Abstract
Background
It has been hypothesized that atrial lesions must be transmural to successfully cure atrial fibrillation (AF). However, ablation lines often do not extend completely across the atrial wall.
Objective
The purpose of this study was to determine the effect of residual gaps on conduction properties of atrial tissue.
Methods
Canine right atria (n=13) were isolated, perfused, and mounted on a 250-lead electrode plaque. The atria were divided with a bipolar radiofrequency ablation clamp, leaving a gap that was progressively narrowed. Conduction velocities at varying pacing rates and AF frequencies were measured before and after ablations. AF was induced with an extra stimulus and acetylcholine.
Results
Gap widths from 11.2mm to 1.1mm were examined. Conduction velocities through gaps were dependent cycle length (p=0.002) and gap size(p<0.001). Overall, 253/260 (97%) of all gaps allowed paced propagation. 51/56(91%) of gaps 1–3mm permitted paced propagation, as did 202/204(99%) of gaps ≥3.0mm. Similarly, 253/260 (97%) of all gaps allowed propagation of AF. For AF, 51/55(93%) of gaps 1–3mm allowed AF to pass through, as did 202/205(99%) of gaps ≥3.0 mm. Gaps as small as 1.1mm conducted paced and AF impulses.
Conclusions
Conduction velocities were slowed through residual gaps. However, propagation of wave fronts during pacing and AF occurred through the majority of residual gaps, down to sizes as small as 1.1mm. Leaving viable tissue in ablation lines for the treatment of AF could account for failures.
Keywords: Atrial fibrillation, Radiofrequency ablation, Ablation gap, Transmural
Introduction
Atrial Fibrillation (AF) is the most common sustained arrhythmia in the world, occurring in 0.4–2.0% of the general population1. The Cox-Maze procedure (CMP) was developed in the 1980s and has the highest reported long-term success for the treatment of AF2. Because the CMP was technically challenging and time-consuming to complete, few surgeons chose to incorporate it into their daily practice. Recent advancements in technology have made it possible to replace many of the incisions with linear lines of ablation, making the procedure both shorter and less technically challenging. Various energy sources, including radiofrequency ablation, microwave, laser, cryoablation, and high-frequency ultrasound have been used.3
There are many contributing factors that can determine success in ablation for either catheter based or surgical treatment of AF. These include the number and location of the lesions, the mass of atrial tissue ablated, the successful isolation of triggers, and the effectiveness of the particular technology in terms of ablation depth and width. An important question that has arisen is whether having transmural or complete lesions is important or necessary. In a chronic canine model, Ishii et al.4 found that conduction block rarely occurred in gaps larger than 5 mm, and decremental slowing was seen in gaps between 5 mm and 15 mm. In this model where gaps were left in surgical incision lines, complete block was seen in gaps smaller than 5 mm. In another canine model in which gaps were left in lines created by unipolar radiofrequency, conduction block was rarely seen with a gap greater than 5 mm, and was variable at 2–5 mm of gap5. Conduction was demonstrated down to a gap of 0.1 mm in an acute canine laser model, substantiating that even very small breaks in ablation lines have the ability to conduct paced impulses6. In an in vivo study done by Inoue and Zipes7, conduction was found in atrial tissue with a cross-sectional area as little as 1 mm2.
This study was designed to test the following hypotheses: Paced conduction would be slowed and eventually blocked with a decrease in gap size and block of conduction of atrial fibrillation through gaps would occur with decreasing gap size.
Methods
All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996). Normal mongrel dogs (n=13), weighing between 20 and 30 kg, were intravenously anesthetized with 7.5 mg/kg of propofol, intubated, and placed on a positive pressure respirator with 2% to 3% isoflurane used throughout the procedure. A median sternotomy was performed, and the heart was cradled in the pericardium. The inter-atrial groove was dissected, separating the left atria and right atria. The heart was then arrested using cold cardioplegia and the right atrium was removed, as described previously8. The right atrium was divided through the superior vena cava down to the inferior vena cava to facilitate mounting of the tissue on the electrode plaque. The right coronary artery was cannulated with a 16-gauge catheter.
