Abstract
Epidemiological studies report a high prevalence of type 2 diabetes and metabolic syndrome in the island nation of Mauritius. The Mauritius Family Study was initiated to examine heritable factors that contribute to these high rates of prevalence and consists of 400 individuals in 24 large extended multigenerational pedigrees. Anthropometric and biochemical measurements relating to the metabolic syndrome were undertaken in addition to family and lifestyle based information for each individual. Variance components methods were used to determine the heritability of the type 2 diabetes and metabolic syndrome related quantitative traits. The cohort was made up of 218 females (55%) and 182 males with 22% diagnosed with type 2 diabetes and a further 30% having impaired glucose tolerance or impaired fasting glucose. Notably BMI was not significantly increased in those with type 2 diabetes (P=0.119), however a significant increase in waist circumference was observed in these groups (P=0.02). The heritable proportion of trait variance was substantial and greater than values previously published for hip circumference, LDL and total cholesterol, diastolic and systolic blood pressure and serum creatinine. Height, weight and BMI heritabilities were all in the upper range of those previously reported. The phenotypic characteristics of the Mauritius Family cohort are similar to those previously reported in the Mauritian population with a high observed prevalence rate of type 2 diabetes. A high heritability for key type 2 diabetes and metabolic syndrome related phenotypes (range 0.23 to 0.68), suggest the cohort will have utility in identifying genes that influence these quantitative traits.
Keywords: Human Genetics, Type 2 Diabetes Mellitus, Metabolic Cardiovascular Syndrome, Heritable Quantitative Traits, Multifactorial Inheritances
The prevalence of type 2 diabetes and obesity continues to increase in both developed and developing countries presenting a major public health issue impacting a wide variety of social and economic measures (Zimmet et al., 2001). The number of affected individuals with type 2 diabetes worldwide is currently estimated at 246 million with a predicted increase to 380 million by 2025 (Sicree et al., 2006). Disease development involves defects in both insulin production through pancreatic β-cell dysfunction and insulin action through peripheral insulin resistance. Type 2 diabetes frequently clusters with central (upper-body) obesity, hypertension, and dyslipidemia comprising a condition collectively termed the metabolic syndrome. While a combination of genetic and environmental factors are thought to underlie the epidemic, the underlying molecular mechanism of pathogenesis and etiology of the disease remains poorly understood (Florez et al., 2003; Stumvoll et al., 2005).
There exists substantial evidence in the literature from genetic epidemiology based familial relative risk estimates and twin studies that support the role of genetic factors in development of the disease together with modifying effects of the environment (Diamond, 2003; Kahn et al., 1996). However, insight into the genetic basis of the disease is limited to the rare monogenic forms of the disease that exhibit a clear Mendelian pattern of inheritance (de Fronzo, 1988; Florez et al., 2003; Kahn, 1994; Owen & McCarthy, 2007) . The genetic architecture underpinning predisposition to the more common forms of type 2 diabetes have remained elusive (Elbein et al., 2002).
While a number of studies have been undertaken, sometimes inconsistent and conflicting results have been obtained possibly contributed to by allelic and locus heterogeneity, inappropriate study designs leading to low power to detect genes and variable phenotyping methodologies (Blangero, 2004; Florez et al., 2003; Walder et al., 2003). We have adopted a study design using large multigenerational pedigrees to reduce genetic heterogeneity, increase the signal to noise ratio and improve statistical power of gene detection. The families were ascertained from a subpopulation within Mauritius that, due to geographic and cultural factors, may exhibit reduced genetic and environmental heterogeneity, and have the potential to maximally expose the phenotypic variation influenced by underlying genetic factors (Arcos-Burgos & Muenke, 2002; Peltonen et al., 2000; Sheffield et al., 1998; Varilo et al., 2003). We centre our analysis on a range of quantitative trait measures underlying the obese and diabetic phenotypes of the metabolic syndrome. Given the reports of high prevalence of diabetes (Dowse et al., 1990) and increasing incidence of type 2 diabetes in Mauritius (Soderberg et al., 2005; Soderberg et al., 2004), the present study is especially urgent.
