Abstract
Acute and prolonged methamphetamine (METH) exposure has been reported to moderate the function of N-methyl-d-aspartate type glutamate receptors (NMDAr) in the hippocampus. These effects have been found to be associated with enhanced NMDAr-dependent release of Ca2+ from IP3-sensitive intracellular stores. The present studies were designed to extend these findings and examine the role of the endoplasmic membrane (ER) bound orphan receptor, the sigma 1 receptor, in NMDA-induced neuronal injury and METH withdrawal-potentiated NMDA-induced neuronal injury. Organotypic hippocampal slice cultures were exposed to METH (0 or 100 µM) for 6 days and withdrawn for 7 days, then exposed to NMDA (0 or 5 µM) for 24 hrs. Additional cultures were also exposed to this regimen and were co-incubated with BD1047 (100 µM), a specific inhibitor of ER-bound sigma 1 receptors, for the 24 hr NMDA exposure. Cytotoxicity was assessed by analysis of propidium iodide uptake. These studies demonstrated that protracted METH exposure and withdrawal significantly potentiated the neuronal injury produced by NMDA exposure. Further, co-exposure to BD1047 with NMDA markedly attenuated neuronal injury in METH-naïve and METH- withdrawn organotypic cultures. As a whole, these data demonstrate that prolonged METH exposure, even at non-toxic concentrations, significantly alters glutamate receptor signaling. Inhibition of sigma 1 receptor-dependent Ca2+ release from the ER entirely prevented NMDA-induced toxicity in METH-naïve cultures and markedly reduced METH-potentiated toxicity. These findings demonstrate the importance of Ca2+-induced intracellular Ca2+ release in excitotoxic insult and suggest that blockade of glutamatergic overactivity may represent a therapeutic target in the treatment of METH withdrawal.
Keywords: NMDA, METHAMPHETAMINE, HIPPOCAMPUS, SIGMA-1 RECEPTOR
1. Introduction
Methamphetamine (METH) abuse has increased substantially in the United States in the last decade. Chronic METH use has been associated with decreases in dopaminergic transporters (DAT), D2 receptors, and hippocampal volume as well as cognitive deficits, including attention/psychomotor speed, learning & memory, and fluency [1, 12, 38]. While DAT measures displayed improvement after protracted abstinence (~9 months) from METH, this does not appear to correlate with improvements on behavioral deficits (time gaiting and delayed recall tests) [40].
Mechanisms identified in METH neurotoxicity primarily involve the oxidation and depletion of monoamines [3, 27]. However, several studies have suggested that METH may interact with glutamatergic systems, particularly the N-methyl-D-aspartate (NMDA) receptor system [23, 31, 32, 41, 42]. Previously our lab has reported that METH may act as a functional NMDA receptor (NMDAr) antagonist, and possibly as a competitive NMDAr antagonist [31]. These findings suggest that chronic antagonism of the NMDAr by METH may result in multiple adaptive responses that could increase the likelihood of neuronal excitability during withdrawal. Specifically, Moriguchi et al. (2002) reported increased NMDAr mediated EPSP amplitudes even upon hyperpolarization of the cell in neostriatal slices following long-term withdrawal (7 days) from METH. Furthermore, previous studies have suggested that long-term withdrawal from chronic METH results in a potentiated neurotoxic response to NMDA in CA1 pyramidal cells, which requires the co-activation of NMDAr and inositol triphosphate receptors (IP3), since antagonism of either receptor alleviates the potentiated response [32]. Currently, it is unknown how METH may interact with the IP3 receptor to produce the observed potentiated neurotoxic response. However, previous studies have suggested that the IP3 receptor is linked to the sigma-1 receptor and that METH may exert its effects on IP3 receptor-mediated Ca2+ release via interactions with the sigma-1 receptor [5, 36, 40].