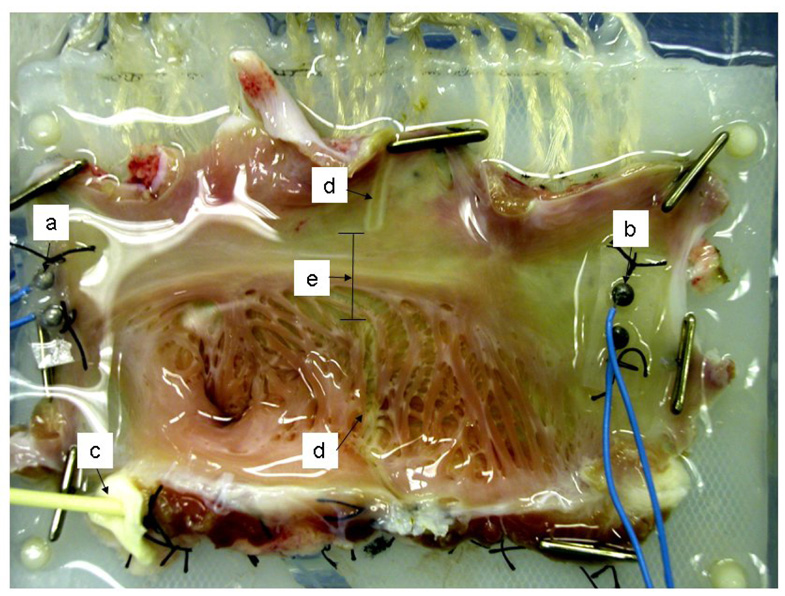
The epicardial surface was mounted on a flat electrode platform containing 256 unipolar electrodes with an inter-electrode distance of 5 mm. The atrial appendage of each preparation was placed into a slot in the electrode template that allowed the atrium to lie flat (Figure 1). For purposes of calculating directional conduction, the atrium that was nearest the superior vena cava was labeled as “superior” or upper atrium, and the tissue nearest to the inferior vena cava was labeled “inferior” or lower atrium.
Figure 1.
Isolated right atrial preparation, mounted to 250-lead electrode plaque in the bath, epicardial surface down. a. superior pacing electrode; b: inferior pacing electrode; c. 16-gauge perfusion catheter inserted in the origin of the right coronary artery; d. ablation lines created by bipolar radiofrequency clamp; e. gap measured between ablation lines.
The preparation was kept in a temperature-controlled bath at 37°C and perfused with a modified Krebs-Henseleit solution at a rate of 8 to 10 mL/min (perfusion pressure was maintained at 40 mm Hg). The composition of the Krebs-Henseleit solution was as follows (mmol/L): Na+, 143; K+, 4.7; Cl−, 128; Ca2+ 1.25, HCO3−, 25; Mg2+, 1.2; and dextrose, 11.1. The solution was oxygenated with 95% O2 and 5% CO2 (pH=7.4). The preparation was also continuously superfused with Krebs-Henseleit (Figure 1).
Continuous S1S1 pacing was used to calculate conduction velocities at 300 ms, 250 ms, 200 ms, 175 ms, 160 ms, 150 ms, 140 ms, 130ms, 120ms, or until one to one capture no longer occurred. The effective refractory period was determined at each pacing site by pacing with eight S1 stimuli (S1S1=300ms), followed by a single S2 stimulus, which was incrementally decreased by 5 ms until the S2 beat did not capture. The effective refractory period was defined as the shortest S1S2 interval that captured the atrium. At least 15 seconds were allowed between each determination.
After the baseline effective refractory period values were determined at each pacing site, the solution was switched to Krebs-Henseleit solution with acetylcholine (ACh) at a concentration of 10−4.5 M to decrease the effective refractory period9. The electrical activity of the atria was recorded using an in-house designed data acquisition and analysis system (Figure 2). AF was induced by a single extra stimulus. If the preparation continuously fibrillated for more than 30 seconds, it was considered a sustained episode of AF (Figure 2). The perfusion solution was then switched to Krebs-Henseleit alone until the arrhythmia terminated.
