Abstract
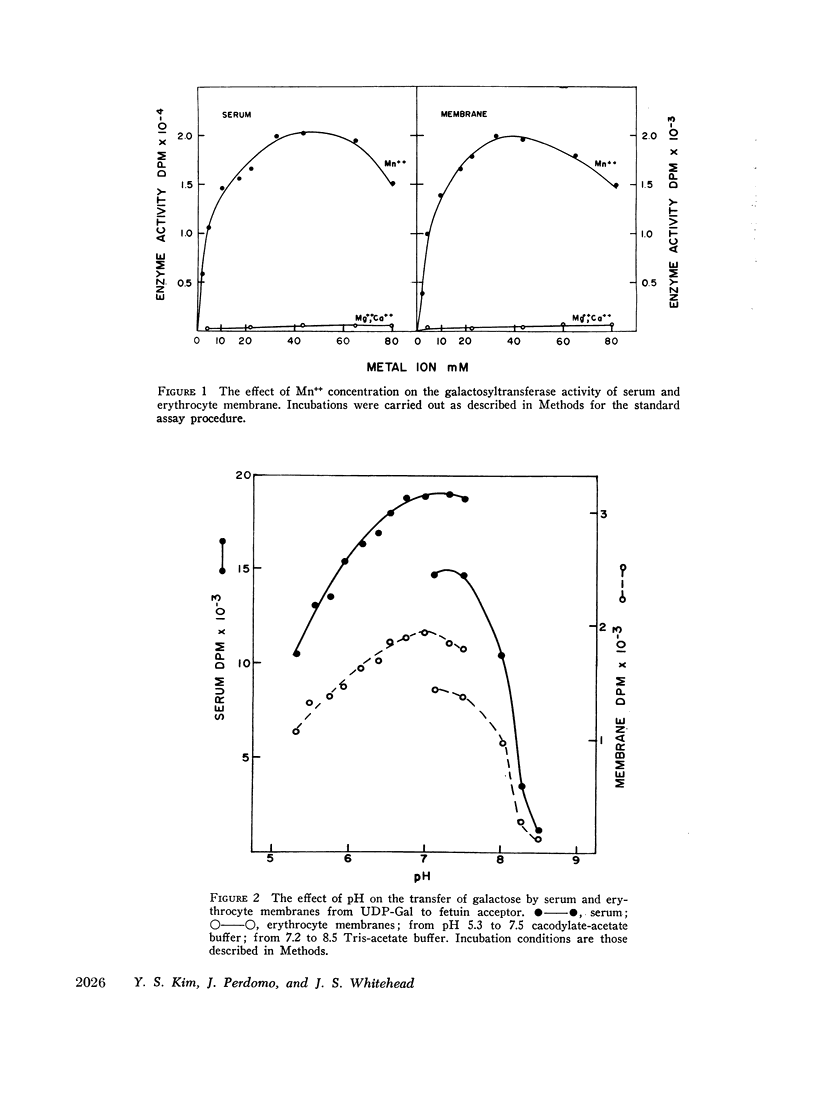
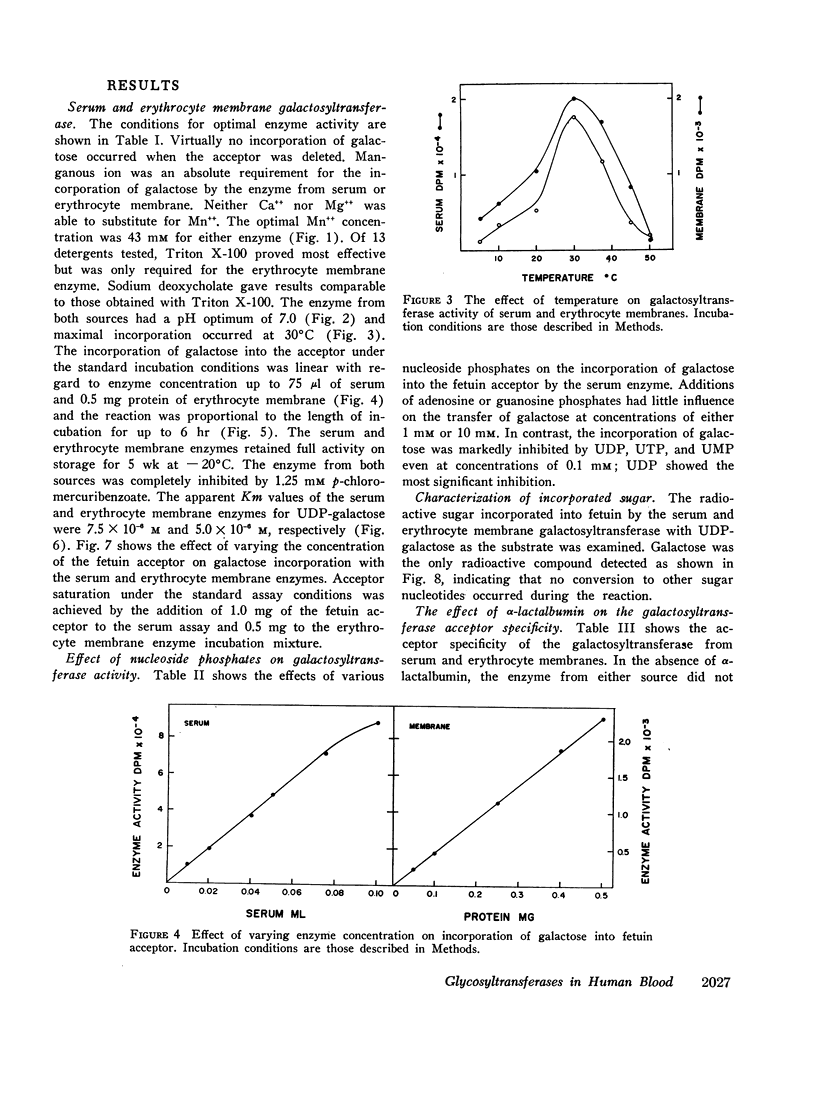
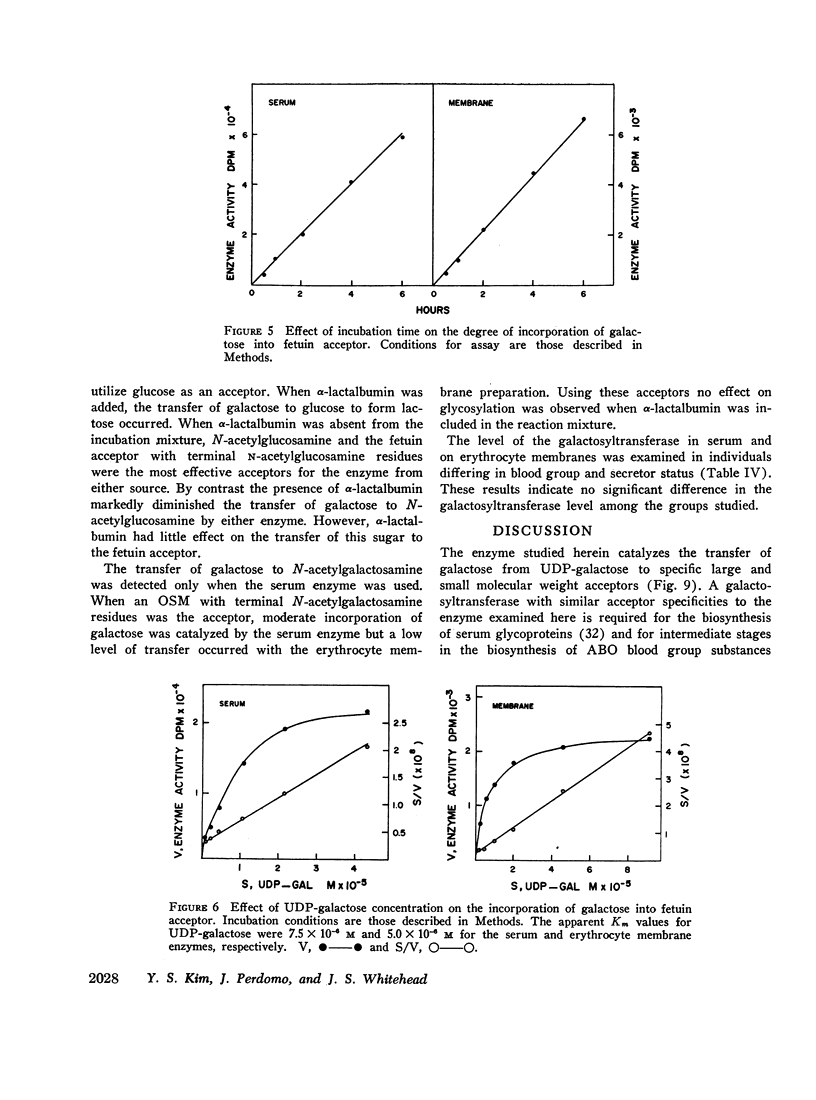
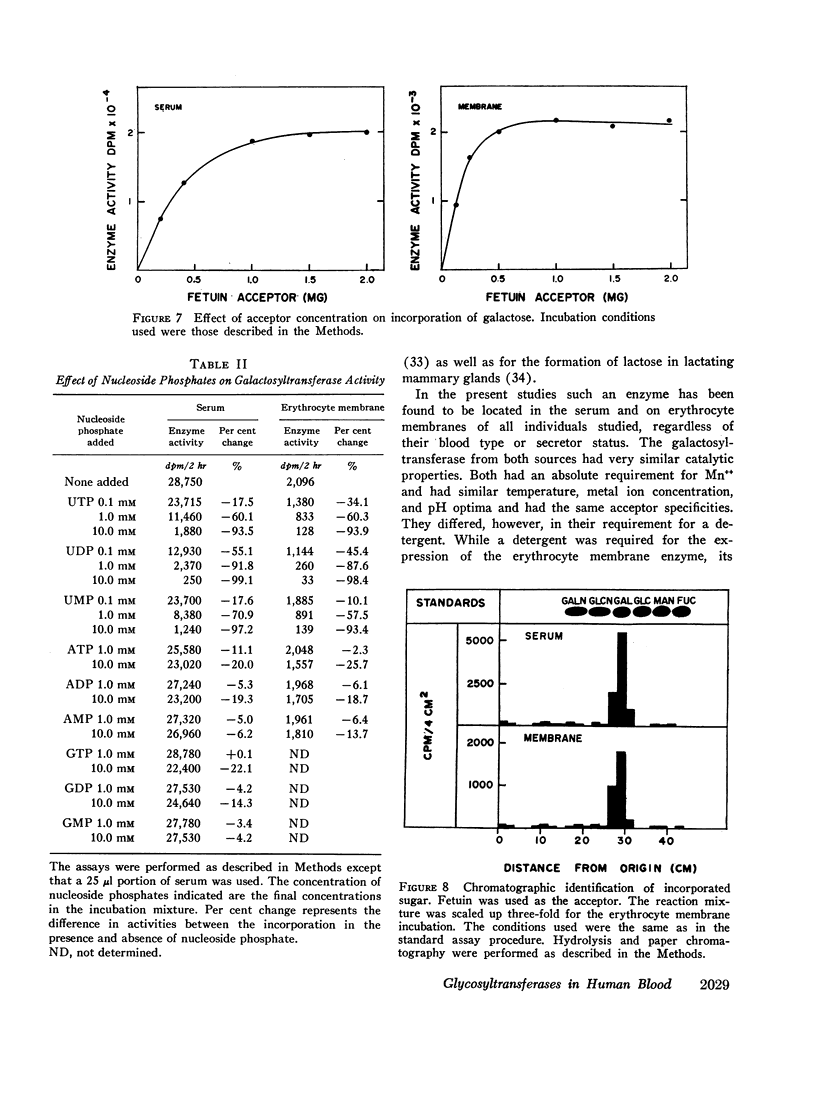
Human serum and hemoglobin-free erythrocyte membranes were found to contain a galactosyltransferase which catalyzes the transfer of galactose from UDP-galactose to specific large and small molecular weight acceptors. The requirements for enzyme activity were found to be similar for the enzymes from both sources. However, the membrane-bound enzyme depended on a detergent for maximal activity. Mn++ was an absolute requirement for transfer and uridine nucleoside phosphates were inhibitors. The most effective acceptor for galactose was a glycoprotein containing N-acetylglucosamine residues in the terminal position of its oligosaccharide side chains, N-acetylglucosamine was also an acceptor. While the presence of α-lactalbumin in the incubation medium resulted in a significant decrease in the transfer of galactose to N-acetylglucosamine, glucose, which was not an acceptor for galactose in the absence of α-lactalbumin, became an excellent acceptor. The serum enzyme catalyzed the transfer of 54 nmoles of galactose per milliliter of serum per hour and its apparent Km for UDP-galactose was 7.5 × 10-6M. The membrane enzyme had a similar apparent Km. Using a quantitative assay system the enzyme was found to be present in all individuals studied, regardless