SUMMARY
Anti-HER2/neu antibody therapy is reported to mediate tumor regression by interrupting oncogenic signals and/or inducing FcR-mediated cytotoxicity. Here, we demonstrate that the mechanisms of tumor regression by this therapy also require the adaptive immune response. Activation of innate immunity and T cells, initiated by antibody treatment, was necessary. Intriguingly, the addition of chemotherapeutic drugs, while capable of enhancing the reduction of tumor burden, could abrogate antibody-initiated immunity leading to decreased resistance to re-challenge or earlier relapse. Increased influx of both innate and adaptive immune cells into the tumor microenvironment by a selected immunotherapy further enhanced subsequent antibody-induced immunity, leading to increased tumor eradication and resistance to re-challenge. Therefore, this study proposes a model and strategy for anti-HER2/neu antibody-mediated tumor clearance.
Highlights.
The tumor regression by anti-HER2/neu antibody is T cell dependent
FcR dependent stress by antibody is required to prime adaptive immune cells
Some chemotherapy drugs could abrogate antibody-mediated immunity
A selected immunotherapy could further enhance antibody-mediated immunity
SIGNIFICANCE.
While anti-HER2/neu antibody is an effective adjuvant therapy targeting HER2+ breast cancers, relapse often occurs even after prolonged treatment. Current understanding holds that this antibody therapy interrupts oncogenic signals and induces FcR-mediated cytotoxicity. This study reveals that the therapeutic effect of anti-HER2/neu antibody treatment also depends on adaptive immunity. Furthermore, this study demonstrates an interesting antibody-mediated mechanism whereby danger signals are required to mobilize and activate innate cells and prime the adaptive immune system for increase tumor clearance. However, antibody-initiated tumor regression can be impaired by certain chemotherapy regimens. Therefore, this study has important clinical impact since various chemotherapy drugs have been used before or after antibody treatment.
INTRODUCTION
The human epidermal growth factor receptor 2 (HER2, HER2/neu, or ErbB-2) is overexpressed in 20–30% of breast carcinomas and is associated with aggressive disease, a high recurrence rate, and reduced patient survival (Hudis, 2007; Kiessling et al., 2002; Meric-Bernstam and Hung, 2006; Slamon et al., 1987). The use of trastuzumab (Herceptin), a humanized monoclonal antibody that binds the extracellular, juxtamembrane domain of HER2, has proved to be an effective treatment in animal and human studies (Hudis, 2007; Moasser, 2007). Many groups have demonstrated that anti-HER2/neu antibody can efficiently stop or slow the growth of HER2/neu+ tumors in vitro (Hudis, 2007; Kiessling et al., 2002; Meric-Bernstam and Hung, 2006). Growth inhibition is mainly due to the induction of G1 cell cycle arrest and is closely tied to increased p27Kip1 expression, and reduced cyclin E expression (Le et al., 2005; Mittendorf et al., 2010). In addition, antibody treatment was shown to inhibit the ability of tumor cells to repair damaged DNA (Pegram et al., 1999). The combination of antibody treatment with multiple chemotherapeutic agents showed additive and synergistic effects in in vitro studies and in vivo xenograft tumor models (Pegram et al., 1999; Pegram et al., 2004). As a result, interference with HER2 oncogenic signaling and increased susceptibility to chemotherapy-induced apoptosis (chemosensitization) have been proposed as the central mechanisms responsible for the clinical efficacy of trastuzumab (Hudis, 2007; Moasser, 2007; Pegram et al., 2004). Based on the convincing preclinical studies, clinical trials were conducted and demonstrated the benefits of combining chemotherapy administration with trastuzumab (Hudis, 2007; Piccart-Gebhart et al., 2005; Romond et al., 2005). Despite of the initial clinical success of antibody plus chemotherapy treatment for Her2+ tumors, relapse has been reported after cessation of this treatment.
Considering reports that inhibition of oncogenic signals by anti-HER2/neu antibody controls tumor growth in vitro, it was surprising that the therapeutic effect of this antibody was diminished in the absence of Fc receptor (FcR) signaling in vivo (Clynes et al., 2000). The role of FcRs in the efficacy of antibody treatment is further supported by evidence that Fcr polymorphisms are associated with the clinical outcome in breast cancer patients (Musolino et al., 2008). These data raise the possibility that antibody-dependent cellular cytotoxicity (ADCC) may play a major role in the anti-tumor effects of antibody therapy. Consistently, an increase of tumor-infiltrating leukocytes, especially FcR+ cells such as NK cells, has been observed in tumor tissue after antibody treatment (Arnould et al., 2006; Varchetta et al., 2007). Furthermore, it was reported that patients with partial or complete remission after antibody treatment had higher in situ infiltration of leukocytes and an increased capacity to mediate in vitro ADCC activity (Gennari et al., 2004 ) Endogenous anti-HER2 antibodies after vaccine can be detected in some patients and can effectively suppress HER2 kinase activity and downstream signaling to inhibit the transformed phenotype of HER2-expressing tumor cells (Montgomery et al., 2005). However, most models, including xenografts used for preclinical evaluation, fail to account for adaptive immunity in the antibody-mediated therapeutic effect. Therefore, the essential role of T and B cells in anti-HER2/neu antibody-mediated tumor regression remains unclear.
RESULTS
Adaptive immunity is essential for the therapeutic effect of antibody treatment
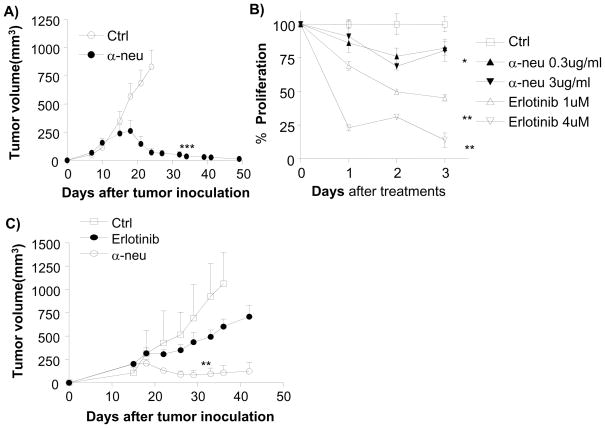
To evaluate whether targeted antibody treatment of HER2/neu+ breast cancer could reduce tumor burden in syngeneic wild type (Wt) mice, we used the well-characterized anti-neu (rat homologue of human HER2) monoclonal antibody 7.16.4 (Zhang et al., 1999). This antibody competes with 4D5 (the original mouse anti-HER2/neu antibody which was humanized to trastuzamab) for binding to human HER2 and inhibition of tumor growth. BALB/c mice bearing established TUBO tumors, a neu overexpressing cell line derived from a spontaneous carcinoma in neu-transgenic mice (Rovero et al., 2000), were treated with anti-neu antibody. Impressively, without the addition of chemotherapy, the majority (28/35) of Balb/c mice from several experiments rejected tumors completely 4 weeks after treatment, while control Ig-treated mice had to be sacrificed due to large tumor burden (Fig. 1A, Table 1).
