Abstract
Rationale and objective
Studies in laboratory animals strongly suggest reciprocal modulation of the opioidergic and endocannabinoid systems, a relationship that has not been demonstrated in humans. This study sought to clarify this interaction by assessing how a range of naltrexone doses altered the subjective, cognitive, and cardiovascular effects of marijuana.
Material and methods
Daily marijuana smokers (n=29) participated in this within-subject, randomized, double-blind, placebo-controlled study. Naltrexone (0, 12, 25, 50, or 100 mg) was administered before active or inactive marijuana (3.27 or 0% THC) was smoked.
Results
Active marijuana increased subjective ratings of marijuana ‘Strength,’ ‘High,’ and positive subjective ratings of marijuana quality and drug effect including ‘Liking,’ ‘Good,’ and ‘Take Again’ compared to inactive marijuana. Naltrexone alone decreased ratings of ‘Liking,’ ‘Take Again,’ and ‘Stimulated’ compared with placebo, but increased ratings of drug ‘Strength,’ ‘High,’ ‘Good,’ ‘Liking,’ ‘Stimulated,’ and ‘Take Again’ when administered under active marijuana conditions. Active marijuana did not affect performance on cognitive tasks relative to inactive marijuana, whereas naltrexone decreased performance when administered alone or in combination with active marijuana. Active marijuana increased heart rate compared to inactive marijuana under placebo naltrexone conditions. Although naltrexone alone decreased heart rate, it further increased marijuana's cardiovascular effect.
Conclusions
In heavy marijuana smokers opioid-receptor blockade enhanced the subjective and cardiovascular effects of marijuana, suggesting that endogenous opioids dampen cannabinoid effects in this population. These findings demonstrate that a broad range of clinically used doses of naltrexone potentially increases the abuse liability and cardiovascular risks of cannabinoids.
Keywords: Marijuana, THC, Opioid, Cannabinoid, Opioid receptor, Naltrexone
Introduction
The cannabinoid 1 (CB1) receptor directly mediates the behavioral and physiological effects of cannabinoid agonists across species (Fattore et al. 2001; Braida et al. 2004; Vlachou et al. 2005; Järbe et al. 2006; Zangen et al. 2006; Huestis et al. 2007; Justinova et al. 2008). Behavioral studies in non-human animals provide compelling evidence of a bidirectional modulatory relationship between the opioid and cannabinoid systems, and complementary neuroanatomical and molecular data further substantiate the reciprocal modulatory roles that these receptor systems have on one another (Robledo et al. 2008). However, there is growing evidence that in humans the functional relationship between the systems is not as clear as the non-human studies would have predicted (Greenwald and Stitzer 2000; Wachtel and de Wit 2000; Haney et al. 2003; Haney 2007; Lile et al. 2009).
In both rodents and non-human primates, opioidergic modulation of the reinforcing and rewarding effects of delta-9-tetrahydrocannabinol (THC) and synthetic cannabinoids has been demonstrated. Mu-opioid agonists facilitated reinstatement of ‘drug-seeking’ behavior for CB1 agonists in rodents (Spano et al. 2004) and monkeys (Justinova et al. 2008), and opioid antagonists decreased cannabinoid self-administration in rats (Braida et al. 2001; Navarro et al. 2001) and monkeys (Justinova et al. 2004). Similarly, the rewarding effects of THC, as assessed by the conditioned place-preference model, were reversed by administration of the opioid antagonist, naloxone, in rodents (Braida et al. 2004). Contrary to these findings, a clinically utilized dose of the opioid antagonist, naltrexone (50 mg), enhanced the positive subjective and reinforcing effects of oral THC (30 mg) in heavy marijuana smokers. A lower naltrexone dose (12 mg) blocked some of the subjective effects of a lower dose of oral THC (20 mg) in heavy marijuana smokers; whereas this dose of naltrexone enhanced the subjective effects of oral THC in non-marijuana smokers (Haney 2007).
Studies in rodents assessing opioidergic contribution to the discriminative-stimulus effects of cannabinoids showed that the mu-opioid agonist, morphine (Solinas and Goldberg 2005), and the endogenous opioid peptide, beta-endorphin (Solinas et al. 2004), did not substitute for THC in a drug-discrimination paradigm, but both potentiated its discriminative-stimulus effects. Furthermore, naloxone (Solinas et al. 2004) and naltrexone (Solinas and Goldberg 2005) decreased THC's discriminative-stimulus effects. In non-human primates, heroin and morphine did not affect the discriminative-stimulus effects of THC in rhesus monkeys (Li et al. 2008), and the mu-opioid agonist hydromorphone did not substitute for oral THC in humans (Lile et al. 2009). Neither of these studies assessed how opioid antagonists affect the discriminative-stimulus effects of THC; it is therefore difficult to conclude to what extent opioids contribute to the discriminative-stimulus effects of cannabinoids across species.
