Abstract
There have been several studies on the maternal administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and effects in the reproductive tract of male offspring, subsequent to risk assessments undertaken in 2001. This review compares the methodology and results to examine key methodological features, and consistency in reported outcomes. Maternal dosing at > 0.8 μg TCDD/kg causes lethality and weight loss, and it is difficult to distinguish between direct and indirect effects of TCDD at these dose levels. Statistically-significant effects of maternal doses of <1 μg TCDD/kg (i.e. the dose levels relevant for risk assessment) on prostate weight or epididymal sperm counts in offspring were reported in the minority of studies. The pharmacokinetics of TCDD differs considerably between acute and chronic dosing, and with dose level of TCDD. On the basis of body burden, TCDD had different potency at inducing adverse effects in the only comparison study between acute and chronic dosing. Understanding of the pharmacokinetics of TCDD and relationship to adverse effects in offspring is required. These analyses identify key features of TCDD developmental toxicity in male offspring, and identify data needs for future risk assessment.
Keywords: Dioxin, TCDD, developmental, reproductive, toxicity, spermatogenesis
1. Background
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a potent and persistent toxicant, which is representative of a family of related compounds, including polyhalogenated dibenzo-dioxins and furans, and polyhalogenated biphenyls (Poland and Knutson, 1982). The high potency of these compounds, combined with their presence in food and the environment, makes a compelling case for a robust assessment of the risk that they pose to human populations. Data on the effects of TCDD on human populations are an inadequate basis for a risk assessment (e.g. Collins et al., 2009), and so several risk assessments are based upon the most potent adverse effects of TCDD that are seen in experimental animals (COT, 2001, JECFA, 2002, SCF, 2001).
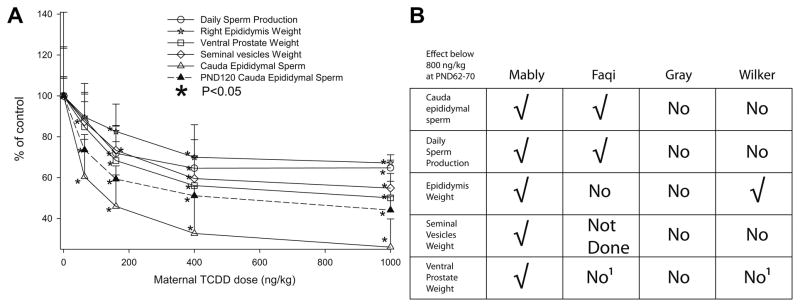
Developmental exposure of rats to as little as 64 ng TCDD/kg was found to cause toxicity in F1 male offspring (Mably et al., 1992a, b, c). Exposure of dams on gestational day (GD)15 to TCDD resulted in a >60% reduction in cauda epididymal sperm levels on post-natal day (PND)63, and these reductions in epididymal sperm levels persisted to PND120 (Fig. 1A), at ~50% of control values. At PND63, the decrease in sperm and spermatid numbers was associated with reductions in weight of the epididymis, ventral prostate and seminal vesicles (Fig. 1A). Effects on accessory sex organs by other chemicals have been causally linked to effects on spermatogenesis through an anti-androgenic mechanism, (e.g. Mylchreest et al., 1998), and so the effect of TCDD on multiple endpoints appears to corroborate the effect on sperm levels.
Fig. 1. Male reproductive effects of TCDD after developmental administration.
A. Some of the effects noted by Mably et al (1992). Holtzman rats were dosed on GD15 with the indicated dose of TCDD (ng/kg), and daily sperm production (circle), right epididymis weight (star), ventral prostate weight (square), seminal vesicle weight (diamond) and cauda epididymal sperm number (triangle) measured in the F1 males on PND 63 (open symbols) or PND120 (closed symbols). Results are normalised to control (set as 100%), and are presented as mean and SD. * indicates P<0.05; note that at 64 ng/kg, right epididymis weight, seminal vesicles weight and ventral prostate weight are not significantly different from control. B. Comparison of effects of TCDD on F1 males at PND62-70, for a maternal TCDD dose of approximately 500 ng/kg and below. Data from (Mably et al., 1992c), (Mably et al., 1992a), (Faqi et al., 1998), (Gray et al., 1997) and (1996) (Wilker et al., 1996) are compared, on the basis of stated statistically significant results. √ means that there was a statistically significant effect. No means no statistically significant effect, Not Done means that the measurement is not reported, and 1 refers to measurements on whole prostate, rather than ventral prostate weight.
However, the constellation of effects originally noted by Mably et al (1992) was not reproducible in three subsequent studies (Faqi et al., 1998, Gray et al., 1997, Wilker et al., 1996); for example, Fig. 1B summarises the results of replication at the PND62-70 time point with doses of <800 ng TCDD/kg. There was a statistically significant effect of TCDD doses <800 ng/kg on F1 epididymal sperm levels in (Faqi et al., 1998) and (Gray et al., 1997), but not in (Wilker et al., 1996). The single consistent effect of TCDD in decreasing epididymal sperm levels in F1 males is the most potent adverse effect of TCDD (Mably et al., 1992a, b, c, Faqi et al., 1998) and it was consequently used as the basis of some risk assessments (COT, 2001, JECFA, 2001, SCF, 2001).
Since these risk assessments were undertaken an additional seven studies examining the effects of developmental exposure of rats to TCDD have been published (Bell et al., 2007b, Bell et al., 2007c, Ikeda et al., 2005, Ohsako et al., 2001, Ohsako et al., 2002, Simanainen et al., 2004, Yonemoto et al., 2005). This review seeks to examine critical aspects of the conduct, design and analysis of studies on developmental exposure to TCDD, and to identify key aspects required for interpretation of these studies. These include statistical aspects of the analyses, differences in measurement parameters between different laboratories, and understanding the differences between acute and chronic dosing regimes in terms of TCDD pharmacokinetics and body burden. The consistency of outcomes from these studies is critically evaluated, and implications for risk assessment are considered.
2. Maternal pharmacokinetics of TCDD
Pharmacokinetics can explain the concentration of TCDD in a target tissue, and how this could be affected by variables in the published papers such as dose, dose frequency, etc. After an acute dose of TCDD to adults, there is a high initial concentration of TCDD in liver, which then redistributes to adipose tissue (Weber et al., 1993). High dose levels of TCDD induce hepatic CYP1A2, which acts as a low-affinity, high-capacity binder of TCDD, causing TCDD to be sequestrated in the liver (Poland et al., 1989a, b). Consequently, the ratio of the concentration of TCDD in the liver, relative to adipose tissue, is a measure of the induction of cytochrome P450 (Diliberto et al., 2001). Markedly different dose levels of TCDD are required to attain an equivalent body burden of TCDD after acute and chronic administration, and these different dose levels can also affect induction of cytochromes P450. However, the relevance of these concepts to fetal exposure to TCDD has been unclear until recently.