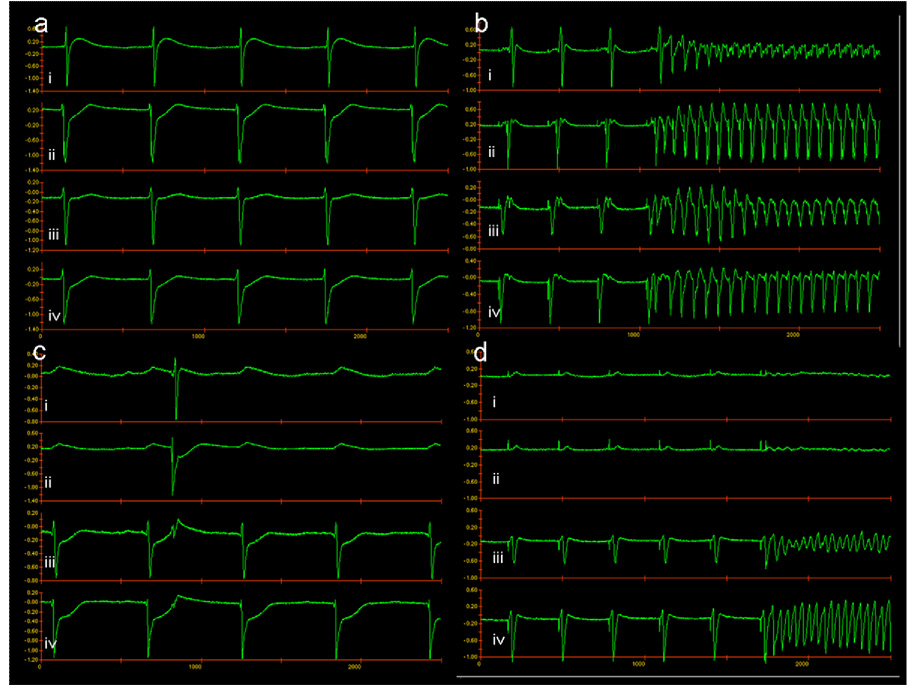
Figure 2.
Electrograms are presented from recordings near the pacing sites. (a) NSR. (b) Initiation of AF in the entire preparation. (c) NSR in the preparation after complete closure of the gap. (d) Initiation of AF in one half of the preparation after closure of the gap. “i” and “ii” represent recordings near the superior pacing electrode, “iii” and “iv” represent recordings near the inferior pacing electrode.
A total of 13 isolated RA were studied. In six preparations, one lesion with a varying gap was made. In the remaining seven RA, three separate linear lesions with gaps were created, which allowed more data to be collected from a single preparation. This resulted in 28 separate lesions. Two lesions had to be omitted from data analysis because perfusion of dye following the experiment showed one or more regions adjacent to the lesion was not adequately perfused. The net result was that there were 26 lesions included in the study.
Ablation lines were created utilizing a bipolar radiofrequency clamp (Atricure Isolator, Atricure Inc., Cincinnati, OH) across the atrium perpendicular to the crista terminalis. Bipolar radiofrequency is a technology well-suited to test these hypotheses because it creates discrete, narrow lesions, and does not damage the microvasculature.8 The atrial tissue was held between the jaws of the clamp and the radiofrequency energy was applied until termination of the algorithm which indicated transmurality was reached. An initial gap was purposefully left between the two ablations. Due to variations in atrial sizes and placement of the clamp across the atrium, the initial gap varied between 11.2 and 5.3 mm. The gap was progressively narrowed after each set of pacing and AF measurements with repeated applications of the clamp until conduction block occurred or the gap was completely closed. After each successive application of the clamp and narrowing of the gap, the effective refractory periods were determined on both sides of the ablation line with and without acetylcholine. The atrial preparations, including reference calipers for measurement, were photographed with a high-resolution digital camera after each ablation.