Two subtypes of sigma receptors have been identified [4]. An endogenous ligand has yet to be determined for these receptors, however, a variety of endogenous and pharmacological compounds have displayed binding affinity for the sigma-1 receptor, including psychomotor stimulants [7, 10, 26, 34, 35]. In fact, previous studies have reported that cocaine and METH preferentially bind the sigma-1 receptor (METH: σ1- 2.16 µM ± 0.25; σ2- 46.67 µM ± 10.34) rather than the sigma-2 receptor [11, 25, 30]. Furthermore, sigma-1 receptor antagonists have been shown to attenuate behavioral sensitization and locomotor effects of cocaine and METH as well as modulating cocaine state dependent learning [18, 19, 25, 28, 29, 37]. These results suggest that sigma-1 receptors uniquely contribute to psychomotor stimulant behavioral profiles.
Chronic exposure to METH may also affect expression and function of sigma-1 receptors. Following chronic self-administration of METH (5 days per week, for 5 weeks; 0.1 mg/kg), a significant upregulation of sigma-1 receptor mRNA in the frontal cortex and hippocampus was observed [33]. Furthermore, both METH self administering animals and yoked controls have displayed a significant upregulation of the sigma-1 receptor in the ventral tegmental area and substantia nigra after chronic exposure (5 days per week, for 5 weeks; 0.1 mg/kg) [9]. Changes in sigma-1 receptor expression following METH exposure may play a role in IP3 receptor expression and IP3 receptor mediated Ca2+ release, since sigma-1 receptors are linked to IP3 receptors via the cytoskeleton protein, ankyrin [6]. Following activation by agonists, including cocaine, the sigma-1 and ankyrin complex dissociates from the IP3 receptor, resulting in increased IP3 receptor mediated Ca2+ release [5, 6]. Given the similar sigma-1 receptor binding and behavioral profiles of METH and cocaine as well as the potential interactions with IP3 receptor mediated Ca2+ release, the current studies examined the effects of sigma-1 receptor antagonism following chronic METH exposure on NMDA-induced neurotoxicity in the hippocampus.
2. Materials and methods
2.1 Preparation of hippocampal explants
Eight day old, male and female, Sprague-Dawley rat pups (Harlan Laboratories: Indianapolis, ID, USA) were sacrificed, and their brains were aseptically removed. After extraction, brains were transferred to ice-cold dissecting medium (Minimum Essential Medium (MEM: Gibco BRL, Gaithersburg, MD), 25 mM HEPES, 20 mM glutamine, and 50 µM streptomycin/penicillin). Bilateral hippocampi were then removed and cleaned of extra tissue. Upon completion of cleaning, hippocampi were transferred to ice-cold culture medium (dissecting media with the addition of 36 mM glucose, 25% Hanks’ balanced salt solution (HBSS: Gibco BRL, Gaithersburg, MD), and 25 % heat inactivated horse serum (HIHS: Sigma, St. Louis, MO)). Afterwards unilateral hippocampi were sectioned coronally at 200 µm using a McIllwain Tissue Chopper (Campden Instruments Ltd; Lafayette, ID, USA). Once sectioned three intact hippocampi slices were plated onto Millicell-CM biopore membrane inserts, with one milliliter of pre-incubated culture medium added to the bottom of each well. Plates were then placed in an incubator, at 37°C (gas composition 5% CO2, 21% oxygen, and 74% nitrogen) for five days to allow hippocampi to affix to the silicon membrane. Care of all animals was carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23).
2.2 NMDA and BD1047 Exposure
METH-naïve cultures were aged to 20 days in vitro (DIV), and medium was changed every 5 days to mimic treatment of METH-exposed and –withdrawn tissue described below. At 19 DIV, METH-naïve slice cultures were exposed to cell culture medium with the addition of propidium iodide (PI; 3.74 µM: Molecular Probes, Eugene, OR, USA), PI-medium with the addition of NMDA (5 µM), PI- medium with the addition of the sigma receptor antagonist BD1047 (100 µM) or PI-medium with the addition of NMDA and BD1047 for 24 hrs. Following PI exposure, all cultures were imaged and cytotoxicity was assessed as described below. All drugs (NMDA: Sigma, St. Louis, MO, USA; BD1047: TOCRIS, Bristol, UK) were dissolved in cell culture medium and applied to the bottom of culture plate well in one ml increments.