of their blood type, secretor status, or sex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babad H., Hassid W. Z. Soluble uridine diphosphate D-galactose: D-glucose beta-4-D-galactosyltransferase from bovine milk. J Biol Chem. 1966 Jun 10;241(11):2672–2678. [PubMed] [Google Scholar]
- Bella A., Jr, Kim Y. S. Inhibition of rat small-intestinal alpha-(1 leads to 2)-fucosyltransferase. Biochem J. 1971 Dec;125(4):1157–1158. doi: 10.1042/bj1251157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld O. O. The proteins of the erythrocyte membrane obtained by solubilization with aqueous pyridine solution. Biochem Biophys Res Commun. 1968 Jan 25;30(2):200–205. doi: 10.1016/0006-291x(68)90471-3. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodbeck U., Ebner K. E. Resolution of a soluble lactose synthetase into two protein components and solubilization of microsomal lactose synthetase. J Biol Chem. 1966 Feb 10;241(3):762–764. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Den H., Kaufman B., Roseman S. Properties of some glycosyltransferases in embryonic chicken brain. J Biol Chem. 1970 Dec 25;245(24):6607–6615. [PubMed] [Google Scholar]
- Glick J. L., Goldberg A. R., Pardee A. B. The role of sialic acid in the release of proteins from L1210 leukemia cells. Cancer Res. 1966 Aug;26(8):1774–1777. [PubMed] [Google Scholar]