Methods
Subjects
The island of Mauritius lies in the Indian Ocean approximately 800 km East of Madagascar. Its multi-ethnic population of 1.1 million inhabitants is predominantly made up of Indo Mauritians (68%), Creole (27%), Sino Mauritians (3%) and Franco Mauritians (2%). This diverse ethnic composition reflects the history of migration of slaves, indentured laborers and merchants from India, Madagascar, Africa, and Asia. Limited admixture between these subpopulations has been noted (Manrakhan, 1984), suggesting that cultural and religious isolates may exist within the geographical population isolate with the potential to contribute to increased levels of genetic and environmental homogeneity among the population. This combined with a cultural preference for large families is expected to improve the statistical power for detection of genes influencing quantitative measures underlying the development of type 2 diabetes, obesity and the metabolic syndrome.
The Mauritius Family Diabetes Study was a collaborative effort between the International Diabetes Institute, the Mauritius Ministry of Health and Quality of Life and the University of Mauritius SSR Centre for Medical Studies and Research. A total of 400 individuals were included in the study in 24 extended pedigrees.
Phenotyping
Phenotypes related to type 2 diabetes, obesity and traits known to cluster with the metabolic syndrome were obtained from each individual during the course of a medical exam. Four phenotypes directly related to obesity and body composition were assessed in the Mauritian sample. These included waist/hip ratio (WHR), body mass index (BMI), fat mass (in kilograms) and relative fat mass. Height was measured to the nearest centimetre and weight measured to the nearest 0.1 kg in light clothes and without shoes. BMI was calculated as weight in kilograms divided by height in metres squared. The waist measures were taken at the midpoint between the iliac crest and the lower rib margin, while the hip measurement was taken around the maximum circumference of the buttocks posteriorly and the symphisis pubis anteriorly. Waist and hip circumferences were measured in duplicate to the nearest 0.5 cm with a measuring tape while the participant was standing relaxed and wearing a single layer of light clothing. If the first two measures differed by more than 2 cm, a third measure was taken. The mean of the closest two measures were used to calculate the waist circumference and waist hip ratios (WHR). Body composition measures were taken using a single frequency bioelectrical impedance meter (BIM4 UniQuest Ltd, St Lucia, Australia), with the participants rested and lying horizontally on a bed.
Blood pressure was measured with a standard mercury sphygmomanometer in the right arm of participants who had been seated for 5 min. Using the first and fifth Korotkoff sounds, blood pressure was recorded twice to the nearest 2 mmHg and the mean value calculated.
To measure aspects of glucose metabolism, we obtained measures of fasting plasma glucose using a YSI Glucose Analyser (Yellow Springs, OH), and fasting levels of insulin using a standard microparticle enzyme immunoassay method (IMx, Abbott Diagnostics, Illinois). Participants who had not previously been diagnosed with diabetes and whose fasting plasma glucose levels were < 10 mmol/l on the morning of the medical exam (determined by a Hemocue blood glucose analyser; B-Glucose Analyzer; HemoCue AB, Angelholm, Sweden) were asked to complete an oral glucose tolerance test (OGTT) (75 g dextrose monohydrate in 250ml water) followed by a second blood sample 120 minutes later. Glucose tolerance status was determined according to 1999 WHO criteria (WHO, 1999).
Total cholesterol and triglycerides were determined on fasting plasma by manual enzymatic methods. All plasma measures were done in duplicate and quality control procedures implemented to maintain accuracy across batches.
Leptin concentrations in human plasma were measured using a commercially available human leptin RIA kit (Linco Research Inc., St Charles, USA). This kit utilised 125I-labelled leptin and a human leptin antiserum to determine plasma levels by the double antibody technique. In the procedure, 50 ul of human plasma was added to 50 ul of 125I-human leptin (tracer) and 50 ul of rabbit anti-human leptin antiserum ('first antibody') and left to bind overnight (20–24 hours) at 4°C. A 0.5 ml aliquot of cold (4°C) goat anti-rabbit IgG antibody ('second antibody') was then added, which was highly specific for the ‘first antibody' and served as a carrier. Binding proceeded at 4°C for 20 mins, after which tubes were centrifuged at 3500 g for 30 mins, to separate the bound from the free leptin. Each tube was then aspirated, leaving the bound leptin in a pellet in the bottom of the tube. The radioactivity of the solid phase pellet was determined using a gamma counter. The concentration of each unknown sample was then computed by comparing the competitive capacity of the plasma leptin with standards of known concentrations (0, 0.5, 1, 2, 5, 10, 20, 50, and 100 ng/ml). A spline-shaped curve described the relationship between the ratio of bound to unbound 125I-labelled leptin and the log of the leptin concentration. Under standard conditions, the gradient and linearity of the curve were maximal in the central region of the curve (approximately 10–100 ng/ml). Samples of high leptin concentration were diluted further and re-assayed where necessary to enable measurement in the linear region of the curve. Two quality controls (Linco Research Inc, St Charles, USA) were incorporated into each assay with low (expected range: 2.1–3.9 ng/ml), and high (expected range: 16.4–24.6ng/ml) concentrations.
