Abstract
To explore the role of spinal cord plasticity in motor learning, we evaluated the effects of H-reflex operant conditioning on GABAergic input to rat spinal motoneurons. Previous work indicated that down-conditioning of soleus H-reflex increases GABAergic input to soleus motoneurons. This study explored the effect of H-reflex up-conditioning on GABAergic input. Of nine rats exposed to H-reflex up-conditioning, up-conditioning was successful (H-reflex increase ≥ 20%) in seven and failed (change < 20%) in two. These rats and eight naive control (i.e. unconditioned) rats were injected with cholera toxin subunit B-conjugated Alexa fluor 488 into the soleus muscle to retrogradely label soleus motoneurons. Sections containing soleus motoneurons were processed for GAD67 [one of the two principal forms of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD)] with an ABC-peroxidase system. Two blinded independent raters counted and measured GABAergic terminals on these motoneurons. Unlike successful down-conditioning, which greatly increased the number of identifiable GABAergic terminals on the motoneurons, up-conditioning did not significantly change GABAergic terminal number. Successful up-conditioning did produce slight but statistically significant increases in GABAergic terminal diameter and soma coverage. These results are consistent with other data indicating that up- and down-conditioning are not mirror images of each other, but rather have different mechanisms. Although the marked changes in GABAergic terminals with down-conditioning probably contribute to H-reflex decrease, the modest changes in GABAergic terminals associated with up-conditioning may be compensatory or reactive plasticity, rather than the plasticity responsible for H-reflex increase. As a variety of spinal and supraspinal GABAergic neurons innervate motoneurons, the changes found with up-conditioning may be in terminals other than those affected in successful down-conditioning.
Keywords: activity-dependent plasticity, learning, memory, motor control, spinal cord
Introduction
The role of activity-dependent spinal cord plasticity in motor learning remains largely unknown. Simple experimental models are essential. The H-reflex, the electrical analog of the spinal stretch reflex (SSR), is the simplest behavior of the vertebrate central nervous system. It is mediated primarily by a two-neuron, monosynaptic pathway composed of the primary afferent fiber, its synapse on the alpha motoneuron and the motoneuron itself (Magladery et al., 1951; Matthews, 1972; Baldissera et al., 1981; Henneman & Mendell, 1981; Brown, 1984). Because it is influenced by descending input from the brain, this pathway can be operantly conditioned. In response to an operant conditioning protocol in which reward depends on reflex size, monkeys, humans, rats and mice can gradually decrease or increase the H-reflex or the SSR (Wolpaw et al., 1983, 1986; Wolpaw, 1987; Evatt et al., 1989; Chen & Wolpaw, 1995; Carp et al., 2006; reviewed in Wolpaw, 1997, 2001, 2007; Wolpaw & Chen, 2008). By a standard definition of ‘skill’ as ‘an adaptive behavior acquired through practice’, operantly conditioned changes in reflex size are simple motor skills, and can serve as simple models for studying the processes of skill acquisition and maintenance.
Operant conditioning of the H-reflex depends on the corticospinal tract (CST) (Chen & Wolpaw, 1997, 2002; Chen et al., 2002), and is associated with multi-site plasticity that includes changes in the spinal motoneuron, in several different synaptic terminal populations on the motoneuron, and probably in spinal interneurons and supraspinal areas as well (Carp & Wolpaw, 1994, 1995; Feng-Chen & Wolpaw, 1996; Carp et al., 2001; Chen & Wolpaw, 2005; Wolpaw & Chen, 2006; reviewed in Wolpaw & Tennissen, 2001; Chen et al., 2007; Wolpaw, 2007). A recent study (Wang et al., 2006) showed that successful down-conditioning of the rat soleus H-reflex markedly increases the number of identifiable GABAergic terminals on soleus motoneurons. Combined with other data (Carp & Wolpaw, 1994, 1995; Feng-Chen & Wolpaw, 1996; Chen & Wolpaw, 1997, 2002, 2005; Wolpaw & Chen, 2006), this result suggests that these terminals convey the CST influence that induces the change in motoneuron firing threshold that appears to be largely responsible for down-conditioning (Carp & Wolpaw, 1994; Halter et al., 1995).
The present study explored the effects of H-reflex up-conditioning on GABAergic terminals on motoneurons. The results provide new insight into the complexity and specificity of the spinal cord plasticity associated with acquisition of the simple skill of a larger or smaller H-reflex.
Materials and methods
Subjects were nine adult (4 months old) male Sprague–Dawley rats weighing 439 ± 25 g at the beginning of the study. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (National Academy Press, Washington, DC, 1996) and had been reviewed and approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. The protocols for H-reflex conditioning, motoneuron labeling and immunohistochemical processing are fully described elsewhere (Wolpaw & Herchenroder, 1990; Chen & Wolpaw, 1995; Wang et al., 2006). They are summarized here.
