Abstract
Cell adhesion molecules have been implicated in the colonization of cancer cells to distant organs. Prostate cancer (PCa) has a propensity to metastasize to bone and cadherin-11, which is an osteoblast cadherin aberrantly expressed in PCa cells derived from bone metastases, has been shown to play a role in the metastasis of PCa cells to bone. However, the mechanism by which cadherin-11 is involved in this process is not known. Here, we show that expression of cadherin-11 in cadherin-11-negative C4-2B4 cells increases their spreading and intercalation into an osteoblast layer, and also stimulates C4-2B4 cell migration and invasiveness. Downregulation of cadherin-11 in cadherin-11-expressing metastatic PC3 cells decreases cell motility and invasiveness. Further, both the juxtamembrane (JMD) and β-catenin binding domains (CBS) in the cytoplasmic tail of cadherin-11 are required for cell migration and invasion, but not spreading. Gene array analyses showed that several invasion related genes, including MMP-7 and MMP-15, are upregulated in cadherin-11, but not in cad11-ΔJMD or cad11-ΔCBS, expressing C4-2B4 cells. These observations suggest that cadherin-11 not only provides a physical link between PCa cells and osteoblasts but also increases PCa cell motility and invasiveness that may facilitate the metastatic colonization of PCa cells in bone.
Keywords: cadherin-11, prostate cancer, osteoblast, adhesion, bone metastasis
Introduction
The cadherin family of cell-cell adhesion molecules are involved in all aspects of tissue morphogenesis, including cell movement and sorting into complex organization in tissues and organs (1, 2). Cadherins, especially E-cadherin and N-cadherin, are also involved in the pathogenesis of various cancers (3). Loss of E-cadherin and upregulation of N-cadherin, also known as the cadherin switch, has been observed in many types of cancers including PCa (4, 5). Because of their unique function in mediating cell-cell interaction, cadherin adhesion molecules are also postulated to play a role in organ-specific metastasis (5).
PCa has propensity to metastasize to bone and bone metastasis is the major cause of PCa related mortality (6, 7). Mechanisms leading to preferential metastasis of PCa cells to bone are not clear. We hypothesized that cell adhesion molecules that mediate the interactions between metastatic PCa cells and osteoblasts, a major cell type in bone, may play a role in the metastasis of PCa cells to bone. We identified cadherin-11, also known as OB-cadherin, as one such adhesion molecule (8). Cadherin-11 was originally identified in mouse osteoblasts and has been shown to be involved in bone development possibly through its homophilic cell adhesion function (9). We showed that cadherin-11 was highly expressed in a PC-3 cell line that was derived from bone metastases. In clinical specimens, the level of cadherin-11 expression was found to increase from primary PCa to metastatic disease, especially in bone. In a mouse model of metastasis, intracardiac injection of PC3 cells led to the metastasis of PC3 cells to bone. However, the incidence of PC3 metastasis to bone was reduced greatly when cadherin-11 was knocked down by gene-specific shRNA (8). Consistent with our observations, a recent report by Tamura et al. (10) also showed that bone-tropic MDA-MB-231 (MDA-MB-231-BO) cells expressed high levels of cadherin-11 compared to that of brain-tropic MDA-MB-231 cells (MDA-MB-231-BR). Furthermore, they showed that re-expression of cadherin-11 in MDA-MB-231-BR cells led to an increase in the incidence of metastasis to bone (10). Together, these observations established the role of cadherin-11 in the metastasis of prostate and breast cancer cells to bone.
How cadherin-11 participates in the bone metastasis process remains unclear. Cadherin-11 is a single-membrane-spanning protein with an extracellular domain of five repeats and a conserved cytoplasmic domain (11). Cadherins have been shown to modulate cancer cell activities. While expression of the epithelial cadherin, E-cadherin, leads to suppression of tumor invasion (12), expression of the mesenchymal cadherin, N-cadherin, promotes tumor cell survival, migration, and invasion (13). Whether cadherin-11 mediates interactions between PCa cells and osteoblasts and whether expression of cadherin-11 in PCa cells leads to increases in migration, invasion, proliferation, or survival of PCa cells is unknown.
In this study, we characterized cadherin 11-mediated cellular changes of PCa cells. We found that expression of cadherin-11 enabled PCa cells to intercalate into osteoblasts and increased the migration and invasion of PCa cells. We also identified the cadherin-11 domains and cadherin 11-mediated gene changes that are involved in modulating these PCa cell activities.
Materials and Methods
Cell lines
C4-2B4 and PC3-mm2 cells were kindly provided by Drs. Robert Sikes (University of Delaware) and I. J. Fidler (M. D. Anderson Cancer Center), respectively. All other cells were purchased from the American Type Culture Collection (Manassas, VA). L-cell/vector, L-cell/cad11, and cad11-Fc were prepared as previously described (14). Cad11-ΔJMD and cad11-ΔCBS in pCEP4 vector were kindly provided by Dr. Michael Brenner (Brigham and Woman Hospital, Harvard University). Cad11-Δcyto mutant, with deletion of the entire cytoplasmic domain, was generated by PCR using forward (5′GGATCCCCATGAAGGAGAACTACTGTTTA3′) and reverse (5′GCGGCCGCTTATTTCTTTTGCCTTCTCAGGGTCAC3′) primers.
Cell spreading assay
Coverslips were coated with various concentrations of protein (cad11-Fc or Fc) for 16 h at 4°C. After blocking with 1% BSA, 105 cells were plated onto the coverslips and incubated at 37°C for 1 to 6 h in serum-free medium.