Glycosylated haemoglobin (HbA1c) was measured in whole blood using ion-exchange high pressure liquid chromatography as implemented in the Variant II analysis procedure according to the manufacturer’s protocols (Bio-Rad laboratories, Hercules, USA).
The project was submitted to and approved by two independent ethics committees in both Australia, the sponsoring country and Mauritius, the host country according to the Council for International Organizations of Medical Sciences (CIOMS) and World Health Organization (WHO) guidelines for International Biomedical Research Involving Human Subjects (Geneva 1993). Participation in the study was allowed after informed consent was obtained following an explanation by local community health workers in their natural language.
Statistical Genetic Methods
For all traits, residuals from least squares multiple linear regression, using age, age2, sex, age-by-sex, and age2-by-sex as independent variables, were exactly normalized using an inverse Gaussian transformation in SOLAR (Almasy & Blangero, 1998). The covariates in all of our models were age, age2, sex, age-by-sex, age2-by-sex, occupational physical activity, leisure physical activity, alcohol intake, smoking, menopause, and oral contraceptive use. Heritability was estimated as the ratio of the additive genetic variance to the total phenotypic variance using a maximum likelihood estimation algorithm implemented in SOLAR. As first demonstrated in Duggirala et al. (1997) (Duggirala et al., 1997), SOLAR can be used to estimate the heritabilities of discrete traits (in addition to the standard case as regards continuous traits), such as type 2 diabetes. Thus, SOLAR was used to compute the heritability of type 2 diabetes in the Mauritius population, as well as the heritabilities of the continuous traits.
Results
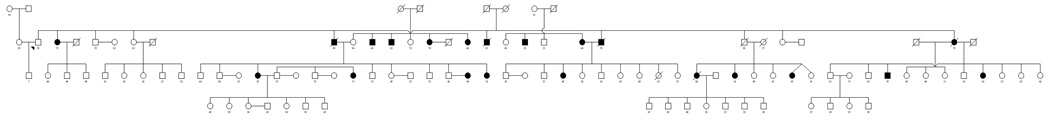
The Mauritius Family Diabetes Study included a total of 400 individuals in 24 extended pedigrees. Probands were identified via questionnaire administered as part of a National randomized cluster based non-communicable diseases prevalence survey conducted in 1998 (Soderberg et al., 2005) and screened for membership of a large multigenerational family. Local community health workers assisted in the gathering of family relationship data and in explanation of the study to potential participants. Those invited to join the study were enthusiastic about participation with an overall participation rate exceeding 70%. The cohort is comprised of a total of 2,409 pairwise relationships with a substantial number of distant relations observed including 755 avuncular and 597 1st cousins (Table 1). The mean sib ship size was 3.8 (range 2–11). This level of extension in the family collection distinguishes this study from a nuclear family or sib-pair based study design and carries increased statistical power to detect genes on a per participant basis (Altmuller et al., 2001; Blangero et al., 2001).
Table 1.
Number of pairwise relationships by type
| Relationship | Pairs (N) |
|---|---|
| Parent-offspring | 205 |
| Siblings | 572 |
| Grandparent-grandchild | 20 |
| Avuncular | 755 |
| Half-siblings | 9 |
| Grandavuncular | 78 |
| Half-avuncular | 12 |
| 1st Cousins | 597 |
| 1st Cousins, once removed | 145 |
| 2nd Cousins | 16 |
| Total | 2409 |
The cohort was examined for a range of anthropometric and biochemical measures related to the risk of development of type 2 diabetes and cardiovascular disease. Basic screening of individuals for complications as a result of diabetes was also undertaken including neuropathy (vibration perception threshold) and retinopathy (visual acuity measurement). Dietary habits, work related and leisure time physical activity levels were also recorded for incorporation into the present and future analyses.