In terms of cannabinoid dependence, there is also little evidence from the human and non-human primate literature demonstrating opioidergic modulation of this effect. Although a mu-opioid antagonist precipitated withdrawal-like behaviors in rodents treated chronically with THC (Hirschhorn and Rosecrans 1974; Kaymakçalan et al. 1977; Navarro et al. 1998, 2001), this effect was not observed in monkeys chronically treated with THC (Beardsley et al. 1986) or in heavy marijuana smokers (Haney et al. 2003).
The primary objective of this within-subject, placebo-controlled, double-blind study was to clarify the regulatory role of the opioid system on cannabinoid-induced effects in humans. Previous studies investigated a single dose of naltrexone in combination with THC (Haney et al. 2003; Haney 2007); thus, to determine dose-dependent effects of the antagonist, a range of doses was used in the current study. While the study's primary objective was neurobiological in nature, it was designed to also address two clinically relevant issues: a) the potential use of naltrexone for marijuana use disorders and b) the effect that current therapeutic use of naltrexone may have on populations that use marijuana (Haney 2007). Therefore, instead of testing oral THC as was done in previous studies, the effects of smoked marijuana were assessed in the current study. The subjective, performance, and physiological effects of marijuana (0.0, 3.27% THC) under placebo and active naltrexone (12, 25, 50, and 100 mg) conditions were measured. To control for drug history, tolerance, and dependence, only heavy marijuana smokers identified as smoking at least four times a week were eligible to participate in the study.
Methods
Participants
Volunteers ages 21–45 years were recruited through newspaper advertisements, and those who met inclusion/exclusion criteria after an initial phone screen were invited to the laboratory for further screening. Prior to enrollment, participants gave written informed consent, received a psychiatric and medical evaluation, and provided a detailed drug use and medical history. Participants were accepted into the study if they were healthy, as determined by a physical examination, electrocardiogram, and urine and blood chemistries. All participants had to currently smoke three marijuana cigarettes at least four times a week for the previous four weeks before screening as determined by urine toxicology and self-report. Volunteers were excluded if they repeatedly used other drugs, with the exception of nicotine, alcohol, or caffeine as determined by urine toxicology and self-report. Those who met the Diagnostic and Statistical Manual (of Mental Disorders), fourth edition, revised criteria for current or past Axis I psychopathology were also excluded from the study. Females were excluded if they were pregnant or nursing. Current use of over-the-counter or prescription medication was also exclusionary. Volunteers were told that (1) the study objective was to determine the effects of commonly prescribed medications on marijuana's effects on mood and physiology, (2) they would receive a capsule containing placebo or one of three medications listed on the consent form, and (3) the capsule would be followed by a cigarette containing active or inactive marijuana, which they would smoke according to instructions from the research staff. Participants were admitted into the study only after written informed consent to participate was given and eligibility criteria were verified. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute and were in accord with the Declaration of Helsinki.
Table 1 describes the demographic characteristic of the 29 participants who completed the study (15 males and 14 females). An additional 20 volunteers enrolled but did not complete the study. Of the 20 that discontinued, seven disliked the medication side effects (one reported drowsiness and six reported gastrointestinal upset including nausea and vomiting), six lost contact, two were noncompliant, and five discontinued for personal reasons (schedule conflict, legal and family issues).
Table 1.
Demographic characteristics of study participants
| Age (years old) | 28±1 |
| Sex (F/M) | 14/15 |
| Race (B/W/M) | 21/6/2 |
| Marijuana | |
| Years regular use | 9.0±1.0 |
| Days/week | 6.7±0.1 |
| $/week | 83.2±13.1 |
| MJ cigarettes/day | 8.3±1.2 |
| Alcohol | |
| % population use | 76.0 |
| Days/week | 1.4±0.3 |
| Standard drinks/occasion | 2.8±0.4 |
| Tobacco | |
| % population use | 83.0 |
| Cigarettes/day | 6.8±0.9 |
Data are presented as means (±SD) or as frequency. Sex is indicated as female (F) and male (M), and race is indicated as Black (B), White (W), and Mixed (M). Two participants reported smoking marijuana in a pipe. Because the size of the pipe was unknown, the equivalent number of marijuana cigarettes per day could not be determined for these two individuals
Design and procedures
The study included 10 outpatient sessions over the course of 4–6 weeks at the New York State Psychiatric Institute. Sessions, which were separated by at least 48 h to prevent medication carryover effects, began around 9 AM and were 5 h in duration. Before study onset, participants were familiarized with computerized tasks and study procedures with 1–2 training sessions, during which, naltrexone and marijuana were not administered.