Under general anesthesia [ketamine HCl (80 mg/kg) and xylazine (10 mg/kg), both i.p.], each rat was implanted with chronic stimulating and recording electrodes in the right hindlimb. To elicit the H-reflex, a nerve-stimulating cuff was placed around the right posterior tibial nerve just proximal to the triceps surae branches. To record soleus electromyographic (EMG) activity, fine-wire electrodes were inserted in the right soleus muscle. The Teflon-coated wires from the nerve cuff and the muscle passed under the skin to a connector plug mounted on the skull.
Data collection started at least 20 days after implantation. Throughout data collection, each rat lived in a standard rat cage with a flexible cable attached to the head connector. The cable, which allowed the rat to move freely about the cage, carried the wires from the electrodes to a commutator above the cage and from there to an EMG amplifier and a nerve-cuff stimulation unit. The rat had free access to water and food, except that during H-reflex up-conditioning it obtained most or all of its food in the form of rewards (as described below). Animal well-being was carefully checked several times each day, and body weight was measured weekly. Laboratory lighting was reduced between 21:00 and 06:00 hours each day.
A computer system continuously (24 h a day, 7 days a week) monitored EMG from the soleus muscle and controlled the nerve-cuff stimulus. If the absolute value of background EMG stayed within a defined range for a randomly varying period of 2.3–2.7 s, a stimulus pulse (typically 0.5 ms in duration) was delivered by the nerve cuff. Pulse amplitude was continually adjusted so that it remained just above M-response threshold. Background EMG level (reflecting soleus motoneuron tone at the time of H-reflex elicitation), M-response size (reflecting the effective strength of the nerve-cuff stimulus) and number of trials/day remained stable throughout data collection.
In the control mode, the computer simply measured the absolute value of soleus EMG for 50 ms following the stimulus. In the up-conditioning mode, it gave a reward (i.e. a 20-mg food pellet) 200 ms after nerve stimulation if EMG amplitude in the H-reflex interval (typically 6.0–10.0 ms after stimulation) was above a criterion value.
In the course of its daily activity, the animal usually satisfied the background EMG requirement, and thus received nerve-cuff stimulation, 3000–8000 times per day. H-reflex size was calculated as average EMG amplitude in the H-reflex interval minus average background EMG amplitude, and was expressed in units of average background EMG amplitude. Each rat was first exposed to the control mode for 20 days to determine its initial H-reflex size and was then exposed to the up-conditioning mode for 50 days. To determine the final effect on H-reflex size of exposure to the up-conditioning mode, average H-reflex size for the final 10 days of the 50-day exposure was calculated as percentage of initial H-reflex size (i.e. the average of the final 10 control-mode days). Successful conditioning was defined as an increase of at least 20% from initial H-reflex size (Wolpaw et al., 1993; Chen & Wolpaw, 1995). [If rats are not exposed to the up-conditioning or down-conditioning mode, and simply continue under the control mode for 50 more days, H-reflex size does not change from its initial value (Chen et al., 2006a).]
H-reflex conditioning was successful [i.e. increase ≥ 20% (Wolpaw et al., 1993; Chen & Wolpaw, 1995)] in seven of the nine up-conditioned rats. This success rate (i.e. 78%) is comparable with those in previous studies (Chen & Wolpaw, 1995, 1997, 2002; Chen et al., 2002, 2003, 2006b). In the seven successfully up-conditioned rats, final H-reflex size averaged 214 ± 41% (SE) of its initial value. In the two failed rats, final H-reflex size was 110.1 and 80.3% of its initial value, respectively. For all rats, background EMG and M-response remained stable throughout data collection.
At the end of the up-conditioning period, the rat was anesthetized and injected in the right soleus muscle with 50 μg (in 50 μL distilled water) of cholera toxin subunit B-conjugated Alexa fluor 488 (CTB-Fluor 488; Molecular Probes, Eugene, OR, USA) as described in Wang et al. (2006). A 100-μL Hamilton syringe with a 33-gauge needle was used. The right soleus muscle was exposed through a small incision in the skin of the lateral aspect of the calf. Under a dissection microscope, the needle was inserted near the distal tendon and advanced carefully to the middle of the muscle. The dye was injected at three points near the middle. Injection was very slow (over 3 min) to prevent leakage along the needle track. After the injection, the needle was left in situ for 2–3 min and then slowly withdrawn. The area was rinsed thoroughly with saline for 5 min to remove any leakage, and the wound was then sutured. Eight naive unimplanted male rats of comparable weight were also anesthetized and injected as controls. Three days later, each rat was killed with an overdose of sodium pentobarbital and perfused intracardially with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3). The lumbosacral spinal cord was removed, postfixed, washed with 0.05 M phosphate buffer containing 137 mM NaCl (PBS, pH 7.4), and infiltrated with 30% sucrose for 24 h. The part of the spinal cord containing the soleus motoneuron pool was blocked, embedded in OCT compound (Tissue-Tek), and frozen on dry ice. Transverse 25-μm frozen sections were cut with a cryostat, mounted onto precoated glass slides (Superfrost; Fisher), and examined by fluorescent microscopy to identify CTB-Fluor 488 retrograde-labeled soleus motoneurons. All identified soleus motoneurons were photographed with a digital camera (Olympus Magnafire™ SP, IKH027801). The slides were then stored in a low-temperature freezer (at −80°C) before further immunohistochemistry processing. Figure 1A shows three CTB-Fluor 488-labeled soleus motoneurons.
