Abstract
Though obesity is common, some people remain resistant to weight gain even in an obesogenic environment. The propensity to remain lean may be partly associated with high endurance capacity along with high spontaneous physical activity and the energy expenditure of activity, called non-exercise activity thermogenesis (NEAT). Previous studies have shown that high-capacity running rats (HCR) are lean compared to low-capacity runners (LCR), which are susceptible to cardiovascular disease and metabolic syndrome. Here, we examine the effect of diet on spontaneous activity and NEAT, as well as potential mechanisms underlying these traits, in rats selectively bred for high or low intrinsic aerobic endurance capacity. Compared to LCR, HCR were resistant to the sizeable increases in body mass and fat mass induced by a high-fat diet; HCR also had lower levels of circulating leptin. HCR were consistently more active than LCR, and had lower fuel economy of activity, regardless of diet. Nonetheless, both HCR and LCR showed a similar decrease in daily activity levels after high-fat feeding, as well as decreases in hypothalamic orexin-A content. The HCR were more sensitive to the NEAT-activating effects of intra-paraventricular orexin-A compared to LCR, especially after high-fat feeding. Lastly, levels of cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) in the skeletal muscle of HCR were consistently higher than LCR, and the high-fat diet decreased skeletal muscle PEPCK-C in both groups of rats. Differences in muscle PEPCK were not secondary to the differing amount of activity. This suggests the possibility that intrinsic differences in physical activity levels may originate at the level of the skeletal muscle, which could alter brain responsiveness to neuropeptides and other factors that regulate spontaneous daily activity and NEAT.
Keywords: non-exercise activity thermogenesis, NEAT, economy, orexin, brain, food intake, PEPCK-C, skeletal muscle
Introduction
Obesity continues to increase worldwide (Prentice, 2006; Wyatt et al., 2006), along with the health problems associated with it (Wyatt et al., 2006). Over the past several decades, physical activity levels in Western populations have steadily decreased at the same time as obesity has increased (Kruger et al., 2007a; Kruger et al., 2007b; Livingstone et al., 1991). This trend exists despite the plethora of treatments and programs available for body weight management, most of which focus on the roles of diet and exercise. The importance of everyday physical activity to total daily energy expenditure (TDEE) is increasingly being recognized. The energy spent while engaging in activities of daily living, called non-exercise activity thermogenesis (NEAT), can have a significant contribution to TDEE, energy balance, body weight, and health (Church et al., 2007; Dauncey, 1990; Johannsen et al., 2008; Levine et al., 1999; Levine et al., 2005), and differences in physical activity can contribute to obesity propensity and weight gain (Mustelin et al., 2008). There is a large amount of inter-individual variation in the amount of activity people engage in as well as their NEAT, and these correlate with leanness, health, and fitness (Hamilton et al., 2007; Levine et al., 1999; Levine et al., 2005). Moreover, changes in diet composition or caloric intake can alter NEAT (Bjursell et al., 2008; Novak et al., 2006a), and the ability to fend off weight gain in the face of increased caloric intake also varies between individuals (Levine et al., 1999).
Although social, cognitive, and environmental factors can surely impact physical activity levels, the innate, biological influences may be equally important. Studies examining physical activity levels in twins revealed a highly heritable component (Joosen et al., 2005; Kaprio et al., 1981). As in humans, there is a large amount of inter-individual variation in physical activity levels in rats, and lower levels of physical activity are associated with higher body weight and obesity (Dauncey, 1986; Dauncey and Brown, 1987; Novak et al., 2006a; Teske et al., 2006a; Tou and Wade, 2002). Previously, we have shown that rats selectively bred for diet-induced obesity (DIO) are less physically active than their diet-resistant (DR) counterparts after obesity induced by a high-fat diet (Novak et al., 2006a). Investigating energy balance in selectively-bred animals may yield insights into the neural and endocrine underpinnings of obesity and physical activity that are relevant to human physiology. Another interesting and highly relevant animal model that contrasts for metabolic disease risk has been developed by selectively breeding rats for high and low intrinsic aerobic capacity measured by distance run to fatigue on a treadmill test. The high-capacity runners (HCR) remain lean on a calorie-dense diet (Britton and Koch, 2001; Noland et al., 2007; Wisloff et al., 2005). The low-capacity runners (LCR), on the other hand, readily develop insulin resistance and symptoms of cardiovascular disease (Wisloff et al., 2005). Though these rats were not bred to be lean or obese per se, the HCR/LCR rats are a highly useful model of leanness vs. obesity (Wisloff et al., 2005). Recently, we have demonstrated that HCR are consistently more active than LCR, and that this is not secondary to body mass (Novak et al., 2009). HCR also engage in more voluntary activity on a running wheel (Burghardt et al., 2006; Waters et al., 2008), though this type of activity is much more variable between rats, specifically between HCR rats (Burghardt et al., 2006). Thus, high daily activity levels seem to be an inherent part of the lean, high-intrinsic-endurance phenotype (Novak et al., 2009). Moreover, the link between innate endurance capacity and high spontaneous activity appears to extend to humans (Novak et al., 2009).
Alterations in diet also affect NEAT (Bjursell et al., 2008; Levine et al., 1999; Rosenbaum et al., 2003). In fact, access to a high-fat Western diet can suppress activity levels within only a few days (Bjursell et al., 2008). Long-term, obesity-prone DIO rats show a decrease in their daily activity levels when on a high-fat diet, whereas their diet-resistant counterparts do not (Novak et al., 2006a). It is possible that the lean, highly active phenotype is also resistant to diet-induced suppression of physical activity levels. Using rats artificially selected for high and low intrinsic aerobic capacity, we first sought to determine whether individual differences in activity can be modulated by high-fat feeding by comparing spontaneous physical activity and energy expenditure before and after one month on a high-fat diet. Energy expenditure of activity was also measured directly while rats walked on a treadmill, allowing for the dissection of this component of energy expenditure.
Several central neuropeptides and peripheral circulating hormones affect physical activity and NEAT (Kotz et al., 2008; Novak et al., 2006a; Novak and Levine, 2007; Novak et al., 2006b; Teske et al., 2008). What is not known, though, is which of these factors might help explain the individual variations in activity levels noted in those with tendencies for obesity or leanness. Our next objective was to identify biological factors that could potentially underlie the high and low activity levels seen in association with obesity propensity. First, we started by examining peripheral and circulating factors that are both altered in obesity and associated with altered levels of physical activity, including adiponectin (Bjursell et al., 2008), leptin (Choi et al., 2008), and corticosterone (Cador et al., 1993; Veldhuis et al., 1982). Second, we tested the potential role of the enzyme cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) in skeletal muscle. In mice, genetically enhancing PEPCK-C in skeletal muscle results in a lean, active, high-endurance mouse (Hakimi et al., 2007; Hanson and Hakimi, 2008). We have previously demonstrated that the lean HCR have heightened skeletal muscle PEPCK-C levels and enzymatic activity (Novak et al., 2009), implicating this enzyme as an important factor in the lean, active, high-endurance phenotype.
Third, we investigated potential differences within the brain that may account for the differential levels of physical activity between HCR and LCR (Novak et al., 2009). Others and we have previously demonstrated a potent effect of orexin-A on physical activity, as well as differences in brain orexin associated with obesity and obesity propensity (Kotz et al., 2006; Novak et al., 2006a; Teske et al., 2006a). More recently, alterations in the Snark gene have been found to result in alterations in wheel running in mice (Ichinoseki-Sekine et al., 2009), making this gene an interesting potential candidate mechanism for intrinsic differences in spontaneous physical activity as well. To determine if any of these variables were likely to contribute to the intrinsic differences or diet-related changes in physical activity in these rats, we investigated the effects of phenotype (high and low intrinsic aerobic capacity) in conjunction with diet on physical activity, energy expenditure, and weight gain, as well as potential mediators including central, circulating, and peripheral factors.
Methods
The data described here were generated from four separate studies: (1) Physical activity and energy expenditure (24-hour and acute activity after orexin-A) were measured in male HCR and LCR before and after one month on a high-fat diet (rats approximately 7 months old). (2) To measure the energy expenditure during resting and activity more precisely, calorimetry was conducted while female rats rested and walked on a treadmill. (3) Body weight and food intake were measured in four groups: HCR and LCR on regular and high-fat diets (rats approximately 4 months old). (4) To determine whether the difference in physical activity levels affects skeletal muscle PEPCK-C levels, we compared female HCR and LCR in standard calorimetry acclimation housing with housing of reduced size. Females were specifically chosen for the final studies because female HCR and LCR do not have a large magnitude of difference in body weight and size between groups as males do. In addition, we previously confirmed that female HCR and LCR are nearly identical to males in their behavioral phenotype (Novak et al., 2009); for this reason, the use of female rats was justified in this study. The reproductive cycle of these female rats, however, was not monitored nor accounted for. All studies and procedures were approved by the Mayo Institutional Animal Care and Use Committee. Rats, selectively bred at the University of Michigan, were air shipped to the Mayo Clinic animal facility at 4–6 mo of age. After arrival at the facility, rats were housed on a 12:12 light: dark cycle, without forced treadmill running or access to running wheels, and given food and water ad lib unless otherwise noted.
Daily Physical Activity
Male rats (10 HCR and 10 LCR, generation 20) were individually housed on a 24-hr light:dark cycle and allowed ad lib access to standard chow (Lab Diet 5001) and tap water. Each rat underwent four phases of the study protocol: After implantation of a guide cannula aimed at the paraventricular nucleus of the hypothalamus (PVN), we measured (1) 2-hr orexin-A-induced physical activity and energy expenditure, and (2) 24-hr spontaneous daily activity; (3) after 29 days on a high-fat diet (Research Diets D12492, 60% calories from fat), we measured 24-hr spontaneous daily activity; (4) 2-hr orexin-A-induced activity and energy expenditure was measured a second time. In this way, we could compare spontaneous daily activity with sensitivity to the NEAT-activating effects of orexin-A both before and after one month on the high-fat diet. The high-fat diet contained 5.24 Kcal/g as determined by Atwater values, with 20% Kcal from each protein and carbohydrate. The standard laboratory rodent diet contained 28% Kcal from protein, 12% from fat, and 60% from carbohydrate, with 3.36 Kcal/g as calculated from Atwater values.