At the conclusion of the study, a vital blue dye bolus was injected into the preparation to ensure that there were no perfusion abnormalities. Any sections of the atrium that were not well perfused were excluded from the data analysis. The atrial sections were photographed and permanently fixed in 10% buffered formalin. The digital photos were analyzed (Adobe Photoshop 7.0) to determine the gap size left in the ablation line.
The conduction velocity was measured using two electrodes immediately adjacent to the lesion. These same two were used for all measurements for that preparation. This was done to detect the slowing across the lesion, and minimize the measurement of conduction across the rest of the atrium. Conduction velocity at 300 ms with no lesion was used as the control velocity for an individual lesion. This was then used to normalize all the velocities for that lesion. All ERPs were determined using an S1=300ms. Successful AF propagation was defined as sustained AF on both sides of the gap line after initiation on a single side.
All velocities were normalized to the control (preablation) superior to inferior velocity at a paced cycle length of 300ms for each preparation. A repeated measure analysis of variance model with two factors was used. The repeated measure was the paced cycle length and the factors were gap size and pacing direction. ANOVA was used to compare ERP data with superior or inferior sections as one factor and number of ablations as a second factor.
All data are expressed as mean ± standard error of the mean. A p<0.05 was considered significantly different. Data were analyzed using Systat for Windows version 11.00.1 (Systat Software Inc, 2004, Point Richmond, CA).
Results
Gap widths were examined from a range of 1.1 mm to 11.2 mm. Paced conduction velocity slowed as gap size decreased (p< 0.002; Figure 3). Conduction velocity (figure 4) was dependent on paced cycle length, as decreased cycle lengths had decreased conduction velocity (p=0.001). Pacing direction did not have a significant impact on conduction velocity independent of gap size (p=0.192). The mean control ERP was 166±3.9 ms and the preablation ERP with ACh was 41±3.2ms. After the fifth ablation the ERP ACh was 43±1.1ms (p=0.81 compared to the preablation value). There was no difference in the ERP between the upper and lower sections during control (146±4.7ms vs 154±6.8ms, p=0.30). There was also no difference between the ERP with ACh between the lower and upper sections (39±2.6ms vs 41±2.5ms, p=0.62). The mean cycle length of induced AF was 73±30ms (median: 61ms; range 37–167ms) superior to the lesion and 70±31ms (median: 58ms; range 37–166) inferior to the lesion.
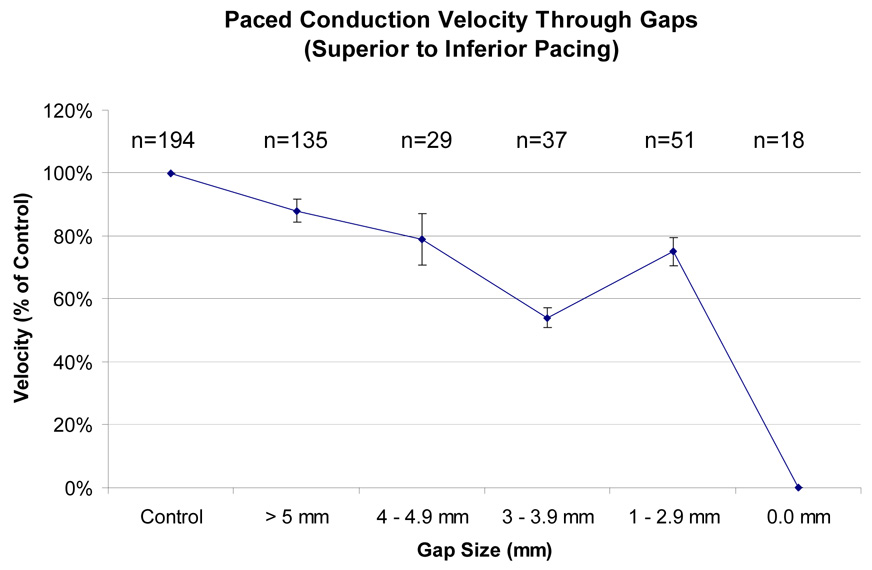
Figure 3.