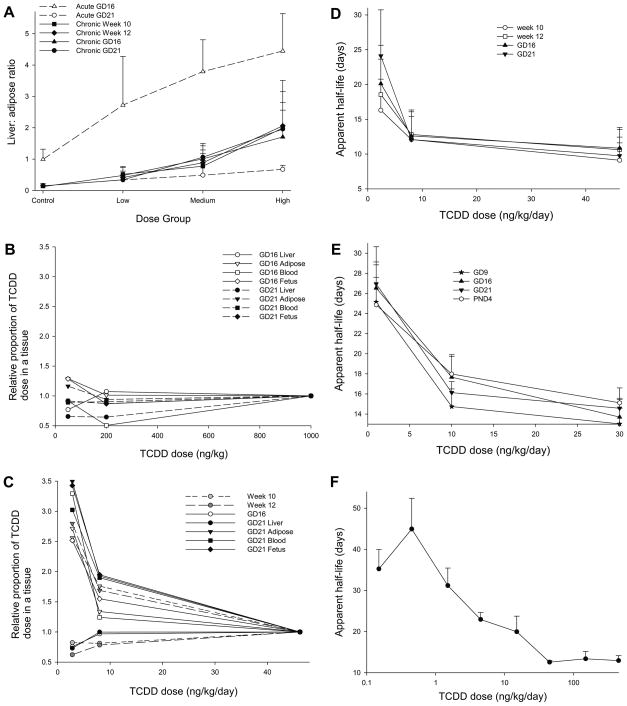
Two independent studies have compared the dosing regimen for TCDD, with either an acute dose on Gestational Day 15 (GD15) or sub-chronic administration before (~ 90 days) and during pregnancy (Bell et al., 2007a, Hurst et al., 2000a, Hurst et al., 2000b). Fig. 2A illustrates the difference in distribution of TCDD between acute and sub-chronic dosing, using the liver: adipose ratio of TCDD concentrations from Bell et al. (2007a). After acute administration of TCDD on GD15, the ratio was > 2.5 in all TCDD dose groups at GD16, but fell to <0.7 in all dose groups by GD21. By contrast, after sub-chronic dosing, there was no difference in the liver: adipose ratio from week 10 of dosing through to GD21. Thus these data demonstrate that there is significant redistribution of TCDD from liver to extrahepatic tissue in the five days after an acute dose, and that this redistribution is not present after sub-chronic dosing.
Fig. 2. Maternal pharmacokinetics of TCDD.
A. The liver: adipose tissue TCDD concentrations from each animal in (Bell et al., 2007b) (Acute study; open symbols) and (Bell et al., 2007c) (Chronic study; filled symbols) were calculated from (Bell et al., 2007a). Data are presented as mean ± SD, for week 10 of dosing (square), week 12 (diamond) GD16 (triangle) and GD21 (circle). B. Relative proportion of TCDD dose in a tissue was calculated as described in materials and methods for the acute study (Bell et al., 2007b). GD16 samples are open symbols, GD21 samples are closed symbols. Liver is shown by circles, adipose by triangles, blood by squares and fetus by diamonds. C. As for B, but for the chronic study (Bell et al., 2007c). Week 10 samples are shown in light grey, and week 12 samples are in dark grey. D. Effect of dose on apparent half-life of TCDD. Data are taken from (Bell et al., 2007a, Bell et al., 2007c), apparent half-life calculated as described in materials and methods, and plotted against dose. The values shown are mean plus an indication of variance, derived from the SD of body burden of TCDD. Data are from week 10 (circle), week 12 (square), GD16 (triangle) or GD21 (inverted triangle). E. As for D, but using the data from (Hurst et al., 2000a). Data are from GD9 (star), GD16 (triangle), GD21 (inverted triangle) and PND4 (circle). F. Effect of dose on apparent half-life of TCDD in mice. As for D, but the data are taken from (Diliberto et al., 2001).
Figs. 2A-C show that acute doses of TCDD approximately 40 ng/kg cause marked hepatic sequestration of TCDD ( Fig. 2B), while the lower dose levels of TCDD used in the chronic administration regime cause a three-fold increase in the proportion of TCDD dose distributing to extrahepatic tissues ( Fig. 2C). This non-linearity of TCDD distribution with dose level is also seen in Hurst et al. (2000a).
A greater proportion of TCDD dose reaches the adipose tissue with lower doses of TCDD, and consequently, the apparent half-life of TCDD increases at lower doses of TCDD, shown in Fig. 2D (Bell et al., 2007a) and Fig. 2E (Hurst et al., 2000a). Both data sets show a marked increase in half-life of TCDD at doses of TCDD below 5 ng/kg/day. Fig. 2F shows the apparent half-life of TCDD in mice (Diliberto et al., 2001) also increases at low doses. Thus increased distribution of TCDD to adipose tissue at low doses of TCDD increases the apparent half-life of TCDD.
These data show that, for the same body burden of TCDD, acute and chronic dosing regimens result in markedly different tissue-specific distribution and pharmacokinetics of TCDD, with high acute doses showing marked hepatic sequestration of TCDD. These high acute doses have reduced delivery of TCDD to extrahepatic tissues (e.g. the fetus, milk), and are consequently less reliable for extrapolation to normal human exposure.
3. Effects of high TCDD dose on perinatal lethality and growth
It is important to choose an appropriate dose level for studies with TCDD, since the gross organ damage arising from frank toxicity can cause secondary effects. Table 2 collates some studies showing the effects of a high dose of TCDD (ca. 1 μg TCDD/kg on GD15, or >40 ng TCDD/kg/day) on perinatal lethality and body growth. Of studies with less than 10 litters per dose group, only one out of six studies showed an effect of TCDD on perinatal pup lethality, with Nishimura et al. (2003) describing a 60% decrease in litter size. Of the five studies with ten or more litters per group, four detect significant decreases in perinatal pup mortality. The finding of perinatal lethality is confirmed in several other studies which have sufficient statistical power, (e.g. Bjerke and Peterson, 1994, Roman et al., 1995, Sommer et al., 1996). Thus we propose that the failure of specific studies to detect lethality in offspring after maternal doses of >0.8–1 μg TCDD/kg is a consequence of inadequate statistical power, and conclude that doses of 0.8–1 μg TCDD/kg cause perinatal lethality affecting ~10–20% of offspring.