- Gold P., Gold M., Freedman S. O. Cellular location of carcinoembryonic antigens of the human digestive system. Cancer Res. 1968 Jul;28(7):1331–1334. [PubMed] [Google Scholar]
- HAKOMORI S. I., JEANLOZ R. W. Isolation and characterization of glycolipids from erythrocytes of human blood A (plus) and B (plus). J Biol Chem. 1961 Nov;236:2827–2834. [PubMed] [Google Scholar]
- Hudgin R. L., Schachter H. Porcine sugar nucleotide: glycoprotein glycosyltransferases. I. Blood serum and liver sialyltransferase. Can J Biochem. 1971 Jul;49(7):829–837. doi: 10.1139/o71-117. [DOI] [PubMed] [Google Scholar]
- KATHAN R. H., WINZLER R. J., JOHNSOM C. A. Preparation of an inhibitor of viral hemagglutination from human erythrocytes. J Exp Med. 1961 Jan 1;113:37–45. doi: 10.1084/jem.113.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSCIELAK J., ZAKRZEWSKI K. Substance from erythrocytes of blood group A. Nature. 1960 Aug 6;187:516–517. doi: 10.1038/187516b0. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Bella A., Jr, Nordberg J. N-acetyl-D-galactosaminyltransferase in human serum and erythrocyte membranes. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1753–1756. doi: 10.1073/pnas.68.8.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Bella A., Jr, Nordberg J. Properties of a CMP-N-acetylneuraminic acid: glycoprotein sialyltransferase in human serum and erythrocyte membranes. Biochim Biophys Acta. 1971 Sep 21;244(3):505–512. doi: 10.1016/0304-4165(71)90067-5. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Nelsestuen G. L., Suttie J. W. Properties of asialo and aglycoprothrombin. Biochem Biophys Res Commun. 1971 Oct 1;45(1):198–203. doi: 10.1016/0006-291x(71)90069-6. [DOI] [PubMed] [Google Scholar]