Effect of gap size on paced conduction velocity (shown as percent of control), with pacing from the upper pole of the atrium. Error bars show standard error of the mean. The conduction velocity was slower with decreasing gap sizes (p < 0.001).
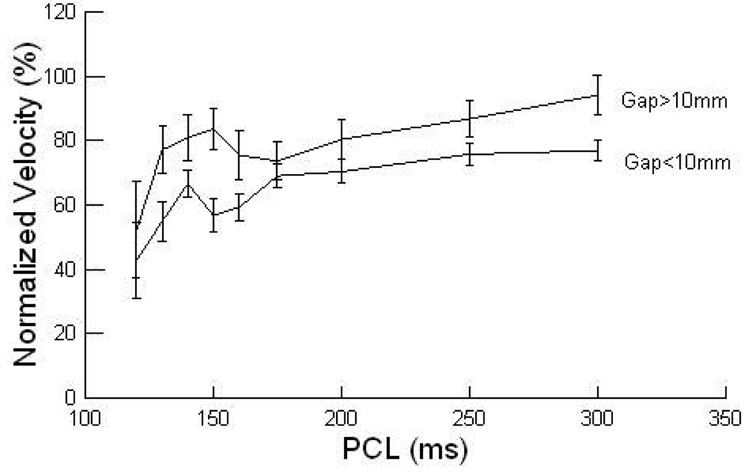
Figure 4.
The dependence of velocity on PCL (paced cycle length) and gap size greater than 10 mm vs less than 10mm is shown.
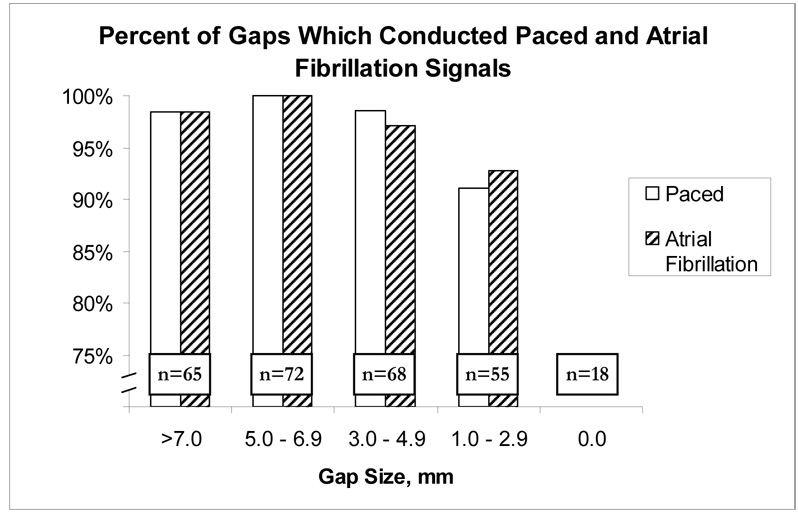
The vast majority of all paced wave fronts passed through even a minimal remaining gap (Figure 5). Overall, 253/260 (97%) of all pacing wave fronts passed through any residual gap. While pacing, 51/56 (91%) of wave fronts passed through gaps between 1–3 mm, and 202/204 (99%) of wave fronts passed through gaps larger than 3.0 mm.
Figure 5.
Percentage of impulses that successfully pass through remaining gap. AF, atrial fibrillation.
The findings for atrial fibrillation propagation through residual gaps were almost identical to the results for paced signals (Figure 5). Overall, 253/260 (97%) of any residual gaps allowed propagation of AF wave fronts. For AF, 51/55 (93%) gaps allowed wave fronts to pass through when the size of the gap was 1–3 mm, and 202/204 (99%) of gaps greater than 3.0 mm allowed AF to propagate. Ablation gaps as small as 1.1 mm conducted both paced and atrial fibrillation wave fronts. When AF propagated through the gaps, the conduction was 1:1 for all gaps greater than 5mm. For gaps less than 5mm, 76% conducted 1:1.