TABLE 2.
Effect of high TCDD dose on perinatal pup mortality.
| Study | Strain | TCDD dose | Perinatal lethality and number of litters | Decreased body weight gain [statistical allowance for litter effects] |
|---|---|---|---|---|
| Bell et al., 2007c | Wistar(Han) | 46.2 ng/kg/day | increase in total litter loss, and number of pups surviving to PND 4 reduced, n=27 | From PND1-120 [yes] |
| Mably et al., 1992c | Holtzman | 1 μg/kg | 8% decrease in live birth index, n=12–16 | From PND 1–63 [yes] |
| Gray and Ostby, 1995 | Long Evans hooded | 1 μg/kg | no effect, n=8 | From PND1-22 [yes; litter means] |
| Wilker et al., 1996 | Sprague-Dawley | 1 μg/kg | no effect, n=3–5 | From PND1-45 [yes; litter means] |
| Gray et al., 1997 | Long Evans hooded | 0.8 μg/kg | pup survival to day 22 reduced by 17%, n=10–12 | From PND1-49 (except PND15) [yes; litter means] |
| Ohsako et al., 2001 | Holtzman | 0.8 μg/kg | no effect, n=6 | no [no] |
| Ohsako et al., 2002 | Sprague-Dawley | 1 μg/kg | not stated, n=4 | no significant effect measured on PND70 [no] |
| Nishimura et al., 2003 | Holtzman | 0.8 μg/kg | 60% decrease in litter size, n=5 | no [no] |
| Simanainen et al., 2004 | Wistar(Han)xLon g Evans crosses | 1 μg/kg | not stated, n=4–7 | From PND 1–49 (A), 1–35, 70 (B), 1–49 (C) [yes; litter means] |
| Yonemoto et al., 2005 | Long Evans | 0.8 μg/kg | no effect, n=12 | From PND 28–56 [no] |
| Bell et al., 2007b | Wistar(Han) | 1 μg/kg | 12% decrease in number of pups alive at PND22, n=15 | From PND1-120 [yes] |
The study, strain and high dose regimen are indicated. Indices of perinatal lethality are shown and effect size indicated, together with the number of litters (n=). The time points of decreases in body weight gain or body weight are shown (decreased body weight gain). The statistical treatment of litter effects is shown in square brackets; yes indicates that the analysis accounted for litter effects, whereas no indicates that there was no allowance for litter effects. Studies with chronic and acute dosing are shown above and below the thick black line, respectively.
The effect of ~1 μg TCDD/kg on body weight gain in offspring can be readily measured, but this is assessed with a variety of endpoints (body weight, or body weight gain) and differing rigour of approach (weight at a single time-point, serial weights of individual animals, failure to account for litter as a variable). Of eleven studies, seven found a significant decrease in body weight or body weight gain (Table 2). Three of the four studies which found no effect on body weight gain in the offspring had six or less litters per treatment group, and consequently, in-sufficient statistical power for detecting effects on body weight.
Thus high doses of TCDD during development cause increased perinatal lethality and decreased body weight gain (Table 2). The use of these dose levels to examine effects on reproductive parameters is potentially confounded by such effects, and so it is not necessarily possible to extrapolate from the effects seen at these high dose levels to those seen at lower dose levels. This review therefore concentrates on dose levels less than 1 μg of TCDD/kg.
4. Variability in measurements of epididymal sperm levels in rat
The results of Mably et al. (1992a) show highly significant effects; for example, a t-test shows that epididymal sperm levels from control versus high-dose TCDD rats on PND120 are significantly different, with P < 10−10. Moreover, Mably et al. (1992a) were able to state that “the LOAEL can be estimated to be substantially lower than 0.064 μg TCDD/kg (the lowest dose tested)”; these high levels of significance arise in part from the low variability in epididymal sperm measurements reported by Mably et al. (1992a). However, for twelve studies on developmental toxicity of TCDD (Bell et al., 2007b, c, Ikeda et al., 2005, Ohsako et al., 2001, Ohsako et al., 2002, Simanainen et al., 2004, Yonemoto et al., 2005, Mably et al., 1992a, Faqi et al., 1998, Gray et al., 1997, Wilker et al., 1996, Gray et al., 1995), the coefficient of variation in cauda epididymal sperm levels ranges from 0.089 (Mably et al., 1992a) to 0.54 (Bell et al., 2007b) at PND120, with a mean of 0.26. The study of Mably et al. (1992a) has the lowest variability in epididymal sperm count measurement. The low variability in sperm counts is not unique to Holtzman rats (the strain used by Mably et al., 1992a–c), since subsequent studies in Holtzman rats had coefficients of variation of 0.42 (Ohsako et al., 2001) and 0.12 (Ikeda et al., 2005). It is difficult to pinpoint any specific methodological difference that could account for the low coefficient of variation in the study of Mably; this study used manual counting of epididymal sperm, with no mention of operator “blinding”, and accounts for litter effects.
Another approach to characterising variability in sperm measurements is to examine mean epididymal sperm counts from the same laboratory. For example, Ohsako et al. (2002) reported counts of 25 ± 2.9 × 106 and 20 ± 1.9×106 (mean ± SEM) in control groups of PND70 males, a ~20% difference. Fig. 3C and D show that after maternal exposure to TCDD, epididymal sperm counts in F1 rats are routinely in the range of 110–140% of control values, presumably reflecting the range of normal variation in mean sperm counts. Thus mean epididymal sperm counts from rats performed in the same laboratory frequently show variation of 10–30%.
Fig. 3. Effect of developmental exposure to TCDD on male F1 reproductive endpoints.