Figure 1. Anti-neu antibody has limited effect in vitro but has strong effect against tumor in vivo.
A) WT BALB/c mice (n=5/group) were injected s.c. with 5 × 105 TUBO cells and treated with 100 μg of anti-neu (α-neu) or isotype control (Ctrl) antibody on days 14 and 21. The tumor growth was measured and compared twice a week. ***, p < 0.005 compared with isotype control group after day 23. One of five representative experiments is shown. B) TUBO cells (1 × 105 cells/well) were plated in a monolayer and incubated with Erlotinib (1–4 μmol/L) or anti-neu antibody (0.3–3 μg/mL). Control groups received isotype-control antibody (Ctrl). Relative proliferation, reflected by metabolic activity, was evaluated at indicated times by MTT assay and graphed as percent of isotype control. Mean±SD; *, p < 0.05; **, p < 0.005 compared with isotype control. One of two representative experiments is shown. C) TUBO bearing BALB/c mice (n=5/group) were treated with four times with 100 μg of anti-neu antibody (α-neu) every other day and with 500 μg of Erlotinib every day for 7 days from day 18. *, p < 0.05 compared with the control group from day 33; **, p < 0.01 compared with erlotinib-treated group after day 26. One of two representative experiments is shown.
Table 1.
The therapeutic effect of combined chemotherapy and antibody treatment on tumor regression and re-challenge
| Antibody Dosagea | Drugb | Chemotherapy Dosage | Schedulec | Rate of tumor regressiond | rejected/total (percent) | Growth after growth/total (%)e |
|---|---|---|---|---|---|---|
| 100μg x2 | None | 28/35 (80%) | 0/19 (0%)F, G | |||
| 100μg x3 | None | 20/24 (83%) | 0/6 (0%) F, G | |||
| 100μg x4 | None | 1/5(80%) | ND F | |||
| 100μg x2 | PTX | 60mg/kg x2 | 3 days after | + | 10/10 (100%) | 7/9 (78%) F |
| ” | ” | 40mg/kg x2 | 3 days after | + | 10/10 (100%) | 7/9 (78%) F |
| ” | ” | 10mg/kg x4 | 3 days after | = | 4/4 (100%) | 0/4 (0%) F |
| ” | PTX | 40mg/kg x1 | 1 day before | + | 12/12 (100%) | 0/4 (0%) F, G |
| ” | ” | 20mg/kg x1 | 1 day before | + | 4/4 (100%) | 2/4 (50%) F, G |
| ” | CTX | 100mg/kg x2 | 3 days after | + | 12/20 (60%) | 2/3 (67%) F |
| ” | DOX | 15mg/kg x2 | Same day | - | 0/5 (0%) | ND G |
| ” | ” | 5 mg/kg x2 | Same day | - | 0/5 (0%) | ND G |
BALB/c mice were implanted with 4–5 × 105 TUBO cells on day 0. Groups of 5–10 mice were injected i.p. with anti-neu antibody at 5–7 day intervals starting on day 12 or day 18 after tumor implantation, with or without chemotherapeutics. Tumors were measured twice a week.
Chemotheraputic used. All drugs were injected ip.
Chemotheraputic administration relative to anti-neu antibody treatment.
Comparison of the incidence of regression to anti-neu antibody treatment (100 μg x2) alone; +, greater than antibody alone; =, similar to antibody alone; −, less than antibody alone.
One month after complete tumor regression, tumor-free mice were re-challenged with 2–5 × 106 TUBO cells and tumor growth was monitored for 30 days. ND, not determined.
The experiments were performed in CAS,
The experiments were performed in UC
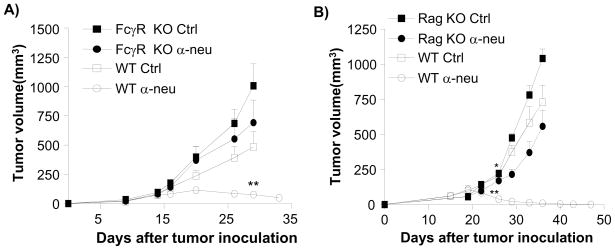
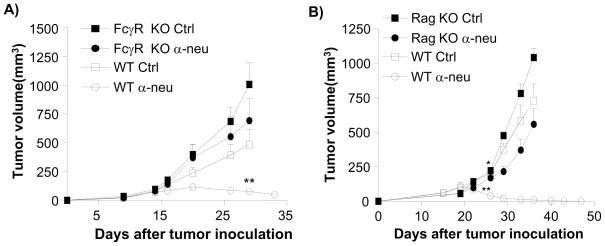
To test the relative contribution of HER2/neu signal interference with anti-neu antibody-mediated tumor regression, we compared the efficacy of this antibody to erlotinib, a tyrosine kinase inhibitor (TKI), in vitro and in vivo. A high dose of anti-neu antibody slightly inhibited TUBO cell proliferation in vitro (approximately 20%), but effectively reversed the growth of or cleared all established tumors in vivo (35/35). On the other hand, erlotinib strongly inhibited TUBO cell proliferation in vitro (more than 80%) but weakly impacted tumor growth rates in vivo without reversal of tumor growth (Fig. 1B, 1C). These data support the recent model that anti-HER2/neu antibody treatment requires ADCC via FcRs for effective in vivo treatment of human HER2/neu+ tumors (Clynes et al., 2000). To test whether ADCC is accountable for this additional reduction of tumor masses in our in vivo model system, WT and Fcγ r KO mice were inoculated with TUBO and established tumors were treated with 7.16.4. Indeed, the therapeutic effect of anti-neu antibody was Fcγ R-dependent, as Fcγ R deficient mice failed to show antibody-induced inhibition (Fig. 2A). Together, using a system modeling human HER2/neu+ tumor growth, our data consistently support the model that FcR+ cells are essential for inhibiting or even eradicating neu+ tumors in response to anti-neu antibody treatment. To further explore the role of ADCC in anti-neu antibody therapy, we inoculated Rag-1−/− mice with TUBO. Though Rag-1−/− mice lack T and B cells, these mice do have a complete population of FcR+ innate cells to mediate ADCC. Thus, we expected these mice to regress established TUBO tumors efficiently after anti-neu antibody treatment. Though WT mice treated with anti-neu antibody consistently demonstrate tumor regression, this antibody treatment had a very limited impact on the growth of established tumors in Rag-1−/− mice (Fig. 2B). Although some Rag-1−/− mice displayed delayed tumor growth lasting, no tumor regression was observed in Rag-1−/− mice (total n=13) after antibody treatment. Thus, these data reveal that the innate immune response alone is not sufficient to mediate the therapeutic effect of anti-neu antibody treatment. Therefore, we considered the role of the adaptive immune response being essential for antibody-mediated tumor reduction.
Figure 2. Anti-tumor effect of antibody depends on both FcR and adaptive immune system.