Fig. 1.
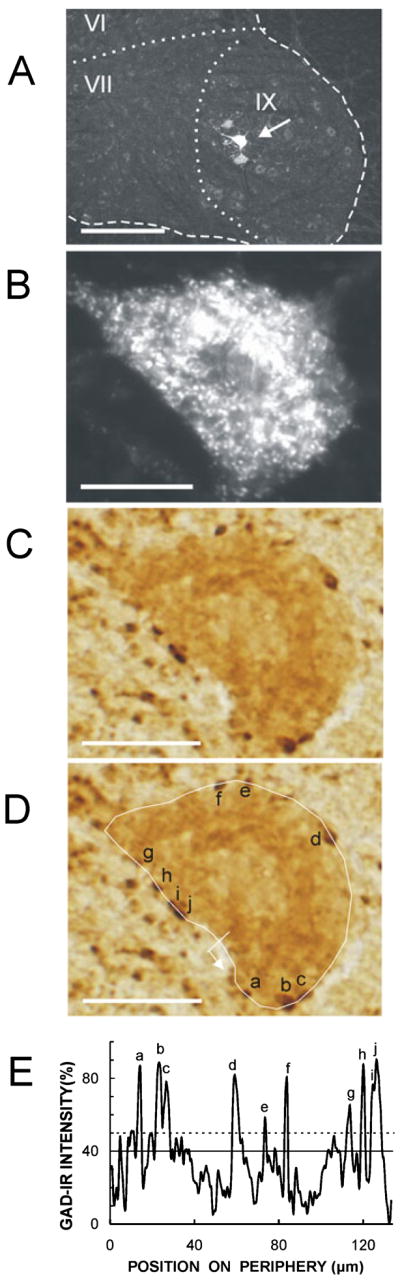
Assessment of glutamic acid decarboxylase-67 immunoreactivity (GAD-Ir). (A) A low-magnification image showing three CTB-Fluor 488-labeled soleus motoneurons in the ventral horn of the rat spinal cord. The arrow indicates the motoneuron shown in B–D. The dashed line shows the border of the ventral horn and the dotted lines separate the laminae, which are labeled. (B) Single soleus motoneuron. (C) The same motoneuron showing GAD-IR. (D) Tracing of the perimeter of the same motoneuron, with identified GABAergic terminals indicated. The line and small arrow indicate the starting point and direction of perimeter luminance measurement. Scale bars: 260 μm in A, 20 μm in B–D. (E) Luminance vs. position on the perimeter of this same motoneuron (luminance of a totally clear slide = 0%, and luminance of a totally opaque slide = 100%). The peaks corresponding to the terminals identified in D are indicated. The solid line is the average value of the perimeter and the dotted line is 10% above this average.
GAD67 [one of the two principal forms of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD)] immunoreactivity was assessed in sections from the portion of the spinal cord containing identified soleus motoneurons using the standard avidin–biotin complex (ABC)–peroxidase system (ABC Elite; Vector Laboratories, Burlingame, CA, USA). We studied every other section from the seven successful and eight naive control rats and three of every four sections from the two failed rats (because there were only two of these rats). Sections from successful, failed and naive control rats were processed together. The sections were washed in 0.05 M phosphate-buffered saline containing 0.1% Triton X-100 (PBST, pH 7.4) three times (10 min each), incubated for 30 min with 5% normal goat serum, and then incubated for 18–20 h in a humid chamber at room temperature in PBST containing 3% bovine serum albumin with rabbit anti-GAD67 polyclonal antibody (K2 antibody; Chemicon, Temecula, CA, USA; 1: 2000 dilution) as the primary antibody. The sections were again washed with PBST, and the biotinylated secondary antibody (goat anti-rabbit, 1: 200 in PBS) was applied for 1.5 h. After endogenous peroxidase activity was quenched by 0.3% H2O2 for 15 min, the sections were reacted for 1.5 h with the ABC (1: 100 in PBS). The sections were washed with 0.05 M Tris–HCl buffer (TBS, pH 7.6) before color development. Finally, the sections were reacted with 0.04% DAB solution and 0.006% H2O2 for 8 min to optimize the signal-to-noise ratio.
Sections processed without primary antibody or with GAD67 antibody diluted 1: 10 000 did not show any GAD67 immunoreactivity in the ventral horn of the spinal cord (although the diluted antibody still showed strong GAD67 immunoreactivity in the superficial laminae of the dorsal horn). The GAD67 specificity of the present study was also confirmed by concomitant processing of cerebellar sections, which showed the expected (Oertel et al., 1981) densely labeled GAD67 terminals on the cell bodies and proximal dendrites of Purkinje cells and in glomeruli in the granular layer.