Generation of cadherin-11-expressing C4-2B4 cells
C4-2B4 cells containing luciferase and a red fluorescence protein (Tomato) (23) was performed as previously described (8). Cad11-ΔJMD and cad11-ΔCBS in pCEP4 vectors were subcloned into pBMN-I-GFP vectors, which were used to generate retroviruses as previously described (8). C4-2B4 cells were infected with retrovirus to generate C4-2B4/vector, C4-2B4/cad11, C4-2B4/cad11-ΔJMD, or C4-2B4/cad11-ΔCBS.
Generation of PC3-mm2 knockdown cells
To knock down cadherin-11, PC3-mm2 cellswere transduced with the lentivirus containing cadherin-11 shRNA (pLKO34, Sigma-Aldrich) or control non-target shRNA (Sigma-Aldrich) and selected with 0.8μg/ml puromycin for 7 days.
Co-culture of C4-2B4 cells with osteoblasts
C4-2B4 cells were seeded at 50,000 cells per well on a confluent MC3T3-E1 osteoblast monolayer, incubated at 37°C for 24 h, fixed with 3.5% paraformaldehyde for 5 min, and permeabilized with 0.1% Triton X-100 in PBS. The slides were incubated with mouse anti-human cadherin-11 mAb 4B6 in 1:25 dilution overnight at 4°C, followed with Cy3-conjugated donkey anti-mouse secondary antibody (Jackson Immuno Research), mounted with Vectashield mounting medium, and observed with an Olympus microscope or a confocal microscope (Leica Microsystems Inc.).
Cell migration assay
The migration inserts (Becton Dickinson Labware) were coated with cad11-Fc or Fc (10 μg/ml) overnight at 4°C. PCa cells (3 × 104 cells) were seeded in the upper chamber in serum free RPMI medium. The lower chamber contained RPMI medium with 10% FBS. After incubation for 24 h, the migrated cells in the bottom part of the insert were labeled with calcein AM. Values for migration were expressed as the average of migrated cells per microscope field (X100). Three microscopic fields per insert were counted.
Cell invasion assay
PCa cells (105 cells) were seeded onto the BioCoat Matrigel coated invasion chamber (BD Bioscience). The lower chamber contained RPMI medium with 10% FBS. After incubation for 24 h, cells on the top of the chamber were removed and cells that invaded through the Matrigel were fixed with cold methanol, stained with DAPI, and counted. Values for invasion were expressed as the average of migrated cells per microscope field (X100). Three microscopic fields per insert membrane were counted.
Immunoprecipitation and Western blot
C4-2B4 cells were lysed in lysis buffer (0.3% NP-40, 25 mM Tris, 0.15 M NaCl, 2 mM EDTA pH7.4) containing protease inhibitors. Cell lysates were immunoprecipitated with goat anti-human cadherin-11 antibody (R&D Systems) and Western blotted with anti-human cadherin-11 monoclonal antibody (Zymed), anti-p120 catenin (BD Bioscience), or anti-β-catenin antibody (Cell Signaling). The signals were detected with SuperSignal West Pico (Pierce).
Microarray
Five-hundred ng of total RNA was used for cDNA synthesis, followed by amplification and biotin labeling (Illumina Inc.). Biotinylated cRNAs (1.5 μg) were hybridized to the Illumina HumanHT-12 Beadchip v.3 microarray. The signal was detected with cyanine 3-streptavidine (GE Healthcare), and the bead chips were scanned with an Illumina BeadArray Reader confocal scanner (BeadStation 500GXDW). The microarray data were extracted with Bead Studio 3.6 (Illumina) and normalized using the quantile normalization method in the Linear Models for Microarray Data (LIMMA) package in R language environment. The expression level of each gene was transformed into a log 2 base for analysis. The random variance t test was applied to identify the genes that are significantly different between two groups.
Statistical analyses
Statistical analysis was performed by using Student’s t test (two-tailed, paired). A P value of less than 0.05 was considered significant. Data are expressed as the mean ± SD.
Results
Expression of cadherin-11 in PCa cells leads to cell spreading on cad11- Fc coated plates
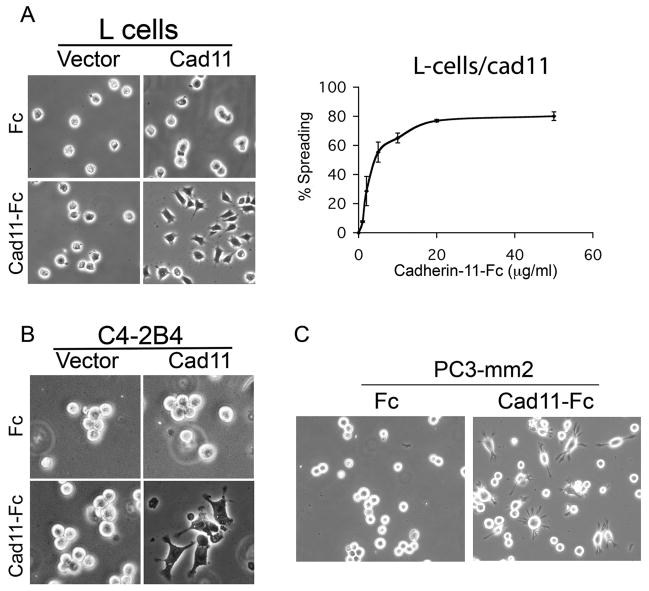
To examine the effect of cadherin-11-mediated adhesion on PCa cells, we first examined whether cadherin-11 expression leads to an increase in cell spreading. L-cells that do not express any of the major cadherins were transfected with cadherin-11 expression vector (L-cell/cad11) or a control vector (L-cell/vector). Cells were plated on coverslips coated with cad11-Fc, an Fc fusion protein containing the extracellular domain of cadherin-11 (14), or with Fc as a negative control. L-cell/vector cells remained in a round shape when plated on either Fc or Cad11-Fc coated coverslips (Fig. 1A). In contrast, L-cell/cad11 cells showed a flattened morphology with lamellipodia/filopodia-like projections upon binding to cad11-Fc, but remained in a round shape when plated on Fc coated coverslips (Fig. 1A). A dose-dependent increase in cell spreading was observed with increasing cad11-Fc on the coverslips (Fig. 1A). Maximal spreading of L-cell/cad11 to cad11-Fc was observed at a concentration of 20 μg/ml and half-maximal at around 3 μg/ml. We thus used 10μg/ml cad11-Fc in our subsequent studies.