2.3 METH Withdrawal, NMDA, and BD1047 Exposure
At 6 DIV, slices were exposed to METH (100 µM; Sigma) dissolved in cell culture medium. Slices were exposed to METH for a total of 6 days with METH cell culture medium replenished at day 3 of exposure. Following 6 days of METH exposure (13 DIV), slices were withdrawn from METH for 7 days. At that time, slice cultures were treated with PI, PI and NMDA, PI and BD1047, or PI, NMDA, and BD1047 as stated above.
2.4 Measurement of cytotoxicity
PI is a polar stain that only penetrates cell membranes of damaged or potentially dying cells. Once in the cell PI binds to nucleic acids to produce a bright red fluorescence at 630 nm [43]. Hippocampi were visualized with SPOT Advanced version 4.0.2 software for Windows (W. Nuhsbaum Inc.: McHenry, IL, USA) at a 5× objective with a Leica DMIRB microscope (W. Nuhsbaum Inc.: McHenry, IL, USA) that is fitted for fluorescent detection (Mercury-arc lamp). The emission wavelength of PI is 620 nm in the visual range, and has a peak excitation wavelength of 536 nm. PI was excited using a band-pass filter exciting wavelengths between 510 and 560 nm. Intensity of PI fluorescence was analyzed by densitometry using NIH Image J software in the pyramidal cell layers of the CA1 and CA3 as well as the granule cell layer of the DG of the hippocampus proper. To minimize variability in PI uptake between replications, each replication was converted to percent control before analysis.
2.5 Statistical analysis
Two-way ANOVAs were initially employed analyzing the effect of treatment [CTRL, CTRL + NMDA, CTRL + BD1047, CTRL + NMDA + BD1047, METH WD, METH WD + NMDA, METH WD + BD1047, METH WD + NMDA + BD1047] and gender in each subregion of the hippocampus proper. However, there were no significant differences observed in regards to gender, so all further statistics collapse gender across treatment groups. One-way ANOVAs were employed examining the effects of treatment in each subregion of the hippocampus proper, since previous studies have demonstrated that the pyramidal cell layer of the CA1 selectively displays NMDA-induced neurotoxicity, particularly following METH withdrawal [32]. Statistical outliers were discovered utilizing Grubb’s test for outliers. Post hoc comparisons were made using the Fisher LSD Method. All significance levels were set at p<0.05.
3. Results
3.1 NMDA and BD1047 Exposure
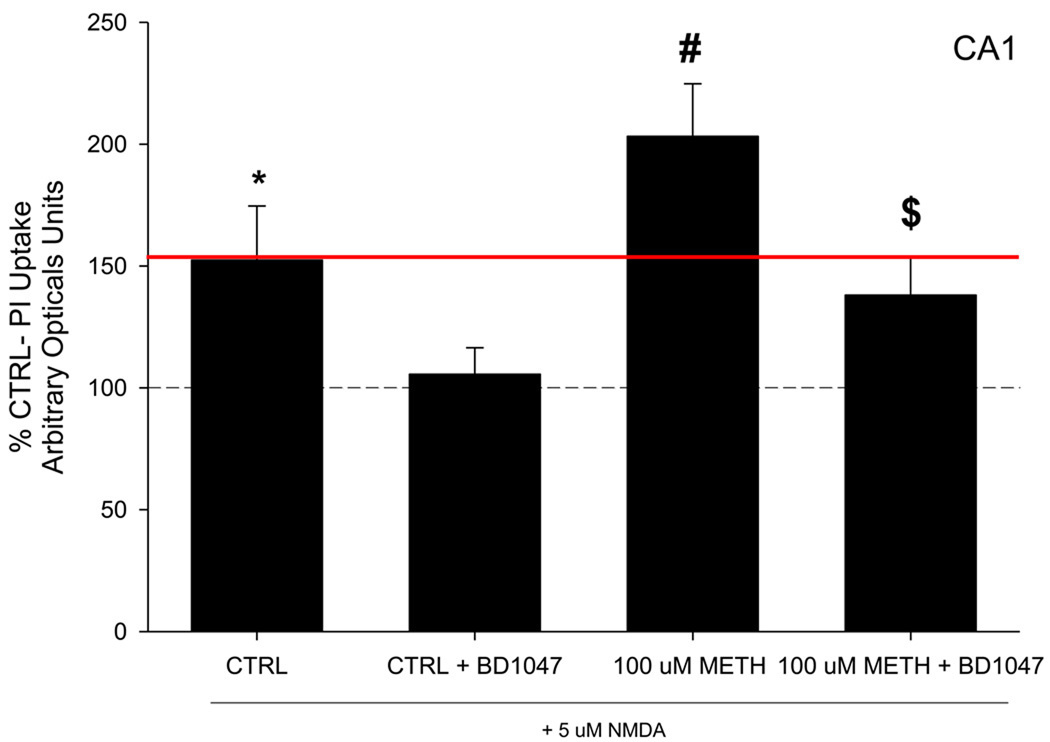
At 19 DIV METH-naïve tissue was exposed to NMDA (5 µM) and PI for 24 hours. NMDA exposure produced a significant increase in PI uptake in the pyramidal cell layer of the CA1 in METH-naive tissue. NMDA treatment increased PI uptake 125% compared to NMDA-naive tissue (Figure 1). Interestingly, co-exposure of BD1047 (100 µM) and NMDA (5 µM) entirely prevented NMDA-induced cytoxicity in CA1 pyramidal cells (F(7, 68)= 7.339, p<0.001; Figure 1, 2). However, exposure to BD1047 alone did not significantly alter PI uptake in any subregion of slice cultures (Table 1).