Measurement of spontaneous physical activity and energy expenditure
Rats were acclimated to the calorimetry room and a simulated calorimetry chamber for at least 24 hrs prior to measurement. For each measurement, the rat was placed in the calorimetry chamber with water (at ambient temperature) and pre-measured food. Air was pumped into the chamber at 3–5 liters per minute (LPM), and expired air was sampled at 0.7 LPM using Oxymax software (Columbus Instruments, Columbus, OH). The calorimetry measurements were made once every minute, except for a 3-min break after every 30 samples to obtain reference values. Physical activity was monitored concurrently with calorimetry, as previously described (Novak et al., 2006a; Novak et al., 2006b, 2007). Opto-M Varimex minor activity monitors (Columbus Instruments), with 17 collimated beams in the X, Y, and Z axes, were used to measure minute-by-minute physical activity. Horizontal and vertical (e.g., rearing) activity were measured in addition to ambulatory (locomotor) counts and repetitive, stationary counts (horizontal minus ambulatory counts). For repeated measurements (e.g., 24-hr tests, or response to orexin-A before and after diet change), efforts were made to make the second set of measurement identical to the first within rat (i.e., order of doses, time of day), as previously described (Novak et al., 2006a; Novak et al., 2006b, 2007).
Measurement of energy expenditure of activity
In a separate group of female rats (8 HCR and 8 LCR, generation 21), we measured NEAT directly. Rats were acclimated to the treadmill and walking procedure prior to the test day. Body composition was measured in each rat before each measurement using EchoMRI (at least one day before testing, as described below). On the test day, during the light phase of the cycle, each rat was placed into the treadmill and energy expenditure was measured using calorimetry with an air flow of 2–3 LPM into the treadmill. The rats were allowed to rest for 90–120 1-min samples before the treadmill was started. Time of resting was confirmed through video monitoring. The rats then walked on the treadmill at 7 meters/min and 60 1-min samples were collected. Rats were re-tested using the same protocol after two weeks on a high-fat diet. For the analysis, we examined the resting energy expenditure (REE) by averaging the lowest 15 data samples. We measured the energy expenditure of activity (EEA) during walking, when rat's energy expenditure was at a steady state for 20 min (the final 20 min of walking); REE was subtracted from this value to yield the final EEA calculation. Thus, in these rats, which did not have access to food for over 2 hours (minimizing the contribution of the thermic effect of food to TDEE), REE+EEA=total energy expenditure. We also determined RER during the same sample intervals as the REE analysis as well as during the entire duration of walking minus the first two samples.
Reduced cage activity
We also measured activity in female rats (8 HCR and 8 LCR, generation 20) divided into two groups: one group was housed in the standard acclimation cage for two weeks, and the other group was housed in a cage of reduced size (“reduced cage”): the cage was the same height but had a diameter of 16cm in order to reduce locomotion and the concomitant effects of locomotion or “exercise training” on skeletal muscle. Total daily activity levels for each rat were measured in either standard or reduced caging.
Stereotaxic surgery
Before the calorimetric measurement, rats underwent stereotaxic surgery for the implantation of a chronic guide cannula aimed at the PVN, as previously described (Novak et al., 2006a; Novak et al., 2006b). Briefly, each rat was sedated with sodium pentobarbital and placed into the stereotaxic frame. The following coordinates were used to aim the guide cannula at the PVN, in relation to the skull and bregma: AP −1.9, ML 0.5, DV −7.3. The guide cannula was affixed to the skull using a sterile Michel wound clip and dental cement. The rats were allowed to recover for at least one week before any measurements were taken. To determine accuracy of cannula placement at the end of the study, after euthanasia, India ink (250 nl) was microinjected through each cannula and the brains were removed, sectioned using a cryostat at 50 μm, mounted onto slides, stained with Cresyl violet, and examined using a microscope. If the tip of the injection needle was not within 250 μm of the PVN, then the data gathered from that rat were not included in the final analysis of orexin-A-induced activity and energy expenditure. This same implantation and injection procedure has been used with other rats for intra-PVN microinjections (Novak et al., 2006a) (also see figure 3 in (Novak and Levine, 2009)).
Orexin-A microinjection
As described previously (Novak et al., 2006a), we measured physical activity and energy expenditure in response to intra-PVN microinjections of orexin-A. After acclimation to the testing room and simulated testing chamber, a rat was weighed then gently restrained and administered a 250 nl microinjection of vehicle or orexin-A (American Peptide Co., Sunnyvale, CA; 0.125, 0.25, 0.5, and 1.0 nmole doses) over 30 sec; the needle was left in place for an additional 30 sec. After microinjections were administered, rats were placed in the calorimeter to record energy expenditure for 2 hours following the infusion. Calorimetry data from the first 20 minutes after the injection were not used. We collected energy expenditure data from two rats (one HCR and one LCR) simultaneously with two calorimeters, and each rat was assigned a calorimeter for all of its measurements, with each calorimeter being used to measure both HCR and LCR. Rats received doses of orexin-A in random order within group, with one HCR and one LCR receiving the same dose in a given testing session and a rat's test taking place at the same time of day for each dose of orexin-A. Each rat was measured at the same time of day and was given the same order of doses for the measurements before and after treatment with the high-fat diet. Though steps were taken to minimize the potential for carryover effects, the experimental design did not eliminate this possibility. The doses were selected for comparison to previous studies which utilized these same doses in different selected lines of rats (Kiwaki et al., 2004; Novak et al., 2006a; Teske et al., 2006a) and showed that these doses covered the dynamic range of orexin-induced physical activity.
Body composition
After euthanasia and tissue removal, each rat was shaved of fur, and the gut contents were removed. We measured body composition using two different methods. Body composition was measured using the EchoMRI-900 (Echo Medical Systems, Houston, TX); results were given in terms of fat mass and lean mass (in g), with the resulting difference from pre-scanning BW representing other non-lean mass, including bone mass. The EchoMRI-900 became available partway through this series of experiments. Before this time, we processed the carcasses using the biochemical method to determine percent carcass adiposity (Leshner et al., 1972), as described previously (Novak et al., 2007). On the one group of carcasses for which we used both methods (EchoMRI-700 on carcasses warmed to 37° C), we compared the two methods using correlation analyses (Pearson's r).
Effects of a high-fat diet
In a second group of male rats, we determined the effects of a high-fat diet on several variables: (1) food intake; (2) body weight; (3) body composition; (4) orexin-A in the perifornical lateral hypothalamic region (PeFLH) containing orexinergic cell bodies, and mRNA expression for orexin, orexin receptor 1 and 2 (OXR1 and OXR2), corticotripin-releasing hormone (CRH), and Snark (Ichinoseki-Sekine et al., 2009) using real-time rt-PCR in other brain regions, namely the PVN, paraventricular nucleus of the thalamus (PVT), and the arcuate nucleus (ARC); (5) circulating hormone levels; and (6) levels of proteins and gene expression in peripheral tissues. In this study, half of the male HCR and LCR rats (total N=36; generation 21) were placed on the high-fat diet for one month, and half remained on the standard diet (n=9 per group). Trunk blood was collected after decapitation within the first five hours after lights-on. Skeletal muscles (gastrocnemius and soleus) were also collected and frozen in liquid nitrogen.
Micropunch and orexin-A radioimmunoassay (RIA)
Brains were rapidly extracted after decapitation and placed in ice-cold saline for 20 min. The brain was then sectioned using a Hatton apparatus. With the optic chiasm as the baseline (“0”) value, a 100μm slice was taken by making cuts at −0.1 mm and −0.2 mm; a second, 200μm slice was then cut. The slices were placed onto slides and frozen on dry ice. From the first slice, we used a 2 mm micropuncher to isolate the PVN (medial punch), a 1 mm medial punch to extract the PVT, and two 1 mm punches to sample the PeFLH bilaterally. Two more punches of the PeFLH were taken on the second (200 μm) slice as well. The punches were quickly placed in liquid nitrogen. One 200μm PeFLH punch was combined with the ipsilateral 500 μm punch; the remaining punches were combined and mRNA was isolated for rtPCR (see below). See Table 7 for a list of sites measured for each gene [though some find only OXR2 and not OXR1 in the PVN, others identify OX1R in the PVN (Backberg et al., 2002; Marcus et al., 2001)].
Table 7.
mRNA levels measured using real-time rt-PCR.
| Tissue | Gene | Site | Fold change, compared to HCR on the regular diet | |||
|---|---|---|---|---|---|---|
| Regular Diet | High-Fat Diet | |||||
| HCR | LCR | HCR | LCR | |||
| Brain | preproorexin | PeFLH | 1.0 | 1.3 | 2.0 | 2.2 |
|
| ||||||
| OXR1 | PeFLH | 1.0 | 1.1 | 1.1 | 11.1 | |
|
| ||||||
| PVN | 1.0 | 1.1 | −1.2 | 1.3 | ||
|
| ||||||
| PVT | 1.0 | 1.1 | −1.0 | 1.7 | ||
|
| ||||||
| OXR2 | PeFLH | 1.0 | 1.4 | 1.3 | 1.3 | |
|
| ||||||
| PVN | 1.0 | 1.3 | −1.0 | 1.2 | ||
|
| ||||||
| PVT | 1.0 | 1.1 | −1.0 | 1.3 | ||
|
| ||||||
| CRH | PVN | 1.0 | 1.2 | −1.3 | −1.3 | |
|
| ||||||
| Snark | PeFLH | 1.0 | 1.6 | 1.3 | 1.5 | |
|
| ||||||
| Arc | 1.0 | 1.1 | 1.1 | 1.3 | ||
|
| ||||||
| PVN | 1.0 | −1 | 1.1 | 1.5 | ||
|
| ||||||
| Skeletal muscle | PEPCK-C | gastrocnemius | 1.0 | 3.3 | 1.2 | −2.1 |
|
| ||||||
| soleus | 1.0 | 2.1 | −1.8 | 2.5 | ||
Orexin receptor 1 (OXR1) and 2 (OXR2); perifornical lateral hypothalamus (PeFLH), Paraventricular nucleus of the hypothalamus (PVN), Paraventricular nucleus of the thalamus (PVT), Arcuate nucleus (Arc). (Backberg et al., 2002; Marcus et al., 2001)
Brain tissue was homogenized and protein was extracted using a total of 700 μl 0.1 M acetic acid. The samples were centrifuged (14,000 g for 15 min at 4° C) and the supernatant was then boiled for 10 min. A portion (35 μl) of the sample was used for later determination of protein concentration with a spectrophotometer (SpectraMax 340) using the absorbance set at 595 nm. The remaining supernatant was frozen. For the protein analysis, 50 μl of this sample was used after it was dissolved in 950 μl distilled water. For the RIA, an orexin-A RIA kit was used (Peninsula Laboratories, San Carlos, CA) with 125I-orexin-A, rabbit anti-orexin-A, and goat anti-rabbit secondary antibody. All samples were run in duplicate. Resulting orexin-A concentrations divided by protein quantities for each sample were used to determine the orexin-A levels for each rat.