- Payza N., Rizvi S., Pigman W. Studies of action of acids and bases on porcine submaxillary mucin. Arch Biochem Biophys. 1969 Jan;129(1):68–74. doi: 10.1016/0003-9861(69)90151-9. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Neutra M., Leblond C. P. Presence of a "cell coat" rich in carbohydrate at the surface of cells in the rat. Anat Rec. 1966 Jan;154(1):41–71. doi: 10.1002/ar.1091540105. [DOI] [PubMed] [Google Scholar]
- Richmond J. E., Glaeser R. M., Todd P. Protein synthesis and aggregation of embryonic cells. Exp Cell Res. 1968 Sep;52(1):43–58. doi: 10.1016/0014-4827(68)90546-6. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Kornfeld S. Hepatic uptake of proteins coupled to fetuin glycopeptide. Biochem Biophys Res Commun. 1971 Nov 5;45(3):622–629. doi: 10.1016/0006-291x(71)90462-1. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Guidotti G. The protein of human erythrocyte membranes. I. Preparation, solubilization, and partial characterization. J Biol Chem. 1968 Apr 25;243(8):1985–1992. [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Schachter H., McGuire E. J., Roseman S. Sialic acids. 13. A uridine diphosphate D-galactose: mucin galactosyltransferase from porcine submaxillary gland. J Biol Chem. 1971 Sep 10;246(17):5321–5328. [PubMed] [Google Scholar]