Fig. 1. Effect of cadherin-11 on cell spreading.
L-cells or C4-2B4 cells transfected with cadherin-11 expression vector or vector only were plated on cad11-Fc or Fc-coated plates. (A) Binding of L-cell/cad11 to cad11-Fc leads to a flattened cell shape in a dose-dependent manner. (B) Binding of C4-2B4/cad11 to cad11-Fc leads to a flattened cell shape and increased lamellipodia or filopodia-like projections. (C) PC3-mm2 cells, which express cadherin-11 endogenously, showed a flattened cell shape and increased filopodia-like projections upon binding to cad11-Fc coated plates.
To determine if the cadherin-11-mediated spreading also occurs in PCa cells, we transfected C4-2B4 cells (15, 16), a subline derived from the serial passage of LNCaP PCa cells in mouse under castration condition. Although C4-2B4 cells were derived from bone metastasis, they do not express cadherin-11 possibly due to the androgen receptor status of the cells (17). We transfected C4-2B4 cells with cadherin-11 expression vector. C4-2B4 cells transfected with vector alone were used as a negative control. Similar to what was observed with L-cell/cad11, binding of C4-2B4/cad11 to cad11-Fc led these cells to exhibit morphological changes characterized by an increase in lamellipodia/filopodia-like projections (Fig. 1B). In contrast, these cells remained in a round shape when plated on Fc coated coverslips. Vector-transfected C4-2B4 cells did not show morphological changes on either Fc or cad11-Fc coated plate (Fig. 1B). We further examined the spreading of PC3-mm2 cells, which express endogenous cadherin-11, on Fc or cad11-Fc coated plates. As shown in Fig. 1C, PC3-mm2 cells exhibited extensive lamellipodia/filopodia-like projections upon seeding onto cad11-Fc, but not to Fc coated coverslips (Fig. 1C). These observations demonstrate that cadherin-11-mediated interaction induces cell spreading through cadherin-11 homophilic interactions.
Cadherin-11 mediates adhesion between PCa cells and osteoblasts
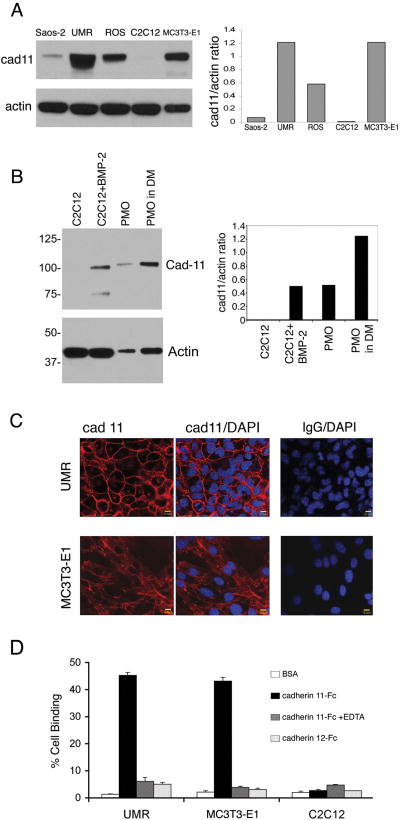
To study the interactions between cadherin-11-expressing PCa cells and osteoblasts, we first examined the expression of cadherin-11 in several osteoblast cell lines, including MC3T3-E1, UMR106, ROS17/2.8 and Saos-2. Western blot analysis showed that UMR106 and ROS17/2.8, which are highly differentiated rat osteoblastic cell lines (18, 19), express high levels of cadherin-11 (Fig. 2A). MC3T3-E1 is an immature but committed osteoblastic cell line (20) that also expresses relatively high levels of cadherin-11. SaOS-2 is an established human osteosarcoma cell line with several osteoblastic features (21) and expresses moderate levels of cadherin-11. Cadherin-11 is absent in uninduced C2C12 cells (Fig. 2A). However, upon induction to differentiate into osteoblasts by BMP-2 (22), C2C12 cells expressed high levels of cadherin-11 (Fig. 2B). Primary mouse osteoblasts (PMO) isolated from mouse calvaria expressed cadherin-11 with a level similar to that in BMP2-induced C2C12 cells (Fig. 2B). The level of cadherin-11 in PMO was further increased by about two-fold when the PMO were cultured in differentiation medium containing ascorbic acid and β-glycerol phosphate (Fig. 2B). Immunochemical staining showed that cadherin-11 was localized at the cell-cell junction between the osteoblasts (Fig. 2C). In a cell-to-substrate adhesion assay, binding of these osteoblast cells to recombinant cad11-Fc correlated with the level of cadherin-11 expression in these cell lines (Fig. 2D). The osteoblast binding to cad11-Fc was abolished by the addition of EDTA (Fig. 2D), suggesting the requirement of calcium for homophilic cadherin-11 interactions. Further, UMR and MC3T3-E1 cells are unable to bind to an unrelated cadherin, cadherin-12-Fc (Fig. 2D). Collectively, these results indicate that cadherin-11 functions as a Ca2+-dependent homophilic cell adhesion molecule in the osteoblast cell lines.
Fig. 2. Expression of cadherin-11 in osteoblast cell lines.