A) TUBO-bearing Fcγ receptor KO and WT BALB/c mice (n=6–8/group) were treated with 100 μg of anti-neu or isotype control antibody on days 14 and 21. **, p < 0.01 compared with WT isotype control group after day 29. One of three experiments is shown. B) TUBO-bearing Wt and Rag-1−/− mice (n=5–7/group) were treated with 100 μg of anti-neu antibody (α-neu) or isotype control (Ctrl) on days 18 and 25. *, p < 0.05; **, p < 0.005 compared to isotype control groups of each mouse strain as A. One of three experiments is shown. Increase of lymphocytes inside tumor is seen in Figure S1.
Anti-neu antibody-induced CTL and immunological memory are required for protection
CD8+ cytotoxic lymphocytes are a major adaptive immune cell population involved in controlling tumor growth. We observed increased lymphocytes, especially CD8+ cells, in TUBO tumor tissues 1–2 weeks after antibody treatment (Figure S1). To determine whether CD8+ T cells are essential for anti-neu antibody-mediated tumor regression, TUBO-bearing BALB/c mice were treated with an anti-CD8α-depleting antibody (YTS169.4.2) in conjunction with anti-neu antibody treatment. Initially, tumors in antibody-treated mice continued to regress, but relapsed rapidly in the absence of CD8+ T cells (Fig. 3A). Anti-CD8α antibody had no detectable impact on tumor growth in control Ig treated mice. In addition, depletion of CD8 α + cells using another clone (53.6.7) that has less impact on CD8 α + DC displayed the same phenotype but antibody-mediated effect is still dependent on CD8+ cells (Figure S2A and B). To completely separate the direct role of CD8 α + DC and CD8+ T cells, specific antibody to CD8β chain or mice deficient of CD8 α DC might be useful.
Figure 3. The therapeutic effect of anti-neu antibody treatment requires CD8+ cells and induces memory T cell responses.
A) WT BALB/c mice (n=5–10/group) were injected s.c. with 5 × 105 TUBO and treated with 100 μg of anti-neu antibody (α-neu) on days 10, 17, and 24. CD8-depleting antibody (YTS169.4.2, 200μg/mouse) was administered every 3 days, starting on day 9. *, p < 0.05; **, p < 0.005 compared to anti-neu antibody-treated WT mice. One of three experiments is shown. B) Neu Tg F1 mice (n=6/group) were injected with 3 × 105 TUBO cells and treated with 100 μg of anti-neu antibody (α-neu) on days 11 and 18. CD8-dpeleting antibody (YTS169.4.2, 200μg/mouse) was administered on the same days. *, p < 0.05 compared to anti-neu antibody-treated group. One of two experiments is shown. The data from other CD8-depleting antibodies is shown in Figure S2. C) Tumor-free, antibody-treated BALB/c mice (n=14 pooled from two experiments) were re-challanged s.c. with 5 × 106 TUBO cells on different site from primary tumor at least 1 month after complete rejection of primary tumors. All of mice rejected the secondary tumor. One of two experiments is shown. Anti-neu antibody therapy increases IFNγ + cells in WT (D) or Tg mice (E). D) TUBO bearing mice were treated twice with 150ug of either anti-neu (n=3) or mIgG (n=3) on days 11 and 18. Mice were sacrificed 12 days after the final treatment and splecnoytes were isolated for ELISPOT analysis as described in the materials and methods. ***p<0.0001. E. Splenocytes from neu Tg F1 mice (N=3–5) treated with anti-neu or isotype control antibody were stimulated with 3T3/KB, 3T3/NKB, or TUBO cells. The ratio of splenocytes to APC was 10:1. IFN-γ–producing cells were enumerated by ELISPOT assay. Results were expressed as number of spots per 106 splenocytes. *, p< 0.05; **, p< 0.005 compared with isotype control group. One of three experiments is shown for D and E. Increase of tumor infiltrated lymphocytes was also seen in human samples after antibody treatment (see Figure S2C)
This essential role for T cells in antibody-mediated tumor regression is not limited to the TUBO cell line. Anti-neu antibody treatment of mice bearing neu-dependent N202 tumors, derived from neu transgenic mice on the FVB background, also resulted in tumor regression. More importantly, antibody-mediated tumor regression of N202 tumor was also CD8+ T cell dependent (data not shown). As WT Balb/c mice are not tolerized to the neu antigen, we decided to test whether CD8+ T cells contribute to the effect of this antibody in a tolerant model. We used F1 neu transgenic (Tg) mice (BALB/c × FVB/N MMTV-neu), which are tolerant to the neu antigen and resistant to various treatments (Machiels et al., 2001). Whereas TUBO bearing WT mice consistently demonstrate tumor regression after anti-neu antibody treatment, only 20% of tumors implanted in neu Tg mice demonstrate complete regression with the other 80% relapsing a few weeks after antibody cessation (Fig. 3B). Nevertheless, short-term treatment with the anti-neu antibody still resulted in significant reduction of tumor growth in a CD8-dependent fashion. Thus, tumor relapse in neu-Tg mice mimics frequently relapse observed in the clinic. Therefore, in addition to supporting the role of CD8+ T cells in mediating neu-antibody therapy, this data also raises an interesting possibility that anti-neu antibody may transiently break tolerance in neu-Tg mice and generate immunity against HER2/neu+ tumors.
To determine whether the immune response initiated by anti-neu antibody results in memory, the hallmark of adaptive immunity, we evaluated cured mice for long-term protection by tumor re-challenge. Mice that underwent complete tumor regression following antibody treatment (n=14) were re-challenged with 5 × 106 TUBO cells (10 times of the primary tumor inoculation) after primary tumors had not been detected for at least 1 month. Impressively, all mice rejected the re-challenged tumors (Fig. 3C, Table 1). This data strongly supports the idea that anti-neu antibody-mediated tumor regression generates detectable long-term immune memory capable of protecting the host from re-challenge, and presumably against relapse.
To define whether tumor-specific T cell responses are increased by antibody treatment, splenocytes were collected after cessation of antibody treatment, and IFN-γ production from anti-neu or control antibody-treated TUBO bearing WT mice was evaluated by ELISPOT. Antibody-treated group has much higher neu-reactive T cells (Fig. 3D). To further define whether tumor-specific T cell responses are increased by antibody treatment, even in antibody-treated neu-Tg mice, the splenocytes from Tg mice was evaluated by ELISPOT (Fig. 3E). In the antibody-treated group, 658 ± 133/106 neu-specific and 1475 ± 120.7/106 TUBO-specific IFN-γ producing T cells were detected in Tg mice, which was about 7–14 fold more than control Ig-treated group. These data suggest that anti-neu antibody treatment induces neu- and TUBO-specific CD8+ T cell responses.