A. Effects on prostate weight at PND 62–70. All experiments are normalised to control values of 100%, and results are shown as mean ± SD. Statistical significance at P<0.05 is shown by filled symbols. Data are from (Mably et al., 1992c) (circle), (Gray et al., 1997) (triangle), (Faqi et al., 1998) (circle with cross), (Simanainen et al., 2004) lines A (square), B (square with dot) and C (square with cross), (Yonemoto et al., 2005) (diamond with cross), (Ohsako et al., 2002) (diamond), (Wilker et al., 1996) (inverted triangle), (Bell et al., 2007b) (hexagon), and (Bell et al., 2007c) (hexagon with cross). Results are ventral prostate weight, except for (Bell et al., 2007b), (Wilker et al., 1996) and (Faqi et al., 1998), which are prostate weight. Doses greater than 1 μg TCDD/kg are not shown. Studies with chronic dosing of TCDD are shown by the equivalent acute doses, based on tissue concentrations of TCDD (Bell et al., 2007a). B. Effects on prostate weight at PND 120+. All experiments are normalised to control values of 100%, and results are shown as mean ± SD. Statistical significance at P<0.05 is shown by filled symbols. Data are from (Mably et al., 1992c) (circle), (Gray et al., 1997) (triangle), (Faqi et al., 1998) (circle with cross), (Ikeda et al., 2005) (square), (Ohsako et al., 2001) (diamond), (Bell et al., 2007b) (hexagon), and (Bell et al., 2007c) (hexagon with cross). Results are ventral prostate weight, except for (Bell et al., 2007b) and (Faqi et al., 1998), which are prostate weight. C. Effects on epididymal sperm count at PND 62–70. All experiments are normalised to control values of 100%, and results are shown as mean ± SEM. Statistical significance at P<0.05 is shown by filled symbols. Data are from (Mably et al., 1992a) (circle), (Gray et al., 1997) (triangle), (Faqi et al., 1998) (circle with cross), (Simanainen et al., 2004) lines A (square), B (square with dot) and C (square with cross), (Yonemoto et al., 2005) (diamond with cross), (Ohsako et al., 2002) (diamond), (Wilker et al., 1996) (inverted triangle), (Bell et al., 2007b) (hexagon), and (Bell et al., 2007c) (hexagon with cross). Doses greater than 1 μg TCDD/kg are not shown. Studies with chronic dosing of TCDD are shown by the equivalent acute doses, based on tissue concentrations of TCDD (Bell et al., 2007a). D. Effects on epididymal sperm counts at PND 120+. All experiments are normalised to control values of 100%, and results are shown as mean ± SEM. Statistical significance at P<0.05 is shown by filled symbols. Data are from (Mably et al., 1992c) (circle), (Gray et al., 1997) (triangle), (Faqi et al., 1998) (circle with cross), (Ikeda et al., 2005) (square), (Ohsako et al., 2001) (diamond), (Bell et al., 2007b) (hexagon), and (Bell et al., 2007c) (hexagon with cross).
5. Reproducibility of effects of developmental exposure to TCDD on F1 males
The effect of developmental exposure to TCDD on male F1 reproductive endpoints has been examined in eleven studies with doses below 1 μg TCDD/kg. This review will focus on effects at PND62-70 and PND 120+, since these are the basis of the risk assessments that have been performed. The use of sexually mature animals is recommended for evaluating effects on spermatogenesis (Creasy, 2003, Lanning et al., 2002), and so data from immature animals is not considered further. Fig. 3A shows data examining the effect of TCDD on prostate weight at PND 62–70. With the exception of the original report, there were no significant effects of < 1 μg/kg TCDD on prostate weight at PND62-70 in eight other studies. The studies have sufficient statistical power to detect an effect; for example, Bell et al. (2007c) report a 90% power for detecting a 10% decrease in ventral prostate weight, compared with the ~40% decrease in ventral prostate weight reported by Mably et al. (1992a-c).
In F1 rats at PND120+, Mably et al. (1992a-c) reported no significant effect of maternal TCDD treatment on ventral prostate weight below 1 μg TCDD/kg (Fig. 3B). In contrast, a decrease in ventral prostate weight was seen after an acute dose of 200 ng TCDD/kg (Ohsako et al., 2001), or a chronic dose approximately equivalent to an acute dose of 400 ng TCDD/kg (Ikeda et al., 2005); notably, neither of these two studies accounted for litter as a source of variation in the statistical analysis, and the failure to account for litter effects can lead to spuriously inflated estimations of significance (Weil, 1970). Bell et al. (2007c) reported a significant increase in prostate weight after chronic dosing of 8 ng TCDD/kg/day (approximately equivalent to an acute dose of 200 ng TCDD/kg). Thus after developmental exposure to <1 μg TCDD/kg, statistically significant reductions in prostate weight have been reported in only three studies, two of which used inappropriate statistical analysis.
Fig. 3C and D show the effect of maternal exposure to TCDD on epididymal sperm levels in F1 rats, at PND 62–70, and 120+, respectively. Faqi et al. (1998) reported a decrease in epididymal sperm at ~50 ng TCDD/kg (the chronic dose regime used was approximated to an acute dose on the basis of liver TCDD concentration), Gray et al. (1997) saw effects at 200 ng TCDD/kg in adult (but not PND63) rats, and Simanainen et al. (2004) saw a reduction after 300 ng TCDD/kg in line C rats, but not at lower doses (Fig. 3C, D). The magnitude of these reductions in epididymal sperm count was less than that observed by Mably et al. (1992a), and was frequently within 30% of control values, i.e. within the range of normal variation. Bell et al. (2007b) observed a significant increase in epididymal sperm counts of 30–38% at the top two doses of TCDD, noted that these values were not accompanied by an effect on testicular sperm production, were within the range of historical control epididymal sperm counts, and concluded that the statistical significance of these results arose from random variation. Statistically significant reductions in epididymal sperm numbers have been observed in only four out of eleven studies at doses <1 μg TCDD/kg, and in only three studies at doses <300 ng TCDD/kg.
Table 1 shows measurements of developmental delay in the offspring of TCDD-exposed dams, and TCDD-induced delay in balanopreputial separation (BPS, a marker of male puberty) has been demonstrated in each study where it has been measured. After a single dose of TCDD on GD15, Gray et al. (1997) reported a delay after a maternal dose of 200 ng TCDD/kg, Yonemoto et al. (2005) reported a significant delay after a maternal dose of 200 ng TCDD/kg, and Bell et al. (2007b) found no significant effect at 50 or 200 ng TCDD/kg, only at the highest dose of 1000 ng TCDD/kg; however, in this latter experiment, the data at 200 ng TCDD/kg were just above the threshold for statistical significance. After sub-chronic dosing of TCDD, Faqi et al. (1998) reported a significant delay of unspecified magnitude in their medium and high dose groups, and Bell et al. (2007c) showed that all three dose groups (2.4, 8 and 46 ng TCDD/kg/day) caused a significant delay in BPS. Although maternal TCDD administration reduces body weight gain in offspring, the body weight of males at PND 21 or 42 did not correlate with the delay in BPS, thus excluding decreased weight gain as a cause of delayed BPS (Bell et al., 2007c). Thus delay in BPS is consistently found to be an adverse effect in the offspring of animals dosed with TCDD, and may be the most sensitive adverse effect. The direct comparison of acute and chronic dosing of TCDD in the same strain of rats using the same methodology (Bell et al., 2007b, 2007c) provides evidence that chronic dosing of TCDD has more potent effects in offspring. The difference in toxicity between acute and chronic dosing regimens has only been directly compared in one laboratory, and repeating this experiment in an independent laboratory is essential to demonstrate reproducibility.