(A) Western blotting using monoclonal antibody 5B2H5, which recognizes the cytoplasmic domain of cadherin-11 in a panel of rodent osteoblast cell lines. (B) C2C12 cells were treated without or with BMP-2 for 5 days. Primary mouse osteoblasts (PMO), isolated from mouse calvaria, were cultured in the absence or presence of differentiation medium (DM) that contains ascorbic acid and beta-glycerol phosphate as described (37). (C) Immunocytochemical staining using goat anti-cadherin-11 polyclonal antibody (R&D Systems). DAPI counterstain (blue) was used to visualize DNA. Cadherin-11 (red) was expressed on the cell membrane in areas of cell-cell contact in MC3T3-E1 and UMR cells. Bar, 10μm. (D) Binding of MC3T3-E1 and UMR cell lines to cadherin-11-Fc, but not to BSA or cadherin-12-Fc, coated wells or wells treated with EDTA to block cadherin-11 homophilic interactions.
Cadherin-11 facilitates intercalation of PCa cells with osteoblasts
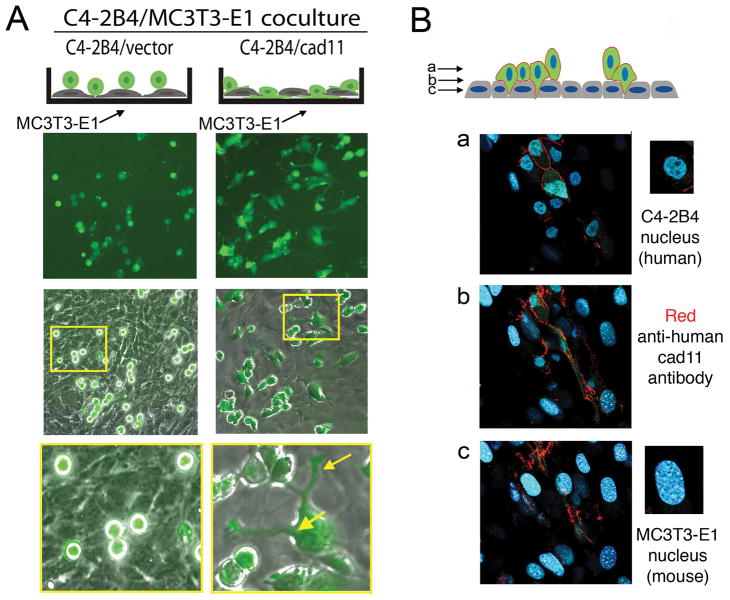
Next, we examined the interactions between cadherin-11-expressing PCa cells and osteoblasts. To test whether cadherin-11 is required on osteoblasts and PCa cells to bind, we transfected the C4-2B4/vector cells with Tomato red fluorescence protein (23) and C4-2B4/cad11 cells with a green fluorescence protein (GFP) as visual markers. C4-2B4/vector or C4-2B4/cad11 cells were mixed with MC3T3-E1 cells in a 2 to 1 ratio. As shown in Supplemental Fig S1A, C4-2B4/vector cells and MC3T3-E1 cells attached to the culture plate independently, while C4-2B4/cad11 cells attached to MC3T3-E1 cells (Supplemental Fig. S1B). In addition, C4-2B4/vector cells showed a more rounded morphology compared to that of C4-2B4/cad11 cells, which showed a flatter morphology and exhibited more cellular extensions. These morphologies are similar to the preferential binding of C4-2B4/cad11 cells to cad11-Fc coated plates observed in Fig 1.
We further examined the morphology of the GFP-labeled C4-2B4 cells when they were plated on top of a confluent layer of MC3T3-E1 cells. As shown in Fig. 3A, control C4-2B4/vector cells remained as round cells lying on top of the MC3T3-E1 cell layer. In contrast, the C4-2B4/cad11 cells exhibited extended/spreading morphology upon interacting with the underlying osteoblasts (Fig. 3A). The extended morphology suggests that C4-2B4 cells may intercalate between the osteoblasts. To further examine such a possibility, we generated a monoclonal anti-cadherin-11 antibody 4B6, which recognizes human cadherin-11 but not mouse cadherin-11 (Supplemental Fig. S2), immunostained cells in the C4-2B4-cad11/MC3T3E1 coculture, and examined the cells by confocal microscopy. In this coculture system, the smaller round nuclei of the PCa cells can be distinguished from the larger, oblong-shaped nuclei of the osteoblasts after DAPI staining (Fig. 3B). C4-2B4/cad11 cells were found to extend numerous lamellipodia/filopodia, as marked by the anti-human cadherin-11 antibody staining (red), that infiltrated into the confluent osteoblast layer, while the nuclei of the C4-2B4/cad11 cells remained positioned on top of the confluent osteoblast layer (Fig. 3B and Supplemental Fig. S3). In contrast, there was no detectable signal for human cadherin-11 in the control C4-2B4 vector/MC3T3E1 coculture (Supplemental Fig. S3). Together, these observations suggest that cadherin-11 is required for PCa cell and osteoblast interaction, and that cadherin-11 promotes filopodial extensions and intercalation of PCa cells with osteoblasts.
Fig. 3. Cadherin-11 facilitates intercalation of PCa cells with osteoblasts.
(A) GFP-labeled human C4-2B4 PCa cells were seeded on a confluent murine MC3T3-E1 osteoblast layer. The C4-2B4/cad11 cells spread on top of the osteoblasts and exhibited filopodia projections that intercalate into the osteoblast layer (arrows in insert). Control C4-2B4/vector cells remained as round cells present on top of the MC3T3-E1 cell layer. (B) Confocal images were acquired as z-stacks a, b and c. C4-2B4/cad11 cells (smaller nuclei in layer a) extended filopodia, as detected by cadherin-11 staining (red, layers b, c), into the osteoblast layer (larger punctate nuclei in layers b, c). Note, the anti-human cadherin-11 4B6 monoclonal antibody only recognizes human cadherin-11 on PCa cells and not mouse cadherin-11 on osteoblasts.
