Abstract
The failure of CD8+ T cells to respond to chronic infection has been termed “exhaustion” and describes the condition in which CD8+ T cells exhibit reduced differentiation, proliferation, and effector function. CD8+ T cell exhaustion has been extensively studied in the murine model of chronic infection, lymphocytic choriomeningitis virus (LCMV). Although LCMV-based studies have yielded many interesting findings, they have not allowed for discrimination between the roles of cytokine- and Ag-driven exhaustion. We have created a system of chronic Ag stimulation using murine influenza A virus that leads to exhaustion and functional disability of virus-specific CD8+ T cells, in the absence of high viral titers, sustained proinflammatory cytokine production and lymphocyte infection. Our findings show that Ag alone is sufficient to drive CD8+ T cell impairment, that Ag-driven loss of virus-specific CD8+ T cells is TRAIL mediated, and that removal of Ag reverses exhaustion. Although programmed death 1 was up-regulated on chronic Ag-stimulated CD8+ T cells, it played no role in the exhaustion. These findings provide a novel insight into the mechanisms that control functional exhaustion of CD8+ T cells in chronic infection.
It is firmly established that during the course of many acute viral infections, the adaptive immune response, in particular CD8+ T cells, is critical for effective viral clearance (1-3). The failure of CD8+ T cells to fully function and eliminate virus may be responsible, in part, for the establishment of chronic viral infection. Indeed, defective CD8+ T cell function has been documented in HIV (4-7), hepatitis B (8, 9) and hepatitis C (10, 11) chronic viral infections. Recognized T cell defects span a broad range of functions, from impaired differentiation, proliferation, and effector function (5, 12-14) to increased susceptibility to apoptosis (15). Given the global significance of chronic viral infections, it is increasingly important to understand the defects that exist in exhausted CD8+ T cells, as well as the mechanisms that lead to this exhaustion.
There are several mouse models of chronic viral infection that lead to the development of functionally inactive, exhausted effector and memory CD8+ T cells. CD8+ T cell exhaustion has been documented following infection with lymphocytic choriomeningitis virus (LCMV)3 (16-20), murine hepatitis (21) and polyomavirus (22). Among these, LCMV infection is one of the most prominently studied chronic infections. During LCMV infection, chronic infection is achieved by infection of wild-type mice with high doses of rapidly replicating virus (18-20), or by infection of immunosuppressed mice that lack functional CD4+ T cells (16, 23), or perforin (24). Under these conditions, there is a profound loss of virus-specific CD8+ T cell expansion, and an accompanying loss of IL-2, IFN-γ, and TNF-α cytokine production by CTLs that do survive (19, 20). Exhaustion impacts multiple facets of virus-specific CD8+ T cell function. Accordingly, exhausted CD8+ T cells also exhibit a loss of cytotoxic function (19), and decreased mitogen induced proliferation (23).
Although many significant findings have been made using LCMV infection, previous studies have been unable to examine the contribution of individual factors to CD8+ T cell exhaustion. LCMV is characterized by the presence of sustained high viral loads, proinflammatory cytokine burst, and lymphocytic viral tropism (25, 26). Their combined presence prevents the delineation of the relative contribution of individual factors to clonal exhaustion, and therefore the capability of any of these individual events to drive exhaustion remains unknown. To address this issue, we created a novel system of chronic Ag stimulation, using the influenza A virus. In this model system, CD8+ T cells are repeatedly stimulated, in vivo, with live influenza virus. Live influenza virus was used, rather than inactivated or repeated peptide stimulation, to conserve all aspects of physiologically relevant priming of CD8+ T cells. The continuous stimulation of CD8+ T cells, in the absence of high viral load and cytokine burst, allowed direct examination of the contribution of chronic Ag stimulation to the development of CD8+ T cell exhaustion. Our data indicate that chronic Ag stimulation alone is sufficient to induce CD8+ T cell exhaustion, as shown by the failure of virus-specific CD8+ T cells to re-expand or produce IFN-γ upon viral rechallenge. Interestingly, the virus-specific CD8+ T cell response was restored either through a period of Ag rest or through inhibition of the TRAIL apoptosis pathway. Taken together, these data indicate that Ag alone is sufficient to drive CD8+ T cell exhaustion and may suggest that during more complex chronic infection, Ag stimulation should be a primary target for control.
Materials and Methods
Mice and reagents
Female, 8-wk-old, C57BL/6 mice were purchased from The Jackson Laboratory and used in all Ag stimulation and intranasal (i.n.) infections described. Female 8-wk-old OT-I TCR transgenic (Tg) mice, a gift from H. Shen (University of Pennsylvania, Philadelphia, PA), were bred at Drexel University College of Medicine (Philadelphia, PA) and used as donors for H1N1 WSN-OVA experiments. For TRAIL and MRL/lpr Fas-deficient studies, 8-wk-old TRAIL knockout mice (Amgen), MRL/lpr (The Jackson Laboratory), or C57BL/6 control mice were Ag-stimulated and rechallenged as described below. All mice were maintained in an American Association of Laboratory Animal Care-accredited facility and acclimatized for 1 wk before use. All experiments were completed following Institutional Animal Care and Use Committee approval. Chronic Ag stimulation and i.n. influenza rechallenge were achieved using H1N1 A/Puerto Rico/ 8/34 (PR8) or H3N2 HKx31 (x31), a gift from W. Gerhardt (University of Pennsylvania, Philadelphia, PA) or recombinant OVA-expressing H1N1 A/WSN/33 (WSN-OVA), a gift from D. Topham (University of Rochester, Rochester, NY).
Chronic Ag stimulation and influenza infection
Female C57BL/6 mice were i.p. primed with 3 × 106 50% tissue culture infective dose (TCID50) influenza A virus H1N1 strain PR8 or 1 × 106 TCID50 WSN-OVA. Control mice received a single i.p. injection on day 0; chronic mice received two injections per week for a minimum of 4 wk. For i.n. rechallenge, mice were anesthetized with Avertin (2,2,2 tribromoethanol) at 240 mg/kg (Acros Organics) and then i.n. infected with 0.2 TCID50 x31 or 40 TCID50 WSN-OVA. For chronic Ag rest studies, mice were chronically i.p. primed with WSN-OVA for 30 days and then Ag rested for 45 days before influenza i.n. rechallenge.
OT-I naive T cell and day 30 virus-specific cell adoptive transfer
A total of 1 × 105 OT-I OVA257–264 TCR Tg T cells were suspended in sterile injectable saline, and i.v. transferred to naive C57BL/6 mice. Transferred mice were then either single or chronic Ag-stimulated as previously described. For rechallenge, splenocytes were isolated from the spleens of WSN-OVA single or chronic Ag-stimulated mice. Splenocytes were surface stained to identify OVA TCR Tg Vα2 and Vβ5.1 double positive (eBioscience) CD8+ T cells, as previously described (27). A total of 5 × 104 day-30 virus-specific CD8+ T cells were then i.v. transferred, in 100 μl of sterile injectable saline solution, to naive C57BL/6 mice. At 24 h after transfer, host mice were i.n. challenged with influenza virus as described. For tracking studies, day-30 virus-specific CD8+ T cells (Thy1.2+) were CFSE-labeled and transferred to naive Thy1.1+ mice. Recipients were i.n. challenged with virus on day 1 after transfer, or remained uninfected.
Determination of influenza viral titers and tissue cytokines
Influenza viral titers and proinflammatory cytokine production were determined in the lung, spleen, kidney, and liver by real-time PCR. Tissues were frozen at −80°C in 1 ml of TRIzol/50 mg tissue (TRI-Reagent; Molecular Research Center). At time of RNA isolation, tissues were thawed on ice and homogenized, and RNA was extracted according to Molecular Research Center specifications. Fragmented RNA was removed using Qiagen RNeasy kit. For viral titers, purified RNA was reverse transcribed to cDNA using random hexamers and matrix protein-specific primer (5′-TCT AAC CGA GGT CGA AAC GTA-3′). The resulting cDNA samples were used, in triplicate, for real-time PCR. For the reverse-transcribed assay, matrix protein-specific primers and FAM probe were used in combination with 2X Taqman Universal PCR mix (Applied Biosystems). Primers used were as follows: 900 nM influenza matrix protein (sense) 5′-AAG ACC AAT CCT GTC ACC TCT GA-3′, (antisense) 5′-CAA AGC GTC TAC GCT GCA GTC C-3′ and 200 nM influenza probe FAM-5′-TTT GTG TTC ACG CTC ACC GT-3′-TAMRA. For cytokine measurements, cDNA was reverse transcribed and assayed in triplicate by real-time PCR. For real-time assay, IL-1-, IL-6-, IL-12-, and TNF-α-specific primers were used in combination with 2X SYBR Universal PCR mix. Primers for cytokine were as follows: IL-1 (sense) 5′-CAA CCA ACA AG GAT ATT CTC CAT G-3′ and (antisense) 5′-GAT CCA CAC TCT CCA GCT GCA-3′; TNF-α (sense) 5′-CCA GAC TCT TCC CTG AGG TG-3′ and (antisense) 5′-CAA GGT AGA GAG GCC AGG TG-3′. For IL-6 and IL-12, RT2 qPCR Primer assay was used (SABiosciences). The specificity of all primer binding was confirmed by SYBR labeling and dissociation analysis. ABI Prism 7900HT sequence detection system with SDS 2.2.1 software (Applied Biosystems) was used for detection and analysis. Viral loads were calculated based on influenza viral stock standard curve and are reported as TCID50 U/tissue. Cytokine production was determined as fold change over healthy tissue using the equation (2CT)−1 influenza dosed/(2CT)−1 healthy control.