TABLE 1.
Effect of maternal TCDD dose on developmental delay.
| Study | Strain | TCDD dosing regime | Developmental delay |
|---|---|---|---|
| Faqi et al., 1998 | Wistar | Loading and weekly maintenance dose | “Age at preputial separation was slightly delayed”, no statistical analysis shown (n=17–22) |
| Ikeda et al., 2005 | Holtzman | Loading and weekly maintenance dose | N.D. |
| Bell et al., 2007c | Wistar(Han) | Chronic dietary dosing for > twelve weeks | Yes, delay in BPS of 1.8, 1.9 and 4.4. days (n=18–25) |
| Mably et al., 1992a, Mably et al., 1992b, Mably et al., 1992c | Holtzman | Single dose on GD15 | Yes, testis descent and eye opening. BPS N.D. (n=9) |
| Bjerke and Peterson, 1994 | Holtzman | Single dose on GD15; also in utero and/or lactational | yes, 2.4 or 3.4 day delay in BPS (n=9–11) |
| Gray et al., 1995 | Long Evans hooded | Single dose on GD15 or GD 8 | Yes, 3.6 day delay in BPS (n=6–8) |
| Roman et al., 1995 | Holtzman | Single dose on GD15 | Yes, 2.1 day delay in BPS (n=30–32) |
| Wilker et al., 1996 | Sprague-Dawley | Single dose on GD15 | N.D. |
| Sommer et al., 1996 | Holtzman | Single dose on GD15 | Yes, 2 day delay in BPS (n=34–39) |
| Gray et al., 1997 | Long Evans hooded | Single dose on GD15 | Yes, delay in eye opening, delay in BPS of 1.5, 3.1 days (n=10–12) |
| Ohsako et al., 2001 | Holtzman | Single dose on GD15 | N.D. |
| Ohsako et al., 2002 | Sprague-Dawley | Single dose on GD15 or GD18 | N.D. |
| Simanainen et al., 2004 | Wistar(Han)xLong Evans crosses | Single dose on GD15 | N.D. |
| Yonemoto et al., 2005 | Long Evans | Single dose on GD15 | Yes, delay in BPS (n=9–12) |
| Bell et al., 2007b | Wistar(Han) | Single dose on GD15 | Yes, delay in BPS of 2.8 days (n=15–21) |
The study, strain and TCDD dosing regimen are indicated. Indices of developmental delay are shown and effect size indicated, together with the number of litters (n=). Balanopreputial separation is BPS, Not Done is N.D. Studies with chronic and acute dosing are shown above and below the thick black line, respectively.
6. Critical periods of susceptibility for exposure to TCDD
It is known that GD16-17 is a critical period of development in the rat, when anti-androgens and phthalates exert effects on the developing male reproductive system (Carruthers and Foster, 2005, Welsh et al., 2008). Given that developmental exposure to TCDD results in adverse effects in the offspring, the developmental timing of susceptibility to TCDD is important, and relevant studies are summarised in Table 3. Gray et al. (1995) show that dosing at GD15, compared to GD8, has a much greater effect on offspring. Ohsako et al. (2002) showed there were numerous significant effects in offspring from rats treated at GD15, but little effect in offspring of rats treated with TCDD on GD18 or PND2. In contrast, Bjerke and Peterson (1994) found that both in utero and lactational exposure caused significant effects in offspring, but in utero exposure had a larger effect than lactational exposure on daily sperm production, day of preputial separation, and suppression of growth. Nishimura et al. (2003) had shown that administration of TCDD to Holtzman rats on GD15 results in thyroid hyperplasia in offspring. Lactational, but not in utero, exposure to TCDD was responsible for effects in offspring (Nishimura et al., 2005). It is challenging to reconcile data showing no or minimal effect of TCDD when administered on GD18 or PND2 (Ohsako et al., 2002) with data showing that lactational exposure of pups alone can have a constellation of effects (Bjerke et al., 1994). The experiments of Nishimura et al. (2005, 2006) do not bear directly on male reproduction, but the profound toxicity caused by lactational transfer of TCDD is likely to affect reproductive physiology. The existing data are contradictory, and do not allow a conclusion as to when developmental exposure to TCDD causes adverse reproductive effects in offspring.
TABLE 3.
Studies on developmental timing of susceptibility to TCDD
| Study | Strain | TCDD exposure | Effects |
|---|---|---|---|
| Gray et al., 1995 | Long Evans hooded rats | GD8 | Decrease in body weight on PND1, ejaculated sperm count, age at BPS |
| GD15 | Decrease in body weight on PND1-22, AnoGenital Distance (AGD), male sexual behaviour, weight of testes, cauda epididymis, seminal vesicles, testis spermatid count, cauda epididymal sperm number, ejaculated sperm count. | ||
| Ohsako et al., 2002 | Sprague-Dawley | GD15 | Decrease in weight of testes, epididymes, ventral prostate, urogenital complex, cauda epididymal sperm count, AGD. |
| GD18 | Decrease in AGD | ||
| PND2 | Increase in right kidney weight | ||
| Bjerke and Peterson, 1994 | Holtzman | in utero | Decrease in crown-rump length, body weight at days 1–63, AGD, age at BPS, plasma testosterone, weight of ventral prostate, seminal vesicles, testes, right caudal epididymis, glans penis, counts for testis spermatid count, cauda epididymal sperm. |
| lactational | Decrease in crown-rump-length, body weight at days 4–63, AGD (day 4 only), plasma testosterone, weight of ventral prostate, seminal vesicles, testes, glans penis, cauda epididymal sperm count. | ||
| in utero and lactational | Decrease in crown-rump length, body weight at days 1–63, AGD, age at BPS, weight of ventral prostate, seminal vesicles, testes, right caudal epididymis, glans penis, counts for testis spermatid count, cauda epididymal sperm. | ||
| Nishimura et al., 2005 | Holtzman | in utero | no effect on pathological parameters |
| lactational | Increase in liver weight, decrease in male thymus weight, decrease in serum thyroxine, increase in serum TSH. | ||
| in utero and lactational | Increase in liver weight, decrease in thymus weight, decrease in serum thyroxine, increase in serum TSH. |
7. Discussion
Mably et al. (1992c) showed that maternal administration of 160 or 400 ng TCDD/kg reduced the weight of prostate in F1 males at PND 63 (and not in adult rats), and these effects were highly significant. However, only two (out of 10 other studies now conducted) show a statistically significant decrease in prostate weight after maternal administration of <1 μg TCDD/kg, and these particular studies (Ikeda et al., 2005, Ohsako et al., 2001) failed to account for litter differences as a source of variation in their statistical analyses (Weil, 1970). After maternal doses of <1 μg TCDD/kg, eight of eleven studies show no significant decrease in prostate weight from control values, and so the original report (Mably et al., 1992c) is not reproducible in the majority of laboratories.