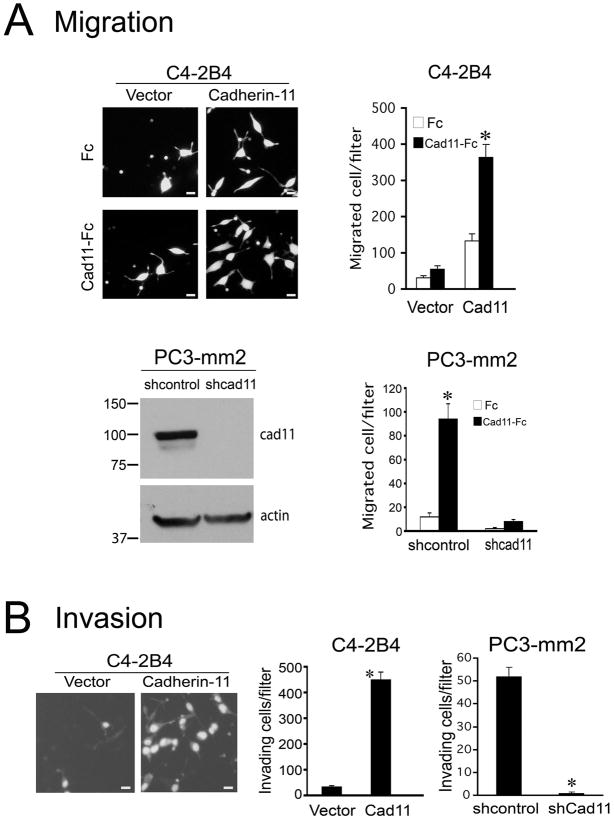
Effect of cadherin-11 on the migration of PCa cells
To determine whether cadherin-11 may induce changes in PCa cell motility, cell migration was examined using a Transwell migration assay, in which cell culture inserts were coated with either cad11-Fc or Fc. C4-2B4/cad11 cells exhibited a higher migration rate compared to control C4-2B4/vector cells when observed in Fc coated chambers (Fig. 4A). The migratory activity of C4-2B4/cad11 cells was further increased when cells were seeded in cad11-Fc coated chambers compared to those in Fc coated chambers (Fig. 4A). We also examined the migration rate of PC3-mm2 cells in which the level of endogenous cadherin-11 was reduced by cadherin-11 shRNA (PC3-shcad11) (Fig. 4A). PC3-mm2 transfected with a scrambled shRNA sequence was used as a control (PC3-shcontrol) (Fig. 4A). A higher migration rate occurred when PC3-shcontrol was seeded on cad11-Fc compared to that on Fc coated chambers (Fig. 4A). In contrast, knockdown of cadherin-11 expression in PC3 – mm2 cells drastically reduced cell migration activity (Fig. 4A). These results indicate that cadherin-11 expression increases the migration of PCa cells.
Fig. 4. Effect of cadherin-11 on cell migration and invasion.
(A) Migratory activity of C4-2B4/vector or C4-2B4/cad11 cells. Left upper panel, cells that migrated to the other side of migration inserts. Right upper panel, number of migrated cells. Left lower panel, levels of cadherin-11 in the PC3-mm2 cells transfected with retroviral vector containing cadherin-11 shRNA or control shRNA detected by Western blot. Actin was used as a loading control. Right lower panel, number of migrated cells. (B) Invasiveness of C4-2B4/cad11, C4-2B4/vector cells and PC3-mm2/shcontrol versus PC3-mm2/shcad11 cells. Bar, 10μm. Data are means ± SD of triplicates. *, P<0.05.
Effect of cadherin-11 on the invasion activity of PCa cells
To examine whether expression of cadherin-11 leads to increase in cell invasion activity, PCa cells were seeded onto Boyden chambers that were coated with Matrigel. C4-2B4/cad11 cells showed a ten-fold increase in the number of cells that have invaded through the Matrigel when compare with C4-2B4/vector control cells (Fig. 4B). Similarly, knockdown of cadherin-11 in PC3-mm2 cells (PC3/shcad11) essentially abrogated the invasiveness of PC3-mm2 cells when compared with PC3/shcontrol cells (Fig. 4B). These results suggest that expression of cadherin-11 leads to an increase in the invasive activity of PCa cells.
Effect of cadherin-11 on the proliferation, survival, sensitivity to docetaxel treatment, and anchorage-independent growth of PCa cells
We examined whether cadherin-11-mediated interactions between PCa cells and osteoblasts affect PCa cell proliferation, survival, or anchorage-independent growth. We found that C4-2B4 cells overexpressed with cadherin-11 proliferated in a similar manner as the control C4-2B4/vector cells, whether these cells were grown on regular tissue culture plates (Supplemental Fig. S4A, left panel) or a confluent layer of MC3T3-E1 osteoblast (Supplemental Fig. S4A, right panel).
In the serum starvation and docetaxel treatment survival tests, overexpression of cadherin-11 did not confer a cell survival advantage over vector-transfected control cells, whether or not these cells were plated on osteoblasts (Supplemental Fig. S4B, S4C). We also found that expression of cadherin-11 did not alter C4-2B4 colony formation activity in anchorage – independent growth assay compared to that of control C4-2B4/vector cells (Supplemental Fig. S4D).