Soleus motoneurons in the GAD67-labeled images were identified by matching them to their CTB-Fluor 488-labeled images using location, somatic features and other landmarks (e.g. Fig. 1A–C). Analysis was confined to those soleus motoneurons that had a nucleus and a clearly defined somatic border. Seventy-four per cent of the CTB-Fluor 488-labeled motoneurons satisfied these criteria. The remainder were either invisible in the sections (9%), lacked a clear somatic border (7%) or lacked a nucleus (10%). These percentages are comparable with those in our earlier study of down-conditioned rats (Wang et al., 2006). In the two rats in which three out of every four sections were examined, whenever a soleus motoneuron with a nucleus was identified in a section, the adjacent sections were examined to determine whether the nucleus appeared in one of them as well. A total of six such motoneurons were found. In these cases, only the image with the bigger nucleus was counted for analysis.
The soleus motoneurons found suitable for analysis were photographed with an Olympus BH2-RFCA microscope (500× magnification with a 40× objective lens, fixed illumination) equipped with an Olympus DP70 digital camera (magnification 10×, standard illumination). Each soleus motoneuron was first examined with a 20× objective lens to identify the perimeter of the motoneuron soma and to count the number of GAD67-labeled terminals on it. It was then examined again with a 40× lens. The 20× and 40× objectives yielded the same number of visible terminals on the membrane. With the help of the DP Controller Program, each motoneuron image was analyzed at a focal plane at which all the terminals on the perimeter of the soma could be seen and were as well focused as possible. The resulting images were then renamed with codes by a person who was not otherwise involved in this study, so that all measurements could be done in a blinded fashion. The GAD67-immunoreactivity (GAD-IR) of each image was then assessed twice, once by each of two independent raters who did not know whether the image came from a rat in which up-conditioning had been successful, from a rat in which up-conditioning had failed or from a naive rat.
As in the down-conditioning study (Wang et al., 2006), each rater measured the soma size and the GAD-IR of the soma and its perimeter as follows. The original microgaphs were enlarged tenfold (i.e. to a total magnification of 5000×), and the perimeter of each motoneuron (excluding proximal dendrites; Wang et al., 2006) was traced with the IMAGE J program (NIH, version 1.29×). The program calculated soma diameter (i.e. Feret’s diameter, equivalent to the long axis of the soma), soma area, average luminance of the entire soma excluding the perimeter and average luminance of the perimeter, and it plotted perimeter luminance vs. location on the perimeter. No corrections were made for tissue shrinkage.
To determine GAD-IR, luminance values were converted into a percentage, with the luminance of a totally clear slide defined as 0% and the luminance of a totally opaque slide defined as 100%. Figure 1C–E shows a representative soleus motoneuron, the tracing of its perimeter and identification of its GABAergic terminals, and the corresponding plot of its perimeter luminance.
Each rater assessed the numbers, sizes and GAD density of the GABAergic terminals on each motoneuron as described previously (Wang et al., 2006). Briefly, GABAergic terminals were identified as punctate or bead-like densities (Border & Mihailoff, 1985) on the perimeter of the motoneuron soma. They were further confirmed by the GAD-IR vs. perimeter location plot, in which each terminal was evident as an abrupt peak with a maximum GAD-IR value that exceeded the average GAD-IR of the perimeter by at least 10%. After the original microgaphs were enlarged 20-fold (i.e. to a total magnification of 10 000×), each confirmed terminal was traced using the IMAGE J program as described above to determine the terminal’s Feret’s diameter, area, average diameter [i.e. 2√(area/π)] and GAD density (i.e. GAD-IR value of the terminal divided by its average diameter to correct for terminal thickness). Motoneuron terminal coverage (i.e. sum of Feret’s diameters of all the terminals on the motoneuron expressed as percentage of the motoneuron’s perimeter) was also calculated.
The analyzed images were decoded and their data were sorted by rat and motoneuron. As in the down-conditioning study (Wang et al., 2006), the data of the two independent raters were found to be very similar, differing by < 3% across the different measurements made. These minor differences were resolved by joint evaluation and discussion (i.e. for differences in terminal identification) or by averaging the two raters’ values (i.e. for quantitative measurements).
The decoded data were collated and evaluated statistically to detect differences between the seven up-conditioning successful (US) rats, the two up-conditioning failed (UF) rats, and the eight naive control (NC) rats. For each measure, groups were compared by one-way ANOVA. When a difference was detected with P < 0.01, it was then confirmed by nested ANOVA, with terminals nested in cells, cells nested in rats and rats nested in groups. Significant (P < 0.01) differences between groups were detected by the Tukey–Kramer HSD test.
Results
Data were gathered from 432 soleus motoneurons and 1745 GABA-ergic terminals from the seven US, two UF and eight NC rats. Table 1 shows the measures obtained from these three groups and indicates with asterisks those US or UF values that differ significantly from NC values.
Table 1.