Likewise, four out of eleven studies show a decrease in epididymal sperm counts after maternal dosing of <1 μg TCDD/kg (Faqi et al., 1998, Gray et al., 1997, Mably et al., 1992a, Simanainen et al., 2004). With the exception of (Mably et al., 1992a), the decreases seen in epididymal sperm count at maternal doses of <1 μg TCDD/kg are less than 30% from control values. Ashby et al. (2003) highlighted the importance of historical control data in interpreting minimalist changes in testicular sperm counts; there must be considerable caution in interpreting differences of 20% or less in epididymal sperm levels as being treatment-related effects, especially when the data show an ambiguous relationship with dose (Faqi et al., 1998, Gray et al., 1997). The fact that seven of the eleven studies (Fig. 3) find no significant decrease in F1 epididymal sperm counts after maternal dosing of <1 μg TCDD/kg, coupled to small effect size and weak dose-response of effects, calls into question whether this effect is reproducible. With the exception of the studies of Mably et al. (1992a, b, c), the studies that found an effect of <1 μg TCDD/kg of TCDD on prostate weight did not find an effect on epididymal sperm levels, and vice versa. The demonstration of adverse effects both on sperm counts and on accessory sexual organs, provides a corroboration that is causally related via mechanism of action, e.g. through anti-androgenic signalling (Howdeshell et al., 2006). Corroboration through effects on multiple endpoints for the endpoints in Fig. 1A is evanescent (Fig. 3, data not shown).
In contrast, high maternal doses of TCDD (>0.8 μg TCDD/kg) cause perinatal lethality, decreased body weight (gain) in the pups (Table 2), and other toxicities (Nishimura et al., 2003, 2005, 2006). After maternal doses of ~1 μg TCDD/kg, it is difficult to determine if any effect in offspring is a direct result of TCDD, or is instead mediated non-specifically through toxic effects on other organs. The use of maternal doses of 1 μg TCDD/kg for mechanistic studies to explain effects seen at 50–200 ng/kg is to be deprecated.
It is challenging to reconcile published reports that examine when TCDD exerts adverse effects on offspring after maternal administration, and consequently, its mechanism of action (Table 3). Some studies show that in utero exposure alone causes toxicity (Ohsako et al., 2002), whereas others show that lactational transfer alone cause toxicity in offspring (Bjerke and Peterson, 1994, Nishimura et al., 2005). Clarification of the mode of action of TCDD is important for two reasons. Firstly, this information is necessary to interpret existing data. For example, Bell et al. (2007b) exposed dams to an acute dose of TCDD on GD15, and found that the LOAEL was 1 μg TCDD/kg, with no significant effects in offspring at 50 or 200 ng TCDD/kg. Chronic dosing of TCDD had a LOAEL of 2 ng TCDD/kg/day for delay in balanopreputial separation (BPS) (Bell et al., 2007c). The TCDD body burdens on GD16 and 21 (Bell et al., 2007a) are roughly equivalent between a chronic dose of 2 ng/kg/day and an acute dose of 50 ng/kg/day. Given that GD16-18 is the time period when TCDD exerts its effects on the developing embryo that lead to reproductive effects, it should follow that there is an approximately equivalent potency between chronic and acute dosing of TCDD. In fact, chronic dosing is approximately 20-fold more potent (based on TCDD body burden) than acute dosing of TCDD at inducing delay in BPS in F1 males. This analysis suggests that GD16-18 is not the time when TCDD is exerting the toxic effect that leads to a delay in BPS. An alternative explanation is that TCDD exerts its toxic effects either before GD15, or after parturition, when the body burden of TCDD would be markedly different between acute and chronic dosing regimens.
The second reason for understanding the timing and mechanism of action is that the risk assessments use a pharmacokinetic comparison between human and rat. One assumption is that exposure in utero at GD16-18 mediates the effects of developmental TCDD exposure, but this assumption is questionable (Table 3). Moreover, if lactational transfer of TCDD is a key determinant of TCDD toxicity in offspring, the consequences for human risk assessment would be difficult to predict. Physiologically-based pharmacokinetic models of TCDD distribution have varying predictivity (Emond et al., 2004, Aylward et al., 2005, Evans and Andersen, 2000), do not yet predict lactational transfer of TCDD, and the apparent half-life of TCDD in children is lower than in adults (Kreuzer et al., 1997, Kerger et al., 2006, Leung et al., 2006). Thus determining whether TCDD exerts effects in utero, or via lactation, will have ramifications for risk assessments of dioxins that rely upon the assumption that TCDD exerts its effects in utero (COT, 2001, JECFA, 2002, SCF, 2001). This is therefore a key uncertainty in risk assessment.
8. Conclusions
In conclusion, there has been a failure to replicate the magnitude or variety of responses caused by maternal doses of <1 μg TCDD/kg in the original work of Mably et al. (1992a, b, c) since the risk assessments in 2001. While there are reports of adverse effects in offspring after maternal administration of < 1 μg TCDD/kg, these reports are in the minority for prostate weight and epididymal sperm counts, and are frequently within the range of historical variation seen in other laboratories. It is unclear why it has not been possible to replicate these findings, despite extensive efforts. Effects on developmental milestones (BPS) are consistently found, and the potency of TCDD to induce these effects appears to be much greater after chronic dosing, compared with acute dosing. Maternal pharmacokinetics of TCDD vary considerably between acute and chronic dosing, and these two differing dosing regimens have been shown to impact upon the potency of TCDD at inducing adverse effects. Thus understanding how and when TCDD operates to cause adverse effects in F1 animals after low dose maternal exposure is a key research need, with consequences for current risk evaluations of dioxins and dioxin-like compounds.
Acknowledgments
Practical studies on the toxicology and disposition of TCDD were funded by a contract (T01034) from the UK Food Standards Agency. We thank Declan Brady for excellent technical support. We thank the editor and anonymous reviewers for constructive and insightful comments.