Figure 1.
PI uptake following long term withdrawal from METH, and NMDA exposure. Data is presented as percent control, 100% line represents CTRL tissue (naïve to METH, NMDA, and BD1047). Significant PI uptake following NMDA exposure was observed in all tissue. METH w/d tissue displayed a potentiated response to NMDA exposure, beyond CTRL tissue exposed to NMDA (above the red line). Co-exposure to BD1047 reduced NMDA toxicity, regardless of treatment. *= p<0.01 vs. CTRL; # = p<0.01 vs. CTRL + NMDA; $ = p<0.01 vs. 100 uM METH + NMDA
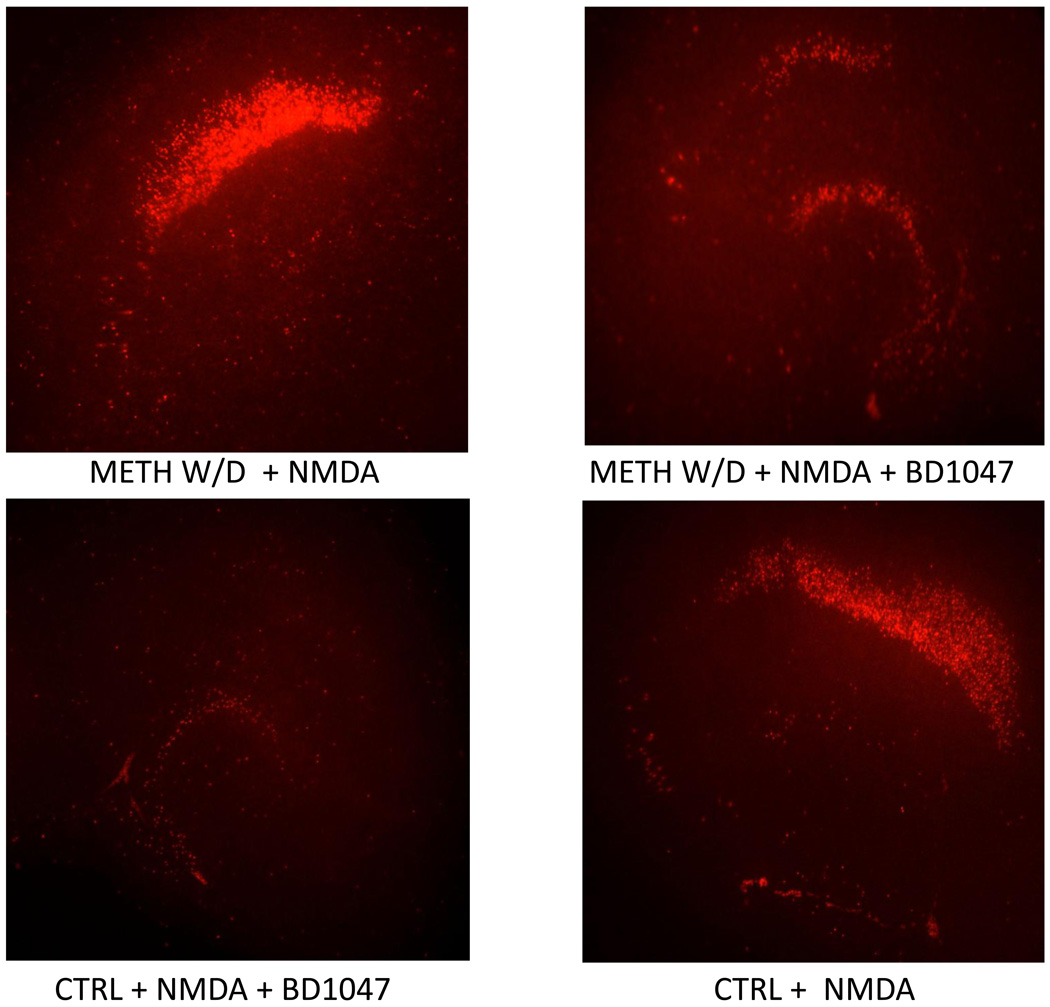
Figure 2.