Methylene blue infusion confirmed intact microcirculation in all atria, as atrial tissue stained uniformly throughout the atria even with completely closed gaps. Two lesions were excluded because one segment did not stain uniformly on both sides of the lesion.
Discussion
This study demonstrated that despite a correlation of decreased conduction velocity with smaller gaps in the ablation line, electrical impulses were still propagated even at very narrow gap widths. The results were similar for paced wave fronts and atrial fibrillation, with consistent conduction persisting in residual gaps greater than the smallest obtainable gap size in this model (~1 mm). In the vast majority of samples, complete ablation was required to prevent conduction of both paced and AF electrical signals. This demonstrates the importance of creating complete, continuous and transmural lesions for the treatment of AF.
Previous studies evaluating conduction through residual gaps have shown similar results to this study. Thomas et al, using a canine model with precise ablations, showed conduction in minimal residual cardiac tissue down to 0.1 mm6. Mitchell et al found that conduction block was variable in gaps 2–5 mm for both paced and AF wave fronts using a unipolar radiofrequency ablation device5. These two models support the contention that any residual viable tissue has the potential to conduct AF. Ishii et al, however, found that gaps larger than 5 mm allowed electrical propagation but gaps less than 5 mm blocked wave fronts using an intact canine model with gaps left in incision lines4. The conduction through these slightly larger gaps in incision lines and conduction through larger gaps in unipolar ablation lines may be due to factors such as scar formation in the case of the chronic model or tissue stunning with lateral thermal injury in the unipolar ablation model. These models still showed that small amounts of remaining tissue conducted paced and AF wave fronts.
Despite inconsistency in the creation of transmural ablation lines, success rates of 60% to 92% have been reported in various studies of unipolar ablation with either radiofrequency or microwave energy10–12. It has thus been suggested that continuous transmural lesions may not be necessary to successfully cure atrial fibrillation13, 14. The results of this study support the contention that continuous, transmural ablation lines are needed to reliably block electrical propagation. Although narrow gaps have been shown to conduct slowly, the majority are still able to allow for effective conduction both of normal paced rhythm or AF. This is not surprising given the intrinsic conductive properties of cardiac tissue. The finding that propagation of paced signals correlates closely with that of atrial fibrillation supports previous results5, 6 and can be of important clinical significance in determining the adequacy of a lesion. In this study, pacing was a reliable indicator of AF conduction across gaps.
Incomplete lesions have previously been associated with the development of post-operative atrial flutter in both surgical and radiofrequency ablation15–18, and require repeat ablation for treatment. Slow conduction can facilitate reentry arrhythmias, so a very small gap in an ablation line actually may increase the chances of recurrent AF. Creating incomplete lesions to treat atrial fibrillation, therefore, have the potential of not only failure but of producing a reentrant tachyarrhythmia such as atrial flutter.
In the case of ablative catheter treatment for AF, the short-term success of non-transmural ablation may be due in part to a limited length of follow-up, which averaged less than 12 months in many studies19. It may also be due to an overall decrease in the critical geometry of tissue with widespread ablation of the atria, decreasing the remaining tissue mass which is necessary to support AF20. Recent work in our laboratory supports the latter hypothesis as the ability to sustain AF declined in a model as atrial area and mass was decreased8. Even with residual gaps that maintain the ability to conduct normally paced impulses, AF would be unable to be sustained due to a lack of critical mass after large areas of ablation were completed.
Limitations
This model has several limitations. The canine atria were normal, not diseased, and thus results may not be directly comparable to the diseased human atria. However, the canine is an excellent model for testing AF, and has been used previously for this purpose21. This model evaluated residual gaps that included full-thickness intact atrial tissue, which may be different than residual endocardial or epicardial rims of tissue. However, wide superficial ablation lines with residual endocardial or epicardial tissue (which often leave up to 50% of the thickness of the atrium intact), such as that which occurs in unipolar sources on the beating heart22, would likely give similar results of conduction.