Brain and muscle gene expression
Tissue was homogenized and RNA was isolated in preparation for analysis by real-time RTPCR. To isolate the RNA, the 2-mm thick portions of the PeFLH were combined and homogenized using 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA) and separated using 200 μl chloroform. The pellet was then washed with 75% ethanol and dissolved in 10 μl DEPC-treated water. The pellet was treated with rDNase I (Ambion, Austin, TX) to remove DNA from the sample, and brought up to 20 μl volume with DEPC-treated water. We then measured the RNA concentration using a spectrometer, and 0.5μg (or 0.5μg/8μl) was used for cDNA synthesis. Approximately 25ng cDNA was used for qRT-PCR. The annealing temperature was 60°C with 40 cycles. All samples were run in triplicate on an Applied Biosystems (Foster City, CA) Sequence Detection System 7900. Table 1 lists the methods used for qRT-PCR. To analyze these data, L32 was used as the reference gene, and the 2−ΔΔCT analysis was used to determine fold change in each target gene compared to the reference gene, with 1-fold defined as the values calculated from HCRs on a regular diet (Livak and Schmittgen, 2001).
Table 1.
Methods used for real time rt-PCR.
| Gene | Size (bp) | Primer | Sequence (5' to 3') | Annealing (°C) | Melting peak (°C) | Reference |
|---|---|---|---|---|---|---|
| Orexin | 143 | sense | CATCCTCACTCTGGGAAA | 60 | 88 | (Teske et al., 2006b) |
| antisense | AGGGATATGGCTCTAGCT | |||||
| OXR1 | 244 | sense | AGAGAGCAGAGAGCGTTGTAAACC | 60 | 84 | (Teske et al., 2006b) |
| antisense | TTCACAGGGACACATTGGTGC | |||||
| OXR2 | 257 | sense | TGTTCAAGAGCACAGCCAAACG | 60 | 82 | (Teske et al., 2006b) |
| antisense | GCCAATACCATAAGACACAGGGG | |||||
| CRF | 150 | sense | CTCTCTGGATCTCACCTTCCAC | 60 | 80 | (Chance et al., 2007) |
| antisense | CTAAATGCAGAATCGTTTTGGC | |||||
| Snark | 294 | sense | CTGTACATCCTGGTGCATGG | 60 | 85.5 | NM 001007617 |
| antisense | ATGACCTTGCCCTTATGCT | |||||
| PEPCK | 252 | sense | GAGACCACAGGATGAGGAA | 60 | 85 | (Azzout-Marniche et al., 2007) |
| antisense | ATGACCTTGCCCTTATGCT | |||||
| L32 | 122 | sense | CGGAAGTTTCTGGTCCACAATGTC | 60 | 79 | (Teske et al., 2006b) |
| antisense | GCTCTTTCTACGATGGCTTTTCGG |
Circulating hormone levels
We measured circulating leptin, adiponectin, and corticosterone. Serum hormone levels were analyzed using an ELISA. Commercially-available enzyme immunoassay kits were used to quantify the plasma levels of leptin (Assay Designs, Ann Arbor, MI), corticosterone (MP Biomedicals, Cleveland, OH), and adiponectin (Alpco Diagnostics, Salem, NH), according to the manufacturer's instructions.
Western blots
PEPCK-C and SIRT-1 were examined in skeletal muscles (gastrocnemius and soleus) from male HCR and LCR on regular and high-fat diets. Muscle tissue was homogenized in ice-cold RIPA buffer (25 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 150 mM NaCl, 1% Sodium deoxycholate, 1 mM sodium orthovanadate, 5 mM NaF, and a protease inhibitor cocktail; Roche, Mannheim, Germany). Homogenates were incubated at 4° C for 20 minutes under constant agitation. Homogenates were then centrifuged for 10 minutes at 10,000 g at 4° C. Supernatant was collected and the protein concentration was determined using the Bradford method (protein assay; Bio-Rad Laboratories, Richmond, CA), with BSA as a standard. Proteins (70 μg/lane) were resolved by SDS-PAGE and transferred by electroblotting onto a PVDF membrane, which was probed with specific antibodies. PEPCK-C antibody was obtained from Cayman Chemical Company (Ann Arbor, MI, USA), tubulin antibody was from Abcam, Inc. (Cambridge, MA, USA), and the antibody for SIRT-1 was from Upstate Biotechnology Inc. (Lake Placid, NY). Western blots were developed using SuperSignal™ West Pico Chemoluminiscent substrate (Pierce, Rockford, IL, USA). Films were scanned and bands quantified by densitometry using Image J (NIH, USA). Tubulin was used as the control, with results expressed as a ratio of the target protein (PEPCK-C or SIRT-1) to tubulin.
Statistical analyses
Body weight change was analyzed using a 2×2×2 repeated-measures ANOVA with group and diet as the between-animal independent variable and body weight as the within-animal independent variable. To compare activity and TDEE before and after the high-fat diet in HCR and LCR, we used a two-way (2×2) mixed ANOVA with group (HCR or LCR) as the between-animal independent variable and pre- vs. post-high-fat diet as the within-animal independent variable. Because energy expenditure is predominantly determined by body mass (Goran, 2005), it is difficult to directly compare energy expenditure between rats of different sizes, especially since “correction” factors commonly overcorrect for body mass (Packard and Boardman, 1999). Though mass-corrected and uncorrected data were analyzed and reported here, we used analysis of covariance (ANCOVA), with body weight as the covariate (Packard and Boardman, 1988), to determine the effects of diet and selected line on energy expenditure. For these rats, horizontal activity and body weight were compared using a correlation (Pearson's r). ANCOVAs were also used to analyze REE and EEA after the treadmill test in HCR and LCR, using body weight and lean mass as covariates. We also used ANCOVAs to analyze the effect of selected line, diet, and fat mass on leptin, with fat mass as the covariate. For orexin-induced activity, we again used a mixed 2×5 ANOVA with orexin-A dose as the within-animal independent variable and group (HCR, LCR) as the between-animal independent variable. To compare the effects of orexin-A on activity before and after the high-fat diet, we used the mixed two-way ANOVA at each dose of orexin-A with group and diet as the independent variables; paired t-tests were used for pairwise comparisons. An independent samples 2×2 ANOVA was used to compare body weights, body composition, hormone levels, brain orexin-A concentrations, and peripheral measures (PEPCK, and serum leptin, adiponectin, and cortisol) in the HCR and LCR on either the high-fat or regular diets; Fisher's PLSD test was used for pairwise comparisons. The same analysis was used for activity and muscle PEPCK data in the female rats in the reduced and regular-sized cages, with Fisher's PLSD used for the post-hoc analysis.
We also compared the variance in the activity data (horizontal counts) in the HCR and LCR to another rat model of obesity, namely male diet-induced obese (DIO) and diet-resistant (DR) rats previously measured in the same lab (Novak et al., 2006a). Data from each group of rats were compared under the same three conditions: on a regular diet, then again both before and after one month on a high-fat diet (Novak et al., 2009; Novak et al., 2006a). Lastly, Pearson's correlation coefficients were calculated to determine the relationship between body weight and activity levels (mean horizontal beam breaks per minute) between rats within group. In all cases, significance was determined by p<0.05.
Results
LCR are more sensitive than HCR to the deleterious effects of a high-fat diet
We investigated the effects of intrinsic aerobic capacity and a high-fat diet on body weight, circulating hormones, and the levels of central and peripheral genes, peptides, and enzymes that could potentially affect physical activity, energy expenditure, and NEAT. Data from one rat (HCR fed a regular diet) were omitted from analysis because of unexplained weight loss in the final weeks of the study that suggested potential illness (>2 SD below the mean body weight change for HCR on the control diet).
For body weight gain, the HCR and LCR responded differently to the high-fat diet (Figure 1A). The LCR on the high-fat diet gained significantly more weight than LCR on the regular diet as well as HCR on the same diet, and LCR on the regular diet gained more weight than HCR on the regular diet. In other words, the high-fat diet had a greater effect on body weight in the LCR compared to HCR. Fat mass as percent of carcass weight using the biochemical method also showed an interaction between selected line and diet: though the high-fat chow resulted in greater body fat in both HCR and LCR compared to their chow-fed controls, LCR gained significantly more fat than HCR (Figure 1B). In addition, chow-fed HCR had significantly lower body fat than chow-fed LCR.