To correlate this data with clinical observations, we compared pre- and post-treatment breast tissue biopsies from patients with HER2+ and HER2− tumors treated with trastuzumab and chemotherapy or chemotherapy alone respectively. There was no significant difference in tumor-infiltrating lymphocytes (TIL) between HER2+ vs. HER2− tumor tissues before treatment, with few cases having high TIL numbers before treatment. In our medical center, obtaining paired pre and post-antibody samples is rare since trastuzumab treatment is reserved mainly for patients with potential metastasis after primary tumors have been surgically removed. However, we were able to obtain multiple post-treatment samples and observed that primary human HER2+ breast cancers had increased lymphocyte infiltrates post trastuzumab treatment while no detectable increase in lymphocyte infiltrates was observed in the chemotherapy alone group. Though both groups received chemotherapy, the addition of trastuzumab significantly increased lymphocyte, especially CD8+ infiltrates (Figure S2C). Defining the role of adaptive immunity initiated trastuzumab alone in human studies will be challenging because standard care requires that trastuzumab be combined with chemotherapy after surgery, and additional biopsy of metastasis requires strict IRB approval. Thus, formal clinical trials are needed to determine whether and when trastuzumab alone can increase TIL in better controlled cases.
Anti-neu antibody induces HMGB-1 release for strong innate responses
To test whether the MyD88 pathway is essential to tumor regression after antibody treatment, TUBO-bearing WT and Myd88−/− mice were treated with anti-neu antibody. The therapeutic effect of anti-neu antibody was abolished in Myd88−/− mice (Fig. 4A). Recent studies have shown that HMGB-1 can function as an endogenous danger signal that stimulates DC cross-priming in a MyD88-dependent fashion (Apetoh et al., 2007; Burgdorf et al., 2008). To determine whether antibody-mediated tumor regression is HMGB-1 dependent, free HMGB-1 was neutralized by administration of an anti-HMGB-1 antibody (3B1) in conjunction with anti-neu antibody treatment. Anti-HMGB-1 alone had no significant impact on tumor growth (data not shown) while co-injection of anti-HMGB-1 antibody partially diminished the therapeutic effects of anti-neu antibody (Fig. 4B). These data indicate that HMGB-1, an endogenous danger signal, is essential for antibody-mediated tumor regression. It is conceivable that anti-neu antibody induces HMGB-1 release in the tumor microenvironment which enhances innate responses via the MyD88 pathway.
Figure 4. The therapeutic effect of anti-neu antibody depends on endogenous danger signals.
A) WT and Myd88−/− BALB/c mice (n=5–7/group) were injected s.c. with 4 × 105 TUBO cells and treated with 100 μg of anti-neu (α-neu) or isotype control (Ctrl) antibody on days 21 and 28. *, p<0.01 compared to anti-neu antibody treated Myd88−/− mice. One of two experiments is shown. B) TUBO-bearing WT BALB/c mice (n=4/group) were treated with 100 μg of anti-neu antibody (α-neu) and 100 μg of neutralizing anti-HMGB-1 antibody on days 14 and 21. **, p < 0.005, compared to anti-HMGB-1 antibody-treated group. One of four experiments is shown.
Combination treatments of anti-neu antibody with chemotherapeutics
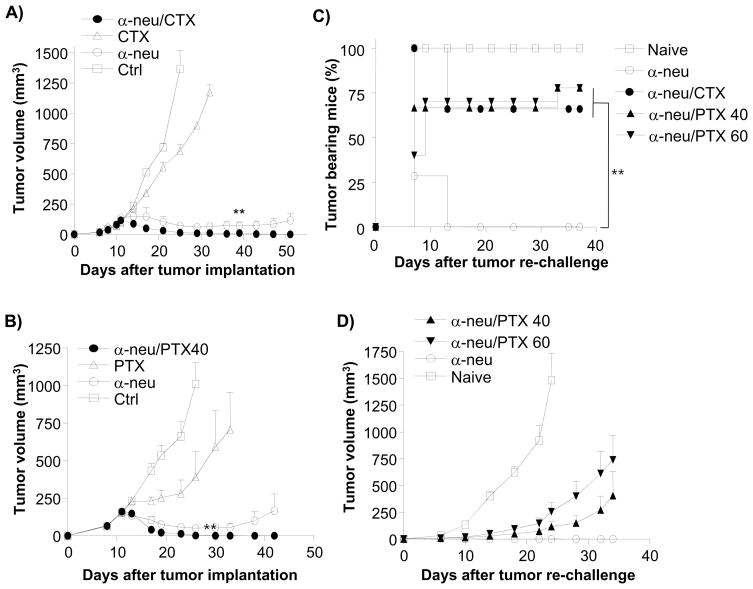
The essential role of T cells in anti-neu antibody-mediated tumor regression warrants reevaluation of current anti-HER2/neu treatment protocols since many current combination treatments of antibody plus chemotherapy may have negative effects on the host immune response to tumor antigens. For instance, high dose chemotherapy is used clinically to significantly reduce tumor burden; yet this treatment could possibly inhibit immune-mediated tumor regression by limiting immune responses. To test whether chemotherapy drugs used for breast cancer synergize with or antagonize anti-neu antibody, this treatment was combined with clinically equivalent doses of doxorubicin (DOX), cyclophophamide (CTX) or paclitaxel (PTX). These chemotherapeutic agents are combined with anti-HER2/neu antibody in the clinic and are effective for TUBO regression in the presence or absence of antibody (Machiels et al., 2001). CTX (100mg/kg) or PTX (40–60mg/kg) was administered 3–5 days after anti-neu antibody, and accelerated tumor mass regression was observed compared to anti-neu antibody treatment alone (Fig. 5A, B, and Table 1). On the contrary, DOX treatment (5 or 15 mg/kg) concomitant with anti-neu antibody resulted in slower tumor regression than antibody alone, and mice receiving this combined treatment demonstrated tumor relapse while the antibody-alone treated group eradicated tumors (Table 1). The lack of a strong effect by drugs is not due to an insufficient dose, since the highest doses of DOX (15 mg/kg) and PTX (60 mg/kg) increased morbidity and mortality in a fraction of tumor bearing hosts (data not shown).
Figure 5. Administration of chemotherapeutics after antibody treatment enhances primary tumor reduction but reduces immunity induced by anti-neu antibody.
WT BALB/c mice (n=5–10/group) were injected s.c. with 5 × 105 TUBO cells and treated with 100 μg of anti-neu antibody (α-neu) on days 11 and 16. Select chemotherapeutic agents were injected i.p. at different time points. One of three experiments is shown. (A) 100 mg/kg of Cyclophosphamide (CTX) was injected i.p. on days 16, 23, and 33. (B) 40 mg/kg of Paclitaxel (PTX) weas injected i.p. on days 14 and 19. Treated, tumor-free mice were re-challenged with 2 × 106 TUBO cells when primary tumor was not detected for at least 30 days. Percent tumor-bearing mice (C) and mean tumor volume (D) are shown. Reduced T cell proliferation was seen in Figure S3.