Representative images of PI uptake following long term w/d from METH, and NMDA and/or BD1047 exposure in the hippocampus.
Table 1.
Percent control PI uptake in CTRL, CTRL + BD1047, 100 µM METH w/d, 100 µM METH w/d + BD1047 treated tissue in the CA1 region of the hippocampus.
| CA1 | Percent CTRL PI Uptake |
|---|---|
| CTRL | 100 ± 5.215 |
| CTRL + BD1047 | 99.9 ± 5.477 |
| 100 µM METH w/d | 129.4940 ± 4.3210 |
| 100 µM METH w/d + BD 1047 | 126.7 ± 9.107 |
3.2 METH Withdrawal, NMDA, and BD1047 Exposure
Following 7 days of withdrawal from METH exposure, there were no observable differences in PI uptake in slice cultures (Table 1). However, PI uptake was significantly increased following NMDA exposure in the CA1 region pyramidal cell layer; furthermore, METH withdrawn tissue displayed a significantly potentiated response to NMDA exposure when compared to METH naïve tissue (F(7, 68)= 7.339, p<0.001; Figure 1). The potentiated NMDA effect in METH withdrawn tissue resulted in an 80% increase in PI uptake when compared to METH-naive tissue also treated with NMDA, and a 200% increase in PI uptake when compared to METH and NMDA-naïve cultures (Figure 1). However, exposure to BD1047 during long-term METH withdrawal significantly reduced the NMDA-induced potentiated response in the CA1 pyramidal cell layer (F(7, 68)= 7.339, p<0.001; Figure 1). Representative images of PI uptake in these slice cultures are shown in Figure 2.
4. Discussion
Release of sequestered intracellular Ca2+ into cytoplasm may be involved or even required for manifestation of many neurotoxic insults. Previous studies have suggested that progesterone may be neuroprotective against NMDA exposure because it attenuates NMDA receptor-dependent Ca2+-induced intracellular Ca2+ release [1]. This effect is thought to be mediated through sigma-1 receptor antagonism, since the sigma-1 receptor antagonist, NE 100, mimicked the neuroprotective effects of progesterone. The current studies also support this hypothesis, given that exposure to the sigma-1 receptor antagonist, BD1047, reduced NMDA toxicity in both METH-naïve and METH-withdrawn tissue. The present studies also may demonstrate the importance of intracellular Ca2+ regulation during METH withdrawal, and may provide a possible mechanism involved in the activation of the hippocampus during METH withdrawal. During withdrawal, craving is consistently reported by METH users and activation of the hippocampus has been postulated to be involved in this phenomenon, particularly as it relates to cue-induced drug craving [2, 15, 20, 24].