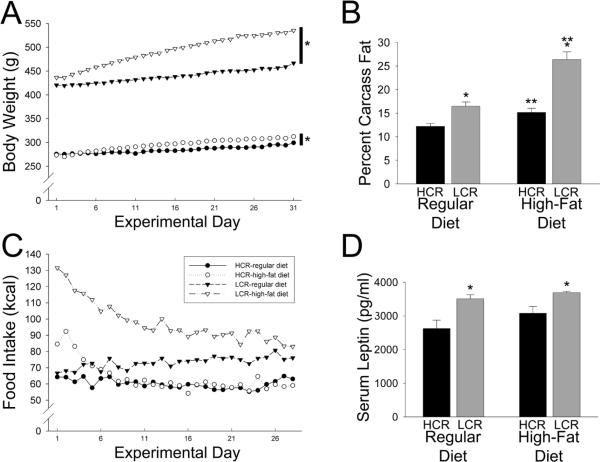
Figure 1.
Low-capacity runners (LCR, n=9 rats on each diet) were more sensitive to the deleterious effects of a high-fat diet than high-capacity runners (HCR; n=9 on rats the high-fat diet, 8 rats on the regular diet). (A) LCR gained significantly more weight over one month on the high-fat or regular diets than HCR; *different from chow-fed controls. (B) LCR on a high-fat diet gained significantly more body fat than HCR compared to controls; *greater than HCR on same diet; **greater than same group on a regular diet. (C) Whereas LCR on the high-fat diet ate significantly more kilocalories than LCR on a regular diet for several weeks, HCR on the high-fat diet returned to baseline consumption within one week. (D) LCR had significantly higher circulating leptin levels than HCR, regardless of the diet; no significant effect of diet on leptin was seen in HCR or LCR; *greater than HCR.
We also examined daily food intake over 27 days on the high-fat diet (Figure 1C) to determine the extent to which food intake affects body weight and body fat gain in HCR and LCR. In order to compare food consumption in the high-fat and regular chow fed groups, food intake was converted to kcal using Atwater values (3.36 kcal/g for the regular chow, and 5.24 kcal/g for the high-fat chow). There was a significant interaction where, compared to chow-fed controls, LCR ate significantly more calories on the high-fat diet but HCR did not (Figure 1C). Even on the standard chow, LCR ate significantly more kcal HCR. When the food intake for each day (in kcal) was divided by the daily body weight for each rat, a similar pattern was seen, with rats on a high-fat diet showing daily intakes comparable to their chow-fed controls after 5 days (for HCR) or 14 days (for LCR), and actually eating fewer kcal per gram body weight than control rats on the final 6 days (for HCR) or 2 days (for LCR).
For serum leptin concentrations, LCR had significantly higher levels than HCR in all conditions (Figure 1D). There was no interaction, and a non-significant but marginal effect of the high-fat diet on serum leptin (p=0.0674)—neither group of rats significantly increased their leptin levels in response to a high-fat diet. The ANCOVA revealed that HCR had higher leptin levels than LCR after fat mass (covariate) was factored out statistically (i.e., body fat mass was the covariate, and there was not a significant interaction between diet and selected line, so the model was recalculated without the interaction); diet had no significant effect on leptin, but there were significant effects of both fat mass and selected line on leptin. No differences were detected in adiponectin levels or corticosterone, potentially because of the high level of variability between animals within each group (Table 2).
Table 2.
Serum adiponectin and corticosterone concentrations in male high- and low-capacity rats (HCR and LCR) after one month on a standard diet or a high-fat diet. Mean ± SEM
| Strain | Diet | Serum hormone concentrations | |
|---|---|---|---|
| Adiponectin (μg/ml) | Corticosterone (ng/ml) | ||
| HCR | standard | 10.9 ± 1.2 | 101.4 ± 44.8 |
| high-fat | 10.9 ± 1.1 | 282.2 ± 132.4 | |
| LCR | standard | 11.0 ± 0.7 | 56.4 ± 22.4 |
| high-fat | 12.0 ± 0.6 | 164.1 ± 82.5 | |
High-fat diet decreases physical activity in both HCR and LCR
In order to determine how body weight gain on a high-fat diet may be affected by physical activity and NEAT in these rats, we measured daily activity and energy expenditure in HCR and LCR on regular and high-fat diets (a group separate from the rats measured in Figure 1). On average, the rats had a small change in body weight during the calorimetry day (−1.89±0.55g), which may have been partially due to the time of weighing (rats were weighed at a later time during the light phase compared to the day prior). The food intake during the calorimetry days were comparable to mean daily intakes for the male HCR and LCR in the study previously described (Figure 1): HCR before high-fat diet, 19.7±1.6g (66.2±5.4 kcal); HCR on high-fat diet, 13.9±1.5g (72.8±7.9 kcal); LCR before high-fat diet, 25.2±3.0g (84.7±10.1 kcal); LCR on high-fat diet, 16.7±3.1g (87.5±16.2 kcal). After one month on a high-fat diet, both HCR and LCR showed a small but significant 10–15% decrease in their activity levels (Figure 2, Table 3). The effect of diet on activity did not differ between HCR and LCR. When oxygen consumption per body weight was compared before and after high-fat feeding, HCR did not change their oxygen consumption, but LCR showed a significant decrease with high-fat feeding. Energy expenditure expressed per kg0.75 body weight was greater in HCR than LCR, regardless of diet, but decreased to a similar degree in both HCR and LCR on the high-fat diet (Figure 2B). When analyzed using ANCOVA, the covariate (body weight), but not selected line, significantly affected total energy expenditure; there was not a significant interaction (p=0.099). Body fat was significantly greater in the LCR than HCR after high-fat feeding using two methods of measurement (Table 4).
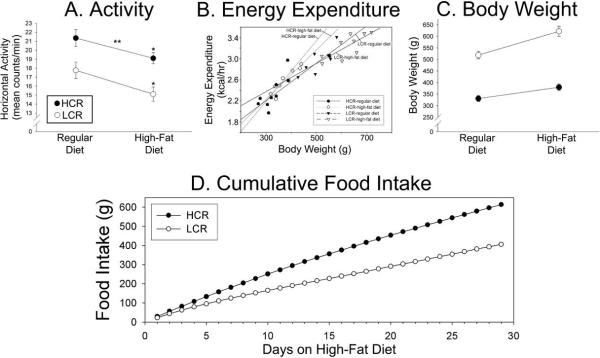
Figure 2.
Activity and energy expenditure in high- and low-capacity running rats (HCR and LCR, n=10/group). (A) HCR were consistently more active than LCR regardless of diet, and the high-fat diet significantly decreased 24-hr physical activity levels similarly in HCR and LCR. *significant decrease compared to regular diet. **greater than LCR. (B) Daily energy expenditure according to body weight of HCR (gray regression lines) and LCR (black regression lines) on regular and high-fat diets (diet × selected line interaction, p=0.099). Body weight gain (C) and cumulative food intake (D) over the course of high-fat feeding in HCR and LCR.
Table 3.
24-hour activity and energy expenditure in male high- and low-capacity rats (HCR and LCR) before and after one month on a high-fat diet. Mean ± SEM; n=9 HCR and 10 LCR.
| Energy Expenditure | Activity (counts/min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| strain | high-fat diet | VO2 (ml/kg/hr) | TDEE (kcal/hr) | TDEE (kcal/kg0.75/day) | RER | vertical | ambulatory | stationary | % min active |
| HCR | before | 1444±39* | 2.36±0.09* | 2.01±0.08* | 0.92±0.01 | 0.61±0.14 | 8±1 | 14±1* | 59±1* |
| after | 1424±25* | 2.58±0.07*† | 1.80±0.06*† | 0.79±0.01† | 0.72±0.09* | 7±1* | 12±1*† | 55±1† | |
| LCR | before | 1165±24 | 2.98±0.08 | 1.31±0.05 | 0.92±0.01 | 0.39±0.03 | 7±1 | 11±1 | 55±1 |
| after | 1089±25† | 3.23±0.08† | 1.09±0.04† | 0.79±0.01† | 0.37±0.08 | 6±1† | 9±1† | 54±1 | |
% min active: the number of 1-min bins during which the rat showed any beam breaks, divided by the total number of minutes sampled.
TDEE=total daily energy expenditure.
different from LCR on same diet (p<0.05).
different from same rats before the high-fat diet (p<0.05).
Table 4.
Mean percent fat ± SEM using the EchoMRI-900 (EchoMRI) and the biochemical method (Biochem). Fat mass data using these methods were significantly positively correlated in both groups of rats, and no bias was detected according to group.
greater than HCR, p<0.0001.
Low variability in activity compared to other obese rat lines
When measuring daily physical activity in HCR and LCR, it was noted that there was low intra-group variability in activity. As shown in Table 5, both HCR and LCR had very low variance in their measured daily activity levels compared to other artificially-bred lean and obese rats (Novak et al., 2006a). The correlation (r) between activity and body weight in rats on a regular diet was −0.30 (p=0.21) for all rats, 0.37 for HCR (p=0.30), and 0.76 for LCR (p<0.05); for energy expenditure (kcal/hr), the correlation was 0.92 for all rats, 0.80 for HCR, and 0.81 for LCR (p<0.05 for all). On the high fat diet, the correlation between activity and body weight was −0.554 for all rats (p<0.05), 0.22 for HCR (p=0.55), and 0.32 for LCR (p=0.41); for energy expenditure, the correlation was r=0.95 for all rats, r=0.90 for HCR, and r=0.78 for LCR (p<0.05 for all).
Table 5.