It was unclear whether the accelerated tumor regression observed by combining anti-neu antibody treatment with CTX or PTX was attributed to enhanced killing of tumor cells directly by the chemotherapy or to enhance activation of the immune system. To test the impact of chemotherapeutics on the immune response and subsequent memory generated by anti-neu antibody treatment, mice were rechallenged with 5 × 106 TUBO cells (10 times more than primary tumor inoculum) 1–2 months after tumor masses were undetectable. All mice whose primary tumor underwent complete regression following anti-neu antibody treatment alone rejected the tumor re-challenge (Table 1). Surprisingly, a majority of the mice treated with anti-neu antibody and chemotherapy were susceptible to tumor re-challenge (66% of CTX combination mice; 77% of 40 mg/kg of PTX combination mice) (Fig. 5C, D, and Table 1). When a higher dose (60 mg/kg) of PTX was used, the treated mice more effectively controlled the growth of the primary tumor burden but were less resistant to re-challenge (Fig. 5D, Table 1). Although the addition of chemotherapeutic agents induce a more robust control of the primary tumor, they also confer a loss of protection from tumor re-challenge, a potential problem related to late relapse observed in the clinic once primary tumor is diminished after treatment. Reduced white blood cell count is a common side-effect in patients undergoing chemotherapy. Though we did not observe significant differences in the numbers or percentages of T and B cells in blood, LN, and the spleen after PTX treatment, the number of Ki67+ cells was reduced in PTX treated group, suggesting early immune suppression (Figure S3). We have previously shown that 20 mg/kg of PTX could suppress the priming of antigen-specific CD8+ T cell response initiated by local radiation of primary tumor (Lee et al., 2009). Similar suppression of Ki67 on lymphocytes was detected in DOX treated group (data not shown). CTX and DOX have been implicated in immunosupression leading to fulminant hepatitis B hepatitis after chemotherapy for non-Hodgkin's lymphoma in an HBV carrier(Aomatsu et al., 2010). However, same drugs can also boost immune responses, likely depending on doses and patients’ immune status(Emens et al., 2001). More studies are needed to address those complicated issues.
We speculated that there might only be a window of time when chemotherapy drugs may effectively reduce tumor burden without inhibiting antibody-induced immunity, as the half-life of these drugs is rather short. To test whether PTX given before anti-neu antibody also inhibited immune memory, an identical dose of PTX (40 mg/kg) was injected 24 hours before antibody instead of 3 days after antibody treatment. Impressively, the combination of PTX at this time and dose not only synergized with anti-neu antibody to control the primary tumor, but also preserved the ability of the host to clear a lethal tumor re-challenge (Fig. 6A and B, and Table 1). Therefore, a simple alteration of drug administration or dose could have a major impact on the immunological memory response to tumor antigens. It is likely that different doses and schedules related to drugs and antibody as well as patient immune status might be critical for overall anti-tumor effect, more studies, including animal models and clinical trials, are needed to optimize the anti-tumor effects by various protocols in the future.
Combination treatments of anti-neu antibody with Ad-LIGHT
Although anti-neu antibody therapy induces initial immunity against tumor, sustained immunity could be transient or diminished if the tumor is able to bolster immune suppressive mechanisms. One major hurdle for antibody treatment of an established tumor is the tumor barrier that prevents effective infiltration of antibody, FcR+ cells (NK and macrophages), DC, and T cells. Expression of LIGHT intra-tumorally can attract various immune cells, including substantial numbers of FcR+ cells, DC, and T cells (Fan et al., 2006; Wang et al., 2005; Yu et al., 2004; Yu et al., 2007). We reasoned, therefore, that given the necessity of the adaptive immune system in anti-neu antibody therapy, targeting tumors with an adenovirus expressing LIGHT (Ad-LIGHT) by intra-tumoral injection could enhance tumor barrier breakdown, attract more immune cells, and amplify or sustain the therapeutic effect of anti-neu antibody treatment. To test this hypothesis and determine the contribution of Ad-LIGHT, mice bearing established TUBO tumors (18 days after inoculation) were treated with a suboptimal dose of anti-neu antibody in close combination with intra-tumoral injection of Ad-LIGHT. Neither suboptimal anti-neu antibody (two injections of 50 μg/mice), nor Ad-LIGHT alone was sufficient to control tumors (Supplement Fig 6A). Tumor growth in the Ad-LIGHT-treated group was only delayed by 1–2 weeks, while all mice in the anti-neu antibody-treated group showed relapse 4–6 weeks after the end of antibody treatment. In contrast, combination treatment of anti-neu antibody and Ad-LIGHT resulted in rejection of all transplanted TUBO tumors, and no relapse was detected up to 8 weeks after the completion of treatment (Figure S4A). Notably, this combination treatment generated immunity sufficient to protect from lethal re-challenge of TUBO but not 4T1, a neu− mammary carcinoma (Figure S4B), demonstrating that this combination treatment induced neu-specific immunity. The combination of anti-neu antibody and Ad-LIGHT was also tested in neu Tg mice that frequently relapse after anti-neu antibody single treatment. Impressively, the combination treatment of anti-neu antibody and Ad-LIGHT rapidly controlled tumor growth in 80% of tolerant mice, while neither of the single treatment was able to control tumor growth effectively at the same dose (Figure S4C, D). These data support the necessity of the adaptive immune system in anti-neu mediated tumor regression and suggest that proper immunotherapy can amplify or maintain antibody-initiated anti-tumor immunity to clear residual cancer and prevent relapse, even in a tolerant environment.
Discussion
Over the past two decades, several studies aimed at understanding the mechanism(s) of anti-HER2/neu antibody therapy have shown that this therapy can efficiently slow the growth of HER2/neu+ tumors in vitro and in vivo, suggesting the importance of oncogenic signaling blockade in antibody-mediated tumor reduction. However, FcR+ cells were also shown to be essential for mediating the therapeutic effects of the HER2/neu antibody (Clynes et al., 2000). Though not mutually exclusive, these data promoted ADCC as the major mechanism for the in vivo effects of antibody treatment. Here, we demonstrate that T cells are necessary for the tumor reduction by anti-neu antibody alone. Our current findings support the critical role of T cells through the following findings: 1) the therapeutic effect of HER2/neu antibody treatment on tumor growth is greatly reduced in T cell-deficient mice; 2) WT mice depleted of CD8+ T cells show rapid relapse of tumor; 3) increased anti-neu reactive T cells can be measured by ELISPOT after antibody treatment; 4) antibody-treated, tumor-free mice are subsequently resistant to high dose tumor re-challenge, suggesting the presence of immune memory; 5) increased T cell infiltration, especially CD8+ cells, can be detected in tumor tissue of mice treated with anti-neu and patients treated with adjuvant HER2/neu antibody, compared to untreated controls; and 6) CD8-dependency occurs in both WT and tolerized neu-transgenic mice. This study reveals an essential role for the adaptive immune system in the therapeutic effect of antibody treatment on HER2/neu tumors.