Lymphocyte preparation
Lung, mediastinal lymph node (MLN), and spleen single cell suspensions were prepared as previously described, by Halstead et al. (28). In brief, lung tissue was digested with 3 mg/ml collagenase A and 150 μg/ml DNase I in RPMI 1640 containing 5% FBS, for2hat 37°C. Digested tissue was filtered over a 40-μm nylon mesh, the mononuclear cell population was purified over Lympholyte-M density gradient (CedarLane Laboratories). MLN were disrupted by passing through a 40-μm nylon mesh and filtered over 20-μm nylon mesh. The spleen was disrupted under 20-μm nylon mesh. Cells were washed one time in RPMI 1640 containing 5% FBS. RBC were lysed through addition of 900 μl of sterile dH2O to the splenocyte pellet. Lysis was stopped with 100 μl of sterile 10X PBS and 9 ml of RPMI 1640 containing 5% FBS; cells were washed and filtered before counting. Femur bones were harvested and flushed with RPMI 1640 containing 3% to remove lymphocytes. Cells were washed, and RBC were lysed as described for spleen. One lobe of liver was harvested and mechanically disrupted under mesh, then digested in 3 mg/ml collagenase I for 1 h at 37°C. The cells were then purified by Percoll gradient. Live and dead counts of mononuclear cells from all tissues was performed by acridine orange (3 μg/ml) ethidium bromide (5 μg/ml) stain and UV light microscopy.
Flow cytometry
Virus-specific CD8+ T cells were identified by multicolor flow cytometry, by either MHC class I H-2Db nuclear protein (NP)366–374 tetramer staining, provided by and produced with the assistance of J. Altman (Emory University School of Medicine, Atlanta, GA) or TCR-specific anti-Vαl2-FITC or anti-Vα2-allophycocyanin and anti-Vβ5.1-PE (eBioscience) staining. Additional phenotyping was performed using the following surface and intracellular Abs: anti-CD8-PerCP (BD Biosciences), anti-CD8-Alexa Fluor 405 (Caltag Laboratories), anti-CD69-PE-Cy7 (BD Biosciences), anti-CD25-allophycocyanin (eBioscience), anti-CD279-FITC (eBioscience), and anti IFN-γ-allophycocyanin (eBioscience). Cells were stained as previously described (28) and fixed in 1% paraformaldehyde. Stained cells were acquired using a FACSCalibur or FACSAria (BD Biosciences) and analyzed using Flow Jo (Tree Star).
Peptide stimulation
A total of 1 × 106 lung mononuclear cells were cultured for 6 h at 37°C in the presence of 10 μg/ml brefeldin A and the presence or absence of 1 μg/ml OVA257–264 peptide (SIINFEKL) or 1 μg/ml NP366–374 (ASNEN METM). For subdominant epitopes, cells were cultured with M1128–135 (MGLIYNRM) or nonstructural protein NS2114–121 (RTFSFQLI). All peptides were purchased from ANASPEC. Cells were intracellular stained for IFN-γ production and analyzed as described in flow cytometry methods.
Programmed death (PD)-1 studies
Single or chronic stimulated C57BL/6 mice were treated with functional grade, anti-mouse B7H-1, clone MIH5, or with control rat IgG2a isotype (eBioscience). Mice were treated with 100 μg of anti-B7H-1 or isotype control every third day from days 0–30 of single or chronic Ag stimulation. At the completion of Ag stimulation, the frequency and absolute number of day-30 virus-specific memory cells was assessed by flow cytometry. Equal numbers of day-30 virus-specific cells from isotype or anti-B7H-1-treated single or chronic mice were transferred to naive mice. Recipient mice were rechallenged with influenza virus as previously described and harvested at the peak of the secondary response on day 7.
Statistical analysis
JMP statistical analysis program (SAS) was used to perform Student's t test, nonparametric Wilcoxon signed-rank test for paired samples and Shapiro-Wilk W test for normality. A value of p < 0.05 was considered significant.
Results
Chronic influenza A stimulation does not induce systemic infection or inflammation
To examine whether chronic Ag stimulation, in the absence of sustained inflammation, induced physical deletion and/or exhaustion of virus-specific CD8+ T cells we developed a system of chronic Ag stimulation that used murine adapted influenza virus. Control mice received a single injection of influenza A virus, whereas chronic mice received two injections per week for a minimum of four consecutive weeks. For both single and chronic Ag stimulation, virus was delivered by i.p. injection of either 3 × 106 TCID50 murine adapted influenza A strain H1N1 A/PR/8 (PR8) or 1 × 106 TCID50 genetically modified A/WSN/33 expressing Kb-restricted OVA257–264 (WSN-OVA). The i.p. injection was chosen as the route of delivery because it does not result in pulmonary infection and disease.
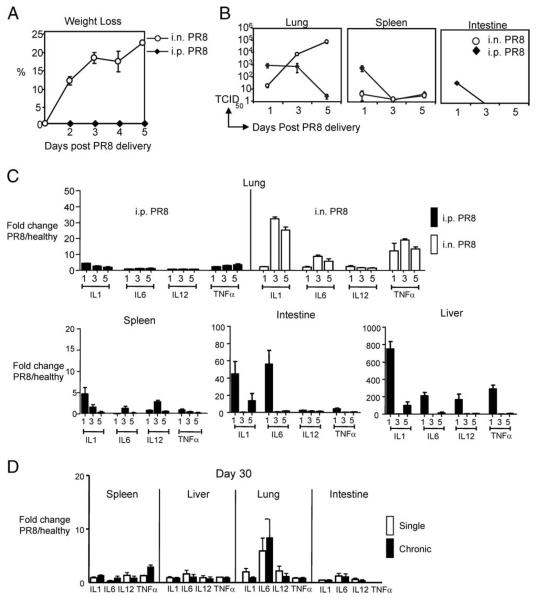
Because live infectious virus was used to achieve chronic Ag stimulation of CD8+ T cells, we first sought to determine whether systemic infection was induced following repeated i.p. delivery of influenza virus. To assess this, mice were either i.p. injected (i.p. primed) or i.n. infected with PR8 virus (3 × 106 TCID50 and 3 TCID50, respectively) and harvested on days 1, 3, and 5 after virus delivery. Weight loss was used as an indicator of productive influenza infection and was recorded daily for both i.n. and i.p. injected mice during the first 5 days following Ag delivery. The i.p. primed mice maintained constant weight, whereas i.n. infected mice rapidly lost up to 22% of their body weight over 5 days (Fig. 1A). The absence of weight loss in i.p. primed mice, in comparison to i.n. infected mice, suggested that i.p. priming did not induce productive infection or inflammation. To directly examine whether or not productive infection was established in i.p. primed mice, the viral load was quantified using real-time PCR. The mRNA was isolated from lungs, spleen, liver, intestine, and kidney at days 1, 3, and 5 after PR8 delivery, and the conserved matrix 1 protein of influenza virus was quantified by real-time PCR to determine the quantity of virus in each tissue. Influenza virus primarily infects pulmonary epithelial cells and alveolar macrophages; therefore as expected, the lungs of i.n. infected mice exhibited a 4 log increase in virus over the 5-day time period, indicating that productive infection and virus replication had occurred (Fig. 1B). In contrast, there was a limited quantity of viral RNA in the first 3 days in the lungs and spleen of mice that received influenza virus through i.p. injection. Due to both the very high 3 × 106 TCID50 i.p. dose used, and the virus decay kinetics, the presence of virus in the lungs on day 3 most likely reflects virus circulating in the lung vasculature and not lung parenchyma infection. By day 5, virus was below 10 TCID50 in all tissues examined, indicating that i.p. injection of influenza virus did not result in a productive systemic infection (Fig. 1B).
FIGURE 1.
The i.p. priming with PR8 did not lead to morbidity, systemic infection, cytokine burst, or chronic inflammation. C57BL/6 mice were i.n. infected or i.p. primed with 3 TCID50 or 3 × 106 TCID50 influenza virus strain PR8. A, Weights of i.n. infected and i.p. primed mice were recorded daily (n = 5 mice/group). B, Viral load was measured by real-time PCR in the lung, spleen, kidney, and intestine on days 1, 3, and 5 after virus delivery (n = 3 mice/group for each time point). C, The level of mRNA was quantified, by real-time PCR, for IL-1, IL-6, IL-12, and TNF-α in the lung, spleen, intestine, and liver of i.p. primed or i.n. infected mice. D, Cytokine production (mRNA) quantified at day 30 after single or chronic Ag stimulation. Data are fold increase over healthy uninfected control lung mRNA (n = 3 mice/group per time point).