The phenotypic characteristics of the Mauritius sample population are reported in Table 2. Overall, 55% of the cohort were women with a mean age (both sexes) of 50 years. Approximately 22% were diagnosed with type 2 diabetes and 30% showed impaired glucose tolerance. These prevalence rates were higher than the National average previously reported in a large cross sectional study undertaken in 1998, 18% for type 2 diabetes and 14% IGT (Soderberg et al., 2005) and consistent with an increased familial risk of type 2 diabetes that has been observed previously in other cohorts. In contrast to many published studies, body mass index (BMI) was not significantly different between euglycemic individuals and those who were hyperglycemic (P=0.119). However, in some populations waist circumference has been proposed as a more accurate predictor of type 2 diabetes (Huxley et al., 2008; Yusuf et al., 2005), although the improvements in efficacy over BMI alone depend on ethnicity, sex and age (Freiberg et al., 2008). In this cohort we observed that waist circumference was significantly higher (P=0.02) in individuals with diabetes as compared to those who were normoglycemic. These observations suggest that development of type diabetes in this cohort is associated with increased waist but not BMI.
Table 2.
Clinical characteristics of study population by glucose tolerance status
| Variable | Euglycemic | IGT and/or IFG | Diabetes |
|---|---|---|---|
| N | 192 | 119 | 89 |
| Age | 47.4 ± 13.2 | 49.49 ± 14.09 | 57.93 ± 11.47 |
| Sex (M/F) | 99/93 | 52/67 | 31/58 |
| Height (cm) | 160.99 ± 9.28 | 158.28 ± 8.59 | 157.86 ± 9.32 |
| Weight (kg) | 62.66 ± 11.41 | 64.68 ± 12.04 | 63.97 ± 12.15 |
| BMI (kg/m2) | 24.21 ± 4.22 | 25.85 ± 4.67 | 25.68 ± 4.46 |
| Waist circumference (cm) | 82.73 ± 9.86 | 85.47 ± 9.57 | 86.59 ± 10.51 |
| Hip circumference (cm) | 98.55 ± 8.40 | 101.62 ± 10.11 | 101.04 ± 9.37 |
| WHR | 0.84 ± 0.07 | 0.84 ± 0.06 | 0.86 ± 0.07 |
| Fat mass (%) | 21.82 ± 8.44 | 24.86 ± 10.66 | 23.67 ± 7.55 |
| Fasting glucose (mmol/l) | 5.87 ± 2.36 | 5.72 ± 0.80 | 10.85 ± 4.25 |
| 2 hour glucose (mmol/l) | 7.93 ± 3.79 | 9.96 ± 2.86 | 13.24 ± 5.22 |
| Fasting insulin (pmol/l) | 12.03 ± 6.41 | 14.03 ± 7.17 | 12.95 ± 6.99 |
| HbA1c | 5.22 ± 1.38 | 5.07 ± 0.53 | 8.06 ± 1.93 |
| HDL cholesterol (mmol/l) | 1.19 ± 0.30 | 1.23 ± 0.31 | 1.18 ± 0.30 |
| LDL cholesterol (mmol/l) | 3.42 ± 0.94 | 3.59 ± 0.90 | 3.66 ± 0.98 |
| Total cholesterol (mmol/l) | 5.35 ± 1.03 | 5.60 ± 1.03 | 5.82 ± 1.19 |
| Triglycerides (mmol/l) | 1.59 ± 0.90 | 1.70 ± 0.87 | 2.03 ± 1.09 |
| Leptin (ng/ml) | 15.13 ± 12.32 | 21.40 ± 17.13 | 18.31 ± 13.43 |
| Diastolic BP (mmHg) | 75.95 ± 14.27 | 77.97 ± 15.30 | 82.48 ± 12.69 |
| Systolic BP (mmHg) | 128.06 ± 23.32 | 133.43 ± 22.25 | 147.11 ± 24.76 |
| Creatinine (mmol/l) | 8.95 ± 5.53 | 7.68 ± 5.27 | 6.99 ± 4.18 |
| Urate (umol/l) | 267.94 ± 80.70 | 286.27 ± 72.35 | 255.98 ± 92.11 |
The average reported incidence of type 1 diabetes in Asian Indian populations in Mauritius is 1.9/100,000, among the lowest observed for any population (Tuomilehto et al., 1993). Nonetheless, we carefully screened participants for early age of diabetes onset and requirement for injected insulin to ensure individuals with type 1 diabetes were not included in the study group. Similarly we screened families where an autosomal dominant pattern of inheritance was observed for type 2 diabetes to minimize inclusion of participants with maturity onset diabetes of the young (MODY).