Motoneuron and GABAergic terminal numbers and properties for up-conditioning successful (US) rats, up-conditioning failed (UF) rats and naive control (NC) rats
| Experimental group |
|||
|---|---|---|---|
| US | NC | UF | |
| Rats (n) | 7 | 8 | 2 |
| Soleus motoneurons (n) | 167 | 191 | 74 |
| Soleus motoneurons per rat | 24 ± 4 | 24 ± 3 | 37 ± 6 |
| Feret’s diameter (μm) | 44 ± 1 | 44 ± 1 | 43 ± 1 |
| Perimeter (μm) | 114 ± 1 | 112 ± 1 | 109 ± 2 |
| Area (μm2) | 845 ± 18 | 887 ± 18 | 822 ± 27 |
| Somatic GAD67-IR (%) | 18.5 ± 0.5 | 17.3 ± 0.5 | 17.2 ± 0.7 |
| Perimeter GAD67-IR (%) | 19.1 ± 0.5 | 17.9 ± 0.4 | 18.9 ± 0.7 |
| GAD67-IR terminals (n) | 727 | 685 | 333 |
| Terminal per cell | 4.3 ± 0.2 | 3.7 ± 0.2 | 4.2 ± 0.3 |
| Feret’s diameter (μm) | 2.07 ± 0.02* | 1.96 ± 0.03 | 2.03 ± 0.03 |
| Area (μm2) | 1.95 ± 0.04 | 1.85 ± 0.05 | 1.79 ± 0.06 |
| GAD density (GAD-IR per diameter) | 20.6 ± 0.3 | 19.5 ± 0.4 | 20.7 ± 0.4 |
| Coverage of soma (%) | 8.5 ± 0.4* | 6.9 ± 0.4 | 8.0 ± 0.5 |
Data are presented as average ± SEM.
P < 0.01 compared with naive control rat (NC) group.
Glutamic acid decarboxylase immunoreactivity (GAD-IR) is in percentage of maximum possible GAD-IR (i.e. GAD-IR of an opaque slide; see text).
With regard to the motoneuron measures, US, UF and NC rats did not differ in number of soleus motoneurons per rat, or in average motoneuron diameter, perimeter or area. Soleus motoneuron sizes were similar to those reported in previous studies (Ishihara et al., 2001; Wang et al., 2006).
With regard to the GABAergic terminal measures, US, UF and NC rats did not differ in terminals per cell, terminal area or GAD density (P > 0.01 for each measure). US rats had slightly but significantly larger Feret’s diameter than NC rats and also had significantly higher GABAergic terminal somatic coverage than NC rats (P < 0.01 for each measure). UF rats did not differ from NC rats in any terminal measure. Nevertheless, it should be noted that UF rats fell between US and NC rats in Feret’s diameter and somatic coverage, and that US and UF rats did not differ significantly from each other in any measure (P > 0.1 for each measure).
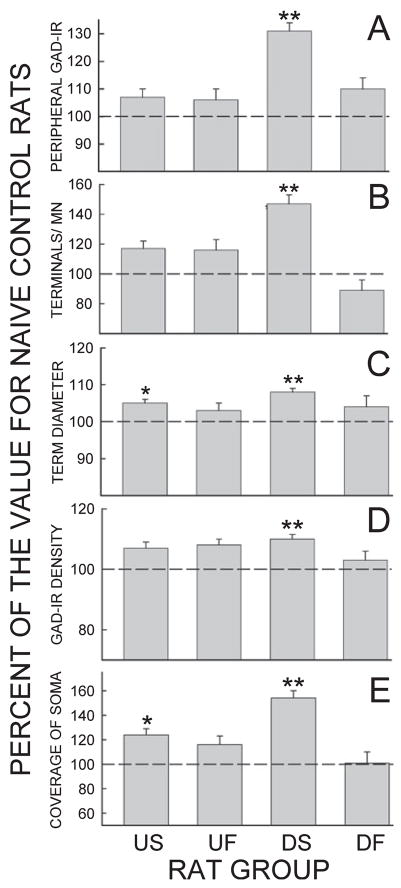
Figure 2 shows, for US and UF rats, the average (± SE) values (in percentage of the values for naive control rats) for GAD-IR intensity of motoneuron perimeter, number of GABAergic terminals per motoneuron, Feret’s diameter of GABAergic terminals, terminal GAD density and GABAergic terminal somatic coverage. Previous data from successful and failed down-conditioning (DS and DF) rats (Wang et al., 2006) are included for comparison.
Fig. 2.
Average (± SE) values for up-conditioning successful (US) and up-conditioning failed (UF) rats of the present study. The values for down-conditioning successful (DS) and down-conditioning failed (DF) rats of our previous study (Wang et al., 2006) are included for comparison. Values are percentage of those for naive control (NC) rats. (Each study had its own group of NC rats.) (A) GAD-IR intensity of motoneuron perimeter. (B) Number of GABAergic terminals per motoneuron. (C) Feret’s diameter of GABAergic terminals. (D) Terminal GAD density (i.e. GAD-IR per diameter, as described in the text). (E) GABAergic terminal coverage of soma (expressed as percentage of perimeter, see text). *P < 0.01 compared with NC rats; **P < 0.001 compared with NC rats.