Because proinflammatory cytokines may induce bystander T cell activation (29), increase dendritic cell (DC) maturation (30), and increase rate of CD8+ T cell contraction (31), we were interested in determining the cytokine profile after priming with PR8 virus. Specifically, we determined transcriptional changes in several proinflammatory cytokines produced during influenza infection, including IL-1, IL-6, IL-12, and TNF-α. The presence of these cytokines was determined by real-time PCR in the spleen and lungs of uninfected healthy control, i.p. primed, and i.n. infected mice. In the lungs of i.n. infected animals, both IL-1 and TNF-α mRNA expression were substantially increased relative to expression in healthy uninfected lungs (32.44 ± 0.34 fold and 19.06 ± 0.64 fold over healthy controls by day 3, for IL-1 and TNF-α, respectively) (Fig. 1C, top right). IFN-α also increased a maximum of 2.74 ± 0.74 fold over uninfected healthy lungs by day 1 (data not shown). The limited increase in IFN-α is not surprising given that influenza virus NS1 protein has been shown to inhibit IFN-α production (32). In striking contrast, there was no significant increase in cytokine production in the lungs of i.p. primed mice (Fig. 1C, top left). This finding is particularly significant because virus was detected in the lungs of i.p. primed mice at days 1 and 3 postinjection. The absence of inflammatory cytokines, despite the presence of virus, most likely indicates an absence of infection in the lungs of i.p. primed mice.
Because virus was i.p. delivered for chronic Ag stimulation, the cytokine profile in peripheral tissues was also determined. For most cytokines and tissues examined, the greatest increase in cytokine mRNA was observed 1 day after virus delivery (Fig. 1C, bottom). The kinetics of IL-6 and IL-12 expression in the spleen varied from other cytokines examined, with IL-6 and IL-12 expression peaking at day 3 (1.31 ± 0.45 and 2.8 ± 0.36, fold increase in IL-6 and IL-12, respectively). Importantly, these early, transient peaks of inflammation were not sustained, and transcript was comparable to naive controls by day 5.
Although i.p. injections of virus induced only transient and small increases in proinflammatory cytokines, it was important to exclude that repeated delivery of virus in chronic stimulated mice did not result in differences in cytokine production. Therefore, we determined the cytokine profile in the peripheral tissues of single and chronic Ag-stimulated mice at day 30. The profile of IL-1, IL-6, IL-12, and TNF-α expression was comparable between single and chronic Ag-stimulated mice, in all tissues examined (Fig. 1D), suggesting that chronic stimulation did not result in a chronic inflammation. With the exception of IL-6 in the lungs of single and chronic Ag-stimulated mice, all cytokine amounts were less than 2-fold increased over uninfected healthy controls. Perhaps most importantly, there was no statistically significant difference in mRNA transcripts between single and chronic Ag-stimulated mice for any of the cytokines examined.
The repeated delivery of virus could impact the number or ability of DC and thus indirectly affect the CD8+ T cell response. Therefore, we determined the frequency and phenotype of DC in the spleens of single and chronic Ag-stimulated mice by flow cytometry. At day 30, DC from both single and chronic Ag-stimulated mice were comparable in terms of frequency and phenotype. The number of CD11c+CD11b+ and CD11c+CD11b− DC in the spleens of chronic Ag-stimulated mice were not statistically different from single prime controls (CD11c+CD11b+: 1.97 ± 0.33 × 105 vs 2.23 ± 0.4 × 105; CD11c+CD11b−: 6.63 ± 1.9 × 105 vs 5.67 ± 1.4 × 105 for chronic vs single, respectively, n = 3 mice/group). Mean fluorescence intensity (MFI) of CD86 expression was also similar between these mice (CD11c+CD11b+: 88.5 ± 12 vs 98.5 ± 17.3; CD11c+CD11b−: 96 ± 15 vs 123 ± 16; chronic and single, respectively, in n = 3 mice/group). The MFI of MHC class II expression on these DC was also comparable between single and chronic Ag-stimulated mice (CD11c+CD11b+: 1260 ± 22.4 vs 1221.3 ± 126.6; CD11c+CD11b−: 2204 ± 152 vs 2123 ± 174; chronic and single, respectively, n = 3 mice/group). These data indicate that the chronic Ag stimulation regiment did not negatively impact DC numbers or phenotype.
Taken together, these results indicate that repeated i.p. delivery of virus did not result in sustained systemic infection or proinflammatory cytokine production and does not alter DC numbers or phenotype. Therefore, this system allowed us to study the effects of chronic Ag stimulation on virus-specific CD8+ T cell development and secondary expansion in the absence of chronic inflammation.
Reduced quantity of virus-specific CD8+ T cells after chronic Ag stimulation
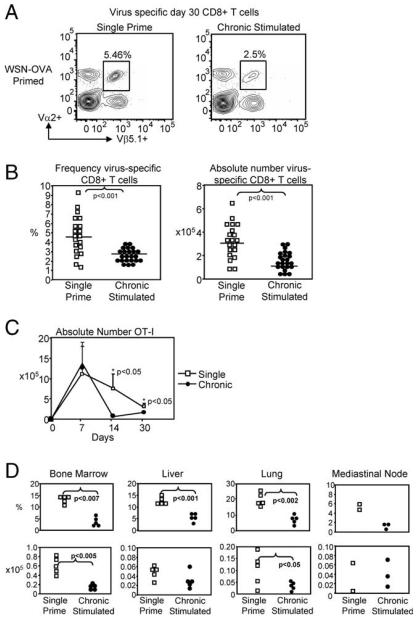
Having demonstrated that i.p. injection of live influenza A virus did not lead to sustained cytokine production or systemic infection, we sought to determine whether chronic Ag stimulation alone could induce defects in the development of virus-specific CD8+ T cells. To test this, 1 × 105 naive CD8+ T cells from TCR Tg OT-I mice were transferred i.v. to naive C57BL/6 mice. Recipients then received either a single i.p. injection of WSN-OVA virus (106 TCID50) or were chronic Ag-stimulated, by i.p. injection two times a week for four consecutive weeks. After 30 days, virus-specific CD8+ T cells in the spleen were examined by multicolor flow cytometry. Interestingly, chronic Ag-stimulated mice exhibited a nearly 2-fold reduction in frequency and number of virus-specific CD8+ T cells, at day 30, when compared with single prime control mice (frequency: 4.94 ± 0.46% and 2.61 ± 0.12% in single and chronic Ag-stimulated, respectively; absolute number: 3.32 ± 0.32 × 105 and 1.54 ± 0.15 × 105 in single and chronic Ag-stimulated, respectively; n = 20 mice/group and n = 23 mice/ group, p < 0.001) (Fig. 2, A and B). Kinetic analysis of virus-specific CD8+ T cells in single and chronic Ag-stimulated mice indicated that significant differences in frequency and absolute number occurred as early as 14 days after initial priming (Fig. 2C). These kinetic data suggest that chronic Ag-stimulated cells may undergo an increased rate of contraction relative to single prime controls. Closer examination of the day 30 virus-specific chronic Ag-stimulated CD8+ T cells showed that they exhibited skewed memory differentiation compared with single prime controls. Specifically, there was a significantly greater frequency of memory, CD44+CD62L+ virus-specific CD8+ T cells in single prime controls compared with chronic Ag-stimulated mice (55.4 ± 4.0% vs 32.9 ± 3.8% single and chronic, respectively; p < 0.001, n = 9 mice/group) (data not shown). Interestingly, chronic Ag-stimulated CD8+ T cells were evenly distributed between CD44+ and CD44− memory cells (32.9 ± 3.8% and 36.1 ± 6.7% CD44− and CD44+ memory, respectively; n = 9 mice/group) (data not shown).
FIGURE 2.
Chronic Ag stimulation reduced the quantity of day-30 virus-specific CD8+ T cells. OT-I transferred C57BL/6 mice were single or chronic stimulated with WSN-OVA. On day 30, the frequency of virus-specific CD8+ T cells was determined by flow cytometry. A, Representative flow cytometry virus-specific CD8+ T cells (gated on CD8+ T cells). B, Reduction in virus-specific CD8+ T cells was observed in pooled frequency and absolute number (n = 15 mice/group, p < 0.001). C, Kinetic analysis of virus-specific OT-I cells during 30 days of priming (n = 3 mice/group). D, Reduction of chronic CD8+ T cells in lymphoid and nonlymphoid tissues indicates migration is not impaired.
Previous studies using LCMV, indicate that virus-specific CD8+ T cells in chronically infect mice have altered migration in both lymphoid and nonlymphoid tissues, when compared with acutely infected animals (20). Therefore, we examined the distribution of virus-specific CD8+ T cells in the MLN, bone marrow, lung, and liver. In contrast to LCMV (20), the decreased number of virus-specific cells in the spleen did not reflect impaired migration of chronically stimulated cells, as similar decreases were observed in the MLN, bone marrow, lung, and liver (Fig. 2D). Importantly, this loss of chronically stimulated cells was observed in both frequency and absolute number when compared with single prime controls.