Abbreviations
- BPS
Balano-Preputial Separation
- CASA
Computer-Assisted Sperm Analysis
- GD
Gestational Day
- PND
Post-Natal Day
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby J, Tinwell H, Lefevre PA, Joiner R, Haseman J. The effect on sperm production in adult Sprague-Dawley rats exposed by gavage to bisphenol a between postnatal days 91–97. Toxicol Sci. 2003;74:129–138. doi: 10.1093/toxsci/kfg093. [DOI] [PubMed] [Google Scholar]
- Aylward LL, Brunet RC, Starr TB, Carrier G, Delzell E, Cheng H, Beall C. Exposure reconstruction for the TCDD-exposed NIOSH cohort using a concentration- and age-dependent model of elimination. Risk Anal. 2005;25:945–956. doi: 10.1111/j.1539-6924.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- Bell DR, Clode S, Fan MQ, Fernandes A, Foster PM, Jiang T, Loizou G, Macnicoll A, Miller BG, Rose M, Tran L, White S. Relationships between tissue levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), mRNAs and toxicity in the developing male Wistar(Han) rat. Toxicol Sci. 2007a;99:591–604. doi: 10.1093/toxsci/kfm179. [DOI] [PubMed] [Google Scholar]
- Bell DR, Clode S, Fan MQ, Fernandes A, Foster PM, Jiang T, Loizou G, Macnicoll A, Miller BG, Rose M, Tran L, White S. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the developing male Wistar(Han) rat I: No Decrease in Epididymal Sperm Count After a Single Acute Dose. Toxicol Sci. 2007b;99:214–223. doi: 10.1093/toxsci/kfm140. [DOI] [PubMed] [Google Scholar]
- Bell DR, Clode S, Fan MQ, Fernandes A, Foster PM, Jiang T, Loizou G, Macnicoll A, Miller BG, Rose M, Tran L, White S. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the developing male Wistar(Han) rat II: chronic dosing causes developmental delay. Toxicol Sci. 2007c;99:224–233. doi: 10.1093/toxsci/kfm141. [DOI] [PubMed] [Google Scholar]
- Bjerke DL, Peterson RE. Reproductive toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male-rats - different effects of in-utero versus lactational exposure. Tox App Pharmacol. 1994;127:241–249. doi: 10.1006/taap.1994.1158. [DOI] [PubMed] [Google Scholar]
- Bjerke DL, Sommer RJ, Moore RW, Peterson RE. Effects of in-utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on responsiveness of the male-rat reproductive-system to testosterone stimulation in adulthood. Tox App Pharmacol. 1994;127:250–257. doi: 10.1006/taap.1994.1159. [DOI] [PubMed] [Google Scholar]
- Carruthers CM, Foster PMD. Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B-Dev Reprod Toxicol. 2005;74:277–285. doi: 10.1002/bdrb.20050. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Bodner K, Aylward LL, Wilken M, Bodnar CM. Mortality rates among trichlorophenol workers with exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Am J Epidemiol. 2009;170:501–506. doi: 10.1093/aje/kwp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COT. Comittee on Toxicity of Chemicals in Food, Consumer Products and the Environment. COT/2001/07. 2001. Statement on the tolerable daily intake for dioxins and dioxin-like polychlorinated biphenyls; p. COT/2001/07. [Google Scholar]
- Creasy DM. Evaluation of testicular toxicology: A synopsis and discussion of the recommendations proposed by the society of toxicologic pathology. Birth Defects Res B-Dev Reprod Toxicol. 2003;68:408–415. doi: 10.1002/bdrb.10041. [DOI] [PubMed] [Google Scholar]
- Diliberto JJ, Devito MJ, Ross GD, Birnbaum LS. Subchronic exposure of [H-3]-2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female B6C3F1 mice: relationship of steady-state levels to disposition and metabolism. Toxicol Sci. 2001;61:241–255. doi: 10.1093/toxsci/61.2.241. [DOI] [PubMed] [Google Scholar]
- Emond C, Birnbaum LS, Devito MJ. Physiologically based pharmacokinetic model for developmental exposures to TCDD in the rat. Toxicol Sci. 2004;80:115–133. doi: 10.1093/toxsci/kfh117. [DOI] [PubMed] [Google Scholar]
- Evans MV, Andersen ME. Sensitivity analysis of a physiological model for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): Assessing the impact of specific model parameters on sequestration in liver and fat in the rat. Toxicol Sci. 2000;54:71–80. doi: 10.1093/toxsci/54.1.71. [DOI] [PubMed] [Google Scholar]
- Faqi AS, Dalsenter PR, Merker HJ, Chahoud I. Reproductive toxicity and tissue concentrations of low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male offspring rats exposed throughout pregnancy and lactation. Toxicol App Pharmacol. 1998;150:383–392. doi: 10.1006/taap.1998.8433. [DOI] [PubMed] [Google Scholar]
- Gray LE, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male long-evans rats and hamsters - reduced ejaculated and epididymal sperm numbers and sex accessory-gland weights in offspring with normal androgenic status. Toxicol App Pharmacol. 1995;131:108–118. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS. In-utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol App Pharmacol. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS, Kelce WR. A dose-response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans Hooded rat offspring. Toxicol App Pharmacol. 1997;146:11–20. doi: 10.1006/taap.1997.8223. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res. 2008;108:168–176. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Devito MJ, Birnbaum LS. Tissue disposition of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in maternal and developing Long-Evans rats following subchronic exposure. Toxicol Sci. 2000a;57:275–283. doi: 10.1093/toxsci/57.2.275. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Devito MJ, Setzer RW, Birnbaum LS. Acute administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in pregnant Long Evans rats: Association of measured tissue concentrations with developmental effects. Toxicol Sci. 2000b;53:411–420. doi: 10.1093/toxsci/53.2.411. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Tamura M, Yamashita J, Suzuki C, Tomita T. Repeated in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure affects male gonads in offspring, leading to sex ratio changes in F-2 progeny. Toxicol App Pharmacol. 2005;206:351–355. doi: 10.1016/j.taap.2004.11.019. [DOI] [PubMed] [Google Scholar]
- JECFA. Evaluation of certain food additives and contaminants (57th report of the Joint FAO/WHO Expert Committee on Food Additives) WHO Technical Report Series. 2002;909:121–149. [Google Scholar]
- Kerger BD, Leung HW, Scott P, Paustenbach DJ, Needham LL, Patterson DG, Gerthoux PM, Mocarelli P. Age- and concentration-dependent elimination half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso children. Env Health Persp. 2006;114:1596–1602. doi: 10.1289/ehp.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer PE, Csanady GA, Baur C, Kessler W, Papke O, Greim H, Filser JG. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Arch Toxicol. 1997;71:383–400. doi: 10.1007/s002040050402. [DOI] [PubMed] [Google Scholar]
