Abstract
The role of the neutrophilic leukocyte in wound healing was investigated by observing the progress of repair in the absence of these cells. Circulating neutrophils were eliminated in guinea pigs by the administration of antineutrophil serum (ANS) 24 hr before wounding and by daily injections throughout a 10 day period of healing. Control animals received normal rabbit serum at the same dose levels and times. The wounds consisted of six linear incisions in the dorsal skin of the animals.
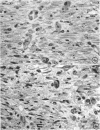
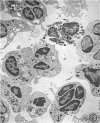
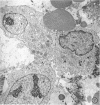
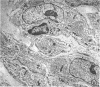
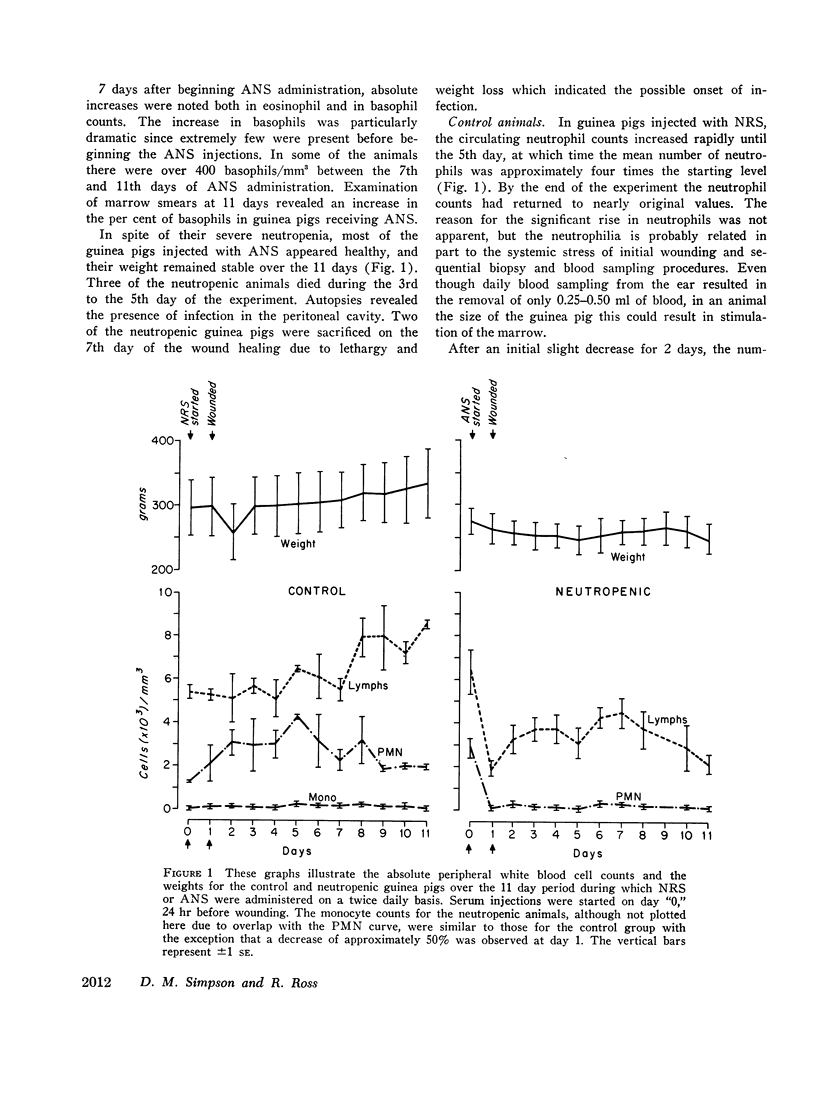
The contents of 24-hr neutropenic and control wounds were compared by quantitating the major cellular and extracellular wound components using a histometric technique. At 24 hr, there were no differences between control and neutropenic wounds in the per cent of total wound volume occupied by mononuclear leukocytes and fibrin. The neutropenic wounds had no neutrophils, had a significantly decreased volume of fluid space, and an increased volume of red cells, as compared with controls. The differences in numbers of erythrocytes and amount of fluid space in these two sets of wounds may be related to substances within neutrophils that promote lysis of erythrocytes or affect vascular permeability.
In spite of the lack of neutrophils in the ANS-treated animals during the 10 days of healing, no differences were observed between the control and neutropenic wounds relative to the rate of wound debridement or the extent of repair. The wounds from the two groups of animals were identical in cellularity and degree of connective tissue formation.