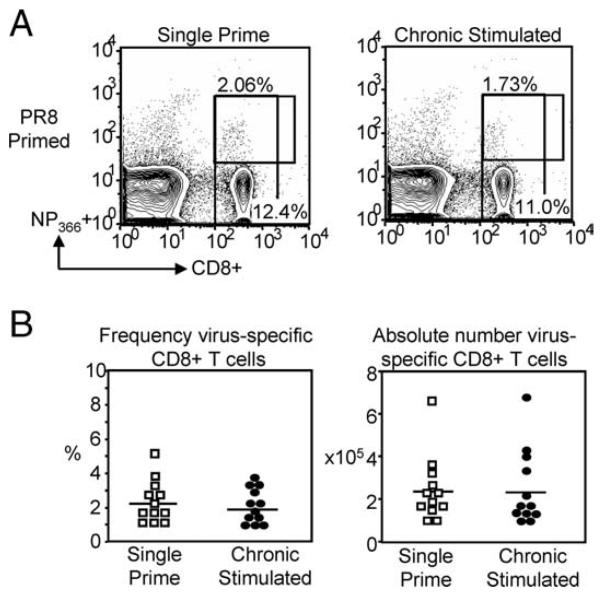
To determine whether the reduction in day-30 virus-specific CD8+ T cells was restricted to the OVA TCR Tg system, endogenous CD8+ T cells underwent chronic Ag stimulation with the influenza A virus strain PR8. After 30 days, the virus-specific population in the spleen was identified by tetramer staining for influenza immunodominant epitope-specific NP366-positive T cells. Interestingly, there was no significant decrease in the frequency (Fig. 3) or absolute number (Fig. 3B) of NP366-positive CD8+ T cells (frequency: 2.08 ± 0.32% and 1.89 ± 0.3% single and chronic, respectively; absolute number: 2.49 ± 0.44 × 105 and 2.44 ± 0.51 × 105 single and chronic, respectively; n = 12 mice/ group). There are several possible reasons for this disparity. Previous studies indicate that the initial precursor frequency may dictate the rate of development and phenotype of memory CD8+ T cells (33). Therefore, transfer of OT-I TCR Tg T cells at a higher precursor frequency, may have changed the rate of expansion and contraction of the virus-specific CD8+ T cell population, compared with priming of endogenous CD8+ T cells. A second possibility may be the inability of the host to generate new OVAspecific CD8+ T cells during the chronic stimulation. During chronic Ag stimulation with PR8 virus, new thymic CD8+ T cell emigrants can be generated, and replenish dying chronic Ag-stimulated cells. In contrast, there is a finite population of transferred OT-I TCR Tg cells that can be stimulated by WSN-OVA. If stimulation induces death, the population cannot be replenished by endogenous CD8+ T cells, and will be reduced. Indeed, a recent report by Vezys et al. (34) elegantly showed that chronic infection results in a heterogeneous population of virus-specific CD8+ T cells that is maintained by the continuous release of naive cells from the thymus.
FIGURE 3.
Chronic Ag stimulation of endogenous CD8+ T cells with PR8 did not significantly reduce the day-30 population. C57BL/6 mice received either a single i.p. injection or chronic Ag stimulation with influenza A virus strain PR8. After 30 days of stimulation, spleens were harvested and the frequency and absolute number of NP366-positive CD8+ T cells was assessed by multicolor flow cytometry. A, Representative FACS plots (left) show that there was no significant reduction in the frequency of day-30 virus-specific CD8+ T cells in chronic Ag-stimulated mice compared with single prime controls. Pooled frequency (right) indicates a trend toward a decrease in chronic stimulated CD8+ T cells, though no significance is reached. B, Pooled absolute numbers indicate that independent of chronic Ag stimulation the number of day 30 virus-specific CD8+ T cells remains comparable.
Deficient quality of virus-specific CD8+ T cells following chronic Ag stimulation
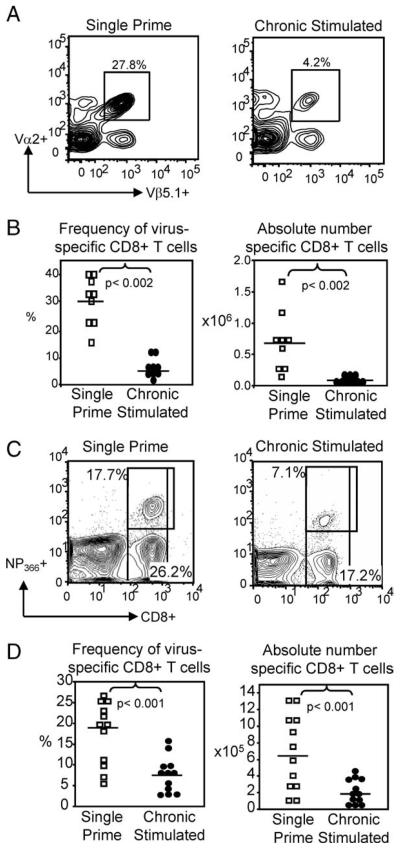
Having observed only limited changes to the quantity of day-30 virus-specific CD8+ T cells, in both frequency and number, we were interested in determining the effect that chronic Ag stimulation had on the quality of these cells. Specifically, we sought to determine whether chronic Ag stimulation altered the ability of virus-specific CD8+ T cells to respond to secondary viral challenge. Because the size of the memory population directly influences the magnitude of the secondary response (35), and chronic Ag stimulation with WSN-OVA resulted in a 2-fold reduction in the number of day 30 virus-specific CD8+ T cells, it was necessary to correct for the difference in population size before influenza rechallenge. Normalization was achieved by transferring an equal number (5 × 105) of day-30 chronic Ag-stimulated or single prime virus-specific CD8+ T cells to naive C57BL/6 mice. Recipient mice were then i.n. challenged with 40 TCID50 WSN-OVA. Mice were harvested at the peak of the secondary response on day 7, and the virus-specific CD8+ T cells in the lung, draining MLN, and spleen were examined by multicolor flow cytometry.
Despite transfer of an equal number of day-30 single or chronic Ag-stimulated virus-specific CD8+ T cells, there was a significant reduction of virus-specific CD8+ T cells in the lungs of chronic Ag-stimulated mice, at the peak of the secondary response. In single prime controls, 30.2 ± 2.89% of the CD8+ T cells in the lung were virus-specific, whereas chronic Ag-stimulated mice had only 5.2 ± 1.3% virus-specific CD8+ T cells (n = 9 mice/group, p < 0.002) (Fig. 4, A and B). Correction for absolute numbers illustrated that the defect was not only in frequency, but also in number, as the lungs of single prime control mice contained 5.35 ± 0.88 × 105 virus-specific CD8+ T cells, whereas the lungs of chronic mice had 10-fold less, with only 0.5 ± 0.89 × 105 virus-specific CD8+ T cells (n = 9 mice/group, p < 0.001) (Fig. 4B). The population was similarly reduced in the draining MLN and the spleen (data not shown). The reduction in the secondary lymphoid tissues and lung confirmed that the loss of chronic Ag-stimulated CD8+ T cells, relative to single prime controls, was not due to altered migration.
FIGURE 4.
Chronic Ag stimulation reduced the quality of virus-specific CD8+ T cells. An equal number of single or chronic Ag-stimulated day-30 virus-specific CD8+ T cells were transferred to naive C57BL/6 mice. Recipient mice were i.n. challenged with 40 TCID50 WSN-OVA and harvested at the peak of the secondary response. Representative flow cytometry plots (A) and pooled frequencies and absolute numbers (B), showing a significant decrease in virus-specific CD8+ T cells in the lung of chronic mice compared with single prime mice. Each symbol represents a single animal. Representative flow cytometry plots (C) and pooled frequencies and absolute numbers (D) show that chronic Ag stimulation with PR8 also results in a significant reduction in responding virus-specific CD8+ T cells relative to single prime controls, following i.n. viral rechallenge.
Importantly, the significant reduction in the frequency and absolute number of the day-7 effector population was observed whether WSN-OVA or PR8 was used for chronic Ag stimulation. Therefore, this event was not TCR Tg-restricted. An equal number of PR8 single or chronic Ag-stimulated CD8+ T cells were transferred to naive C57BL/6 mice. As before, mice were then i.n. rechallenged with influenza A virus and harvested at the peak of the secondary response on day 7. In the lungs of single prime recipients, virus-specific CD8+ T cells represented 17.56 ± 2.2% of the total CD8+ T cells, whereas in chronic mice, only 7.45 ± 1.22% of the CD8+ T cells were virus-specific (n = 12 mice/ group, p < 0.0001) (Fig. 4, C and D). Additionally, the absolute number of chronic-stimulated virus-specific CD8+ T cells was reduced greater than 3-fold when compared with single prime controls (6.77 ± 1.31 × 105 and 2.01 ± 0.43 × 105 single and chronic stimulated, respectively, n = 12 mice/group, p < 0.001) (Fig. 4D). Given that an equal number of day-30 virus-specific CD8+ T cells were transferred to naive mice, these data strongly indicated that chronic Ag-stimulated virus-specific CD8+ T cells were defective in quality.