Discussion
GAD67 is one of the two principal forms of the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD). In the spinal cord, the two forms are present in large numbers of boutons and are frequently co-localized. In the rat, P boutons, which form axoaxonic synapses in lamina IX (and lamina VII), have been shown to contain GAD65 but have not as yet been shown to contain GAD67 (Hughes et al., 2005). In contrast, GAD67 is more evident in terminals directly on motoneurons (McLaughlin et al., 1975; Mackie et al., 2003; Hughes et al., 2005). In addition to contacting motoneurons directly, GABAergic terminals also contact other terminals on motoneurons presynaptically (Rudomín et al., 1987; Rudomín, 1990; Maxwell & Riddell, 1999). Because terminals that receive presynaptic contacts (i.e. primary afferent terminals) are absent or rare on the motoneuron soma (Holstege & Calkoen, 1990; d’Incamps et al., 1998; Watson & Bazzaz, 2001), we are confident that the GABAergic terminals identified in this light-microscopic study are largely or wholly on the motoneuron rather than on other terminals.
Successful up-conditioning (US rats) was associated with modest but significant increases in the average Feret’s diameter of identified GABAergic terminals and in GABAergic terminal coverage of the motoneuron somatic membrane. Whereas up-conditioning failed (UF) rats did not show significant changes in these values, their values did tend to change in the same direction, and US and UF values were not significantly different from each other. Thus, the GABAergic terminal changes noted in US rats may not be responsible for H-reflex increase; rather they may result simply from exposure to the up-conditioning mode. This possibility is supported by the fact that the increases in GABAergic terminal diameter and somatic coverage are ostensibly inconsistent with the probability that up-conditioning results from decreased oligosynaptic inhibition (and/or increased oligosynaptic excitation) from spinal interneurons excited by primary afferent input (Carp & Wolpaw, 1995; Wolpaw & Chen, 2001). However, it is possible that the increased GABAergic terminal size and coverage are reactive responses to decreased activity in these inhibitory interneurons. That is, they may reflect reactive hypertrophy comparable with that found in primary afferent terminals on motoneurons after long-term suppression of afferent excitation (e.g. Webb & Cope, 1992).
The present results stand in clear contrast to those from down-conditioned rats (Wang et al., 2006), and are additional evidence that up-conditioning and down-conditioning do not have the same mechanisms, i.e. that they are not mirror images of each other. Using the same labeling and analysis methodology as the present study, Wang et al. (2006) found that successful down-conditioning (DS) rats markedly increased the number, size and GAD density of identified GABAergic terminals on soleus motoneurons. These changes were large: terminal number increased more than 54% and total synaptic coverage increased 60%. Down-conditioning failed (DF) rats did not show similar changes, and did not even show insignificant changes in the same direction. As discussed elsewhere (Wang et al., 2006) and further supported by recent data (Chen et al., 2007), these GABAergic terminals may originate in interneurons in spinal lamina VI–VII that receive CST input, and may convey to the motoneurons the critical CST influence responsible for the positive shift in motoneuron firing threshold that largely accounts for the H-reflex decrease found in successfully down-conditioned rats.
H-reflex conditioning is associated with plasticity at multiple spinal and supraspinal sites (Wolpaw & Chen, 2008). Although some of this plasticity [e.g. the positive shift in motoneuron firing threshold in successfully down-conditioned animals (Carp & Wolpaw, 1994)] appears to be responsible for the conditioned reflex change, other plasticity is likely to be compensatory or reactive (Wolpaw & Tennissen, 2001; Wolpaw, 2007). That is, it may be compensatory plasticity that preserves other motor skills despite the impact of the plasticity responsible for the H-reflex change, or it may be reactive plasticity caused by plasticity elsewhere (Wolpaw & Tennissen, 2001; Wolpaw, 2007). Thus, the changes in GABAergic terminals in up-conditioned rats may not contribute to the H-reflex increase. It is important to note that GABAergic terminals are abundant on motoneurons and come from a variety of spinal and supraspinal areas (Holstege, 1991; Todd & Maxwell, 2000; Alvarez et al., 2005; Hughes et al., 2005). The changes noted with up-conditioning could well involve a population of GABAergic terminals different from the population changed by down-conditioning.
Spinal cord GABAergic function is affected by lesions of various kinds, including peripheral nerve injury, rhizotomy and spinal cord transection (Dumoulin et al., 1996; Lindå et al., 2000; Tillakaratne et al., 2000; Moore et al., 2002). It is also changed by locomotor training after spinal cord transection, and in this context is thought to contribute to improvements in performance (Tillakaratne et al., 2002). The GABAergic plasticity induced by H-reflex conditioning differs from that described after spinal cord transection in that it occurs in intact rats and is presumably the result of conditioning-induced changes in descending activity, especially in the CST. Nevertheless, it is likely that the mechanisms of the spinal GABAergic plasticity that occurs in the course of skill acquisition in intact animals are related to those of the spinal GABAergic plasticity that results from central and peripheral lesions.
Acknowledgments
We thank Ms Lu Chen and Ms Rongliao Liu for excellent technical assistance, Dr David L. Martin for important methodological advice, and Drs Jonathan S. Carp, Dennis J. McFarland and Elizabeth Winter Wolpaw for valuable comments on the manuscript. We are grateful to Dr Niranjala J. K. Tillakaratne of the Department of Physiological Sciences and Neurology at the University of California, Los Angeles, for her advice and for providing us with K2 antibody. This work was supported in part by grants from the National Institutes of Health [HD36020 (X.Y.C.) and NS22189 (J.R.W.)], the New York State Spinal Cord Injury Trust Fund (X.Y.C.), the International Spinal Research Trust (J.R.W.), and the Christopher Reeve Paralysis Foundation (X.Y.C.).