These observations support the notion that neither wound debridement nor the formation of granulation tissue are dependent upon the presence of neutrophils. A neutrophil response in early wounds is not an essential antecedent to the infiltration of monocytes, as suggested by previous investigations.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Bossert J. E., McClellan M. A., Altemeier W. A. Stimulants of cellular proliferation in wounds. Arch Surg. 1971 Aug;103(2):167–174. doi: 10.1001/archsurg.1971.01350080083012. [DOI] [PubMed] [Google Scholar]
- CHAN B. S., YOFFEY J. M. The basophil cells of guinea-pig bone marrow and their response to horse serum. Immunology. 1960 Jul;3:237–243. [PMC free article] [PubMed] [Google Scholar]
- Calnan J., Davies A. The effect of methotrexate (amethopterin) on wound healing: an experimental study. Br J Cancer. 1965 Sep;19(3):505–512. doi: 10.1038/bjc.1965.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor C. W., Yaron M. Leukocyte-connective tissue cell interaction. II. The specificity, duration, and mechanism of interaction effects. Arthritis Rheum. 1969 Aug;12(4):374–386. doi: 10.1002/art.1780120405. [DOI] [PubMed] [Google Scholar]
- DUNPHY J. E., UDUPA K. N. Chemical and histochemical sequences in the normal healing of wounds. N Engl J Med. 1955 Nov 17;253(20):847–851. doi: 10.1056/NEJM195511172532002. [DOI] [PubMed] [Google Scholar]
- Dale D. C., Wolff S. M. Skin window studies of the acute inflammatory responses of neutropenic patients. Blood. 1971 Aug;38(2):138–142. [PubMed] [Google Scholar]
- EDWARDS L. C., DUNPHY J. E. Wound healing. I. Injury and normal repair. N Engl J Med. 1958 Jul 31;259(5):224–233. doi: 10.1056/NEJM195807312590506. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. The mechanism of Arthus reactions. II. The role of polymorphonuclear leucocytes and platelets in reversed passive reactions in the guinea-pig. Br J Exp Pathol. 1955 Jun;36(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Cellular mediators of immunological tissue injury. J Reticuloendothel Soc. 1970 Aug;8(2):124–138. [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971 Apr 1;133(4):885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- Janoff A. Mediators of tissue damage in leukocyte lysosomes. X. Further studies on human granulocyte elastase. Lab Invest. 1970 Mar;22(3):228–236. [PubMed] [Google Scholar]
- Janoff A., Schaefer S., Scherer J., Bean M. A. Mediators of inflammation in leukocyte lysosomes. II. Mechanism of action of lysosomal cationic protein upon vascular permeability in the rat. J Exp Med. 1965 Nov 1;122(5):841–851. doi: 10.1084/jem.122.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Zeligs J. D. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science. 1968 Aug 16;161(3842):702–704. doi: 10.1126/science.161.3842.702. [DOI] [PubMed] [Google Scholar]
- Lawrence J. S., Craddock C. G., Jr, Campbell T. N. Antineutrophilic serum, its use in studies of white blood cell dynamics. J Lab Clin Med. 1967 Jan;69(1):88–101. [PubMed] [Google Scholar]
- Lazarus G. S., Brown R. S., Daniels J. R., Fullmer H. M. Human granulocyte collagenase. Science. 1968 Mar 29;159(3822):1483–1485. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- Miller G. L., Wilson J. E. A study of variables in the assay of cytotoxic antisera. J Immunol. 1968 Nov;101(5):1068–1073. [PubMed] [Google Scholar]
- PAGE A. R., GOOD R. A. A clinical and experimental study of the function of neutrophils in the inflammatory response. Am J Pathol. 1958 Jul-Aug;34(4):645–669. [PMC free article] [PubMed] [Google Scholar]
- ROSS R., BENDITT E. P. Wound healing and collagen formation. I. Sequential changes in components of guinea pig skin wounds observed in the electron microscope. J Biophys Biochem Cytol. 1961 Dec;11:677–700. doi: 10.1083/jcb.11.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranadive N. S., Cochrane C. G. Isolation and characterization of permeability factors from rabbit neutrophils. J Exp Med. 1968 Oct 1;128(4):605–622. doi: 10.1084/jem.128.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Odland G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J Cell Biol. 1968 Oct;39(1):152–168. doi: 10.1083/jcb.39.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STALEY C. J., KUKRAL J. C., PRESTON F. W. Effect of anticancer drugs on experimental wound healing. Surg Forum. 1962;13:35–36. [PubMed] [Google Scholar]
- Scott R. E., Horn R. G. Ultrastructural aspects of neutrophil granulocyte development in humans. Lab Invest. 1970 Aug;23(2):202–215. [PubMed] [Google Scholar]
- Selye H., Cunnington J., Somogyi A., Côté G. Acceleration and inhibition of wound healing by topical treatment with different types of inflammatory irritants. Am J Surg. 1969 May;117(5):610–614. doi: 10.1016/0002-9610(69)90391-2. [DOI] [PubMed] [Google Scholar]
- Stein J. M., Levenson S. M. Effect of the inflammatory reaction on subsequent wound healing. Surg Forum. 1966;17:484–485. [PubMed] [Google Scholar]
- Van Winkle W., Jr The tensile strength of wounds and factors that influence it. Surg Gynecol Obstet. 1969 Oct;129(4):819–842. [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I., Krakauer K. Arthritis induced in rabbits by lysates of granulocyte lysosomes. Arthritis Rheum. 1969 Apr;12(2):103–116. doi: 10.1002/art.1780120207. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Coote E., Turk J. L. Complement in acute inflammation. J Pathol. 1969 Feb;97(2):295–305. doi: 10.1002/path.1710970215. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Spector W. G. Inflammation in agranulocytotic rats. Nature. 1968 Sep 21;219(5160):1258–1258. doi: 10.1038/2191258a0. [DOI] [PubMed] [Google Scholar]