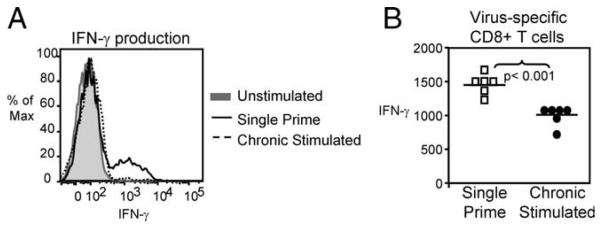
During influenza infection, CTLs exert several important functions including production of IFN-γ. Following ex vivo OVA257-264 peptide stimulation, the frequency of IFN-γ-producing day-7 pulmonary effectors from chronic Ag-stimulated mice was significantly reduced compared with single prime controls (data not shown), confirming the tetramer- and TCR-specific surface staining results. More importantly, however, chronic Ag-stimulated CD8+ T cells exhibited significantly decreased IFN-γ production on a per cell basis when compared with single prime controls (MFI = 1447 single prime Ag-stimulated and MFI = 995 chronic Ag-stimulated CD8+ T cells, n = 6 mice/group, p < 0.001) (Fig. 5). This finding indicated that qualitative defects exist beyond reduced expansion or survival because the surviving virus-specific CD8+ T cells also exhibited a significant impairment in IFN-γ cytokine production.
FIGURE 5.
Chronic Ag-stimulated day 7 secondary effector CD8+ T cells produced less IFN-γ. IFN-γ production by chronic Ag-stimulated virus-specific CD8+ T cells was defective compared with controls, after ex vivo stimulation with OVA257–264 peptide. A, Representative histogram (gated on CD8+ T cells) shows the reduction in IFN-γ production by chronic Ag-stimulated CD8+ T cells relative to single prime controls. B, Each symbol represents a single animal, pooled MFI of IFN-γ-producing cells (gated on CD8+ T cells) indicates decreased function of chronic Ag-stimulated CD8+ T cells compared with single prime controls (n = 6 mice, p < 0.001).
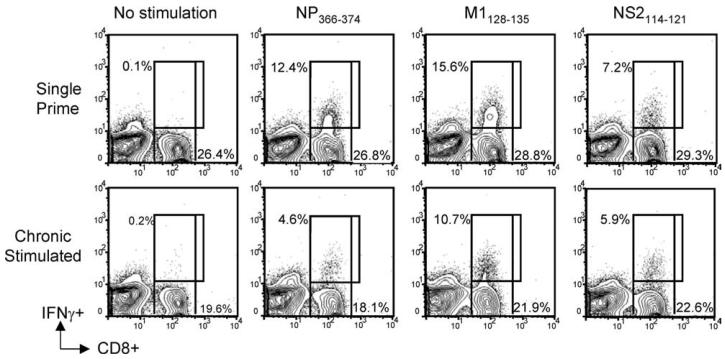
Beyond characterizing the response to the dominant epitope, we also determined the effect of chronic Ag stimulation on subdominant epitopes. In some chronic infections, skewing of dominance in CD8+ T cell responses has been observed, whereby immuno-dominant responses are lost and subdominant responses become more prevalent (19, 21, 36). When chronic Ag stimulation alone was used to induce exhaustion of CD8+ T cells, both immuno-dominant and subdominant responses were suppressed, although the most profound effect was observed in the immunodominant NP366–374 epitope. Indeed, ex vivo peptide stimulation of day-7 pulmonary effectors with peptides for conserved M1128–135 and nonstructural protein NS2114–121 resulted in robust IFN-γ production by single prime CD8+ T cells, whereas a markedly reduced response was observed in chronic Ag-stimulated CD8+ T cells (Fig. 6).
FIGURE 6.
Chronic stimulation resulted in a loss of dominant and subdominant epitope responses. C57BL/6 mice received either a single i.p. prime or chronic stimulation for 30 days with 3 × 106 TCID50 PR8. An equal number of single or chronic stimulated virus-specific CD8+ T cells were transferred to naive mice. Recipient mice were i.n. rechallenged with x31 and harvested at the peak of the secondary response on day 7 (n = 3 mice/group). Pulmonary lymphocytes were stimulated ex vivo with peptides to dominant (NP366–374) or subdominant epitopes (M1128–135 or NS2114–121) for 6 h and intracellular stained for IFN-γ production. Representative flow cytometry plots show that both dominant and subdominant epitope specific responses were reduced in chronic mice (bottom) compared with single prime controls (top).
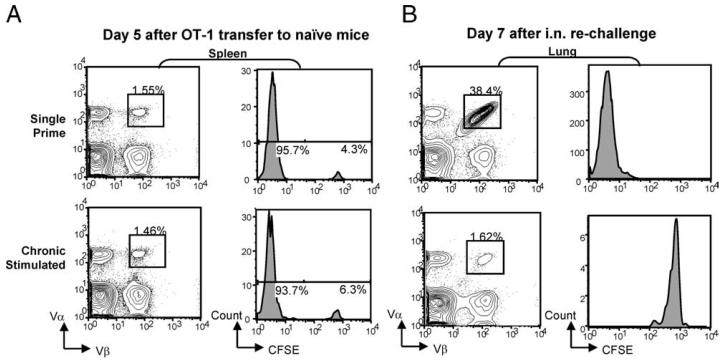
To confirm that chronic CD8+ T cells did not simply undergo apoptosis immediately after transfer, day-30 CD8+ T cells from single and chronic Ag-stimulated mice were CFSE-labeled and transferred to naive hosts. New recipients were i.n. rechallenged with influenza virus or left uninfected. In the absence of viral infection, the frequency of CFSE-positive OT-I+ T cells in the spleen of single and chronic recipient mice was comparable, up to 5 days after transfer (Fig. 7A). The low frequency of CFSE-positive cells, 4–6% of OT-I population, found at day 5 is reasonable given that the endogenous frequency of Vα+Vβ+ cells of the naive host is ~1%. The identification of comparable CFSE-positive populations between the two groups suggests that, at least early after transfer, the survival is similar. After rechallenge, all donor OT-I+ cells found in the lungs of single prime mice were CFSE-negative, indicating proliferation, whereas in the lungs of chronic mice donor OT-I+ cells, although capable of surviving, fail to robustly expand in response to restimulation (Fig. 7B).
FIGURE 7.
Comparable survival of single and chronic stimulated CD8+ T cells after transfer to naive recipients. Day 30 single or chronic Ag-stimulated CD8+ T cells were CFSE-labeled and transferred to naive recipients. Recipients remained uninfected or were rechallenged i.n. with WSN-OVA virus. Mice were harvested on days 5 (healthy controls) and day 7 (virus-infected). A, Comparable frequency of CFSE-positive OT-I+ T cells were found in the spleens of naive recipients receiving either single or chronic stimulated CD8+ T cells. B, In the lungs of day 7 rechallenged mice, single prime CD8+ T cells exhibited robust expansion, resulting in an absence of CFSE-positive cells. Chronic Ag-stimulated CD8+ T cells failed to expand in number, and did not divide. Histograms depict CFSE stain on cells are gated on donor OT-I+ T cells based on Thy1.2 and Vα/Vβ expression.
Recovery of function by Ag rest of chronic Ag-stimulated day-30 cells
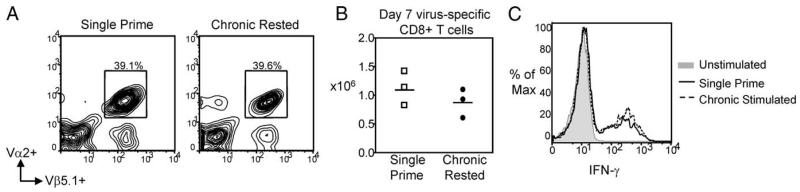
If the loss of CD8+ T cell quantity and function is Ag-driven, we reasoned that removal of Ag might reverse the virus-specific CD8+ T cell exhaustion. Previous studies in LCMV (37) and HIV (38-40) show that in the absence of Ag, CD4+ and/or CD8+ T cell responses can be partially restored, and suggest that the impairment may not be permanent. To address the issue of reversibility in the chronic Ag influenza system, 1 × 105 OT-I Tg T cells were transferred to naive C57BL/6 mice. Recipient mice were then either single or chronic Ag-stimulated with WSN-OVA, as previously described. After 30 days of Ag stimulation, the chronic delivery of virus was discontinued and mice were “Ag-rested” for 45 days. Mice were harvested after 45 days of rest, and an equal number of virus-specific CD8+ T cells were transferred to naive C57BL/6 mice. Recipient mice were rechallenged i.n. with 40 TCID50 WSN-OVA, and harvested at the peak of the secondary response on day 7. Following Ag rest, virus-specific CD8+ T cells in the lungs of single prime and chronic Ag-rested mice were comparable both in frequency (37.1 ± 2.39% and 38.1 ± 4.88%; n = 3 mice/group) (Fig. 8A) and number (1.35 ± 0.2 × 106 and 1.06 ± 0.2 × 106; n = 3 mice/group) (Fig. 8B). Equivalent responses, in single and chronic Ag-rested CD8+ T cells, were also observed in the draining MLN and spleen of infected mice (data not shown). Additionally, the quality of the virus-specific CD8+ T cells was recovered in chronic rested mice. When pulmonary lymphocytes were ex vivo stimulated with OVA257-264 peptide, and intracellular stained for IFN-γ, there was no significant difference between single prime and chronic rested CD8+ T cells in the frequency or MFI of IFN-γ-producing cells (Fig. 8C). These data suggested that the exhausted state of CD8+ T cells in this model is not irreversibly programmed and can be recovered following Ag rest.