Conclusion
Propagation of paced signals occurred through narrow gaps left in ablation lines on the atrium, even when conduction velocities were significantly slowed. In addition to propagation of paced signals, the majority of narrow residual gaps conducted atrial fibrillation. A close correlation was seen between the propagation of paced signals and atrial fibrillation, suggesting that pacing can be used as a surrogate to ensure adequacy of a lesion. To guarantee complete conduction block in this model, continuous transmural lines of ablation were needed.
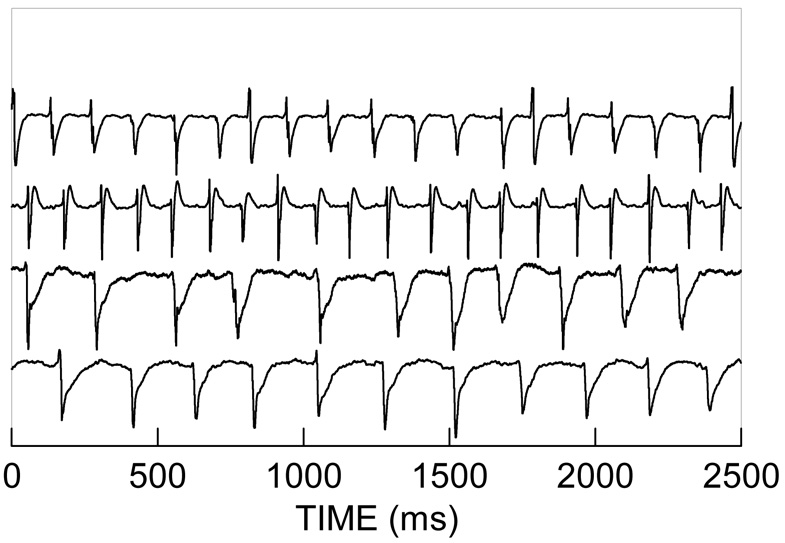
Figure 6.
Electrograms are presented from recordings near the pacing sites; the top two represent recordings near the superior pacing electrode, the bottom two represent recordings near the inferior pacing electrode. Atrial fibrillation was initiated at the superior pacing electrode, propagated through a gap to the inferior portion of the atrium and was sustained at a 2:1 ratio.
Acknowledgments
Funded in part from National Institutes of Health Grants RO1-HL032257, R01-HL085113,and F32 HL078136-01.
Dr. Damiano is a consultant for Atricure, Inc., and Medtronic Corporation. Dr. Schuessler is a consultant for Atricure, Inc.
Abbreviations
- AF
Atrial Fibrillation
- CMP
Cox-Maze procedure
- ERP
Effective Refractory Period
- PCL
Paced Cycle Length
- ACh
Acetylcholine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995 Mar 13;155(5):469–473. [PubMed] [Google Scholar]
- 2.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. Journal of Thoracic & Cardiovascular Surgery. 2003;126(6):1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 3.Melby SJ, Lee AM, Damiano RJ. Advances in surgical ablation devices for atrial fibrillation. In: Wang PJ, editor. New Arrhythmia Technologies. Blackwell Futura; 2005. pp. 233–241. [Google Scholar]
- 4.Ishii Y, Nitta T, Sakamoto S, et al. Incisional atrial reentrant tachycardia: experimental study on the conduction property through the isthmus. J Thorac Cardiovasc Surg. 2003 Jul;126(1):254–262. doi: 10.1016/s0022-5223(02)73603-9. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell MA, McRury ID, Everett TH, et al. Morphological and physiological characteristics of discontinuous linear atrial ablations during atrial pacing and atrial fibrillation. J Cardiovasc Electrophysiol. 1999 Mar;10(3):378–386. doi: 10.1111/j.1540-8167.1999.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SP, Wallace EM, Ross DL. The effect of a residual isthmus of surviving tissue on conduction after linear ablation in atrial myocardium. J Interv Card Electrophysiol. 2000 Apr;4(1):273–281. doi: 10.1023/a:1009838201448. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Zipes DP. Conduction over an isthmus of atrial myocardium in vivo: a possible model of Wolff-Parkinson-White syndrome. Circulation. 1987 Sep;76(3):637–647. doi: 10.1161/01.cir.76.3.637. [DOI] [PubMed] [Google Scholar]