During each session, one capsule containing placebo or naltrexone (12, 25, 50, and 100 mg ReVia; Dupont Pharma) was administered to the participant in a size 00 opaque capsules with lactose filler, prepared by the New York State Psychiatric Institute Research Pharmacy. A marijuana cigarette (0 or 3.27% THC; ca. 800 mg) provided by the National Institute on Drug Abuse was smoked 45 min after naltrexone administration, the time at which naltrexone levels peak (Wall et al. 1981). Cigarettes were stored frozen in an airtight container and humidified at room temperature for 24 h prior to the session. A within-subject design was used in which all participants received all strengths of naltrexone in combination with active and inactive marijuana. The order of dosing was randomized.
Experimental session
Participants were instructed to not eat breakfast before each session and to not drink alcohol or smoke marijuana or cigarettes after midnight the night before each session. Upon arrival to the laboratory, carbon monoxide levels were measured to confirm no recent smoking, breath alcohol levels were assessed, and use of illicit drugs other than marijuana was determined by a urine toxicology screen (please refer to Table 2 for schedule of session events). If carbon monoxide levels indicated that the participant had smoked marijuana or a cigarette prior to arrival (8 ppm or higher) the session did not proceed, and the volunteer was sent home. Pregnancy tests were also run before the first and fifth sessions for female participants. A standardized breakfast was provided prior to the session.
Table 2.
Session events
| Time (min) | Events |
|---|---|
| −75 | Urine toxicology screen, carbon monoxide levels, balance task |
| −60 | Vitals, Task Battery, SE-VAS |
| −45 | Capsule administration |
| −15 | CRF |
| 0 | Marijuana smoked |
| 15 | Vitals, MRF, SE-VAS |
| 30 | Vitals, Task Battery, MRF, SE-VAS |
| 60 | Vitals, MRF, SE-VAS, CRF |
| 75 | Vitals, Task Battery, MRF, SE-VAS |
| 120 | Vitals, MRF, SE-VAS |
| 180 | Vitals, Task Battery, MRF, SE-VAS, CRF |
| 210 | Vitals, SE-VAS |
Vitals, Heart rate and blood pressure; Task Battery, repeated acquisition task, DAT, DSST, RIT, and DRT; SE-VAS, Subjective Effect-Visual Analog Scale; CRF, capsule rating form; MRF, marijuana rating form
Before drug administration, the subjective-effects questionnaires and performance tasks were completed, and heart rate and blood pressure were measured using a Sentry II vital signs monitor (Model 6100: NBS Medical Services, Costa Mesa CA). After capsule administration, a marijuana cigarette was smoked according to a cued-smoking procedure that has been shown to produce reliable increases in heart rate and plasma THC levels (Foltin et al. 1987). During the smoking procedure, the experimenter observed the participant behind a one-way mirror and instructed participants through an intercom to ‘inhale’ (5 s), ‘hold smoke in lungs’ (10 s), and ‘exhale’. Participants continued to smoke according to this procedure until 70% of the cigarette was pyrolized (3–7 puffs) with a 40 s interval between puffs. Vital signs (heart rate and blood pressure) were monitored throughout the session. A cognitive task battery and subjective ratings of mood and drug effect were also completed at specified time points after smoking. Timing of each measurement was scheduled to capture the full time course of naltrexone and marijuana effects and to allow for consistent intervals between each event. Cigarette smokers were permitted to smoke at predetermined intervals throughout the session in order to minimize nicotine withdrawal symptoms. At the end of each session (about 3 h after smoking), participants were free to go home once sobriety was determined using field sobriety and balancing tasks.
Subjective-effects questionnaires and performance tasks
All subjective effects were measured using visual analog scales (VAS), a series of 100 mm long lines labeled ‘not at all’ at one end (0 mm) and ‘extremely’ at the other end (100 mm). Participants were instructed to rate their subjective experiences on the line according to how they felt at that particular moment.
Marijuana rating form
Subjective marijuana-related drug effects were assessed using a 5-item VAS asking participants to rate the strength of the drug effect, good effect, bad effect, drug liking, and willingness to take the drug again.