The treatment of WT mice with anti-neu antibody did result in more potent anti-tumor effects than treatment of neu-Tg mice. Modeling human HER2+ cancer poses many complications. On one hand, humans are partially tolerant to the HER2 tumor antigen, so using neu transgenic mice provide alternative mdoel. On the other hand, transgenic mice greatly overexpress the neu antigen in every mammary epithelial cell soon after birth and potentially induce stronger tolerance observed in humans (Park et al., 2008). Endogenous anti-HER2 antibodies have been detected and shown to suppress HER2 kinase activity and to inhibit the transformed phenotype of HER2-expressing tumor cells (Montgomery et al., 2005). Moreover, a recent study demonstrated that treatment of neu transgenic mice with a combination of anti-DR5 and anti-ErbB-2 monoclonal antibodies induced complete responses in a majority of the transgenic mice(Park et al., 2008). Notably, depletion of CD8+ T cells provoked primary and secondary tumor relapse, revealing the induction of antitumor immunity by the combination treatment (Stagg et al., 2008). Since anti-DR5 antibody mainly kills tumor cells, this study raises the possibility that anti-neu antibody can also induce immunity even in Tg mice. Therefore, using both WT and transgenic models to assess anti-neu antibody treatment of Her2/neu+ tumors is complementary .
Since FcR-deficient mice fail to reduce or eradicate tumor after antibody treatment, ADCC was proposed as the ultimate mechanism for tumor clearance. Our study, however, raises the possibility that ADCC might not be the only mechanism dependant on FcR+ cells. In addition to their cytotoxic effects, FcR+ cells can also produce cytokines and/or danger signals in response to signals received via FcR. Indeed, various FcR+ cells can release HMGB-1 that increases cross-priming and activation of DC in both mice and humans (Apetoh et al., 2007; Urbonaviciute et al., 2008). We have shown that the blockade of even this one danger signal greatly reduces the efficacy of antibody treatment. Thus, danger signals might coordinate with FcR signaling to activate DC and NK cells. Furthermore, APCs may utilize FcR to internalize antigens for enhanced presentation. Several studies have shown that anti-tumor specific antibody treatment enhances cross-priming of CD8+ T cells through FcR-mediated phagocytosis, especially through the formation of immune complexes (Dhodapkar et al., 2002; Kalergis and Ravetch, 2002; Rafiq et al., 2002). Given the importance of danger signals in initiating immunity, we propose that blocking oncogenic signals by the anti-HER2/neu antibody may be an important initiator of, and positive-feedback loop for, adaptive immunity. For instance, antibody treatment of a neu+ transfected tumor line, that is not dependent on a HER2/neu signal for its growth, does not reverse tumor growth in vitro and fails to demonstrate any effect on in vivo tumor growth (Whittington et al., 2008). We also observed that tyrosine kinase inhibitor treatment is very potent in blocking oncogenic signals in vitro, but anti-neu antibody is more potent in vivo, presumably because of the additional FcR-mediated effect. More studies are needed to explore the mechanisms and efficacies of the two different treatment strategies to develop better combination protocols.
Despite the initial clinical success of trastuzumab plus chemotherapy treatment for Her2+ tumors, in metastatic and adjuvant settings, relapse still occurs. Frequently, this relapse is thought to be acquired resistance to the antibody, but our data suggest that this may also be the result of reduced T cell responses by chemotherapy. It has been shown previously that agents traditionally used for tumor reduction can have both positive and negative effects on host immunity (Emens et al., 2001). Most conventional chemotherapeutic drugs inhibit rapidly dividing cells, including some tumor cells and high proliferative T cells. However, small numbers of tumor cells might undergo dormancy or a very slow growth rate resulting in “drug resistance”. These cells could be the source of relapse. Thus far, the traditional combination of anti-HER2/neu antibody with chemotherapeutic agents has shown additive and synergistic effects in in vitro studies and in vivo xenograft tumor models (Hudis, 2007; Pegram et al., 1999; Pegram et al., 2004). Chemotherapeutic agents can not only reduce the size of the primary tumor, but also alter immune responses. Recent studies showed that the success of some protocols using low dose drug administration for anticancer therapy might stimulate innate and adaptive antitumor immune responses (Apetoh et al., 2007) or enhance vaccine-mediated immunity (Emens et al., 2001). During low dose chemotherapy, DCs require signaling through TLR4 and its adaptor MyD88 for efficient processing and cross-presentation of antigen from dying tumor cells. Patients with breast cancer who carry a Tlr4 loss-of-function allele relapse more quickly after radiotherapy and chemotherapy than those carrying the normal Tlr4 allele (Apetoh et al., 2007). Anti-neu antibody treatment could play a unique role for combination with low dose chemotherapy since it can significantly reduce tumor burden while boosting immune responses. A recent study showed that combined therapy with anti-DR5 and anti-ErbB-2 monoclonal antibodies significantly enhanced suppression of the growth of advanced spontaneous tumors in ErbB-2/neuT transgenic mice, even when treatment was delayed until tumors were palpable (Stagg et al., 2008). Recent clinical study showed that anti-HER-2/neu humoral responses significantly increased during combinational chemo and trastuzumab therapy, supporting our clinical observation(Taylor et al., 2007). Thus, current commonly uses of xenograft to evaluate anti-tumor effect by various treatments and protocols have their limitation and could have a biased selection of direct anti-tumor effect but ignore immune responses. As more preclinical and clinical studies are conducted, taking immune responses into consideration for future combination treatments will be necessary.
Based on numerous preclinical studies, clinical trials were conducted and demonstrated benefits of chemotherapy administration with trastuzumab over chemotherapy alone. Thus, anti-HER2/neu antibody therapy is currently administered in conjunction with chemotherapy. However, tests done by cell culture or xenograph of human tumors into T cell deficient mice, may have been biased in favor of high dose chemotherapy. It is unclear whether chemotherapy plus anti-HER2/neu antibody is always better than antibody alone (Hudis, 2007; Piccart-Gebhart et al., 2005; Romond et al., 2005). Thus it remains to be determined if, when, and how anti-HER2/neu antibody should be combined with chemotherapy. It is likely that careful monitoring of the effect of chemotherapy on the tumor and the individual’s immune response. Our study suggests that sequential administration of anti-HER2/neu antibody after chemotherapy may allow chemotherapy to enhance the antibody mediated anti-tumor effect. Yet, formal clinical trials are needed to revisit this issue and to address whether and which chemotherapy drugs can enhance antibody mediated anti-cancer effect in neoadjuvant, adjuvant and metastasis settings.
Optimal impact of anti-neu antibody treatment on tumor growth likely depends in part on the amount of antibody, and the number of FcR+ cells antigen presenting cells inside the tumor microenvironment. Therefore, combining this antibody treatment with an immunotherapy that can break tumor barriers and attract immune cells may have great impacts on the efficacy of anti-HER2/neu antibody treatment. Ad-LIGHT has multiple potential effects for enhancing antibody-mediated immunity. We have previously shown that intra-tumoral delivery of LIGHT, but not extra-tumoral delivery, can increase the expression of chemokines and adhesion molecules within the tumor, which then attract various immune cells, including T cells, NK, and DC (Fan et al., 2006; Ishihara et al., 2004; Wang et al., 2005; Yu et al., 2007). Recruiting FcR+ cells to the tumor might facilitate the FcR-mediated effect of HER2/neu antibody. In addition, LIGHT can also activate NK and T cells via the HVEM receptor to enhance anti-tumor cytotoxicity (Fan et al., 2006). Finally, driving high expression of LIGHT in the tumor by Ad-LIGHT may elaborate lymphatic vessels and alter the permeability of tumor vasculature to favor infiltration of both antibody and immune cells. LTβ R signaling on vessels has been shown to promote the neogenesis of vasculature and regulate LN hypertrophy (Liao and Ruddle, 2006). Thus, the synergy we observe from intra-tumoral injection of Ad-LIGHT during anti-neu antibody treatment may result from increased infiltration of FcR+ cells and enhanced interactions between antibody and FcR+ cells leading to antibody-mediated tumor clearance and the generation and local accumulation of T cells for the increase of host resistance to re-challenge.