FIGURE 8.
Chronic Ag-stimulated virus-specific CD8+ T cells recovered function after Ag rest. A total of 1 × 105 OT-I TCR Tg T cells were transferred to naive C57BL/6 mice. Mice were single or chronic stimulated with 1 × 106 TCID50 WSN-OVA, by i.p. injection, for 30 days. On day 30, chronic animals were Ag rested for 45 days, and an equal number of single or chronic Ag-rested virus-specific CD8+ T cells were transferred to naive C57BL/6 mice, which were then i.n. challenged with WSN-OVA and harvested on day 7. A, Representative flow cytometry (gated on CD8+ T cells) from lungs of single or chronic rested infected mice is shown. As indicated by Vα2+Vβ5.1+ cells, chronic Ag-rested CD8+ T cells expanded equivalently to single prime animals. B, Pooled absolute numbers indicate that Ag rest recovers not only frequency, but also the number of chronic rested virus-specific CD8+ T cells (n = 3 mice/group). C, Representative flow cytometry histogram (gated on CD8+ T cells) shows equivalent MFI of IFN-γ production after ex vivo stimulation with OVA254–267-specific peptide.
Inhibitory signaling through PD-1 does not mediate Ag-induced CD8+ T cell exhaustion
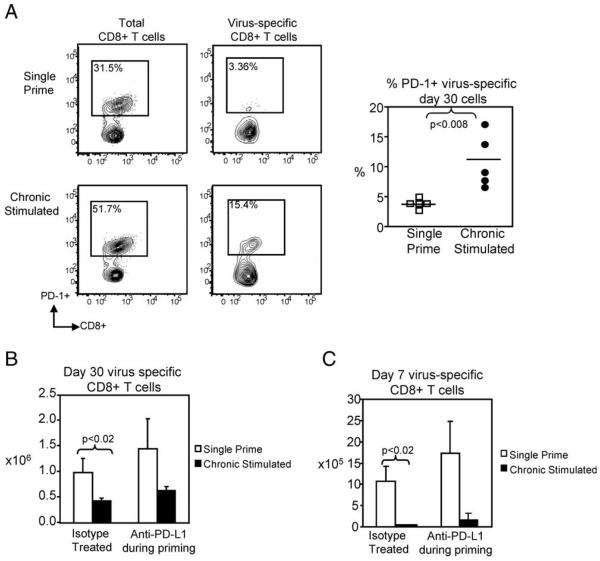
In response to activation, CD8+ T cells up-regulate inhibitory signaling molecules to block continued activation and proliferation responses. Chronic infection can result in similar up-regulation of inhibitory molecules, and prominent among them is PD-1. Recent evidence suggests that enhanced PD-1 expression on virus-specific CD8+ T cells may be partially responsible for the CD8+ T cell exhaustion observed in LCMV (41) and HIV (42-45) infections. Therefore, we sought to determine whether under conditions of chronic Ag stimulation alone, the impairment in virus-specific CD8+ T cell function and expansion was due to enhanced expression of PD-1. Examination at day 30 showed that PD-1 expression was significantly increased, in comparison to single prime controls, on both total and virus-specific chronic Ag-stimulated CD8+ T cells (Fig. 9A). Because CD8+ T cells from chronic Ag stimulation mice exhibited increased PD-1 expression, PD ligand-1 (PDL1) binding to PD-1 was blocked by treating both single and chronic Ag-stimulated mice with anti-B7H-1 (anti-PD-L1) during the first 30 days of Ag stimulation. The efficiency of anti-PD-L1 treatment was confirmed by examining the bulk lymphocyte cell absolute number. After PD-L1 blockade, lymphocytes in single and chronic Ag-stimulated recipients were significantly increased compared with isotype-treated controls (data not shown). Despite blockade of PD-1 signaling, there was no significant increase in the size of the virus-specific chronic Ag-stimulated CD8+ T cell population at day 30 (Fig. 9B). Additionally, when rechallenged, there was no recovery of expansion or function of the anti-B7H-1-treated chronic Ag-stimulated virus-specific CD8+ T cells in comparison to anti-B7H-1 single primed CD8+ T cells or isotype-treated chronic stimulated controls (n = 5 mice/group, p < 0.005) (Fig. 9C). Although PD-1 expression increased following chronic Ag stimulation, PD-1 inhibitory signaling did not appear to mediate the defects observed in secondary rechallenge. These findings may indicate an important distinction between the role PD-1 plays when chronic stimulation occurs under an inflamed vs a noninflamed state.
FIGURE 9.
PD-1 did not mediate chronic Ag-induced CD8+ T cell exhaustion. To assess the functional significance of enhanced PD-1 expression on chronically stimulated CD8+ T cells, 1 × 105 OT-I TCR Tg T cells were transferred to naive C57BL/6 mice. After transfer, mice received either single or chronic Ag stimulation with WSN-OVA virus along with 100 μg of rat IgG2a isotype or anti-B7H-1 Ab every third day during the 30-day stimulation period. A, Representative flow cytometry plots show significantly increased PD-1 expression on both total and virus-specific chronic Ag-stimulated CD8+ T cells. B, After 30 days, the absolute number of virus-specific cells was determined by flow cytometry. There is no significant recovery in the spleen number of anti-B7H-1-treated chronic Ag-stimulated CD8+ T cells in comparison to single prime controls. C, Following transfer and i.n. rechallenge, we observed no significant recovery of chronic Ag-stimulated CD8+ T cells that were treated with anti-PD-L1. Lung numbers shown.
TRAIL signaling mediates deletion of virus-specific CD8+ T cells
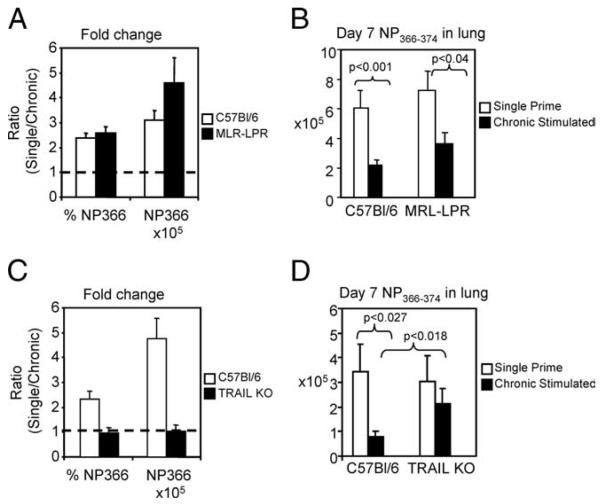
Because the elevated expression of PD-1 had no functional role in the depression of chronic Ag-stimulated CD8+ T cells, we wanted to determine whether these cells were more susceptible to death than their single prime counterparts. Specifically, we examined the death-inducing pathways, TRAIL and Fas. To address the role of these pathways in the defects observed during rechallenge, C57BL/6, Fas-deficient MRL/lpr, or TRAIL knockout mice were single or chronic Ag-stimulated with influenza virus strain PR8. After 30 days, single or chronic stimulated virus-specific CD8+ T cells were transferred to naive C57BL/6 mice. Transferred mice were rechallenged i.n. with 0.2 TCID50 x31 influenza A virus and harvested, on day 7 at the peak of the secondary response. As previously shown, in the lungs of C57BL/6 mice, there was a greater than 2-fold change in frequency between single and chronic stimulated virus-specific CD8+ T cells (Fig. 10A). A coordinate 3-fold change in the absolute number was observed when single and chronic stimulated virus-specific populations were compared (10.09 ± 1.36 × 105 and 3.24 ± 0.4 × 105 single and chronic, respectively, n = 6 mice/group, p < 0.001) (Fig. 10, A and B). On day 7 of secondary rechallenge, Fas signaling-deficient mice also exhibited a 2-fold change virus-specific CD8+ T cells frequency when single and chronic stimulated animals were compared (Fig. 10A). Correction for absolute number showed that Fas-deficient chronic Ag-stimulated virus-specific CD8+ T cells were still significantly reduced relative to Fas-deficient single prime controls (7.24 ± 1.29 × 105 and 3.66 ± 0.75 × 105 single and chronic, respectively, n = 7 mice/group, p < 0.04) (Fig. 10, A and B). This reduction was confirmed by ex vivo stimulation with influenza-specific peptide NP366–374, which resulted in significantly less IFN-γ production by the chronic Ag-stimulated CD8+ T cells from C57BL/6 or Fasdeficient mice, compared with their single prime counterparts (data not shown). On the basis of our data, it did not appear that Fas-mediated death had a substantial role in the loss of chronic-stimulated CD8+ T cells.
FIGURE 10.