Subjective Effect-Visual Analog Scale (SE-VAS)
Participants were asked to rate their mood and physical symptoms on a modified 44-item VAS intended to measure affective and physical subjective drug effects (see Haney et al. 1999 for description of the original 50-question version).
Capsule rating form
Subjective capsule-related drug effects were assessed using a 5-item VAS asking participants to rate the strength of the drug effect, good effect, bad effect, drug liking, and willingness to take the drug again. Participants were also asked to indicate whether they thought the capsule contained placebo or active medication.
Task battery
Task batteries consisted of a 3-min repeated acquisition task, 10-min divided attention task (DAT), 3-min digit-symbol substitution task (DSST), and an immediate and delayed digit-recall task (DRT). The battery was designed to measure attention, psychomotor ability, learning, and memory (Foltin et al. 1993; Haney et al. 1997; Hart et al. 2001). Briefly, for the repeated acquisition task, four buttons corresponding to positions on the keypad were illuminated on the computer screen, and participants were required to learn and then enter a 10-response sequence as quickly as possible in the given time limit. The DAT required the participants to track a moving target on a computer screen using a mouse while signaling when a brief stimulus appeared in one of the four corners. Accurate tracking of the target increased its speed. The DSST measured psychomotor performance by presenting nine 3 × 3 matrices of blocks, with a single blackened square in each row; below each matrix was an identifying number (1–9). A number appeared at the bottom of the screen indicating to the participant which pattern of highlighted boxes from the above matrices should be replicated using a 9-key keypad as accurately and quickly as possible in the given time limit. The DRT measured both immediate and delayed recall by displaying an 8-digit sequence on a computer screen, which the participant was required to enter accurately when the number was on the screen and when it disappeared (immediate recall). Participants were then asked to recall and recognize one of the sequences at the end of the task battery (delayed recall/recognition).
Data analysis
Repeated measures analysis of variance (ANOVA) with planned comparisons was used to assess naltrexone's intrinsic effects and its effect on marijuana's subjective, cognitive, and cardiovascular effects. There were with three within-group factors (naltrexone dose, marijuana strength, and time point). Dependent variables included subjective measures, as assessed with the CRF, MRF, and SE-VAS scales, heart rate, blood pressure, and task battery endpoints (refer to Foltin et al. 1993 and Haney et al. 1997). For each dependent measure, nine planned comparisons were completed. Marijuana's effects were determined by comparing the active and inactive marijuana conditions when paired with the placebo naltrexone condition (one comparison). Naltrexone's intrinsic effects were assessed by comparing placebo and each active dose of naltrexone (12, 25, 50, and 100 mg) under the inactive marijuana condition (four comparisons). Finally, the active marijuana–placebo naltrexone condition was compared to the active marijuana–active naltrexone conditions (four comparisons). Results were considered statistically significant when p values were equal to or less than 0.01 using Huynh–Feldt corrections.
Results
Demographic characteristics
Table 1 describes the demographic information of the participants who completed the study.
Subjective ratings
Marijuana ‘Strength’ and ‘ High’
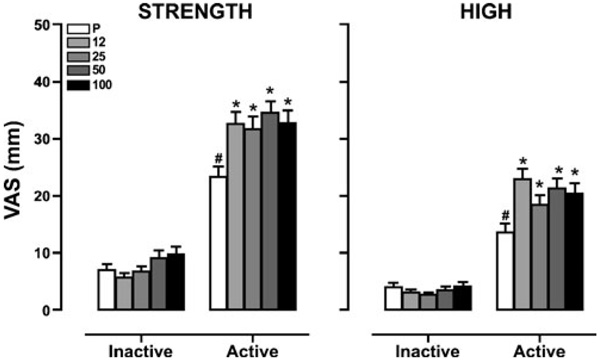
Figure 1 depicts subjective ratings of marijuana ‘Strength’ and ‘High’ as a function of naltrexone dose and marijuana strength. Naltrexone alone had no effect on subjective ratings of ‘Strength’ and ‘High’ compared to placebo, whereas active marijuana alone increased these ratings compared to inactive marijuana (p≤0.01). All doses of naltrexone increased the effects of active marijuana on these ratings compared to placebo (p≤0.01).
Figure 1.