Heritabilities of the measured quantitative and dichotomous traits were calculated using SOLAR in the family cohort (Table 3). The majority of traits exhibited heritabilities that were within the range published by other studies, however four traits (leptin, percentage fat mass, urate and HDL cholesterol) showed slightly lower heritability than those previously observed. Conversely, six traits had heritabilities above the range previously published, these were hip circumference (0.55), LDL (0.68) and total cholesterol (0.66), diastolic (0.32) and systolic blood pressure (0.54) and serum creatinine (0.45). A further three traits, height (0.84), weight (0.67) and from these calculated BMI (0.64), showed a level of heritability that was well above the mean of the previously reported range. The observation that the majority of the observed traits exhibited higher heritability measures than the mean of other published studies suggests the cohort is well suited for the identification of genetic factors contributing to variance in the complex disease traits.
Table 3.
Heritability estimates of diabetes and cardiovascular disease related quantitative traits in Mauritius
Discussion
We report here the ascertainment of a large extended pedigree based cohort as part of the Mauritius Family Diabetes Study of type 2 diabetes and cardiovascular disease. The cohort is made of up 400 participants in 24 families comprising a total of 2409 pair-wise relationships with a substantial number being represented in extended relationships within the family. Extensive anthropometric and biochemical quantitative traits related to type 2 diabetes and cardiovascular disease were measured and recorded in addition to dietary habits and physical activity levels. The prevalence of type 2 diabetes was higher in this family cohort as compared to the general population in Mauritius consistent with the widely observed familial predisposition to the disease.
In contrast to the published observations on many cohorts, we did not observe a significantly increased BMI among individuals with type 2 diabetes as compared to subjects with normal glucose tolerance. This is consistent with the findings of our previously reported data from a cross-sectional survey that observed a leaner phenotype as measured by BMI among individuals with type 2 diabetes in contrast to the phenotypes reported in the Pima Indian cohort (Kriska et al., 2001; Soderberg et al., 2005). We did observe however a modestly increased waist circumference in individuals with diabetes as compared to normoglycemic individuals, an alternate measure of obesity that has found utility in prediction of type 2 diabetes (Huxley et al., 2008; Yusuf et al., 2005). Interestingly it has been noted that there exists a high prevalence of type 2 diabetes among the Asian Indian population in Mumbai, India despite the relatively low rates of obesity (Marita et al., 2005). The participants of this cohort are direct descendants of Asian Indian migrants to Mauritius, and appear to have retained the phenotypic attributes of type 2 diabetes of that population.
The heritability estimates of the recorded quantitative traits were in general consistent with those of prior published studies. However we observed in approximately one third of the traits measured, a heritability level that exceeded the range of earlier reported cohorts. This suggests a potential reduction in trait variation due to environmental and random factors and an increased influence of genetic or heritable factors, making the cohort well suited for the identification of trait influencing genes.
In summary, we report on the ascertainment of a large family based cohort for the identification genes influencing risk of type 2 diabetes and cardiovascular disease in Mauritius. The large sib ship size, combined with the relatively low mobility of this population, allowed for considerable collateral and vertical expansion of pedigrees, permitting the capture of substantial meiotic information. Given the complexity of the genotype-phenotype relationship for quantitative traits related to diabetes/obesity, we suggest that future localization efforts should focus on large extended pedigrees in order to obtain the maximum power required for mapping these important susceptibility genes. In general an increased proportion of variance due to heritable factors (heritability) was observed in this cohort as compared to previously published studies indicating the suitability of this cohort for future gene discovery research.
Figure 1.
Acknowledgments
This study was undertaken with the support and collaboration of the Mauritius Ministry of Health and Quality of Life, their health community workers and staff at the SSR Centre, University of Mauritius without which this study would not have been possible. The authors further extend their gratitude to the individuals and families who participated in the study.
Funding
This study was supported in part by a research grant from ChemGenex Pharmaceuticals. The SOLAR statistical genetics computer package is supported by a grant from the US National Institute of Mental Health (MH059490). The supercomputing facilities used for this work at the AT&T Genetics Computing Center were supported in part by a gift from the AT&T Foundation.
Footnotes
Competing interests
All authors declare they have no duality of interest with the exception of PZ who declares he was chairman of the Scientific Advisory Board of ChemGenex Pharmaceuticals during the period this study was undertaken and whose family owns shares in ChemGenex Pharmaceuticals.