TRAIL-induced apoptosis mediated the loss of chronic stimulated virus-specific CD8+ T cells in rechallenge. C57BL/6, TRAIL knockout or MRL/lpr Fas-deficient mice were single or chronic stimulated for 30 days with PR8. After 30 days, mice were harvested and an equal number of NP366–374 tetramer-specific CD8+ T cells were transferred to naive C57BL/6 mice, which were rechallenged and harvested on day 7. A, The NP366-positive CD8+ T cell response in single primed C57BL/6 and MRL/lpr Fas-deficient mice was increased 2-fold and greater than 3-fold in frequency and absolute number when compared with chronic Ag-stimulated animals. Data are shown as the ratio of single prime stimulated to chronic Ag-stimulated CD8+ T cells, where 1 is an equivalent response between single and chronic stimulation. B, Pooled absolute numbers indicate that the absence of Fas does not restore chronic CD8+ T cells (n = 7 mice/group, p < 0.04). C, Comparison of the ratio of single prime to chronic stimulated frequency and absolute number of virus-specific CD8+ T cells indicates that in the absence of TRAIL, chronic CD8+ T cells recover; 1 is an equivalent response between single and chronic stimulation. D, Pooled absolute number indicates that in the absence of TRAIL, chronic Ag-stimulated virus-specific CD8+ T cells are comparable to single prime controls (n = 6 mice/group).
Chronic Ag stimulation in TRAIL knockout mice yielded substantially different results. Following transfer of day 30 virus-specific CD8+ T cells, and i.n. rechallenge, chronic Ag-stimulated CD8+ T cells from C57BL/6 wild-type controls were significantly decreased in frequency and absolute number compared with single prime controls. Comparison of the frequency and absolute number of single prime and chronic Ag-stimulated virus-specific CD8+ T cells show that single prime responses were 2.33 ± 0.35 and 4.82 ± 0.81 fold greater than responses from chronic stimulated animals, respectively (Fig. 10C). Direct comparison of the absolute number of T cells shows that chronic stimulated virus-specific CD8+ T cells were significantly lower than single prime controls (n = 9 mice/group for chronic stimulated and n = 6 mice/group for single CD8+ T cells, p < 0.03) (Fig. 10D). Interestingly, in the absence of TRAIL, chronic stimulated CD8+ T cells were no longer significantly reduced in frequency or number when compared with TRAIL-deficient single prime controls. Indeed, the ratio of single prime to chronic stimulated virus-specific cells, in frequency and absolute number, was near 1, indicating that the responses were comparable (0.98 ± 0.21 and 1.03 ± 0.28, frequency and absolute number, respectively) (Fig. 10C). Direct comparison of the absolute number confirms this, as the virus-specific population from chronic stimulated TRAIL knockout mice was 3.04 ± 1.06 × 105 and from single primed mice was 2.13 ± 0.62 × 105 (n = 6 mice/group) (Fig. 10D). TRAIL knockout chronic stimulated virus-specific CD8+ T cells not only responded to rechallenge at levels comparable to single prime controls, but was significantly greater than the chronic Ag-stimulated virus-specific CD8+ T cell from C57BL/6 mice (Fig. 10D). Ex vivo stimulation of TRAIL knockout single prime and chronic Ag-stimulated CD8+ T cells resulted in comparable IFN-γ production, confirming the previously presented tetramer data (data not shown). The findings in rechallenged TRAIL knockout and Fas signaling-deficient virus-specific CD8+ T cells strongly indicate that the inability of chronically stimulated virus-specific CD8+ T cells to respond to rechallenge is mediated by TRAIL-induced apoptosis.
Discussion
During the course of several different chronic infections, virus-specific CD8+ T cells become functionally exhausted and incapable of clearing virus from the infected host (4-11, 13-15, 46). Although much effort has been exerted in determining the phenotype and functional deficits of exhausted CD8+ T cells, there is a paucity of studies examining the individual factors that contribute to CD8+ T cell exhaustion. Indeed, several classic mouse models of chronic infection used to characterize exhausted CD8+ T cells are marked by high viral loads, sustained proinflammatory cytokine production, and/or lymphocyte infection (16-22), all of which may contribute to exhaustion. Studies using these infections have led to many important findings that define our understanding of exhausted CD8+ T cells (16-24). However, these models are unable to provide information about the significance of Ag stimulation alone in the development of exhausted CD8+ T cells. In this study, we used murine-adapted influenza A virus to provide chronic Ag stimulation to CD8+ T cells, in the absence of lymphocyte infection, sustained high viral loads, and overt inflammation. Importantly, the chronic Ag stimulation we achieved is distinct from repeatedly stimulating with inactivated virus or peptide alone. Although it has been shown, in vitro, that heat- or UV-inactivated influenza virus can be used for CTL priming (47, 48), the most efficient mechanism of CTL priming is through the use of live virus (47, 48). The use of repeated peptide stimulations was avoided because it prohibits exploration of the CTL response to subdominant epitopes. Additionally, with both inactivated and peptide induced stimulation, cross-presentation appears to be a primary mechanism of priming (47, 48). Through use of live infectious virus, we could preserve classical Ag processing, costimulation events, and examination of responses to multiple viral epitopes.
The repeat virus injections did not result in chronic inflammation, as determined by proinflammatory cytokine production, and did not affect the phenotype and number of DC. There was no difference in IL-1, IL-6, IL-12, and TNF-α expression in any of the tissues examined after a single i.p. injection or after 30 days of repeated stimulation. Because DC expression of CD86 and MHC class II did not differ in chronically stimulated animals, it is likely that DC are not functionally impaired. Although our studies demonstrate that transferred chronically stimulated Ag-specific CD8+ T cells in naive hosts exhibit expansion defects, suggesting that these defects are intrinsic to the CD8+ T cells, we cannot exclude that the some function of DC is affected in chronically stimulated animals and contributes to the CD8+ T cell defect. Such a function of DC could be required to keep CD8+ T cells “healthy,” but it does not appear to relate to maturation or CD86 expression of DC.
Using this chronic stimulation system, we determined that TCR stimulation by chronic Ag alone was sufficient to induce exhaustion of CD8+ T cells, as exhibited by their inability to respond to viral rechallenge. Chronic Ag-stimulated CD8+ T cells displayed defects in expansion and cytokine production following in vivo rechallenge with influenza virus and suggest a reduced quality of the virus-specific CD8+ T cells. When equal numbers of single prime or chronic Ag-stimulated day-30 virus-specific CD8+ T cells were transferred into naive mice and i.n. rechallenged, chronic virus-specific CD8+ T cells exhibited a profound reduction in frequency and absolute number at the peak of the secondary response. The impairment in virus-specific CD8+ T cell population extended beyond a decrease in number, and also resulted in the functional loss of IFN-γ production. This loss was identified in CTLs responding to both dominant and subdominant epitopes. It is important to note, that the loss of subdominant epitope responses may actually be an underestimate. Because subdominant responses were only identified by cytoplasmic staining for IFN-γ, a loss in MFI would prevent their identification as subdominant responders. The defects observed after chronic Ag stimulation alone are similar to those documented during other chronic infections (16, 17, 19, 20, 23), and may indicate that much of what is driving the clonal exhaustion of CD8+ T cells during chronic infection is Ag and continuous TCR stimulation. Our findings in mice are supported by our studies in SIV-infected macaques, which suggest that Ag is driving SIV-specific CD8+ T cell exhaustion (49).
Although many defects observed were consistent with previous observations (16, 17, 19, 20, 23), we also observed unique properties of chronic stimulated CD8+ T cells distinct from previously published reports. Chief among the distinctions was the ability of impaired chronic stimulated CD8+ T cells to completely recover after Ag rest and respond to rechallenge at levels comparable to single prime controls. This suggests that the capability to generate fully functional memory CD8+ T cells is not irreversibly lost by the exhausted CD8+ T cells, and that once TCR stimulation is removed, memory can be generated. Our data suggest that during chronic Ag stimulation the effector phase is perpetuated. Although long-term maintenance of these effectors most likely requires the presence of constant Ag (50, 51), we have shown that once Ag is removed, productive memory can be generated. In a recent report, neither cytokine production nor inflammation was sufficient to sustain homeostatic proliferation of chronic CD8+ T cells in the absence of Ag (50), and it was concluded that in the absence of Ag, chronic CD8+ T cells cannot be maintained. However, despite the absence of homeostatic proliferation of chronic CD8+ T cells, a small surviving population of virus-specific CD8+ T cells remained (50). The capacity of these surviving Ag-independent chronic memory CD8+ T cells to respond to viral rechallenge has never been examined (50, 51). Our findings of functional recovery after Ag rest suggest that despite a reduction in number, in the absence of Ag chronic memory, CD8+ T cells developed during LCMV infection may still recover function. Thus the inability of chronic CD8+ T cells to undergo homeostatic proliferation in the absence of Ag may not necessarily correlate with an inability of surviving cells to adequately respond to viral rechallenge.