Our data demonstrate that T cells are necessary for anti-HER2/neu antibody-mediated tumor reduction. Since the antibody mediated blockade of oncogenic signals and the induction of ADCC have been previously demonstrated, we propose that both of these outcomes can induce a significant release of danger signals to alarm/activate DC and promote cytokine production. FcR-mediated signaling can induce additional cytokine/danger signal production, and FcR-mediated phagocytosis and MyD88-enhanced cross-presentation may more effectively activate the adaptive immune system for enhanced tumor control. However, even with efficient blockade of oncogenic signals and induction of proper danger signals, antibody-initiated immunity may still be transient and weak, making further combination immunotherapy necessary to reach clinical significance. We demonstrate that timing of chemotherapeutic doses has a distinct impact on antibody-initated immunity, and that proper immunotherapy can synergize with antibody treatment. Thus, this study provides insight into an interesting anti-tumor mechanism of anti-HER2/neu antibody that promotes cooperation between innate and adaptive immunity and warrants the use of combination therapies promoting antibody-initiated anti-tumor immune responses.
EXPERIMENTAL PROCEDURES
Mice
Balb/c, Balb/c Rag-1 and Rag-2 KO and FVB/N-Tg (MMTV-neu) mice were purchased from Jackson Laboratory and Balb/c Fcrγ −/− mice were purchased from Taconic at 6 to 7 weeks of age. BALB/c MyD88−/− mice were kindly provided by Dr. Anita Chong, University of Chicago. Neu Tg F1 (FVB/N-Tg/MMTV-neu × BALB/c) were bred and housed at the University of Chicago. All mice were maintained under specific pathogen free conditions and used between 6–16 weeks of age in accordance to the animal experimental guidelines set by the Institutional Animal Care and Use Committee. The study has been approved by the Institutional Animal Care and Use Committee of the University of Chicago and Institute of Biophysics and all experiments conform to the relevant regulatory standards.
Cell lines and reagents
TUBO was cloned from a spontaneous mammary tumor in a BALB Neu Tg mouse (Rovero et al., 2000) while N202 was cloned from a spontaneous mammary tumor in a FBV Neu Tg mice. Both cell lines are gift from Joseph, Lustgarten J. Mayo, Arizona. TUBO-HA was selected after transfection of pSEB-GFP-HA plasmid with 2 μg/ml of Blasotcidin (InvtiroGen). TUBO and TUBO-HA were cultured in 5% CO2, and maintained in vitro in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Sigma), 10% NCTC 109 medium, 2 mmol/L L-glutamine, 0.1 mmol/L MEM nonessential amino acids, 100 units/mL penicillin, and 100 μg/mL streptomycin.
APC 3T3/KB and 3T3/NKB were provided by Dr. Wei-Zen Wei, Wayne State University (Wei et al., 2005). Anti-CD8 depleting antibody 53.6.7 was purchased from BioXcell (West Lebanon, NH). Anti-neu mAb 7.16.4, anti-CD8 depleting antibody YTS 169.4.2 (ATCC) and and anti-HMGB-1 neutralizing mAb 3B1, were produced in house. Anti-neu antibody (7.16.4) recognizes the juxtamembrane region of rat neu and competes with 4D5, the precursor of trastuzumab, for binding and inhibition of tumor grwoth (Zhang et al., 1999). Anti-HMGB-1 mAb is capable of neutralizing HMGB-1 in vivo (Chen et al., 2009). Chemotherapeutic agents; erlotinib (TarcevaTM, Genentech, CA), cyclophosphamide (CTX, Baxter, IL), paclitaxel (PTX, Mayne Pharma Inc. NJ), and doxorubicin (DOX, Teva Parenteral Medicine, CA) were purchased and prepared according to the Manufacturers’ recommendations. All antibodies for FACS were purchased from BD Biosciences. The generation of Ad-LIGHT was described previously (Kim et al., 2007). Endotoxin inside antibody was measured by the limulus amebocyte lysate assay (Cambrex inc. MD). For all mAb preparations, the amount of endotoxin was determined to be < 0.2 E.U./mg mAb (limit of detection).
In vivo treatments
3–5 × 105 TUBO cells were injected s.c. in the back of 6 to 8-week-old mice. Tumor volumes were measured along three orthogonal axes (a, b, and c) and calculated as tumor volume = abc/2. Mice were treated with two or three i.p. injections of 80–100 μg of anti-neu antibody (7.16.4). For CD8 depletion experiments, 200 μg of anti-CD8 antibody (YTS 169.4.2 or 53.6.7) was injected i.p. at the same time as anti-neu antibody treatment. For the HMGB-1 neutralizing experiment, 100 μg of mouse anti-HMGB-1 antibody (3B1) was injected i.p. on the day of anti-neu antibody treatment. For chemotherapeutic agent combination, 500 μg/mouse of erlotinib, 100 mg/kg of CTX, 40–60 mg/kg of PTX, or 5–15 mg/kg of DOX were administered i.p. at the indicated times.
Proliferation assay
Cell proliferation was measured indirectly by mitochondria metabolic activity using a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) assay. Briefly, quadruplicate wells of cells were plated in flat-bottomed 96-well plates at 10,000 cells/well. Approximately 16 h after plating, when cells reached 40% to 50% of confluence, dilutions of anti-neu mAb 7.16.4 or erlotinib were added. Replicate plates were terminated at 24, 48, and 72 hours post-treatment. At each time point, 10 to 20 μL of 5 mg/mL MTT in PBS was added and incubated for 4 h at 37° C before the stop reagent (Isopropanol with 0.04N HCl) was added and the absorbance measured at 600 to 650 nm.