Subjective ratings of marijuana ‘Strength’ and ‘High’ as a function of naltrexone dose ( , placebo;
, placebo;  , 12 mg;
, 12 mg;  , 25 mg;
, 25 mg;  , 50 mg;
, 50 mg;  , 100 mg) and marijuana strength (0 mm=‘not at all’; 100 mm=‘extremely’). Data are presented as average values across all post-smoking time points. Differences in ratings between active and inactive marijuana under placebo naltrexone conditions determined from average post-smoking time points are indicated as #, p≤0.01, and differences between naltrexone and placebo under inactive and active marijuana conditions determined from average post-smoking time points are indicated as *, p≤0.01
, 100 mg) and marijuana strength (0 mm=‘not at all’; 100 mm=‘extremely’). Data are presented as average values across all post-smoking time points. Differences in ratings between active and inactive marijuana under placebo naltrexone conditions determined from average post-smoking time points are indicated as #, p≤0.01, and differences between naltrexone and placebo under inactive and active marijuana conditions determined from average post-smoking time points are indicated as *, p≤0.01
Positive subjective drug effects
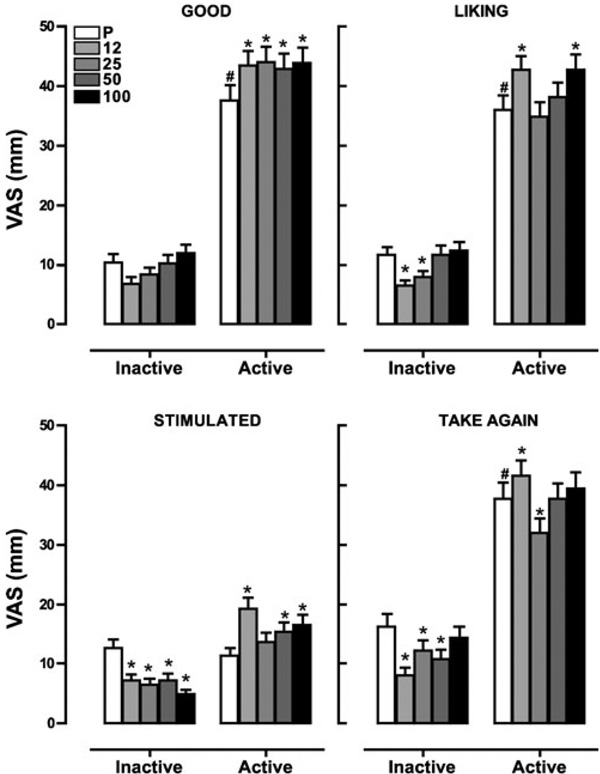
Figure 2 portrays ratings of positive subjective drug effects including ‘Good Effect,’ ‘Liking,’ ‘Stimulated,’ and ‘Take Again’ as a function of naltrexone dose and marijuana strength. Under inactive marijuana conditions, naltrexone alone decreased ratings of ‘Liking’ (12 and 25 mg, p≤0.01), ‘Stimulated’ (12, 25, 50, and 100 mg, p≤0.01), and ‘Take Again’ (12, 25, and 50 mg, p≤0.01) compared with placebo. Active marijuana alone increased ratings of ‘Good Effect,’ ‘Liking,’ and ‘Take Again’ compared with inactive marijuana (p≤0.01) but had no effect on ratings of ‘Stimulated.’ When naltrexone was combined with active marijuana, increased subjective ratings of ‘Good Effect’ (12, 25, 50, and 100; p≤0.01), ‘Liking’ (12 and 100 mg; p≤0.01), ‘Stimulated’ (12, 50, 100; p≤0.01), and ‘Take Again’ (12 mg, p≤0.01) were observed relative to placebo naltrexone. However, a single dose of naltrexone decreased ratings of ‘Take Again’ compared to placebo under active marijuana conditions (25 mg, p≤0.01).
Figure 2.
Subjective ratings of ‘Good,’ ‘Liking,’ ‘Stimulated,’ and ‘Take Again’ as a function of naltrexone dose and marijuana strength. Data are presented as average values across all post-smoking time points. Differences in ratings are indicated as explained in the figure legend for Fig. 1
Capsule rating form
Changes in subjective ratings of capsule effects under naltrexone alone, active marijuana alone, and the combination of naltrexone and active marijuana were small in magnitude and not likely to be clinically relevant (mean values <15 mm).