Abbreviations
- CST
corticospinal tract
- DF
down-conditioning failed
- DS
down-conditioning successful
- GAD
glutamic acid decarboxylase
- IR
immunoreactivity
- NC
naive control
- SSR
spinal stretch reflex
- UF
up-conditioning failed
- US
up-conditioning successful
References
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology Section I: The Nervous System Vol 2 Motor Control. Part I. Williams and Wilkins Co; Baltimore, MD: 1981. pp. 509–595. [Google Scholar]
- Border BG, Mihailoff GA. GAD-immunoreactive neural elements in basilar pontine nuclei and nucleus reticularis tegmenti pointis of the rat. I Light microscopic studies. Exp Brain Res. 1985;59:600–614. doi: 10.1007/BF00261352. [DOI] [PubMed] [Google Scholar]
- Brown WF. The Physiological and Technical Basis of Electromyography. Butterworths; Boston, MA: 1984. [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron properties after operantly conditioned increase in primate H-reflex. J Neurophysiol. 1995;73:1365–1373. doi: 10.1152/jn.1995.73.4.1365. [DOI] [PubMed] [Google Scholar]
- Carp JS, Chen XY, Sheikh H, Wolpaw JR. Operant conditioning of rat H-reflex affects motoneuron axonal conduction velocity. Exp Brain Res. 2001;136:269–273. doi: 10.1007/s002210000608. [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol. 2006;96:1718–1727. doi: 10.1152/jn.00470.2006. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995;73:411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Dorsal column but not lateral column transection prevents down conditioning of H-reflex in rats. J Neurophysiol. 1997;78:1730–1734. doi: 10.1152/jn.1997.78.3.1730. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in rats. J Neurophysiol. 2002;87:645–652. doi: 10.1152/jn.00391.2001. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Ablation of cerebellar nuclei prevents H-reflex down-conditioning in rats. Learn Mem. 2005;12:248–254. doi: 10.1101/lm.91305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Corticospinal tract transection prevents up-conditioning of H-reflex in rats. Exp Brain Res. 2002;144:88–94. doi: 10.1007/s00221-002-1026-8. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Wolpaw JR. Conditioned H-reflex increase persists after transection of the main corticospinal tract in rats. J Neurophysiol. 2003;90:3572–3578. doi: 10.1152/jn.00264.2003. [DOI] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Chen L, Wolpaw JR. Sensorimotor cortex ablation prevents H-reflex up-conditioning and causes a paradoxical response to down-conditioning in rats. J Neurophysiol. 2006a;96:119–127. doi: 10.1152/jn.01271.2005. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen Y, Chen L, Tennissen AM, Wolpaw JR. Corticospinal tract transection permanently abolishes H-reflex down-conditioning in rats. J Neurotrauma. 2006b;23:1743–1750. doi: 10.1089/neu.2006.23.1705. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wang Y, Pillai S, Wolpaw JR. H-reflex down-conditioning increases the number of identifiable GABAergic spinal interneurons in rats. Soc Neurosci Abstr. 2007:404.4. doi: 10.1016/j.neulet.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Alonso G, Privat A, Feldblum S. Biphasic response of spinal GABAergic neurons after a lumbar rhizotomy in the adult rat. Eur J Neurosci. 1996;8:2553–2563. doi: 10.1111/j.1460-9568.1996.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: preliminary studies. Neurosci Lett. 1989;105:350–355. doi: 10.1016/0304-3940(89)90646-0. [DOI] [PubMed] [Google Scholar]
- Feng-Chen KC, Wolpaw JR. Operant conditioning of H-reflex changes synaptic terminals on primate motoneurons. Proc Natl Acad Sci USA. 1996;93:9206–9211. doi: 10.1073/pnas.93.17.9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter JA, Carp JS, Wolpaw JR. Operantly conditioned motoneuron plasticity: possible role of sodium channels. J Neurophysiol. 1995;73:867–871. doi: 10.1152/jn.1995.73.2.867. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and inputs. In: Brooks VB, editor. Handbook of Physiology Section I: The Nervous System Vol 2 Motor Control. Part I. Williams and Wilkins Co; Baltimore, MD: 1981. pp. 423–507. [Google Scholar]
- Holstege JC. Ultrastructural evidence for GABAergic brain stem projections to spinal motoneurons in the rat. J Neurosci. 1991;11:159–167. doi: 10.1523/JNEUROSCI.11-01-00159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, Calkoen F. The distribution of GABA in lumbar motoneuronal cell groups. A quantitative ultrastructural study in rat. Brain Res. 1990;530:130–137. doi: 10.1016/0006-8993(90)90669-3. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci USA. 2005;102:9039–9043. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Incamps BL, Destombes J, Thiesson D, Hellio R, Lasserre X, Kouchtir-Devanne K, Jami L, Zytnicki DL. Indications for GABA-immnuoreactive axo-axonic contacts on the intraspinal arborization of Ib fiber in cat: a confocal microscope study. J Neurosci. 1998;23:10030–10036. doi: 10.1523/JNEUROSCI.18-23-10030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Ohira Y, Tanaka M, Nishikawa W, Ishioka N, Higashibata A, Izumi R, Shimazu T, Ibata Y. Cell body size and succinate dehydrogenase activity of spinal motoneurons innervating the soleus muscle in mice, rats, and cats. Neurochem Res. 2001;26:1301–1304. doi: 10.1023/a:1014245417017. [DOI] [PubMed] [Google Scholar]