Cadherin11-mediated migration and invasion of PCa cells are dependent on its cytoplasmic domain
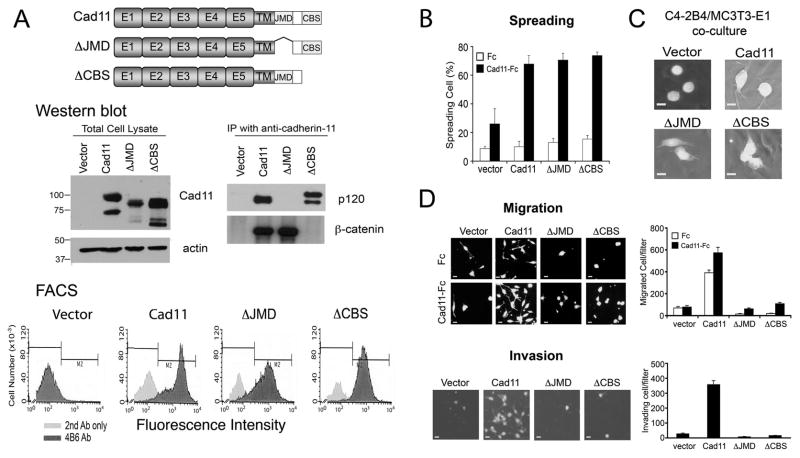
Previous studies on E-cadherin have established that p120-catenin and β-catenin bind to the juxtamembrane domain (JMD) and C-terminus of E-cadherin (24–27), respectively. To examine whether these domains play a role in cadherin-11-mediated cell migration and invasion, C4-2B4 cells were transfected with cadherin-11 mutants containing deletions in the juxtamembrane domain (cad11-ΔJMD) or the C-terminus catenin-binding sequence (cad11-ΔCBS) (Fig. 5A). Western blot of total cell lysates showed that these cadherin-11 deletion mutants have decreased apparent molecular weights compared to that of wild type cadherin-11 (Fig. 5A). Anti-cadherin-11 antibody positive proteins with corresponding smaller molecular masses were observed in wild type and mutant cadherin-11 expressing cells, possibly due to proteolysis. Fluorescence activated cell sorting (FACS) analysis of cells stained with the 4B6 antibody showed a significant fluorescence increase compared with those stained with only secondary antibody, suggesting that both cadherin-11 mutant proteins are expressed on the cell surface (Fig. 5A). Immunoprecipitation followed with Western blot showed that wild type cadherin-11 binds to both p120-catenin and β-catenin, while Cad11-ΔJMD and Cad11-ΔCBS lost the ability to bind to p120-catenin andβ-catenin, respectively (Fig. 5A).
Fig. 5. Effect of cadherin-11 cytoplasmic domains on cadherin11-mediated cell spreading, migration, and invasion in C4-2B4 cells.
(A) Characterization of C4-2B4 cells expressing wild type or mutant cadherin-11. Schematic diagram of cadherin-11 mutants. E1-5, extracellular cadherin repeats 1-5. TM, transmembrane domain. JMD, juxtamembrane cytoplasmic domain. CBS, β-catenin-binding sequence. Middle left panel, western blot of the total cell lysates showed that cad11-ΔJMD and cad11-ΔCBS have reduced apparent molecular weights compared to that of the wild type. Middle right panel, immunoprecipitation of cadherin-11 followed with Western blot with anti-p120- catenin or anti-β-catenin antibodies. Fluorescence activated cell sorting using anti- cadherin-11 antibody indicated that wild type and mutant cadherin-11 were expressed on the cell surface. (B) Cell spreading of cells in (A) on cad11-Fc or Fc-coated plates. (C) Cells plated on a confluent layer of MC3T3-E1 osteoblasts. The C4-2B4/cad11, C4- 2B4/ΔJMD and C4-2B4/ΔCBS cells exhibited filopodia projections while control C4- 2B4/vector cells remained as round cells on top of the MC3T3-E1 osteoblast layer. (D) Migration or invasion of cells in (A) through migration chambers coated with Fc or cad11-Fc or through BioCoat Matrigel invasion chambers, respectively. Quantification of migrated or invading cells is shown on the right. Bar, 10 μm.
We examined the effects of JMD and CBS domains on cadherin-11-mediated functions, including spreading, migration and invasion. C4-2B4 cells expressing either ΔJMD or ΔCBS mutant showed a similar extent of spreading on cad11-Fc compared to cells expressing full-length cadherin-11 (Fig. 5B), suggesting that cadherin-11-mediated spreading is not dependent on either the JMD or CBS domain. A cadherin-11 mutant with deletion of the entire cytoplasmic domain (cad11-Δcyto) was also capable of mediating cell spreading on cad11-Fc (Supplemental Fig. S5), suggesting that cadherin 11-mediated spreading is independent of its cytoplasmic domain. When GFP-labeled cadherin-11 mutant-expressing C4-2B4 cells were seeded onto a confluent layer of MC3T3-E1 osteoblast, both C4-2B4/ΔJMD and C4-2B4/ΔCBS cells exhibited extended spreading morphology upon interacting with the osteoblast layer while control C4-2B4/vector cells remained as round cells (Fig. 5C). In contrast, deletion of either the JMD or CBS domain abolished cadherin-11-mediated cell migration and invasion (Fig. 5D). These results suggest that both JMD and CBS domains are involved in the cadherin-11-mediated signal transduction that leads to PCa cell migration and invasion.