Performance effects
Table 3 depicts the effects of marijuana, naltrexone, and the combination of the two drugs on attention, psychomotor function, and delayed recall as measured by performance on the DAT, DSST, and DRT tasks. Naltrexone and marijuana did not affect performance on the repeated acquisition task. However, naltrexone alone impaired vigilance, defined on the DAT by the average speed of the moving target to be tracked by the participant (12, 25, 50, and 100 mg, p≤0.01), accuracy of psychomotor performance, reflected in decreased number of trials completed during the DSST (100 mg, p≤0.01), and delayed recognition on the DRT (25 mg, p≤0.01) compared with placebo. Although active marijuana alone had no effect on task performance, naltrexone combined with marijuana decreased psychomotor performance on the DSST relative to placebo (50 and 100 mg, p≤0.01).
Table 3.
Performance on selected psychomotor and cognitive tasks (mean±SEM) as a function of placebo, naltrexone dose, and marijuana strength
| Naltrexone dose | ||||||
|---|---|---|---|---|---|---|
| Variable | MJ strength | 0 mg | 12 mg | 25 mg | 50 mg | 100 mg |
| DAT: average speed (cm/s) | Inactive | 7.3 (0.2) | 6.6 (0.2)* | 6.9 (0.2)* | 6.7 (0.2)* | 7.0 (0.2)* |
| Active | 7.0 (0.2) | 7.0 (0.2) | 7.1 (0.2) | 6.9 (0.2) | 6.9 (0.2) | |
| DSST:Total attempts | Inactive | 86.2 (1.8) | 86.7 (1.7) | 83.4 (1.7) | 83.6 (1.8) | 82.1 (1.6)* |
| Active | 86.1 (1.7) | 84.7 (1.6) | 83.5 (1.6) | 82.1 (1.4)* | 83.0 (1.7)* | |
| DRT: delayed recognition (% correct) | Inactive | 66.7 (5.1) | 57.5 (5.3) | 49.4 (5.4)* | 60.9 (5.3) | 63.2 (5.2) |
| Active | 66.7 (5.1) | 57.5 (5.3) | 57.5 (5.3) | 59.8 (5.3)* | 66.7 (5.1) | |
Data are presented as average values (±SEM) across post-smoking time points (30, 75, and 180 min after smoking). Significant differences between active and placebo naltrexone are indicated (*p≤0.01)
Cardiovascular effects
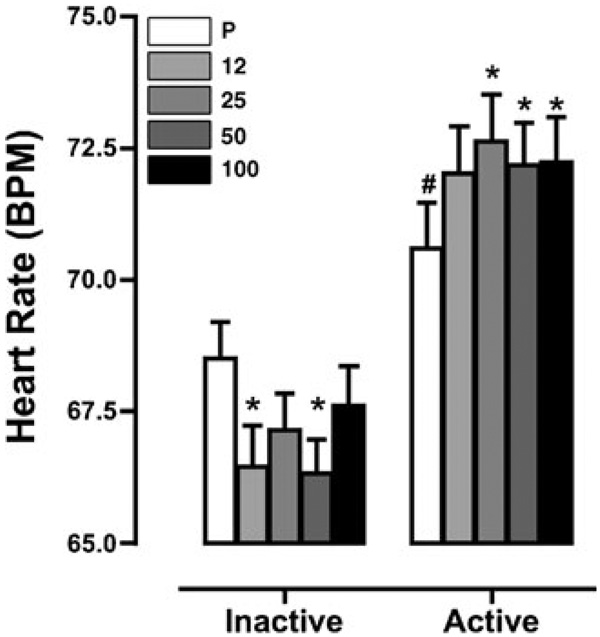
Figure 3 portrays changes in heart rate as a function of naltrexone dose and marijuana strength. Naltrexone alone decreased heart rate (12 and 50 mg, p≤0.01) compared with placebo, whereas active marijuana alone increased heart rate (p≤0.01) compared with inactive marijuana. Naltrexone administered under active marijuana conditions further increased heart rate compared to placebo (25, 50, and 100 mg, p≤0.01).
Figure 3.
Heart rate as a function of naltrexone dose and marijuana strength. Data are presented as average values across all post-smoking time points. Differences in ratings are indicated as explained in the figure legend for Fig. 1
Discussion
The present study was designed to assess endogenous opioid modulation of the subjective, cognitive, and cardiovascular effects of marijuana by blocking the opioid receptor with naltrexone. The results reported herein demonstrate that opioid receptor blockade enhanced cannabinoid effects in heavy marijuana smokers. Naltrexone-induced potentiation of marijuana's effects, though not dose-dependent, was particularly striking given that naltrexone and active marijuana produced predominantly opposing effects when administered independently. That is, naltrexone alone tended to decrease measures of abuse liability (drug liking, willingness to take again, stimulated) and heart rate. Yet, naltrexone consistently enhanced these same ratings and heart rate when combined with marijuana.