Male rats selectively bred for high and low intrinsic aerobic capacity (HCR and LCR) showed significantly less variability in daily activity levels within group compared to male rats selectively bred for resistance to weight gain on a high-fat diet (DR) or diet-induced obesity (DIO) (Novak et al., 2006a).
| Selected Line | Lean or Obese | N | Variance |
|---|---|---|---|
| HCR | Lean | 30 | 5.081 |
| LCR | Obese | 28 | 6.742 |
| DR | Lean | 26 | 55.197 |
| DIO | Obese | 30 | 40.859 |
HCR have low fuel economy of activity
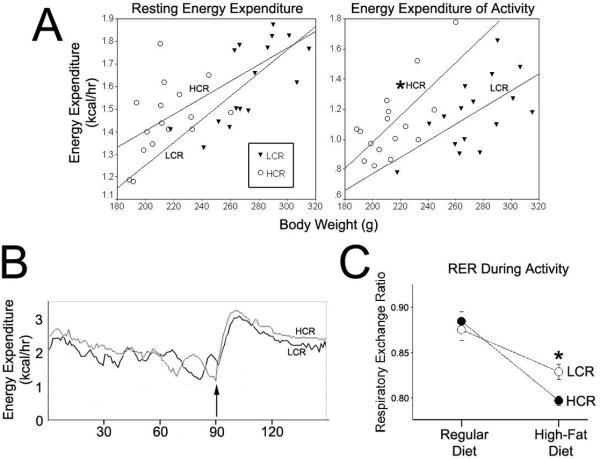
To more precisely assess NEAT in these rats, we measured energy expenditure during rest and walking. Though a trend was apparent for differential REE between HCR and LCR, this difference was not significant when assessed using ANCOVA with body weight as the covariate. Energy expenditure of walking was greater in HCR than LCR (Figure 3A,B). The same results were found when REE and EEA were analyzed using lean mass as the covariate. Diet had no significant effect on REE or EEA, though the high-fat diet predictably decreased RER in all rats during rest and activity. There was a significant interaction for RER during activity where HCR showed a significantly lower RER compared to LCR during walking (Figure 3C). There was no significant interaction between diet and line for RER during resting.
Figure 3.
Energy expenditure of activity (EEA) and resting energy expenditure (REE) were measured in high-capacity rats (HCR) and low-capacity rats (LCR). (A) Energy expenditure during resting and activity in HCR and LCR (collapsed across diet) according to body weight. Energy expenditure of activity was significantly greater in the HCR (*significantly different effect of selected line after body weight, the covariate, was accounted for), indicating that they had a lower fuel economy of activity than LCR. (B) The energy expenditure during resting in the treadmill and while waling at 7 meters/min (arrow indicates start time) in one HCR (gray line; 216 g) and one LCR (black line; 218g); final EEA = EEA+REE − REE. (C) On the high-fat diet, respiratory exchange rate (RER) was significantly lower in the HCR than HCR on the high-fat diet, indicating increased use of lipid as fuel during activity in these rats.
Orexin-induced activity is greater in HCR than LCR
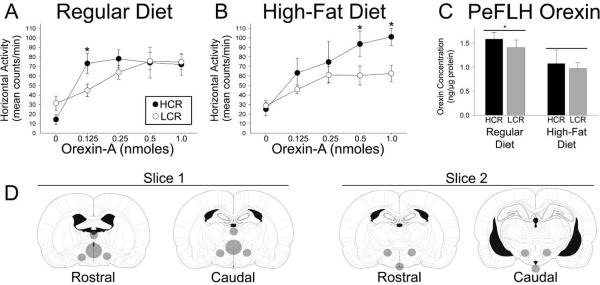
We tested the hypothesis that intrinsic differences in orexin sensitivity, or diet-related changes in orexin sensitivity, could underlie the differences in physical activity found in HCR and LCR. In rats fed a regular diet, orexin-A increased activity and energy expenditure in both HCR and LCR (Figure 4A; Table 6). The HCR were significantly more active at the lowest dose of orexin-A given (0.125 nmoles/250 nl). There was no difference between groups at the higher doses. After one month on the high-fat diet, on the other hand, the HCR were more active than the LCR at the highest doses of orexin-A (0.5 and 1.0 nmoles/250 nl). This was due to a marginal increase in the amount of activity after 0.5 nmoles orexin-A in the HCR (p=0.050147) in addition to a more sizeable and significant decrease in the activity of the LCR at the highest two doses of orexin-A.
Figure 4.
Intra-paraventricular (PVN) microinjections of orexin-A at increasing doses resulted in heightened physical activity in high- and low-capacity runners (HCR and LCR; n=5 and 9, respectively). (A) On a regular diet, HCR were slightly more sensitive to intra-PVN orexin-A than LCR (HCR>LCR at 0.125 nmoles, p<0.05). (B) After one month on a high-fat diet, HCR were significantly more responsive to the intra-PVN orexin-A. *HCR were more active than LCR at the same dose *(HCR>LCR at 0.5 nmoles, p<0.05; HCR>LCR at 1.0 nmoles 0.01). (C) In a separate study, orexin-A concentrations in the perifornical lateral hypothalamic region (PeFLH) were greater in rats on a regular diet compared to a high fat diet. *greater than rats on a high-fat diet. No differences were seen between high- and low-capacity runners (HCR and LCR; n=8 HCR and 9 LCR on the regular diet, 9 HCR and 9 LCR on the high-fat diet). (D) Atlas figures [plates 42/49 and 49/66 from (Paxinos and Watson, 2005)] representing the micropunches taken from brain slices.
Table 6.
Intra-PVN orexin-A-induced increases in activity and energy expenditure in high- and low-capacity rats (HCR and LCR) before and after one month on a high-fat diet. Mean ± SEM.
| Energy Expenditure | Activity (counts/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| strain | diet | Orexin-A (nmoles) | VO2 (ml/kg/hr) | EE (kcal/hr) | EE (kcal/kg0.75/hr) | RER | vertical | ambulatory | stationary | % time active |
| HCR | regular | vehicle | 1285±92‡ | 2.25±0.19‡ | 0.030±0.002 | 0.91±0.03 | 0.31±0.17 | 8±3 | 9±3 | 52±8 |
| 0.125 | 1636±98‡* | 2.86±0.26* | 0.035±0.002* | 0.88±0.02‡ | 2.55±0.53* | 36±5* | 41±7*‡ | 94±4* | ||
| 0.25 | 1698±112‡* | 3.00±0.16*‡ | 0.036±0.005* | 0.91±0.04 | 3.15±1.64* | 35±5* | 42±8* | 99±1* | ||
| 0.5 | 1654±132‡* | 2.85±0.25*‡ | 0.035±0.006* | 0.86±0.03 | 2.45±0.85* | 40±9* | 43±8* | 98±2* | ||
| 1.0 | 1605±111‡* | 2.74±0.20*‡ | 0.034±0.002* | 0.85±0.02 | 1.86±0.47* | 33±6* | 42±7* | 98±0.6* | ||
| high-fat | vehicle | 1372±64‡ | 2.59±0.19 | 0.029±0.001‡ | 0.77±0.01‡ | 0.47±0.27* | 11±4* | 14±4* | 65±11* | |
| 0.125 | 1614±21‡* | 3.06±0.20*‡ | 0.034±0.001* | 0.79±0.01† | 1.94±0.76* | 27±6* | 37±11* | 97±1* | ||
| 0.25 | 1841±108‡* | 3.53±0.29* | 0.039±0.002*‡ | 0.79±0.02*† | 3.87±0.72*‡ | 37±10* | 38±12* | 97±1* | ||
| 0.5 | 1720±91‡* | 3.24±0.21* | 0.037±0.002* | 0.80±0.01† | 3.51±0.38*‡ | 43±5*‡ | 51±9*‡ | 96±4* | ||
| 1.0 | 1860±113‡* | 3.50±0.25* | 0.040±0.002*‡ | 0.80±0.01† | 3.25±0.43†‡ | 48±5*†‡ | 53±5*‡ | 100±0*† | ||
| LCR | regular | vehicle | 1117±26 | 2.83±0.15 | 0.026±0.001 | 0.90±0.02 | 0.45±0.24 | 13±3 | 19±5 | 70±6 |
| 0.125 | 1308±56* | 3.52±0.17* | 0.030±0.001* | 0.86±0.02 | 1.50±0.30* | 21±3.43* | 24±4* | 88±3* | ||
| 0.25 | 1369±38* | 3.56±0.08* | 0.033±0.001* | 0.87±0.01 | 1.61±0.35* | 31±4* | 33±4* | 95±3* | ||
| 0.5 | 1423±55* | 3.55±0.19* | 0.033±0.001* | 0.85±0.02* | 2.42±0.56* | 38±6* | 38±4* | 97±3* | ||
| 1.0 | 1370±56* | 3.48±0.20* | 0.032±0.001* | 0.89±0.02 | 2.22±0.50* | 37±5* | 38±4* | 99±1* | ||
| high-fat | vehicle | 1011±63 | 2.99±0.15 | 0.024±0.001 | 0.79±0.01† | 0.23±0.12 | 12±3 | 15±3 | 75±4 | |
| 0.125 | 1201±41*† | 3.55±0.13*† | 0.028±0.001* | 0.78±0.01† | 1.10±0.53* | 22±2* | 24±3* | 92±6* | ||
| 0.25 | 1293±65* | 3.80±0.2*† | 0.030±0.001* | 0.79±0.01† | 1.11±.032* | 29±5* | 28±4* | 92±4* | ||
| 0.5 | 1231±63*† | 3.68±0.24* | 0.029±0.002*† | 0.79±0.01† | 1.33±0.27*† | 29±5*† | 30±4*† | 92±3* | ||
| 1.0 | 1304±70* | 3.92±0.67*† | 0.031±0.002* | 0.78±0.01† | 1.50±0.29*† | 34±6* | 28±3*† | 96±2* | ||
HCR n=5; LCR n=9.
different from vehicle value in same group on same diet (p<0.05).
different from same dose in same group on regular diet (p<0.05).
different from corresponding data point (same diet and dose) in LCR group (p<0.05).
A high-fat diet decreases orexin-A concentration in the hypothalamus
In order to ascertain the mechanisms of phenotypic or diet-related differences in orexin responsiveness, we measured gene expression and peptide levels in brain regions that affect are part of the brain orexin system. As illustrated in Figure 4C, orexin-A concentrations (in ng/μl) in the PeFLH were significantly decreased by the high-fat diet but not different between groups. In short, the high-fat diet decreased the concentration of orexin-A to a similar extent in both HCR and LCR; no differences were seen between HCR and LCR, either on the regular or the high-fat diet.
We found no significant effect of group or diet on any gene expression variable measured in any brain region (OXR1, OXR2, orexin, CRH, Snark), though there was a trend for increased preproorexin expression in the PeFLH of high-fat-fed rats compared to rats on the standard diet in (Table 7).