In the current studies, long term withdrawal from METH did not result in any overt toxicity in any region of the hippocampus. But a potentiated NMDA-induced PI uptake during long-term METH withdrawal was observed in the CA1 pyramidal cell layer, replicating earlier findings [32]. NMDA-induced neurotoxicity within the CA1 region was not surprising, due to the abundance of NMDA receptors located in this region of the hippocampus, making the CA1 especially vulnerable to NMDA-mediated insults [32]. Exposure to the sigma-1 receptor antagonist, BD1047, also did not result in any overt toxicity in any region of the hippocampus, regardless of prior exposure regiment. However, co-exposure to BD1047 significantly reduced the potentiated NMDA toxicity within the CA1 pyramidal cell layer. Previous studies have reported that IP3 receptors are linked to sigma-1 receptors and that dissociation of sigma-1 receptors results in a potentiated release of Ca2+ from IP3 receptor mediated stores [6]. Cocaine has been shown to bind to sigma-1 receptors resulting in dissociation from IP3 receptors [6]. METH also binds to sigma-1 receptors, however, it has not been confirmed if this results in dissociation of IP3 receptors. It is unknown why exposure to sigma-1 receptor antagonists during protracted METH withdrawal attenuates the observed potentiated NMDA toxicity. Although, METH may be sequestered in brain lipids, resulting in an extended time period to achieve complete clearance of METH [21]. Exposure to BD1047 during this period may inhibit dissociation of sigma-1 receptors from IP3 receptors attenuating intracellular Ca2+ release. Furthermore, co-exposure to BD1047 significantly reduced NMDA toxicity within the CA1 pyramidal cell layer. While this result was unexpected, it does emphasize the important role of intracellular Ca2+ stores in NMDA-induced toxicity.
Sigma-1 receptor antagonists appear to regulate some of METH’s actions on the CNS. However, in the current study a general effect to reduce NMDA-induce neurotoxicity in the hippocampus by the sigma-1 receptor antagonist, BD1047, was found. This suggests that these antagonists may have a generalized effect on intracellular calcium regulation, which may or may not help to explain changes induced by psychomotor stimulant exposure in other studies. Regardless of METH exposure, the findings of the current study suggest an important role of the sigma-1 receptor in the regulation of intracellular calcium stores. It also provides further evidence that the potentiated neurotoxicity seen during prolonged withdrawal from METH may, in part, be regulated by release of intracellular calcium. Further studies are required to elucidate the exact mechanism that results in the observed potentiated response to NMDA, following METH exposure and withdrawal. In sum, intracellular calcium stores appear to be an important component of NMDA-induced toxicity. Furthermore, potentiated NMDA-induced neurotoxicity during METH withdrawal may, in part, result from a modification of intracellular calcium stores that could influence glutamatergic signaling, and possibly the development of craving.
Acknowledgements
The authors acknowledge the support of T32DA016176 to K.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanism underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55(2):127–138. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Chang L, Haning W. Insights from recent positron emission tomographic studies of drug abuse and dependence. Curr. Opin. Psychiatry. 2006;19(3):246–252. doi: 10.1097/01.yco.0000218594.46431.2f. [DOI] [PubMed] [Google Scholar]
- 3.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 4.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. U.S.A. 1996;93(15):8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi T, Maurice T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors, Ca (2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J. Pharmacol. Exp. Ther. 2000;293(3):788–798. [PubMed] [Google Scholar]
- 6.Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. Proc. Natl. Acad. Sci. U.S.A. 2001;16(2):491–496. [Google Scholar]
- 7.Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18(5):269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Su TP. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77(14):1612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332(3):1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacolo. 1994;268(1):9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 11.Kahoun JR, Ruoho AE. (125I)iodoazidococaine, a photoaffinity label for the haloperidol-sensitive sigma receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89(4):1393–1397. doi: 10.1073/pnas.89.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J. Neuropsychiatry Clin. Neurosci. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- 13.Kaiya H, Takeuchi K, Yoshida H, Kondo T, Sanpei F, Okada Y, Namba M. Effects of subchronic treatment of methamphetamine haloperidol on the rat brain levels of GABA, glutamate, and aspartate. Folia. Psychiatr. Neurol. Jpn. 1983;37(1):107–113. doi: 10.1111/j.1440-1819.1983.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 14.Kekuda R, Prasad PD, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (nSigmaR1) Biochem. Biophys. Res. Commun. 1996;229(2):553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- 15.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Gaber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 16.Kitanaka J, Kitanaka N, Tatsuta T, Hall FS, Uhl GR, Tanaka K, Nishiyama N, Morita Y, Takemura M. Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice. Psychopharmacology. 2008;203(3):781–792. doi: 10.1007/s00213-008-1425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197(3):517–532. [PubMed] [Google Scholar]