- 8.Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation. 2005 Aug 30;112(9 suppl):I7–I13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 9.Schuessler RB, Grayson TM, Bromberg BI, et al. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circulation Research. 1992;71(5):1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 10.Maessen JG, Nijs JF, Smeets JL, et al. Beating-heart surgical treatment of atrial fibrillation with microwave ablation. Ann Thorac Surg. 2002 Oct;74(4):S1307–S1311. doi: 10.1016/s0003-4975(02)03908-5. [DOI] [PubMed] [Google Scholar]
- 11.Raman JS, Seevanayagam S, Storer M, et al. Combined endocardial and epicardial radiofrequency ablation of right and left atria in the treatment of atrial fibrillation. Ann Thorac Surg. 2001 Sep;72(3):S1096–S1099. doi: 10.1016/s0003-4975(01)02963-0. [DOI] [PubMed] [Google Scholar]
- 12.Sueda T, Nagata H, Shikata H, et al. Simple left atrial procedure for chronic atrial fibrillation associated with mitral valve disease. Ann Thorac Surg. 1996 Dec;62(6):1796–1800. doi: 10.1016/s0003-4975(96)00613-3. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell MA, McRury ID, Haines DE. Linear atrial ablations in a canine model of chronic atrial fibrillation: morphological and electrophysiological observations. Circulation. 1998 Mar 31;97(2):1176–1185. doi: 10.1161/01.cir.97.12.1176. [DOI] [PubMed] [Google Scholar]
- 14.Deneke T, Khargi K, Muller KM, et al. Histopathology of intraoperatively induced linear radiofrequency ablation lesions in patients with chronic atrial fibrillation. Eur Heart J. 2005 Sep;26(17):1797–1803. doi: 10.1093/eurheartj/ehi255. [DOI] [PubMed] [Google Scholar]
- 15.Cox JL, Schuessler RB, D'Agostino HJ, Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991 Apr;101(4):569–583. [PubMed] [Google Scholar]
- 16.Gaita F, Riccardi R, Calo L, et al. Atrial mapping and radiofrequency catheter ablation in patients with idiopathic atrial fibrillation. Electrophysiological findings and ablation results. Circulation. 1998 Jun 2;97(21):2136–2145. doi: 10.1161/01.cir.97.21.2136. [DOI] [PubMed] [Google Scholar]
- 17.Haissaguerre M, Jais P, Shah DC, et al. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1996 Dec;7(12):1132–1144. doi: 10.1111/j.1540-8167.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobza R, Hindricks G, Tanner H, et al. Late recurrent arrhythmias after ablation of atrial fibrillation: incidence, mechanisms, and treatment. Heart Rhythm. 2004 Dec;1(6):676–683. doi: 10.1016/j.hrthm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Cappato R, Calkins H, Chen S-A, et al. Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circulation. 2005 March 8;111(9):1100–1105. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 20.Garrey W. The nature of fibrillary contraction of the heart: its relation to tissue mass and form. Am J Physiol. 1914;33:397–414. [Google Scholar]
- 21.Prasad SM, Maniar HS, Diodato MD, et al. Physiological consequences of bipolar radiofrequency energy on the atria and pulmonary veins: a chronic animal study. Annals of Thoracic Surgery. 2003;76(3):836–841. doi: 10.1016/s0003-4975(03)00716-1. discussion 841-832. [DOI] [PubMed] [Google Scholar]
- 22.Gaynor SL, Byrd GD, Diodato MD, et al. Microwave Ablation for Atrial Fibrillation: Dose-Response Curves in the Cardioplegia-Arrested and Beating Heart. Ann Thorac Surg. 2006;81(1):72–76. doi: 10.1016/j.athoracsur.2005.06.062. [DOI] [PubMed] [Google Scholar]