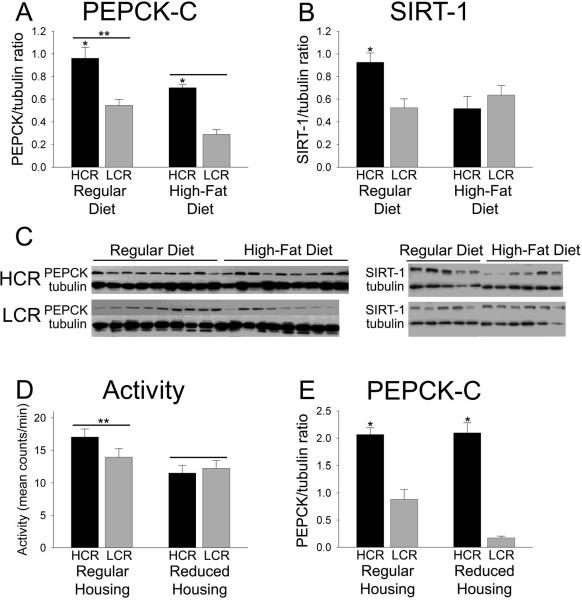
Skeletal muscle PEPCK-C is higher in HCR than LCR
We previously demonstrated that HCR have heightened skeletal muscle PEPCK-C levels and enzymatic activity (Novak et al., 2009). Here, we determined whether diet affected skeletal muscle PEPCK-C enzyme levels and gene expression differentially in HCR and LCR skeletal muscle. PEPCK-C was significantly elevated in HCR compared to LCR (Figure 5A, C). The high-fat diet decreased muscle PEPCK-C in both HCR and LCR (Figure 5A). The levels of PEPCK-C mRNA in the skeletal muscle did not mirror this effect, however: mRNA levels were actually higher in LCR compared to HCR in one case (Table 7). Levels of the NAD-dependent deacetylase SIRT-1 were significantly higher in HCR on the control diet compared to all other groups (Figure 5B, C).
Figure 5.
Skeletal muscle cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) in high-and low-capacity running rats (HCR and LCR). (A) PEPCK-C was higher in the skeletal muscle of HCR compared to LCR and the high-fat diet significantly decrease PEPCK-C in the skeletal muscle in both groups (n=9 in HCR on both diets and LCR on the regular diet, n=8 in LCR on the high-fat diet). *greater than LCR; **greater than rats on a high-fat diet. (B) SIRT-1 was significantly higher in HCR on a control diet compared to all other groups (n=5 HCR and 5 LCR on the regular diet, 6 HCR and 6 LCR on the high-fat diet. *p<0.05). (C) Western blots illustrating the effects of selected line and diet on PEPCK-C and SIRT-1, compared to tubulin. (D) When rats were housed in cages of reduced size, this resulted in reduced activity in HCR but not LCR; **greater than reduced housing. (E) This reduction in activity did not alter skeletal muscle PEPCK-C in HCR, demonstrating that skeletal muscle PEPCK-C was secondary to heightened activity in HCR; *greater than LCR in the same housing condition, n=5 HCR and 5 LCR on the regular diet, 6 HCR and 6 LCR on the high-fat diet.
To determine if differences in skeletal muscle PEPCK-C were secondary to increased physical activity, we placed HCR and LCR females (to minimize the difference in body weight) in housing of reduced size to minimize ambulation. As shown in Figure 5D, the female HCR in the reduced cages were significantly less active than their counterparts housed in the standard activity cages. There was no effect of cage size on activity in LCR. In essence, the reduced cages brought the HCR activity counts down to the approximate level of the LCR. In rats housed in both regular and reduced cages, skeletal muscle PEPCK-C was higher in HCR compared to LCR (Figure 5E).
Discussion
The energy expended through spontaneous activity may confer resistance to obesity and its health consequences, particularly in an obesogenic environment (Church et al., 2007; Hamilton et al., 2007; Johannsen et al., 2008; Levine et al., 1999; Levine et al., 2005; Woolf et al., 2008). Though numerous physiological factors can affect NEAT (Kotz et al., 2008; Novak and Levine, 2007; Teske et al., 2008), the mechanisms underlying individual differences in activity levels associated with obesity propensity are not known. We have previously demonstrated that intrinsic aerobic capacity is a critical predictor of high spontaneous activity in both rats and human participants (Novak et al., 2009), and that low spontaneous activity is not secondary to a high body mass (Novak et al., 2009). This implies that high physical activity levels and a high intrinsic aerobic endurance may be related, characteristic features of the lean phenotype. Here, we investigated the potential mechanisms that modulate daily activity in lean and obese rats and how these are altered on a high-fat diet.
HCR are consistently more active than LCR, both on the regular and high-fat diet (Figure 2A). This is in line with evidence of higher voluntary exercise activity in HCR (Waters et al., 2008). Differences in daily activity are unlikely to be secondary to body mass because (1) the amount of daily activity, as measured by beam breaks, is dependent upon an endurance phenotype but not body size (Novak et al., 2009); (2) within each group, low activity did not correlate with high body mass; and (3) LCR gained much more body weight than HCR, yet both groups showed comparable decreases in daily activity on the high-fat diet (Figure 2A). Taken together, this evidence suggests that the decrease in activity from the high-fat diet was not due primarily to the extra body weight. In short, high daily activity is associated with a lean, high-endurance phenotype rather than a lower body mass per se. Physical activity is notably variable between individuals, even between animals within a relatively homogeneous group, and is sensitive to stressors and other environmental perturbations. The low variance in the HCR and LCR rats' activity levels (Figure 2A)(Thyfault et al., 2009) compared to other artificially-selected rats (Table 5) further supports the idea that the mechanisms underlying aerobic capacity as defined by duration and distance of forced treadmill running (a physiological trait) and spontaneous daily activity (a behavioral trait) may overlap.
A high-fat diet consistently reduces physical activity levels in rodents, including the HCR and LCR rats examined here (Figure 2A)(Bjursell et al., 2008; Novak et al., 2006a). It is interesting that the only type of rodent in which measured physical activity does not follow this pattern is the diet-resistant (DR) rat (Novak et al., 2006a), which were bred specifically for resistance to obesity on a high-fat diet (Levin et al., 1997). In mice, spontaneous activity can be suppressed by a calorie-dense diet within a few days after starting the diet and therefore cannot be attributed to a markedly increased body mass (Bjursell et al., 2008). Instead, dietary fat may modulate locomotor activity through direct actions of circulating lipids on the brain (Elder et al., 2008). It has not been established, however, whether it is the dietary fat per se in the calorie-dense Western diet, or another aspect of the diet such as the increased caloric intake, that is the critical factor that modulates locomotor activity. It is notable that exposure to long-term high-fat feeding dampens daily activity to a similar degree in lean HCR and obese LCR (Figure 2A). This suggests that the highly active HCR phenotype is not exempt from the suppressive effects of the high-fat diet on activity long-term. Because LCR gained much more weight on the high-fat diet than HCR (Figure 1A, 2C), but both groups of rats showed similar decreases in activity after a high-fat diet (Figure 2A), it is unlikely that the amount of physical activity alone accounts for the differential propensity for weight gain seen in these rats.
In an attempt to determine the relative contribution of energy intake and expenditure to body weight gain on a high-fat diet, we measured food intake in HCR and LCR. LCR on a high-fat diet consistently consumed more food (measured in kcal/rat/day), and also showed a steady weight gain, compared to the chow-fed LCR (Figures 1, 2D). The HCR, on the other hand, normalized their energy intake within one week. During this time, the high-fat fed HCR did not show any weight gain relative to the chow-fed HCR. This implies that their energy expenditure effectively compensated for the increased energy intake. Activity and energy expenditure were not measured during the first week of high-fat feeding in this study; such information may shed some light on the underlying cause of the differential weight gain in HCR and LCR at the onset of the change in diet, as would a pair-feeding in these rats. Over several weeks, while HCR on a high-fat diet effectively normalized their food intake relative to controls after about one week, high-fat food intake in the LCR remained elevated for nearly the entire duration of the study (Figure 1). This implies that the mechanisms underlying food intake, particularly for the high-fat diet, are different in LCR compared to HCR, and that heightened food intake is likely to contribute to the long-term weight gain in LCR. This could potentially relate to the hedonic or reward value of such a diet (Kelley et al., 2005), and may differ according to diet, obesity, and phenotype (la Fleur et al., 2007). It is likely that several aspects of energy balance are concurrently modified with changes in intrinsic aerobic capacity.
We know that the lean phenotype seen in HCR in associated with high levels of activity, but the question remains as to what extent this activity energy expenditure is a factor in their resistance to obesity. Answering this question in inherently problematic because body mass is by far the most important factor determining energy expenditure during rest and activity, but the influence of body weight is not linear because of the different energy expenditures of different tissues, for example fat and lean mass. In fact, activity energy expenditure is doubtless even more influenced than basal metabolic rate by body mass regardless of its composition (NEAT is accurately conveyed as a direct function of body weight (i.e., load), at least in humans (Schoeller and Jefford, 2002)). While energy expenditure is higher with increasing body mass, correcting or normalizing energy expenditure for body mass also results in an bias, albeit in the opposite direction, even when allometric factors are used in an attempt to mitigate the biasing effect of body weight (Arch et al., 2006; Packard and Boardman, 1999). No universal standard is followed regarding how to express energy expenditure when body weights and composition differ. Moreover, this makes it extremely complicated to parse out the specific contribution to energy balance from NEAT, which accounts for 40% of TDEE in humans, but much less the in smaller animals. To precisely assess NEAT in these rats, we measured it directly by calculating energy expenditure during rest, then EEA during treadmill walking at a constant speed. The HCR had a lower fuel economy of activity than LCR; that is, they expended more energy walking the same distance carrying the same load (Figure 3), implying differences in skeletal muscle work efficiency (Rosenbaum et al., 2005). Thus, HCR not only are more active, but they also expend more calories moving around a given weight compared to LCR. Diet had no detectable effect on fuel economy of activity in these rats. It is interesting that, on the high-fat diet, HCR had a significantly lower RER during activity than did LCR (Figure 3C), indicating that they were more effectively utilizing lipids as a fuel while active. This is consistent with others' findings regarding skeletal muscle fuel use in these rats (Lessard et al., 2009), and reflects an enhanced metabolic flexibility (Galgani and Ravussin, 2008).