- 18.McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur. J. Pharmacol. 1999;370(3):225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 19.McCracken KA, Bowen WD, Matsumoto RR. Novel sigma receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur. J. Pharmacol. 1999;365(1):35–38. doi: 10.1016/s0014-2999(98)00876-0. [DOI] [PubMed] [Google Scholar]
- 20.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course, and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 21.Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and - methamphetamine on the dopamine terminal. J. Pharmacol. Exp. Ther. 1995;274(1):90–96. [PubMed] [Google Scholar]
- 22.Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol. Cell. 2005;97(12):873–883. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- 23.Moriguchi S, Watanabe S, Kita H, Nakanishi H. Enhancement of N-methyl-D-aspartate receptor-mediated excitatory postsynaptic potentials in the neostriatum after methamphetamine sensitization. Exp. Brain Res. 2002;144:238–246. doi: 10.1007/s00221-002-1039-3. [DOI] [PubMed] [Google Scholar]
- 24.Newton TF, Roache JD, De La Garza R, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31(7):1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (σ) receptors in the acute actions of methamphetamine: Receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13(3):85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 27.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 28.Romieu P, Lucas M, Maurice T. Sigma 1 receptor ligands and related neuroactive steroids interfere with the cocaine-induced state of memory. Neuropsychopharmacology. 2006;31(7):1431–1443. doi: 10.1038/sj.npp.1300885. [DOI] [PubMed] [Google Scholar]
- 29.Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma 1 receptor in the cocaine-induced conditioned place preference. Neuroreport. 2000;11(13):2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- 30.Sharkey J, Glen KA, Wolfe A, Kuhar MJ. Cocaine binding at sigma receptors. Eur. J. Pharmacol. 1988;149(1–2):171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- 31.Smith KJ, Self RL, Butler TR, Mullins MM, Ghayoumi L, Holley RC, Littleton JM, Prendergast MA. Methamphetamine exposure antagonized N-methyl- D-aspartate receptor-mediated neurotoxicity in organotypic hippocampal slice cultures. Brain Res. 2007;1157:74–80. doi: 10.1016/j.brainres.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KJ, Butler TR, Self RL, Braden BB, Prendergast MA. Potentiation of N-methyl-D-aspartate receptor-mediated neuronal injury during methamphetamine withdrawal in vitro requires co-activation of IP3 receptors. Brain Res. 2008;1187:67–73. doi: 10.1016/j.brainres.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma 1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology. 2004;175(1):68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- 34.Su TP. HR 375: a potential antipsychotic drug that interacts with dopamine D2 receptors and sigma-receptors in the brain. Neurosci. Lett. 1986;71(2):224–228. doi: 10.1016/0304-3940(86)90563-x. [DOI] [PubMed] [Google Scholar]
- 35.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240(4849):219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 36.Su TP, Hayashi T. Cocaine affects the dynamics of cytoskeletal proteins via sigma (1) receptors. Trends Pharmacol. Sci. 2001;22(9):456–458. doi: 10.1016/s0165-6147(00)01740-5. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Miwa T, Horikomi K. Involvement of σ1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci. Lett. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- 38.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,2,5-triphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008;283(42):28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J. Pharmacol. Exp. Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- 42.Yeh GC, Chen JC, Tsai HC, Wu HH, Lin CY, Hsu PC, Peng YC. Amphetamine inhibits the N-Methyl-D-Asparate receptor-mediated responses by directly interacting the receptor/channel complex. J. Pharmacol. Exp. Ther. 2002;300(3):1008–1016. doi: 10.1124/jpet.300.3.1008. [DOI] [PubMed] [Google Scholar]
- 43.Zimmer J, Kristensen BW, Jakobsen B, Noraberg J. Excitatory amino acid neurotoxicity and modulation of glutamate receptor expression in organotypic brain slice cultures. Amino Acids. 2000;19(1):7–21. doi: 10.1007/s007260070029. [DOI] [PubMed] [Google Scholar]