A second important distinction between the exhaustion observed in chronic Ag-stimulated CD8+ T cells vs other models of chronic infection was the role of inhibitory molecule PD-1. Recently, a number of reports have indicated that PD-1 expression is increased in chronically stimulated CD8+ T cells and that signaling through PD-1:PD-L1 is responsible, at least in part, for functional deficits of exhausted CD8+ T cells (41-45, 52, 53). Following chronic Ag stimulation alone, we observed a significant increase in expression of PD-1 on both total and virus-specific CD8+ T cells. The increased PD-1 expression on total CD8+ T cells was particularly striking, and could suggest nonspecific activation of CD8+ T cells. Alternatively, the increased expression on total CD8+ T cells may have been induced by IFN-γ produced by Ag-specific effectors (54). Indeed, the link between IFN-γ and PD-1 expression has recently been established in patients with Mycobacterium tuberculosis (54). Despite the increased PD-1 expression, blockade of signaling during the priming phase yielded no significant effect on the ability of chronic stimulated CD8+ T cells to expand or function in response to i.n. rechallenge. Although the frequency of day-30 PD-1-expressing cells was increased in chronic stimulated CD8+ T cells compared with single prime controls, the MFI of PD-1 between the two groups was not statistically different. Perhaps in this case, it is not only the frequency of PD-1-expressing cells that is important, but also the intensity of the receptor expression on those cells. The disparity in findings between our chronic Ag stimulation system and other models of chronic infection may also point to important differences in the development of exhausted CD8+ T cells in the presence or absence of overt inflammation.
Finally, because inhibitory PD-1 expression did not seem to be regulating the loss of chronic Ag-stimulated CD8+ T cell function, we examined the role of TRAIL and Fas death pathways. Following transfer of Fas-deficient single or chronic stimulated CD8+ T cells to naive mice, there was no significant change in the ability of chronic Ag-stimulated CD8+ T cells to respond to viral rechallenge. However, when chronic Ag stimulation was initiated in TRAIL knockout mice, both single and chronic stimulated virus-specific CD8+ T cell responses were comparable. Perhaps even more importantly, TRAIL-deficient chronic CD8+ T cells were significantly greater in absolute number than their wild-type chronic CD8+ T cell counterparts. A role for TRAIL-mediated apoptosis has been suggested in the development of helpless CD8+ T cells (55) and in CD8+ T cell exhaustion (24). Helpless CD8+ T cells, those CD8+ T cells primed in the absence of CD4+ T cells, are frequently compared with exhausted CD8+ T cells as they share a variety of functional defects (56-60) and may have similar mechanisms of exhaustion and deletion. Previous studies indicate that increased susceptibility of helpless CD8+ T cells to apoptosis is TRAIL-mediated (55, 61), at least at early stages of CD8+ T cell memory, and can be overcome in the presence of TRAIL decoy receptors (55). Our findings of increased susceptibility to TRAIL-mediated death following chronic influenza A stimulation, in combination with previous findings, provoke the question of whether chronic Ag stimulation results in a loss of CD4+ T cell help during priming. Although this is one possibility, a recent study refutes the relationship between TRAIL and the development of helpless CD8+ T cell memory (62). Listeria monocytogenes infection of double-deficient (MHC class II−/− TRAIL−/−) mice showed that the absence of TRAIL failed to recover the helpless CD8+ T cell memory response (62). Additionally, because helpless CD8+ T cells exhibit a permanent defect, and chronic Ag-stimulated CD8+ T cells were restored after Ag rest, one could argue that the defect seen in this study is not due to helplessness. Further study will be necessary to determine whether the effects of longer duration of chronic Ag stimulation can be overcome in the absence of TRAIL.
We have shown that chronic Ag stimulation alone is sufficient for the induction of CD8+ T cell exhaustion and subsequent deletion of this population. Contrary to other models of chronic infection in which deletion and exhaustion is induced in the context of inflammation and lymphocyte infection, the exhaustion induced by chronic Ag stimulation alone was reversible by Ag withdraw and not mediated by inhibitory PD-1 signaling. Rather, the loss of virus-specific CD8+ T cells appears to be mediated by TRAIL apoptosis. These data point to a critical importance of understanding the conditions under which exhausted CD8+ T cells are derived. If inflammation and viral load are successfully controlled we may be able to better use current therapies, or develop other therapies, which would allow for recovery of exhausted CD8+ T cell function.
Acknowledgments
We thank Annie Borowski, Constantinos Petrovas, and Duc Do for helpful suggestions and technical support.
Footnotes
This work was supported by Grants R01 AI62437, AI46719, and AI66215 awarded by the National Institutes of Health (to P.D.K.).
Abbreviations used in this paper: LCMV, lymphocytic choriomeningitis virus; DC, dendritic cell; i.n., intranasal; NP, nuclear protein; MLN, mediastinal lymph node; MFI, mean fluorescence intensity; TCID50, 50% tissue culture infective dose; Tg, transgenic; PD-1, programmed death 1; PD-L1, programmed death ligand 1.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 4.Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goepfert PA, Bansal A, Edwards BH, Ritter GD, Jr., Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce γ interferon. J. Virol. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostense S, Vandenberghe K, Joling J, Van Baarle D, Nanlohy N, Manting E, Miedema F. Persistent numbers of tetramer+ CD8+ T cells, but loss of interferon-γ+ HIV-specific T cells during progression to AIDS. Blood. 2002;99:2505–2511. doi: 10.1182/blood.v99.7.2505. [DOI] [PubMed] [Google Scholar]
- 7.Petrovas C, Mueller YM, Katsikis PD. HIV-specific CD8+ T cells: serial killers condemned to die? Curr. HIV Res. 2004;2:153–162. doi: 10.2174/1570162043484960. [DOI] [PubMed] [Google Scholar]
- 8.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 2004;78:5707–5719. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, Klenerman P. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 12.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 13.Kostense S, Ogg GS, Manting EH, Gillespie G, Joling J, Vandenberghe K, Veenhof EZ, van Baarle D, Jurriaans S, Klein MR, Miedema F. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 2001;31:677–686. doi: 10.1002/1521-4141(200103)31:3<677::aid-immu677>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Petrovas C, Mueller YM, Katsikis PD. Apoptosis of HIV-specific CD8+ T cells: an HIV evasion strategy. Cell Death Differ. 2005;12(Suppl. 1):859–870. doi: 10.1038/sj.cdd.4401595. [DOI] [PubMed] [Google Scholar]
- 15.Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD, Katsikis PD. Increased CD95/Fas-induced apoptosis of HIV-specific CD8+ T cells. Immunity. 2001;15:871–882. doi: 10.1016/s1074-7613(01)00246-1. [DOI] [PubMed] [Google Scholar]
- 16.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 2004;172:4204–4214. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 17.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 18.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S, Ou R, Huang L, Price GE, Moskophidis D. Differential tissue-specific regulation of antiviral CD8+ T-cell immune responses during chronic viral infection. J. Virol. 2004;78:3578–3600. doi: 10.1128/JVI.78.7.3578-3600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmann CC, Altman JD, Hinton D, Stohlman SA. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 22.Moser JM, Altman JD, Lukacher AE. Antiviral CD8+ T cell responses in neonatal mice: susceptibility to polyoma virus-induced tumors is associated with lack of cytotoxic function by viral antigen-specific T cells. J. Exp. Med. 2001;193:595–606. doi: 10.1084/jem.193.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou S, Ou R, Huang L, Moskophidis D. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 2002;76:829–840. doi: 10.1128/JVI.76.2.829-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed R, Oldstone MB. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 1988;167:1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topham DJ, Castrucci MR, Wingo FS, Belz GT, Doherty PC. The role of antigen in the localization of naive, acutely activated, and memory CD8+ T cells to the lung during influenza pneumonia. J. Immunol. 2001;167:6983–6990. doi: 10.4049/jimmunol.167.12.6983. [DOI] [PubMed] [Google Scholar]
- 28.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat. Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 29.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 30.Sporri R, Reis e Sousa C. Newly activated T cells promote maturation of bystander dendritic cells but not IL-12 production. J. Immunol. 2003;171:6406–6413. doi: 10.4049/jimmunol.171.12.6406. [DOI] [PubMed] [Google Scholar]
- 31.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 32.Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 2007;81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vezys V, Masopust D, Kemball CC, Barber DL, O'Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr. Opin. Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 36.van der Most RG, Murali-Krishna K, Lanier JG, Wherry EJ, Puglielli MT, Blattman JN, Sette A, Ahmed R. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology. 2003;315:93–102. doi: 10.1016/j.virol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 40.Oxenius A, Price DA, Easterbrook PJ, O'Callaghan CA, Kelleher AD, Whelan JA, Sontag G, Sewell AK, Phillips RE. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA. 2000;97:3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 42.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 43.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 46.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 48.Bender A, Bui LK, Feldman MA, Larsson M, Bhardwaj N. Inactivated influenza virus, when presented on dendritic cells, elicits human CD8+ cytolytic T cell responses. J. Exp. Med. 1995;182:1663–1671. doi: 10.1084/jem.182.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller YM, Petrovas C, Do DH, Altork SR, Fischer-Smith T, Rappaport J, Altman JD, Lewis MG, Katsikis PD. Early establishment and antigen dependence of simian immunodeficiency virus-specific CD8+ T-cell defects. J. Virol. 2007;81:10861–10868. doi: 10.1128/JVI.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, Musella RM, Chuluyan HE, Garcia VE. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 55.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 56.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8+ T-cell responses in CD4-depleted Ig−/− mice. J. Virol. 2000;74:9762–9765. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 58.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 61.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J. Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 62.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J. Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]