C, Mueller YM, Katsikis PD. Apoptosis of HIV-specific CD8+ T cells: an HIV evasion strategy. Cell Death Differ. 2005;12(Suppl 1):859–870. doi: 10.1038/sj.cdd.4401595. [DOI] [PubMed] [Google Scholar]
- 70.Xu XN, Laffert B, Screaton GR, Kraft M, Wolf D, Kolanus W, Mongkolsapay J, McMichael AJ, Baur AS. Induction of Fas ligand expression by HIV involves the interaction of Nef with the T cell receptor zeta chain. J Exp Med. 1999;189:1489–1496. doi: 10.1084/jem.189.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu XN, Screaton GR, Gotch FM, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott JE, McMichael AJ. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]