References
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmuller J, Palmer LJ, Fischer G, Scherb H, Wjst M. Genomewide scans of complex human diseases: true linkage is hard to find. Am J Hum Genet. 2001;69(5):936–950. doi: 10.1086/324069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos-Burgos M, Muenke M. Genetics of population isolates. Clin Genet. 2002;61(4):233–247. doi: 10.1034/j.1399-0004.2002.610401.x. [DOI] [PubMed] [Google Scholar]
- Blangero J. Localization and identification of human quantitative trait loci: king harvest has surely come. Curr Opin Genet Dev. 2004;14(3):233–240. doi: 10.1016/j.gde.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trait loci. Adv Genet. 2001;42:151–181. doi: 10.1016/s0065-2660(01)42021-9. [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Hixson JE, Almasy L, Mitchell BD, Mahaney MC, Dyer TD, Stern MP, MacCluer JW, Blangero J. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet. 1997;15(3):273–276. doi: 10.1038/ng0397-273. [DOI] [PubMed] [Google Scholar]
- de Fronzo RA. Lilly Lecture 1987: The triumvirate: b-cell, muscle, liver: A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Diamond J. The double puzzle of diabetes. Nature. 2003;423(6940):599–602. doi: 10.1038/423599a. [DOI] [PubMed] [Google Scholar]
- Dowse GK, Gareeboo H, Zimmet PZ, Alberti KG, Tuomilehto J, Fareed D, Brissonnette LG, Finch CF Mauritius Noncommunicable Disease Study Group. High prevalence of NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians. Diabetes. 1990;39(3):390–396. doi: 10.2337/diab.39.3.390. [DOI] [PubMed] [Google Scholar]
- Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O'Connell P, Stern MP. Linkage of Type 2 Diabetes Mellitus and of Age at Onset to a Genetic Location on Chromosome 10q in Mexican Americans. Am J Hum Genet. 1999;64(4):1127–1140. doi: 10.1086/302316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggirala R, Williams JT, Williams-Blangero S, Blangero J. A variance component approach to dichotomous trait linkage analysis using a threshold model. Genet Epidemiol. 1997;14(6):987–992. doi: 10.1002/(SICI)1098-2272(1997)14:6<987::AID-GEPI71>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Elbein SC, Chiu KC, Permutt MA. Type 2 Diabetes Mellitus. In: King RA, Rotter JI, Motulsky AG, editors. The Genetic Basis of Common Disease. 2nd ed. Oxford: Oxford University Press; 2002. pp. 457–480. [Google Scholar]
- Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257–291. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- Fox CS, Yang Q, Cupples LA, Guo CY, Larson MG, Leip EP, Wilson PW, Levy D. Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol. 2004;15(9):2457–2461. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
- Freiberg MS, Pencina MJ, D'Agostino RB, Lanier K, Wilson PW, Vasan RS. BMI vs. waist circumference for identifying vascular risk. Obesity (Silver Spring) 2008;16(2):463–469. doi: 10.1038/oby.2007.75. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, Langefeld CD, Freedman BI, Bowden DW, Carr JJ. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res. 2005;13(2):312–319. doi: 10.1038/oby.2005.42. [DOI] [PubMed] [Google Scholar]
- Hsueh W, Mitchell B, Aburomia R, Pollin T, Sakul H, Ehm M, Michelsen B, Wagner M, St. Jean P, Knowler W, Burns D, Bell C, Shuldiner A. Diabetes in the Old Order Amish. Diabetes Care. 2000;23(5):595–601. doi: 10.2337/diacare.23.5.595. [DOI] [PubMed] [Google Scholar]
- Hunt KJ, Lehman DM, Arya R, Fowler S, Leach RJ, Goring HH, Almasy L, Blangero J, Dyer TD, Duggirala R, Stern MP. Genome-wide linkage analyses of type 2 diabetes in Mexican Americans: the San Antonio Family Diabetes/Gallbladder Study. Diabetes. 2005;54(9):2655–2662. doi: 10.2337/diabetes.54.9.2655. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Coon H, Hasstedt SJ, Cawthon RM, Camp NJ, Wu LL, Hopkins PN. Linkage of serum creatinine and glomerular filtration rate to chromosome 2 in Utah pedigrees. Am J Hypertens. 2004;17(6):511–515. doi: 10.1016/j.amjhyper.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Hasstedt SJ, Kuida H, Stults BM, Hopkins PN, Williams RR. Genetic heritability and common environmental components of resting and stressed blood pressures, lipids, and body mass index in Utah pedigrees and twins. Am J Epidemiol. 1989;129(3):625–638. doi: 10.1093/oxfordjournals.aje.a115175. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD. Genetic contribution to renal function and electrolyte balance: a twin study. Clin Sci (Lond) 2002;103(3):259–265. doi: 10.1042/cs1030259. [DOI] [PubMed] [Google Scholar]