How does this relate to leanness in these rats? Thus far, we have identified several traits that are inherent to the lean phenotype (Novak et al., 2009) including endurance capacity and NEAT, which consists of amount of activity and fuel economy of activity. These features are also associated with leanness in humans (Levine et al., 1999; Levine et al., 2005; Novak et al., 2009), suggesting that their underlying mechanisms may be important for maintaining the lean phenotype. Therefore, we investigated the mechanisms underlying these individual differences in physical activity, beginning with brain mechanisms thought to underlie differences and alterations in activity levels.
Brain
To determine how the high-fat diet might alter the brain to affect spontaneous activity, we examined the brain orexin system in HCR and LCR fed different diets. Others and we have demonstrated that obesity-prone rats are less sensitive to the NEAT-promoting effects of central orexin (Novak et al., 2006a; Teske et al., 2006a), implicating sensitivity to central orexin as one factor contributing to differences in NEAT which lead to obesity. Here, we show that on the regular diet, HCR were slightly more sensitive to orexin than were LCR (Figure 4A). After a month on the high-fat diet, though, a more pronounced difference in responsiveness was evident (Figure 4B). The heightened orexin sensitivity seen in HCR on the high-fat diet (Figure 4B) did not translate into a resistance to the high-fat-diet-induced suppression of physical activity, as it did in other lean rats (Figures 2A, 3B)(Teske et al., 2006a). These data do not address potential differences in metabolic efficiency or fuel economy of activity, however. Lastly, orexin has the potential to alter energy expenditure through routes other than physical activity (Cerri and Morrison, 2005; Monda et al., 2007; Wang et al., 2001), which was not measured in these studies.
To probe a mechanism for this differential sensitivity to the locomotor-activating properties of orexin-A, we measured orexin-A levels in the orexin cell body-containing region of the brain. Orexin content in the PeFLH was significantly decreased in rats fed a high-fat diet compared to those on a regular diet, with no differences between HCR and LCR (Figure 4C). This could result from decreased orexin synthesis and/or increased transport and release. Our data are consistent with studies showing no difference in orexin expression in this region in active vs. sedentary rats (Teske et al., 2006a). This implies that the decrease in PeFLH orexin seen in high-fat-fed rats (Figure 4C) may be due to increases in orexin degradation or release rather than decreases in orexin synthesis, though orexin cell number could also differ (Chang et al., 2008). Our findings may also seem inconsistent with studies demonstrating enhancement of orexin expression by exposure to dietary or circulating lipids, either over a few hours or a few weeks (Chang et al., 2004; Wortley et al., 2003). These studies (Chang et al., 2004; Wortley et al., 2003) examined acute, rather than long-term, effects of circulating lipids and high-fat feeding, and also focused on gene expression rather than peptide levels. Unlike other active DR and sedentary DIO rats (Teske et al., 2006b; Thorpe et al., 2005), the HCR and LCR studied here showed no differences in orexin receptor mRNA levels in the PeFLH that might explain the differential sensitivity or responsiveness. In fact, we identified no differences in the expression patterns of several candidate genes that might account for the robust differences in physical activity or orexin sensitivity according to phenotype or diet (Table 7). This included mRNA levels for preproorexin in the PeFLH; OXR1 and 2 in multiple brain regions; PVN corticotrophin-releasing hormone, which increases locomotor activity and interacts with brain orexin (Heinrichs and Koob, 2004; Winsky-Sommerer et al., 2005); and Snark, which interacts with environment to modulate physical activity and energy balance (Ichinoseki-Sekine et al., 2009). These findings led us to examine peripheral factors that could underlie individual and diet-related differences in spontaneous physical activity.
Circulating factors
Leptin levels in the LCR were consistently higher than in HCR (Figure 1D), though HCR had higher leptin levels per gram of body fat. It is noteworthy that there was not a significant effect of diet on leptin levels; in essence, intrinsic aerobic capacity (i.e., the trait used for the artificial selection of HCR vs. LCR) was a stronger predictor of circulating leptin levels than was diet. The HCR were resistant to a diet-induced increase in leptin levels (Figure 1D) even though they had a significant increase in adiposity (Figure 1B). Contrary to others' findings (Noland et al., 2007; Waters et al., 2008), we found no differences in adiponectin or corticosterone between HCR and LCR. This incongruence may be secondary to methodological differences (e.g., time in circadian cycle, fasting vs. fed state, presence of running wheel, presence of stressors) and, in the case of corticosterone, due to high variability in the measurements. While many circulating hormones, including leptin, can affect locomotor activity levels (Choi et al., 2008; Novak and Levine, 2007), our results did not implicate any particular hormone in either the heightened activity seen in HCR or the diet-related decrease in activity seen in all rats.
Skeletal muscle
Because neither circulating hormones nor brain orexin could fully explain the consistent difference in daily activity levels between HCR and LCR, we probed other peripheral systems which modulate daily activity (Novak et al., 2009; Novak and Levine, 2007) and could underlie the differences in activity we observe between HCR and LCR. While exploring these avenues, we observed a robust difference in skeletal muscle PEPCK-C: high PEPCK-C levels and enzymatic activity were associated with high activity and leanness in two strains of inbred rats (Novak et al., 2009) (Figure 5). Moreover, the high-fat diet also decreased skeletal muscle PEPCK in both HCR and LCR (Figure 5). Overall, the relative amount of skeletal muscle PEPCK (Figure 5A) closely resembled daily physical activity levels between groups (Figure 2A). When considering how is skeletal muscle PEPCK-C differentially regulated in these rats, we found it interesting that the same pattern was not found in PEPCK-C mRNA. In fact, quite a different pattern was seen, with PEPCK-C expression higher in the LCR than the HCR in the gastrocnemius (Table 7). This result was unexpected because it is generally accepted that PEPCK levels in liver, the animal tissue in which this enzyme is most thoroughly characterized, is governed through transcriptional regulation.
Daily levels of physical activity can be altered by exercise training; in mice, exercise training enhances skeletal muscle mitochondrial function and also increases daily physical activity (Chow et al., 2007). In people, those with sedentary jobs are less active than those in more active professions, even on their leisure days (McCrady and Levine, 2009). These findings reveal that, though surely influenced by inherited traits (Joosen et al., 2005), spontaneous physical activity and its underlying mechanisms are probably plastic. To ensure that the difference in skeletal muscle PEPCK-C (Figure 5) was not secondary to the increased locomotion in HCR, we limited activity by housing rats in small cages that restricted their locomotor activity. This effectively equalized physical activity levels between HCR and LCR (Figure 5C). Under these conditions, HCR' skeletal muscle still contained more PEPCK than that of LCR (Figure 5D), indicating that this difference is not secondary to hind limb muscle use through locomotion.
Skeletal muscle PEPCK-C has a very interesting potential impact on metabolism (Hakimi et al., 2007; Hanson and Hakimi, 2008; Yang et al., 2009). Mice that overexpress skeletal muscle PEPCK-C show a phenotype much like HCR: they have high endurance, are long-lived, lean, frisky during handling, and resistant to a high-fat diet (Hakimi et al., 2007; Hanson and Hakimi, 2008). Perhaps most interesting from our standpoint, the PEPCK-overexpressing mice are exceptionally active. In fact, their hyperactivity was the defining feature used to distinguish transgenic mice from littermates (Hanson and Hakimi, 2008). The consistently high levels of PEPCK in skeletal muscle in the HCR compared to the LCR seen here (Figure 5A) raises several questions. Are the high muscle PEPCK levels causally related to the high activity levels in the HCR and other similarities between the HCR and the PEPCK-overexpressing mice? If so, is this via its potential effects on skeletal muscle energy flux (Hanson and Hakimi, 2008)? This possibility is supported by heightened energy expenditure of activity found in HCR (Figure 3), as well as the high levels of SIRT-1 found in HCR rat skeletal muscle (Figure 5C). SIRT-1 is a deacetylase that is increased in association with heightened energy expenditure (Feige and Auwerx, 2007; Gates et al., 2007). Through what mechanisms could skeletal muscle affect the brain circuits that underlie locomotor activity levels? One might hypothesize that, because the brains of highly active rats are consistently more sensitive and/or responsive to neuropeptides that stimulate locomotion (Figure 4A, B)(Novak et al., 2006a; Novak et al., 2007; Teske et al., 2006a), a peripheral factor emanating from skeletal muscle could modulate physical activity through its effects on central neuropeptide systems. Other brain systems could also be affected, such as those mediating exercise fatigue (Foley et al., 2006).
In summary, rats selectively bred for high aerobic endurance capacity were consistently more active than rats bred for low endurance, and expended more calories being active as well. A high-fat diet decreased activity similarly in all rats, however. We investigated brain systems that could potentially underlie diet- or endurance-capacity-related differences in physical activity. As with other highly active rats (Novak et al., 2006a; Teske et al., 2006a), sensitivity or responsiveness to central orexin-A was enhanced, but we did not identify the source of this enhanced responsiveness or any other differences in the brain that could potentially underlie the heightened physical activity levels consistently seen in high-endurance rats (Novak et al., 2009). The studies described here further support the possible role for skeletal muscle PEPCK-C in the modulation of daily physical activity. Skeletal muscle PEPCK-C was not directly manipulated and therefore do not imply causal role of skeletal muscle PEPCK-C in the differences in physical activity or other aspects of the HCR phenotype. Taken together with studies in genetically-altered mice (Hakimi et al., 2007), the present results suggest a promising new potential mechanism for individual differences in physical activity. The HCR and LCR provide a physiologically intact platform to investigate the potential role of skeletal muscle PEPCK on spontaneous activity, NEAT, and obesity.