The findings from the current study build upon previous reports demonstrating the potentiation of oral THC (30 mg) effects by naltrexone administration (50 mg) in heavy marijuana smokers (Haney et al. 2003). However, a later study found that a lower dose of naltrexone (12 mg), which was hypothesized to be more selective for the mu-opioid receptor, effectively blocked the positive subjective ratings of low (20 mg) but not high doses (40 mg) of oral THC (Haney 2007). From these two studies, it was concluded that the higher dose of naltrexone (50 mg) enhanced oral THC's effects through a non-mu-opioid mediated mechanism. The current study, which was designed to directly investigate the dose-dependency of naltrexone's effects on smoked marijuana across a wide range of doses (12–100 mg), showed that all doses of naltrexone enhanced marijuana's effects. This suggests that the endogenous opioid contribution to smoked marijuana's effects (3.27%) are likely mediated by the mu-opioid receptor, as these effects were blocked by a low mu-selective dose of naltrexone as well as for higher less opioid-selective naltrexone doses. Testing lower naltrexone doses than those used in the current study would help to determine if the observed enhancement of marijuana's effects is due to a competitive interaction between naltrexone and endogenous opioids.
Naltrexone-induced potentiation of marijuana's subjective and cardiovascular effects as observed in the current study strongly suggests that cannabinoid-induced endogenous opioid release dampens marijuana's effects in heavy smokers. These findings seem incongruent with reports from animal studies investigating opioidergic modulation of the cannabinoid system. However, there is some ambiguity regarding the bidirectional modulation of the cannabinoid and opioid systems in these data. As discussed in the “Introduction”, opioid agonists do not share discriminative-stimulus effects with CB1 agonists in rodents, monkeys, or humans (Solinas and Goldberg 2005; Li et al. 2008; Lile et al. 2009). Opioidergic modulation of the reinforcing effects of CB1 agonists has been demonstrated in rodents (Navarro et al. 2001) and monkeys (Justinova et al. 2004) by naltrexone-induced attenuation of CB1 agonist self-administration. Yet, in rodents, this evidence is derived from naltrexone-induced antagonism of the reinforcing effects of a single dose of CB1 agonist; thus, it is unknown if mu-opioid antagonism shifts the dose-effect function of CB1 agonist self-administration to the left or right. A shift to the left would indicate increasing sensitivity to the reinforcing effects of the agonist, whereas a rightward shift would demonstrate antagonism (Navarro et al. 2001). Similarly, in non-human primates, naltrexone (0.1 mg/kg) flattened, rather than shifted, the dose–response curve for THC self-administration by decreasing responding for the dose of THC that maintained peak responding (4.0 µg/kg/infusion) to rates maintained by the two doses that comprised the ascending and descending limbs of the dose–response function (2.0 and 8.0 µg/kg/infusion). Furthermore, higher and lower doses of naltrexone (0.03 and 0.30 mg/kg) did not affect THC self-administration (Justinova et al. 2004). Finally, the same dose of naltrexone that flattened the dose–response curve for THC self-administration also decreased THC self-administration using a drug-seeking (second-order) schedule of reinforcement. This was a robust but transient effect (Justinova et al. 2008). In marijuana smokers, naltrexone tended to increase self-administration of a high dose of THC and increased subjective effects of THC (Haney et al. 2003). Taking these data together with the currently described findings, it is clear that the opioid system is indeed involved in modulating the reinforcing and subjective effects of cannabinoids. However, the nature of this relationship likely varies across a range of experimental variables including population studied (heavy versus light marijuana smokers), route of administration (oral THC versus smoked marijuana), and naltrexone dose.
Future directions and limitations
Given that a wide range of naltrexone doses generally increased marijuana-induced subjective and cardiovascular effects, it is apparent that in heavy marijuana smokers, blocking the mu-opioid receptor likely shifts the dose–response curve for many cannabinoid effects to the left. That the study was carried out with a study population that was nearly equivalent in the number of males and females increases the generalizability of the results. Testing additional active strengths of marijuana, specifically lower strengths than tested in the current study, would provide a more comprehensive characterization of the nature of this interaction. Also, although findings from this study suggest that naltrexone enhances the abuse potential of marijuana in heavy marijuana smokers, directly assessing naltrexone's effects on marijuana self-administration would elucidate the effects of opioid-blockade on marijuana's abuse liability. Investigating how naltrexone alters cannabinoid effects in non-marijuana smokers would be informative in understanding how much of the observed effects may be due to neurobiological adaptations as a consequence of chronic exposure to cannabinoids. Finally, because naltrexone is administered chronically to treat alcohol and opioid-dependent patients, the current findings also raise questions regarding the effects of chronic naltrexone administration on the abuse liability of marijuana in these populations.