- Lindå H, Shupliakov O, Örnung G, Ottersen OP, Storm-Mathisen J, Risling M, Cullheim S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J Comp Neurol. 2000;425:10–23. [PubMed] [Google Scholar]
- Mackie M, Hughes DI, Maxwell DL, Tillakaratne NJK, Todd AJ. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience. 2003;119:461–472. doi: 10.1016/s0306-4522(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Magladery JW, Porter WE, Park AM, Teasdall RD. Electro-physiological studies of nerve and reflex activity in normal man. IV The two-neuron reflex and identification of certain action potentials from spinal roots and cord. Bull John Hopkins Hosp. 1951;88:499–519. [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and their Central Actions. Williams & Wilkins; Baltimore, MD: 1972. [Google Scholar]
- Maxwell DL, Riddell JS. Axoaxonic synapses on terminals of group II muscle spindle afferent axons in the spinal cord of cat. Eur J Neurosci. 1999;11:2151–2159. doi: 10.1046/j.1460-9568.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin BJ, Barber R, Saito K, Roberts E, Wu JY. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J Comp Neurol. 1975;164:305–322. doi: 10.1002/cne.901640304. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz L, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Schmechel DE, Mugnaini E, Tappaz ML, Kopin IJ. Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neuroscience. 1981;6:2715–2735. doi: 10.1016/0306-4522(81)90115-9. [DOI] [PubMed] [Google Scholar]
- Rudomín P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990;13:499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Rudomín P, Solodkin M, Jiménez I. Synaptic potentials of primary afferent fibers and motoneurons evoked by single intermediate nucleus interneurons in the cat spinal cord. J Neurophysiol. 1987;57:1288–1313. doi: 10.1152/jn.1987.57.5.1288. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJK, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;59:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJK, Leon RDD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Maxwell DJ. GABA in the mammalian spinal cord. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: the View at Fifty Years. Lippincott, Williams & Wilkins; Philadelphia, PA: 2000. pp. 439–457. [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR, Chen XY. Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci. 2006;23:141–150. doi: 10.1111/j.1460-9568.2005.04547.x. [DOI] [PubMed] [Google Scholar]
- Watson AHD, Bazzaz AA. GABA and Glycine-like immunoreactivity at axoaxonic synapses on Ia muscle afferent terminals in the spinal cord of the rat. J Comp Neurol. 2001;433:335–348. doi: 10.1002/cne.1143. [DOI] [PubMed] [Google Scholar]
- Webb CB, Cope TC. Modulation of Ia EPSP amplitude: the effects of chronic synaptic inactivity. J Neurosci. 1992;12:338–344. doi: 10.1523/JNEUROSCI.12-01-00338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol. 1987;57:443–458. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20:588–594. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Spinal cord plasticity in the acquisition of a simple motor skill. In: Patterson MM, Graw JW, editors. Spinal Cord Plasticity: Alterations in Reflex Function. Kluwer Academic Publications; Boston, MA: 2001. pp. 101–125. [Google Scholar]
- Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol. 2007;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. Operant conditioning of rat H-reflex: effects on mean latency and duration. Exp Brain Res. 2001;136:274–279. doi: 10.1007/s002210000609. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. The cerebellum in maintenance of a motor skill: a hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn Mem. 2006;13:208–215. doi: 10.1101/lm.92706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. Operant conditioning of reflexes. In: Squire L, Albright T, Bloom F, Gage F, Spitzer N, editors. Encyclopedia of Neuroscience. Academic Press; Oxford: 2008. in press. [Google Scholar]
- Wolpaw JR, Herchenroder PA. Operant conditioning of H-reflex in freely moving monkeys. J Neurosci Meth. 1990;31:145–152. doi: 10.1016/0165-0270(90)90159-d. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Ann Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in the primate spinal stretch reflex: initial development. J Neurophysiol. 1983;50:1296–1311. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, O’Keefe JA, Noonan PA, Sanders MG. Adaptive plasticity in primate spinal stretch reflex: persistence. J Neurophysiol. 1986;55:272–279. doi: 10.1152/jn.1986.55.2.272. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Herchenroder PA, Carp JS. Operant conditioning of the primate H-reflex: factors affecting the magnitude of change. Exp Brain Res. 1993;97:31–39. doi: 10.1007/BF00228815. [DOI] [PubMed] [Google Scholar]