Acknowledgements
This work was supported by AHA 0635113N, NINDS 55859, and a grant from the Minnesota Obesity Consortium to C.M.N.; DK56650, DK63226, DK66270, and R04-0771 to JAL; grants from the American Federation of Ageing Research, AHA, and Mayo Foundation to E.N.C; grant RR17718 from the National Center for Research Resources of the National Institutes of Health (L.G.K. and S.L.B.); the Office of Naval Research Grant N00014-09-0598 and the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. to H.A.; and the Canonical and Translational Science Award UL1RR024986 as well as the University of Medical Rehabilitation Research Training Program 5T32HD007422-19 to P.B. Lastly, we are grateful to Drs. Antonio Nunez and Laura Smale for their helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 2006;30(9):1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- Azzout-Marniche D, Gaudichon C, Blouet C, Bos C, Mathe V, Huneau JF, Tome D. Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1400–1407. doi: 10.1152/ajpregu.00566.2006. [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15(2):315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Gerdin AK, Lelliott CJ, Egecioglu E, Elmgren A, Tornell J, Oscarsson J, Bohlooly YM. Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2008;294(2):E251–260. doi: 10.1152/ajpendo.00401.2007. [DOI] [PubMed] [Google Scholar]
- Britton SL, Koch LG. Animal genetic models for complex traits of physical capacity. Exerc Sport Sci Rev. 2001;29(1):7–14. doi: 10.1097/00003677-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Britton SL, Koch LG, Watson SJ, Akil H. Selective breeding for intrinsic aerobic capacity alters levels of neuropeptides that modulate energy management. Society for Neuroscience; Atlanta, GA: 2006. pp. 565–561. [Google Scholar]
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993;56(4):981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Cerri M, Morrison SF. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience. 2005;135(2):627–638. doi: 10.1016/j.neuroscience.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Chance WT, Dayal R, Friend LA, Thomas I, Sheriff S. Mediation of burn-induced hypermetabolism by CRF receptor-2 activity. Life Sci. 2007;80(11):1064–1072. doi: 10.1016/j.lfs.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28(46):12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145(8):3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Choi YH, Li C, Hartzell DL, Little DE, Della-Fera MA, Baile CA. ICV leptin effects on spontaneous physical activity and feeding behavior in rats. Behav Brain Res. 2008;188(1):100–108. doi: 10.1016/j.bbr.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Chow LS, Greenlund LJ, Asmann YW, Short KR, McCrady SK, Levine JA, Nair KS. Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function. J Appl Physiol. 2007;102(3):1078–1089. doi: 10.1152/japplphysiol.00791.2006. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. Jama. 2007;297(19):2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Dauncey MJ. Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia. 1986;42(5):547–549. doi: 10.1007/BF01946696. [DOI] [PubMed] [Google Scholar]
- Dauncey MJ. Activity and energy expenditure. Can J Physiol Pharmacol. 1990;68(1):17–27. doi: 10.1139/y90-002. [DOI] [PubMed] [Google Scholar]
- Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1987;72(4):549–559. doi: 10.1113/expphysiol.1987.sp003096. [DOI] [PubMed] [Google Scholar]
- Elder GA, Ragnauth A, Dorr N, Franciosi S, Schmeidler J, Haroutunian V, Buxbaum JD. Increased locomotor activity in mice lacking the low-density lipoprotein receptor. Behav Brain Res. 2008;191(2):256–265. doi: 10.1016/j.bbr.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17(6):292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Foley TE, Greenwood BN, Day HE, Koch LG, Britton SL, Fleshner M. Elevated central monoamine receptor mRNA in rats bred for high endurance capacity: Implications for central fatigue. Behav Brain Res. 2006;174(1):132–142. doi: 10.1016/j.bbr.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Galgani J, Ravussin E. Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 2008;32(Suppl 7):S109–119. doi: 10.1038/ijo.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, Malone JP, Townsend RR, Chakravarthy MV, Semenkovich CF. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6(6):497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Goran MI. Estimating energy requirements: regression based prediction equations or multiples of resting metabolic rate. Public Health Nutr. 2005;8(7A):1184–1186. doi: 10.1079/phn2005803. [DOI] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J Biol Chem. 2007;282(45):32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Hakimi P. Born to run; the story of the PEPCK-Cmus mouse. Biochimie. 2008;90(6):838–842. doi: 10.1016/j.biochi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311(2):427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Ichinoseki-Sekine N, Naito H, Tsuchihara K, Kobayashi I, Ogura Y, Kakigi R, Kurosaka M, Fujioka R, Esumi H. Provision of a voluntary exercise environment enhances running activity and prevents obesity in Snark-deficient mice. Am J Physiol Endocrinol Metab. 2009;296(5):E1013–1021. doi: 10.1152/ajpendo.90891.2008. [DOI] [PubMed] [Google Scholar]
- Johannsen DL, Welk GJ, Sharp RL, Flakoll PJ. Differences in daily energy expenditure in lean and obese women: the role of posture allocation. Obesity (Silver Spring) 2008;16(1):34–39. doi: 10.1038/oby.2007.15. [DOI] [PubMed] [Google Scholar]
- Joosen AM, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Am J Clin Nutr. 2005;82(6):1253–1259. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Sarna S. Cigarette smoking, use of alcohol, and leisure-time physical activity among same-sexed adult male twins. Prog Clin Biol Res. 1981;69(Pt C):37–46. [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286(4):E551–559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006;142(1):29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Kruger J, Bowles HR, Jones DA, Ainsworth BE, Kohl HW., 3rd Health-related quality of life, BMI and physical activity among US adults (>/=18 years): National Physical Activity and Weight Loss Survey, 2002. Int J Obes (Lond) 2007a;31(2):321–327. doi: 10.1038/sj.ijo.0803386. [DOI] [PubMed] [Google Scholar]
- Kruger J, Yore MM, Kohl HW., 3rd Leisure-Time Physical Activity Patterns by Weight Control Status: 1999–2002 NHANES. Med Sci Sports Exerc. 2007b;39(5):788–795. doi: 10.1249/mss.0b013e3180333efc. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 2007;31(8):1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Litwin VA, Squibb RL. A simple method for carcass analysis. Physiol Behav. 1972;9(2):281–282. doi: 10.1016/0031-9384(72)90251-x. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, van Denderen BJ, Watt MJ, Koch LG, Britton SL, Kemp BE, Hawley JA. Impaired Skeletal Muscle {beta}-Adrenergic Activation and Lipolysis Are Associated with Whole-Body Insulin Resistance in Rats Bred for Low Intrinsic Exercise Capacity. Endocrinology. 2009 doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273(2 Pt 2):R725–730. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Livingstone MB, Strain JJ, Prentice AM, Coward WA, Nevin GB, Barker ME, Hickey RJ, McKenna PG, Whitehead RG. Potential contribution of leisure activity to the energy expenditure patterns of sedentary populations. Br J Nutr. 1991;65(2):145–155. doi: 10.1079/bjn19910076. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McCrady SK, Levine JA. Sedentariness at Work: How Much Do We Really Sit? Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano E, Messina G, Tafuri D, De Luca V. Sympathetic and hyperthermic reactions by orexin A: role of cerebral catecholaminergic neurons. Regul Pept. 2007;139(1–3):39–44. doi: 10.1016/j.regpep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 2008 doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293(1):E31–41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, Koch LG, Levine JA. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS ONE. 2009;4(6):e5869. doi: 10.1371/journal.pone.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006a;290(2):E396–403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol. 2007;19(12):923–940. doi: 10.1111/j.1365-2826.2007.01606.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Daily Intra-Paraventricular Orexin-A Treatment Induces Weight Loss in Rats. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.91. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA. Neuromedin U in the paraventricular and arcuate hypothalamic nuclei increases non-exercise activity thermogenesis. J Neuroendocrinol. 2006b;18(8):594–601. doi: 10.1111/j.1365-2826.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA. Sensitivity of the hypothalamic paraventricular nucleus to the locomotor-activating effects of neuromedin U in obesity. Brain Res. 2007;1169:57–68. doi: 10.1016/j.brainres.2007.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard GC, Boardman TJ. The misuse of ratios, indices, and percentages in ecophysiological research. Physiol Zool. 1988;61(1):1–9. [Google Scholar]
- Packard GC, Boardman TJ. The use of percentages and size-specific indices to normalize physiological data for variation in body size: wasted time, wasted effort? Comp Biochem Physiol A. 1999;122:37–44. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5 Edition Elsevier Academic Press; 2005. [Google Scholar]
- Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35(1):93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R183–192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Jefford G. Determinants of the energy costs of light activities: inferences for interpreting doubly labeled water data. Int J Obes Relat Metab Disord. 2002;26(1):97–101. doi: 10.1038/sj.ijo.0801851. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87(2):71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A and spontaneous physical activity in obesity resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006a;294(4):R889–899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006b;291(4):R889–899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005:1–9. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou JC, Wade CE. Determinants affecting physical activity levels in animal models. Exp Biol Med (Maywood) 2002;227(8):587–600. doi: 10.1177/153537020222700806. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, De Kloet ER, Van Zoest I, Bohus B. Adrenalectomy reduces exploratory activity in the rat: a specific role of corticosterone. Horm Behav. 1982;16(2):191–198. doi: 10.1016/0018-506x(82)90018-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Osaka T, Inoue S. Energy expenditure by intracerebroventricular administration of orexin to anesthetized rats. Neurosci Lett. 2001;315(1–2):49–52. doi: 10.1016/s0304-3940(01)02322-9. [DOI] [PubMed] [Google Scholar]
- Waters RP, Renner KJ, Pringle RB, Summers CH, Britton SL, Koch LG, Swallow JG. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav. 2008;93(4–5):1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32(3):285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- Woolf K, Reese CE, Mason MP, Beaird LC, Tudor-Locke C, Vaughan LA. Physical activity is associated with risk factors for chronic disease across adult women's life cycle. J Am Diet Assoc. 2008;108(6):948–959. doi: 10.1016/j.jada.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1454–1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331(4):166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284(40):27025–27029. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]