- Huxley R, James WP, Barzi F, Patel JV, Lear SA, Suriyawongpaisal P, Janus E, Caterson I, Zimmet P, Prabhakaran D, Reddy S, Woodward M. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9 Suppl 1:53–61. doi: 10.1111/j.1467-789X.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Insulin action, diabetogenes, and the cause of Type II diabetes. Diabetes. 1994;43(8):1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Kahn CR, Vicent D, Doria A. Genetics of non-insulin-dependent (type-II) diabetes mellitus. Annu Rev Med. 1996;47:509–531. doi: 10.1146/annurev.med.47.1.509. [DOI] [PubMed] [Google Scholar]
- Knuiman MW, Divitini ML, Welborn TA, Bartholomew HC. Familial correlations, cohabitation effects, and heritability for cardiovascular risk factors. Ann Epidemiol. 1996;6(3):188–194. doi: 10.1016/1047-2797(96)00004-x. [DOI] [PubMed] [Google Scholar]
- Kriska AM, Pereira MA, Hanson RL, de Courten MP, Zimmet PZ, Alberti KG, Chitson P, Bennett PH, Narayan KM, Knowler WC. Association of physical activity and serum insulin concentrations in two populations at high risk for type 2 diabetes but differing by BMI. Diabetes Care. 2001;24(7):1175–1180. doi: 10.2337/diacare.24.7.1175. [DOI] [PubMed] [Google Scholar]
- Manrakhan J. Examination of the slavery-indenture continuum of Mauritius including a scenario that never was. In: Bissoondoyal U, Servansing SBC, editors. Indian Labour Immigration. Moka, Mauritius: Mahatma Ghandhi Institute Press; 1984. pp. 20–72. [Google Scholar]
- Marita AR, Sarkar JA, Rane S. Type 2 diabetes in non-obese Indian subjects is associated with reduced leptin levels: study from Mumbai, Western India. Mol Cell Biochem. 2005;275(1–2):143–151. doi: 10.1007/s11010-005-1204-7. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, Dyke B, Hixson JE, Henkel RD, Sharp RM, Comuzzie AG, VandeBerg JL, Stern MP, MacCluer JW. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation. 1996;94(9):2159–2170. doi: 10.1161/01.cir.94.9.2159. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Szczech R, Winnicki M, Chrostowska M, Pawlowski R, Lysiak-Szydlowska W, Choe I, Kato M, Sivitz WI, Krupa-Wojciechowska B, Somers VK. Heritability of plasma leptin levels: a twin study. J Hypertens. 1999;17(1):27–31. doi: 10.1097/00004872-199917010-00005. [DOI] [PubMed] [Google Scholar]
- Nath SD, Voruganti VS, Arar NH, Thameem F, Lopez-Alvarenga JC, Bauer R, Blangero J, MacCluer JW, Comuzzie AG, Abboud HE. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol. 2007;18(12):3156–3163. doi: 10.1681/ASN.2007040426. [DOI] [PubMed] [Google Scholar]
- North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, Fabsitz RR, Roman MJ, MacCluer JW. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157(4):303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- Owen KR, McCarthy MI. Genetics of type 2 diabetes. Curr Opin Genet Dev. 2007;17(3):239–244. doi: 10.1016/j.gde.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Peltonen L, Palotie A, Lange K. Use of population isolates for mapping complex traits. Nat Rev Genet. 2000;1(3):182–190. doi: 10.1038/35042049. [DOI] [PubMed] [Google Scholar]
- Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance - a population-based twin study. Diabetologia. 1999;42(2):139–145. doi: 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- Rice T, Perusse L, Bouchard C, Rao DC. Familial aggregation of body mass index and subcutaneous fat measures in the longitudinal Quebec family study. Genetic Epidemiology. 1999;16:316–334. doi: 10.1002/(SICI)1098-2272(1999)16:3<316::AID-GEPI7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rotimi C, Luke A, Li Z, Compton J, Bowsher R, Cooper R. Heritability of plasma leptin in a population sample of African-American families. Genet Epidemiol. 1997;14(3):255–263. doi: 10.1002/(SICI)1098-2272(1997)14:3<255::AID-GEPI4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sakul H, Pratley R, Cardon L, Ravussin E, Mott D, Bogardus C. Familiality of physical and metabolic characteristics that predict the development of non-insulin-dependent diabetes mellitus in Pima Indians. Am J Hum Genet. 1997;60(3):651–656. [PMC free article] [PubMed] [Google Scholar]
- Sale MM, Freedman BI, Hicks PJ, Williams AH, Langefeld CD, Gallagher CJ, Bowden DW, Rich SS. Loci contributing to adult height and body mass index in African American families ascertained for type 2 diabetes. Ann Hum Genet. 2005;69(Pt 5):517–527. doi: 10.1046/j.1529-8817.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henriksen JE, Heitmann BL, Sorensen TI. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord. 2004;28(1):39–48. doi: 10.1038/sj.ijo.0802524. [DOI] [PubMed] [Google Scholar]
- Selby JV, Newman B, Quesenberry CP, Jr, Fabsitz RR, Carmelli D, Meaney FJ, Slemenda C. Genetic and behavioral influences on body fat distribution. Int J Obes. 1990;14(7):593–602. [PubMed] [Google Scholar]
- Sheffield VC, Stone EM, Carmi R. Use of isolated inbred human populations for identification of disease genes. Trends Genet. 1998;14(10):391–396. doi: 10.1016/s0168-9525(98)01556-x. [DOI] [PubMed] [Google Scholar]
- Sicree R, Shaw J, Zimmet P. Diabetes and Impaired Glucose Tolerance. Prevalence and Projections. In: Gan D, editor. Diabetes Atlas. 3rd ed. Brussels: International Diabetes Federation; 2006. pp. 16–103. [Google Scholar]
- Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes. 2001;50(12):2858–2863. doi: 10.2337/diabetes.50.12.2858. [DOI] [PubMed] [Google Scholar]
- Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, Gareeboo H, Alberti KG, Shaw JE. Increasing prevalence of Type 2 diabetes mellitus in all ethnic groups in Mauritius. Diabet Med. 2005;22(1):61–68. doi: 10.1111/j.1464-5491.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, Stenlund H, Gareeboo H, Alberti KG, Shaw J. High incidence of type 2 diabetes and increasing conversion rates from impaired fasting glucose and impaired glucose tolerance to diabetes in Mauritius. J Intern Med. 2004;256(1):37–47. doi: 10.1111/j.1365-2796.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Dabee J, Karvonen M, Dowse GK, Gareeboo H, Virtala E, Tiihonen M, Alberti KG, Zimmet PZ. Incidence of IDDM in Mauritian children and adolescents from 1986 to 1990. Diabetes Care. 1993;16(12):1588–1591. doi: 10.2337/diacare.16.12.1588. [DOI] [PubMed] [Google Scholar]
- Varilo T, Paunio T, Parker A, Perola M, Meyer J, Terwilliger JD, Peltonen L. The interval of linkage disequilibrium (LD) detected with microsatellite and SNP markers in chromosomes of Finnish populations with different histories. Hum Mol Genet. 2003;12(1):51–59. doi: 10.1093/hmg/ddg005. [DOI] [PubMed] [Google Scholar]
- Walder K, Segal D, Jowett J, Blangero J, Collier GR. Obesity and diabetes gene discovery approaches. Curr Pharm Des. 2003;9(17):1357–1372. doi: 10.2174/1381612033454739. [DOI] [PubMed] [Google Scholar]
- Watanabe RM, Valle T, Hauser ER, Ghosh S, Eriksson J, Kohtamaki K, Ehnholm C, Tuomilehto J, Collins FS, Bergman RN, Boehnke M. Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators. Hum Hered. 1999;49(3):159–168. doi: 10.1159/000022865. [DOI] [PubMed] [Google Scholar]
- WHO. Geneva: World Health Organisation, Department of Noncommunicable Disease Surveillance; Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. 1999 (No. WHO/NCD/NCS/99.2)
- Yang Q, Guo CY, Cupples LA, Levy D, Wilson PW, Fox CS. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism. 2005;54(11):1435–1441. doi: 10.1016/j.metabol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]