- Schanbacher F. L., Ebner K. E. Galactosyltransferase acceptor specificity of the lactose synthetase A protein. J Biol Chem. 1970 Oct 10;245(19):5057–5061. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Glycoprotein biosynthesis: studies on thyroglobulin. Thyroid galactosyltransferase. J Biol Chem. 1968 Dec 25;243(24):6529–6537. [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Wagner R. R., Cynkin M. A. Glycoprotein metabolism: a UDP-galactose-glycoprotein galactosyltransferase of rat serum. Biochem Biophys Res Commun. 1971 Oct 1;45(1):57–62. doi: 10.1016/0006-291x(71)90049-0. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Kamat V. B. The contribution of sialic acid to the surface change of fragments of plasma membrane and endoplasmic reticulum. J Cell Biol. 1966 Sep;30(3):660–663. doi: 10.1083/jcb.30.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzler R. J., Harris E. D., Pekas D. J., Johnson C. A., Weber P. Studies on glycopeptides released by trypsin from intact human erythrocytes. Biochemistry. 1967 Jul;6(7):2195–2202. doi: 10.1021/bi00859a042. [DOI] [PubMed] [Google Scholar]
- Ziderman D., Gompertz S., Smith Z. G., Watkins W. M. Glycosyl transferases in mammalian gastric mucosal linings. Biochem Biophys Res Commun. 1967 Oct 11;29(1):56–61. doi: 10.1016/0006-291x(67)90540-2. [DOI] [PubMed] [Google Scholar]