Measurement of IFN-γ–secreting T cells by ELISPOT assay
Neu reactive T cells were measured by ELISPOT assay (Jacob et al., 2006). In this assay, the APC were transfected to stably express the MHC-I molecule H2-Kd and B7.1 (3T3KB) or H2-Kd, B7.1 and neu (3T3NKB); thus allowing for measurement of neu-specific CD8+ T cell responses. Spleen or lymph node cells (responder cells) were resuspended in RPMI 1640 supplemented with 10% FCS, 2 mmol/L L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. A total of 1–4 × 105 spleen or lymph node cells were added to each well of a 96-well HTS IP plate (Millipore), which was precoated with 2.5 μg/mL rat anti-mouse IFN-γ (clone R4-6A2; BD-PharMingen). 3T3/NKB cells were added as APC over the spleen cells. 3T3/KB cells were used as control. The ratio of responser cells to APC was 10:1. After 48 h of incubation, cells were removed and 2 μg/mL biotinylated rat anti-mouse-IFN-γ (clone XMG 1.2; BD-PharMingen) was added. Plates were incubated for another 12 h at 4° C, then washed to remove unbound antibody. Bound antibody was detected by incubating the plates with 0.9 μg/mL avidin-horseradish peroxidase (BD-PharMingen) for 2 h at room temperature. The substrate 3-amino-9-ethylcarbazole (AEC; PharMingen) diluted in 0.1 mol/L acetic acid and 0.003% hydrogen peroxide was added, and the plate was incubated for 3 to 5 min. AEC solution was discarded, and the plates were washed six times with water. The visualized cytokine spots were enumerated with the ImmunoSpot analyzer (CTL), and the results were expressed as the number of cytokine producing cells per 106 cells.
Analysis of tumor infiltrating lymphocytes (TIL)
Tumor masses established by TUBO were resected 3 days after antibody. For FACS anslysis, single-cell suspensions were obtained by collagenase digestion and incubated with fluorochrome-conjugaetd mAbs against surface markers. Cells were acquired on FACSCanto flow cytometer (BD Cytomtry Sytems). For immunohistochemiocal staining, resected tumors were embedded in Tissue-Tek (Sakura Finetek, Netherlands) and snap frozen in liquid nitrogen. The following primary antibodies were used for immunohistochemisty of human tissues: CD4 (4B12; Leica Biosystems Newcastle Ltd, UK), CD8 (C8/144B) and CD20cy (L26); DakoCytomation, Carpinteria, CA. Immunostaining was performed on the automated Bond TM system (Leica-Microsystems, Melbourne, Australia) according to the modified manufacturer protocol using Bond TM Polymer Refine Detection system (Leica Biosystems Newcastle Ltd). Peroxidase reaction was developed with 3,3 diaminobenzidine (DAB) provided in the kit
Statistical analysis
Differences between groups were analyzed using the two-tailed Student’s t test or 2way ANOVA. Error bars represent standard deviations (+/− SD). For survival curves, differences between curves were analyzed using the log-rank (Mantel-Cox) test.
Supplementary Material
Acknowledgments
We thank Xiao-Jun Liu for technical assistance. This research was in part supported by US National Institutes of Health grants AI062026, CA115540 and CA97296 to Y.X.F, National Key Basic Research Program 2006CB910901 and Natural Science Foundation Grant C0302050102 to S.W. and by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-013-E00017) and the Korea Science and Engineering Foundation (KOSEF) grant (No.R13-2007-023-00000-0) to S.G.P. N.K.B. was supported by training grant T32AI007090.
Footnotes
Statement
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aomatsu T, Komatsu H, Yoden A, Hosomi A, Miyazaki H, Sogo T, Inui A, Fujisawa T, Tamai H. Fulminant hepatitis B and acute hepatitis B due to intrafamilial transmission of HBV after chemotherapy for non-Hodgkin's lymphoma in an HBV carrier. Eur J Pediatr. 2010;169:167–171. doi: 10.1007/s00431-009-1000-6. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation ofcCellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA, Machiels JP, Reilly RT, Jaffee EM. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001;3:77–84. [PubMed] [Google Scholar]
- Fan Z, Yu P, Wang Y, Wang Y, Fu ML, Liu W, Sun Y, Fu YX. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood. 2006;107:1342–1351. doi: 10.1182/blood-2005-08-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Harada M, Azuma K, Tamura M, Shomura H, Fujii T, Itoh K, Shichijo S. HER2/neu-derived peptides recognized by both cellular and humoral immune systems in HLA-A2+ cancer patients. Int J Oncol. 2004;24:967–975. [PubMed] [Google Scholar]
- Jacob J, Radkevich O, Forni G, Zielinski J, Shim D, Jones RF, Wei WZ. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cell Immunol. 2006;240:96–106. doi: 10.1016/j.cellimm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J Exp Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Wei WZ, Herrmann F, Lindencrona JA, Choudhury A, Kono K, Seliger B. Cellular immunity to the Her-2/neu protooncogene. Adv Cancer Res. 2002;85:101–144. doi: 10.1016/s0065-230x(02)85004-7. [DOI] [PubMed] [Google Scholar]
- Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le XF, Lammayot A, Gold D, Lu Y, Mao W, Chang T, Patel A, Mills GB, Bast RC., Jr Genes affecting the cell cycle, growth, maintenance, and drug sensitivity are preferentially regulated by anti-HER2 antibody through phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem. 2005;280:2092–2104. doi: 10.1074/jbc.M403080200. [DOI] [PubMed] [Google Scholar]
- Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res. 2006;12:6326–6330. doi: 10.1158/1078-0432.CCR-06-1732. [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, Liu Y, Tucker SL, McKenzie T, Qiao N, Akli S, Biernacka A, Meijer L, Keyomarsi K, Hunt KK. A novel interaction between HER2/neu and cyclin E in breast cancer. Oncogene. 2010 doi: 10.1038/onc.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moasser MM. Targeting the function of the HER2 oncogene in human cancer therapeutics. Oncogene. 2007;26:6577–6592. doi: 10.1038/sj.onc.1210478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RB, Makary E, Schiffman K, Goodell V, Disis ML. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- Park JM, Terabe M, Steel JC, Forni G, Sakai Y, Morris JC, Berzofsky JA. Therapy of advanced established murine breast cancer with a recombinant adenoviral ErbB-2/neu vaccine. Cancer Res. 2008;68:1979–1987. doi: 10.1158/0008-5472.CAN-07-5688. [DOI] [PubMed] [Google Scholar]
- Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Rovero S, Amici A, Carlo ED, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, Johnstone RW, Smyth MJ. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci U S A. 2008;105:16254–16259. doi: 10.1073/pnas.0806849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Hershman D, Shah N, Suciu-Foca N, Petrylak DP, Taub R, Vahdat L, Cheng B, Pegram M, Knutson KL, Clynes R. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Menard S, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- Wang YG, Kim KD, Wang J, Yu P, Fu YX. Stimulating lymphotoxin beta receptor on the dendritic cells is critical for their homeostasis and expansion. J Immunol. 2005;175:6997–7002. doi: 10.4049/jimmunol.175.10.6997. [DOI] [PubMed] [Google Scholar]
- Wei WZ, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YC. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Res. 2005;65:8471–8478. doi: 10.1158/0008-5472.CAN-05-0934. [DOI] [PubMed] [Google Scholar]
- Whittington PJ, Piechocki MP, Heng HH, Jacob JB, Jones RF, Back JB, Wei WZ. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Res. 2008;68:7502–7511. doi: 10.1158/0008-5472.CAN-08-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF, Schreiber H, You Z, Kaynor C, Wang X, Fu YX. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. 2007;179:1960–1968. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Q, Montone KT, Peavey JE, Drebin JA, Greene MI, Murali R. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Exp Mol Pathol. 1999;67:15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.