Conclusion
The results from this study demonstrate that the opioid system contributes an integral component to the subjective experience of marijuana intoxication in heavy marijuana smokers. By blocking potential input from this system with naltrexone, the subjective experience and cardiovascular effects of marijuana are enhanced. To further investigate the clinical implications of opioid-receptor contributions to marijuana's subjective effects, naltrexone's effects on marijuana self-administration should be explored.
Acknowledgments
This research was supported by US National Institute on Drug Abuse Grant DA09236. The authors acknowledge and appreciate the exceptional assistance of Roxanne McMorris, Divya Lakhaney and Elyssa Berg in data collection, and Richard Foltin and Gillinder Bedi for statistical assistance.
Contributor Information
Ziva D. Cooper, Email: zc2160@columbia.edu.
Margaret Haney, Email: mh235@columbia.edu.
References
- Beardsley PM, Balster RL, Harris LS. Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther. 1986;239:311–319. [PubMed] [Google Scholar]
- Braida D, Pozzi M, Cavallini R, Sala M. Conditioned place preference induced by the cannabinoid agonist CP 55, 940: interaction with the opioid system. Neuroscience. 2001;104:923–926. doi: 10.1016/s0306-4522(01)00210-x. [DOI] [PubMed] [Google Scholar]
- Braida D, Iosuè S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55, 212-2 in rats. Psychopharmacology. 2001;156:410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Brady JV, Fischman MW, Emurian CS, Dominitz J. Effects of smoked marijuana on social interaction in small groups. Drug Alcohol Depend. 1987;20:87–93. doi: 10.1016/0376-8716(87)90079-2. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pippen PA, Kelly TH. Behavioral effects of cocaine alone and in combination with ethanol or marijuana in humans. Drug Alcohol Depend. 1993;32:93–106. doi: 10.1016/0376-8716(93)80001-u. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Factors influencing marijuana self-administration by humans. Behav Pharmacol. 1997;8:101–112. [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology. 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Hart CL, van Gorp WG, Haney M, Foltin RW, Fischman MW. Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology. 2001;29:158–170. doi: 10.1016/S0893-133X(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Hirschhorn ID, Rosecrans JA. Morphine and delta 9-tetrahydrocannabinol: tolerance to the stimulus effects. Psychopharmacologia. 1974;36:243–253. doi: 10.1007/BF00421806. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heischman SJ, Preston KL, Bonnet D, Le Fur G, Gorelick DA. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology. 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A. Antagonism of discriminative stimulus effects of Δ9-THC and (R)-methanandamide in rats. Psychopharmacology. 2006;184:36–45. doi: 10.1007/s00213-005-0225-y. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology. 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacology. 2008;33:2870–2877. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymakçalan S, Ayhan IH, Tulunay FC. Naloxone-induced or postwithdrawal abstinence signs in delta 9-tetrahydrocannabinol-tolerant rats. Psychopharmacology. 1977;55:243–249. doi: 10.1007/BF00497855. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between Delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology. 2008;199:199–208. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Delta9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Delta9-tetrahydrocannabinol. Psychopharmacology. 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Chowen J, Rocío A, Carrera M, del Arco I, Villanúa MA, Martin Y, Roberts AJ, Koob GF, de Fonseca FR. CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MR, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gomez R, del Arco I, Villanua MA, Maldonado R, Koob GF, Rodriguez de Fonseca F. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Berrendero F, Ozaita A, Maldonado R. Advances in the field of cannabinoid-opioid cross-talk. Addict Biol. 2008;13:213–224. doi: 10.1111/j.1369-1600.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;11:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thirlet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9 tetrahydrocannabinol. Eur J Neurosci. 2004;19:3183–3192. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br J Pharmacol. 2004;143:343–350. doi: 10.1038/sj.bjp.0705932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology. 2005;179:498–508. doi: 10.1007/s00213-004-2050-0. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Naltrexone does not block the subjective effects of oral Delta(9)-tetrahydrocannabinol in humans. Drug Alcohol Depend. 2000;59:251–260. doi: 10.1016/s0376-8716(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Wall ME, Brine DR, Perez-Reyes M. Metabolism and disposition of naltrexone in man after oral and intravenous administration. Drug Metab Dispos. 1981;9:369–375. [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]