Effect of cadherin-11 on gene expression in C4-2B4 cells
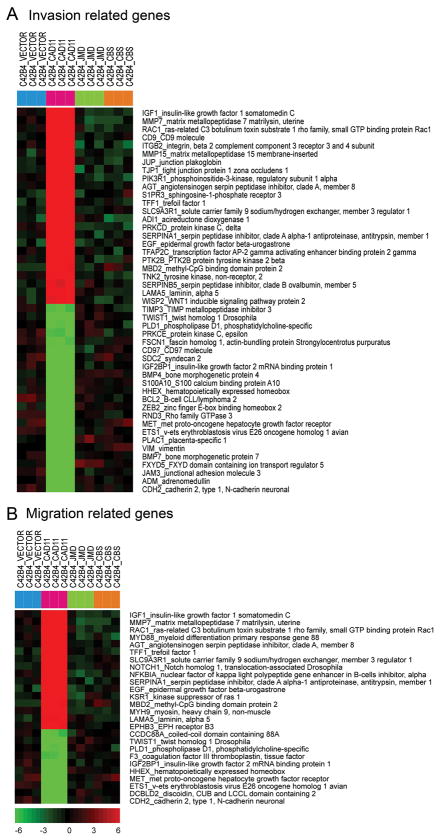
Cadherin-11-mediated signal transduction through its cytoplasmic domain likely results in changes in C4-2B4 cell gene expression that modifies cell activities. Gene array analyses comparing the gene expression profiles of C4-2B4/vector versus C4-2B4/cad11, C4-2B4/ΔJMD or C4-2B4/ΔCBS showed that a unique set of genes were up or down regulated by the expression of cadherin-11, but not ΔJMD or ΔCBS cadherin-11 mutants (Supplemental Fig. S6 and Supplemental Table S1). When the data were analyzed according to invasion related genes, IGF-1, MMP7, Rac1, CD9, integrin beta2, MMP15, and junction plakoglobin were among the top upregulated genes (Fig. 6A). Analysis of the gene array data based on migration related genes showed that myeloid differentiation primary response gene (Myd88) and Notch1 were among those upregulated in addition to the invasion-related genes (Fig. 6B). These results suggest that expression of cadherin-11 in PCa cells leads to the upregulation of several invasion related genes that may result in increased cell invasiveness observed in C4-2B4/cad11 cells.
Fig. 6. Effect of cadherin-11 on C4-2B4 gene expression.
Gene array analyses comparing the gene expression profiles of C4-2B4/vector versus C4-2B4/cad11, C4-2B4/ΔJMD, and C4-2B4/ΔCBS using the Ilumina gene analysis platform. Triplicate samples were used in gene array analysis. The gene array data were analyzed according to invasion related genes (A) or migration related genes (B). Red: upregulated genes. Green: downregulated genes.
Discussion
We previously established that cadherin-11 increases the colonization of PCa cells in bone in an animal model in vivo (8). In this study, we further elucidated the cadherin-11-mediated cellular activities in PCa cells. We demonstrate that expression of cadherin-11 in PCa cells enables the cells to spread, migrate, and invade, resulting in enhanced intercalation of PCa cells into a layer of osteoblasts. In addition, the cytoplasmic domain of cadherin-11 plays a critical role in PCa cell migration and invasion, an observation further supported by microarray analyses that suggest that cadherin-11 may activate a signaling pathway to promote these cellular activities. Based on these observations, we propose that cadherin-11 not only provides a physical link between PCa cells and osteoblasts, but also modulates cellular activities that enhance the ability of PCa cells to migrate into and invade the bone environment. Together, these cadherin-11-mediated cellular functions may enhance the colonization of PCa cells in bone.
Cadherin-11 is a mesenchymal cadherin that is mainly expressed in osteoblasts and synovicytes, and at low levels in the lung, testis and brain tissues (11). Synovial fibroblasts were found to express cadherin-11 that functions in the organization of the synovial lining layer (28). Mice lacking functional cadherin-11 exhibit a reduction in bone density (9). Aberrant expression of cadherin-11 was observed in several cancers of epithelial origin, including breast (29), gastric (30) and prostate (8) cancers. While normal prostate epithelial cells express the epithelial type cadherin or E-cadherin, cadherin-11 was found to be expressed in PCa cells that have metastasized to the lymph node (4) and bone (8). Recently, we have identified that androgen depletion is one of the mechanisms that leads to the upregulation of cadherin-11 in prostate cancer (17). Importantly, our studies show that cadherin-11 expression leads to an increase in cell motility and invasiveness of PCa cells, suggesting that cadherin-11 plays a role in the invasive phenotype of PCa.
That cadherin-11 promotes cell migration has also been reported in other cell types. Cadherin-11 has been shown to modulate migration of vascular smooth muscle cells (31). In the neuronal system, cadherin-11 activation by immobilized cad11-Fc promotes axonal extension of spinal cord explants (32). Overexpression of cadherin-11 in transfected L-cells also increases cell motility (33). In breast cancer cells, overexpression of cadherin-11 in BT-20 cells promotes epithelial cell motility in a manner similar to N-cadherin (34). Thus, cadherin-11 promotes an increase in mesenchymal cell migration under normal conditions and cells of epithelial origin under pathological conditions.
Because cadherin-11-mediated cellular migration and invasion are dependent on its cytoplasmic domain, these cellular activities are likely mediated through the activation of intracellular signal pathways. Most of the studies on cadherin signaling have been focused on E-cadherin. Although both E-cadherin and cadherin-11 bind to p120 and β-catenin, they have fundamental differences in their functions. While E-cadherin suppresses the invasiveness of tumor cells (12, 35, 36), cadherin-11 is associated with an invasive phenotype. The cytoplasmic domains of E-cadherin and cadherin-11 have only 49% similarity (data not shown). This raises the possibility that the differences in cellular functions are due to the recruitment of additional unique proteins. Cadherin-11 likely interacts with a unique set of proteins to modulate cellular activities that promote the invasive phenotype of PCa cells. By microarray analyses, we found that several genes known to be involved in cell motility and invasiveness were increased in C4-2B4/cad11 when compared to C4-2B4/vector cells (Fig. 6). Functional analysis of these cadherin-11 regulated genes will provide insights into its mechanism of action.