- Lanning LL, Creasy DM, Chapin RE, Mann PC, Barlow NJ, Regan KS, Goodman DG. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol Path. 2002;30:507–520. doi: 10.1080/01926230290105695. [DOI] [PubMed] [Google Scholar]
- Leung HW, Kerger BD, Paustenbach DJ. Elimination half-lives of selected polychlorinated dibenzodioxins and dibenzofurans in breast-fed human infants. J Toxicol Environ Health A. 2006;69:437–443. doi: 10.1080/15287390500246886. [DOI] [PubMed] [Google Scholar]
- Mably TA, Bjerke DL, Moore RW, GendronFitzpatrick A, Peterson RE. In utero and lactational exposure of male-rats to 2,3,7,8-tetrachlorodibenzo-para-dioxin. 3 effects on spermatogenesis and reproductive capability. Toxicol App Pharmacol. 1992a;114:118–126. doi: 10.1016/0041-008x(92)90103-y. [DOI] [PubMed] [Google Scholar]
- Mably TA, Moore RW, Goy RW, Peterson RE. In utero and lactational exposure of male-rats to 2,3,7,8-tetrachlorodibenzo-para-dioxin. 2 effects on sexual-behavior and the regulation of luteinizing-hormone secretion in adulthood. Toxicol App Pharmacol. 1992b;114:108–117. doi: 10.1016/0041-008x(92)90102-x. [DOI] [PubMed] [Google Scholar]
- Mably TA, Moore RW, Peterson RE. In utero and lactational exposure of male-rats to 2,3,7,8-tetrachlorodibenzo-para-dioxin. 1 effects on androgenic status. Toxicol App Pharmacol. 1992c;114:97–107. doi: 10.1016/0041-008x(92)90101-w. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, Foster PMD. Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicol Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yonemoto J, Miyabara Y, Sato M, Tohyama C. Rat thyroid hyperplasia induced by gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinol. 2003;144:2075–2083. doi: 10.1210/en.2002-220737. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yonemoto J, Nishimura H, Ikushiro S, Tohyama C. Disruption of thyroid hormone homeostasis at weaning of Holtzman rats by lactational but not in utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2005;85:607–614. doi: 10.1093/toxsci/kfi122. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yonemoto J, Nishimura H, Tohyama C. Localization of cytochrome P450 1A1 in a specific region of hydronephrotic kidney of rat neonates lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. 2006;227:117–126. doi: 10.1016/j.tox.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Ohsako S, Miyabara Y, Nishimura N, Kurosawa S, Sakaue M, Ishimura R, Sato M, Takeda K, Aoki Y, Sone H, Tohyama C, Yonemoto J. Maternal exposure to a low dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppressed the development of reproductive organs of male rats: Dose-dependent increase of mRNA levels of 5 alpha-reductase type 2 in contrast to decrease of androgen receptor in the pubertal ventral prostate. Toxicol Sci. 2001;60:132–143. doi: 10.1093/toxsci/60.1.132. [DOI] [PubMed] [Google Scholar]
- Ohsako S, Miyabara Y, Sakaue M, Ishimura R, Kakeyama M, Izumi H, Yonemoto J, Tohyama C. Developmental stage-specific effects of perinatal 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on reproductive organs of male rat offspring. Toxicol Sci. 2002;66:283–292. doi: 10.1093/toxsci/66.2.283. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-para-dioxin and related halogenated aromatic-hydrocarbons - examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland A, Teitelbaum P, Glover E. [I-125]2-Iodo-3,7,8-Trichlorodibenzo-p-dioxin-binding species in mouse-liver induced by agonists for the ah receptor - characterization and identification. Mol Pharmacol. 1989a;36:113–120. [PubMed] [Google Scholar]
- Poland A, Teitelbaum P, Glover E, Kende A. Stimulation of in vivo hepatic uptake and invitro hepatic binding of [i-125]2-iodo-3,7,8-trichlorodibenzo-p-dioxin by the administration of agonists for the Ah receptor. Mol Pharmacol. 1989b;36:121–127. [PubMed] [Google Scholar]
- Roman BL, Sommer RJ, Shinomiya K, Peterson RE. In-utero and lactational exposure of the male-rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin - impaired prostate growth and development without inhibited androgen production. Toxicol App Pharmacol. 1995;134:241–250. doi: 10.1006/taap.1995.1190. [DOI] [PubMed] [Google Scholar]
- Rose JQ, Ramsey JC, Wentzler TH, Hummel RA, Gehring PJ. Fate of 2,3,7,8-tetrachlorodibenzo-para-dioxin following single and repeated oral doses to rat. Toxicol App Pharmacol. 1976;36:209–226. doi: 10.1016/0041-008x(76)90001-6. [DOI] [PubMed] [Google Scholar]
- SCF. Opinion of the Scientific Committee On Food on the risk assessment of dioxins and dioxin-like pcbs in food. Update based on new scientific information available since the adoption of the SCF opinion of 22nd November 2000. 2001 Adopted on 30 May 2001. [Google Scholar]
- Simanainen U, Haavisto T, Tuomisto JT, Paranko J, Toppari J, Tuomisto J, Peterson RE, Viluksela M. Pattern of male reproductive system effects after in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive rat lines. Toxicol Sci. 2004;80:101–108. doi: 10.1093/toxsci/kfh142. [DOI] [PubMed] [Google Scholar]
- Sommer RJ, Ippolito DL, Peterson RE. In utero and lactational exposure of the male Holtzman rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Decreased epididymal and ejaculated sperm numbers without alterations in sperm transit rate. Toxicol App Pharmacol. 1996;140:146–153. doi: 10.1006/taap.1996.0207. [DOI] [PubMed] [Google Scholar]
- Weber LWD, Ernst SW, Stahl BU, Rozman K. Tissue distribution and toxicokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats after intravenous-injection. Fund App Toxicol. 1993;21:523–534. doi: 10.1006/faat.1993.1129. [DOI] [PubMed] [Google Scholar]
- Weil CS. Selection of valid number of sampling units and a consideration of their combination in toxicological studies involving reproduction, teratogenesis or carcinogenesis. Food Cosmet Toxicol. 1970;8:177–182. doi: 10.1016/s0015-6264(70)80337-6. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PTK, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker C, Johnson L, Safe S. Effects of developmental exposure to indole-3-carbinol or 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive potential of male rat offspring. Toxicol App Pharmacol. 1996;141:68–75. doi: 10.1006/taap.1996.0261. [DOI] [PubMed] [Google Scholar]
- Yonemoto J, Ichiki T, Takei T, Tohyama C. Maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and the body burden in offspring of Long-Evans rats. Environ Health Prev Med. 2005;10:21–32. doi: 10.1265/ehpm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]