Metastasis to bone is a lethal progression of PCa. Our study elucidated that cadherin-11 plays a critical role in one of the earliest metastasis steps, that is, spreading, migration and invasion, towards PCa metastasis to bone. We suggest that the atypical expression of cadherin-11 under pathological conditions activates new signal pathways to enhance the metastasis of PCa cells to bone. Whether cadherin-11 interacts with unique proteins and how cadherin-11 signals to increase cell migration and invasion in PCa remain to be explored.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant Nos CA111479 and P50-CA140388, DK53176, US Department of Defense Grant No. PC093132, and an award from the Prostate Cancer Foundation.
References
- 1.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–27. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–34. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 3.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 4.Tomita K, van Bokhoven A, van Leenders GJ, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–4. [PubMed] [Google Scholar]
- 5.Schmidmaier R, Baumann P. ANTI-ADHESION evolves to a promising therapeutic concept in oncology. Curr Med Chem. 2008;15:978–90. doi: 10.2174/092986708784049667. [DOI] [PubMed] [Google Scholar]
- 6.Tu S-M, Lin S-H. Clinical aspects of bone metastases in prostate cancer. In: Keller ET, Chung LW, editors. The Biology of Bone Metastases. Boston, MA: Kluwer Academic Publishers; 2004. pp. 23–46. [DOI] [PubMed] [Google Scholar]
- 7.Ye XC, Choueiri M, Tu SM, Lin SH. Biology and clinical management of prostate cancer bone metastasis. Front Biosci. 2007;12:3273–86. doi: 10.2741/2311. [DOI] [PubMed] [Google Scholar]
- 8.Chu K, Cheng C-J, Ye X, et al. Cadherin-11 Promotes the Metastasis of Prostate Cancer Cells to Bone. Mol Cancer Res. 2008;6:1259–67. doi: 10.1158/1541-7786.MCR-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi J, Azuma Y, Hoshi K, et al. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J Bone Miner Res. 2001;16:1265–71. doi: 10.1359/jbmr.2001.16.7.1265. [DOI] [PubMed] [Google Scholar]
- 10.Tamura D, Hiraga T, Myoui A, Yoshikawa H, Yoneda T. Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int J Oncol. 2008;33:17–24. [PubMed] [Google Scholar]
- 11.Okazaki M, Takeshita S, Kawai S, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092–8. [PubMed] [Google Scholar]
- 12.Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–85. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariotti A, Perotti A, Sessa C, Rüegg C. N-cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs. 2007;16:451–65. doi: 10.1517/13543784.16.4.451. [DOI] [PubMed] [Google Scholar]
- 14.Lira CB, Chu K, Lee YC, Hu MC, Lin S-H. Expression of the extracellular domain of OB-cadherin as an Fc fusion protein using bicistronic retroviral expression vector. Protein Expr Purif. 2008;61:220–6. doi: 10.1016/j.pep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu TT, Sikes RA, Cui Q, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–94. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y-C, Cheng C-J, Huang M, et al. Androgen Depletion Upregulates Cadherin-11 Expression in Prostate Cancer. J Path. 2010 doi: 10.1002/path.2687. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partridge NC, Alcorn D, Michelangeli VP, Ryan G, Martin TJ. Morphological and biochemical characterization of four clonal osteogenic sarcoma cell lines of rat origin. Cancer Res. 1983;43:4308–14. [PubMed] [Google Scholar]
- 19.Majeska RJ, Nair BC, Rodan GA. Glucocorticoid regulation of alkaline phosphatase in the osteoblastic osteosarcoma cell line ROS 17/2.8. Endocrinology. 1985;116:170–9. doi: 10.1210/endo-116-1-170. [DOI] [PubMed] [Google Scholar]
- 20.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–8. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodan SB, Imai Y, Thiede MA, et al. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987;47:4961–6. [PubMed] [Google Scholar]
- 22.Katagiri T, Yamaguchi A, Komaki M, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–66. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2:905–9. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 24.Finnemann S, Mitrik I, Hess M, Otto G, Wedlich D. Uncoupling of XB/U-cadherin-catenin complex formation from its function in cell-cell adhesion. J Biol Chem. 1997;272:11856–62. doi: 10.1074/jbc.272.18.11856. [DOI] [PubMed] [Google Scholar]
- 25.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–89. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–84. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DM, Kiener HP, Agarwal SK, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–10. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 29.Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–52. [PubMed] [Google Scholar]
- 30.Shibata T, Ochiai A, Gotoh M, Machinami R, Hirohashi S. Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett. 1996;99:147–53. doi: 10.1016/0304-3835(95)04047-1. [DOI] [PubMed] [Google Scholar]
- 31.Monahan TS, Andersen ND, Panossian H, et al. A novel function for cadherin 11/osteoblast-cadherin in vascular smooth muscle cells: modulation of cell migration and proliferation. J Vasc Surg. 2007;45:581–9. doi: 10.1016/j.jvs.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Boscher C, Mège RM. Cadherin-11 interacts with the FGF receptor and induces neurite outgrowth through associated downstream signalling. Cell Signal. 2008;20:1061–72. doi: 10.1016/j.cellsig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Kiener HP, Stipp CS, Allen PG, Higgins JM, Brenner MB. The cadherin-11 cytoplasmic juxtamembrane domain promotes alpha-catenin turnover at adherens junctions and intercellular motility. Mol Biol Cell. 2006;17:2366–76. doi: 10.1091/mbc.E05-08-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–44. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, Van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 36.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 37.Lin S-H, Cheng CJ, Lee Y-C, et al. A 45-kDa ErbB3 secreted by prostate cancer cells promotes bone formation. Oncogene. 2008;27:5195–203. doi: 10.1038/onc.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.