SUMMARY
The degree of lineage stability achieved by pathogen-specific CD4+ T cells in vivo, and how this impacts host defense against infection, remains unclear. We demonstrate that in response to Th1-polarizing intracellular bacterial or viral pathogens, only 80–90% of responding polyclonal T cells become indelibly committed to this lineage. Th1 commitment was nearly invariant in cells that proliferated extensively, but perturbations to the extrinsic cytokine milieu or the pathogen’s ability to enter the cytosol impeded commitment and promoted plasticity for future IL-17 expression. Conversely, cell intrinsic interferon-γ expression and acquisition of permissive chromatin at the Ifng gene during priming predicted heritable Th1 commitment. Importantly, CD4+ T cells that retained plasticity conferred protection against M. tuberculosis, while these protective effects were abolished with Th17 polarization. These findings illustrate the immune signals that induce memory CD4+ T cell responses required for maintaining host defense against infection yet are adaptable in novel environmental contexts.
INTRODUCTION
On initial encounter with cognate antigen, naïve CD4+ T cells proliferate and differentiate into one of several helper subsets. Th1 cells produce interferon-γ and IL-2 and help to combat infection by intracellular pathogens; Th2 cells promote the clearance of multicellular helminths by producing IL-4, IL-5 and IL-13; Th17 cells produce IL-17a, IL-17f, IL-21, IL-22, and (in humans IL-26) and help to protect against extracellular bacteria and fungi, particularly at epithelial surfaces (Zhu and Paul, 2008; Curtis and Way, 2009). T follicular helper lineage, specialized in providing help for B cells, has also been recently proposed (Silver and Hunter, 2008; Vogelzang et al., 2008; Nurieva et al., 2008). Given the specialized role of helper T cells in host defense, stable commitment to each defined helper lineage would ensure recall of qualitatively similar responses upon subsequent re-infection with the same pathogen.
The paradigm of T helper (Th) cell lineage commitment originated from studies showing that when naïve mouse CD4+ T cells are cultured in Th1 or Th2 polarizing conditions in vitro, irreversible helper lineage commitment occurs by the fourth cell division and is associated with silencing of cytokines and transcription factors specific to the opposing helper lineage (Grogan et al., 2001; Mullen et al., 2001). However, the applicability of these results where CD4+ T cells are stimulated by lineage-inducing cytokines in the presence of neutralizing antibodies to lineage-opposing cytokines in vitro to conditions when T cells are primed in vivo is uncertain.
In this regard, recent studies suggesting greater diversity and plasticity of helper cell phenotypes have brought into question the Th cell paradigm of heritable lineage commitment (O’Shea et al., 2008; Reiner et al., 2007). For example, human Th1 and Th2 clones can produce lineage-inappropriate cytokines when stimulated in opposite polarizing conditions (Messi et al., 2002). In the mouse, sustained Th1 immunity against Leishmania major and Toxoplasma gondii is unstable without continuing exposure to IL-12 (Park et al., 2000; Stobie et al., 2000; Yap et al., 2000), while in vitro-derived Th17 cells require continual TGF-β to maintain IL-17 and repress IFN-γ production and revert to a colitis-inducing Th1 phenotype following adoptive transfer into RAG-deficient mice (Lee et al., 2009; Nurieva et al., 2009). Similarly, memory Th cells generated in vivo by immunization with polarized dendritic cells readily produce cytokines associated with other lineages when stimulated in opposite polarizing conditions, while in vitro-derived Th2 cells gain the ability to produce Th1 upon challenge in vivo with LCMV (Krawczyk et al., 2007; Lohning et al., 2007). Based on these results, the immune signals that dictate stability versus plasticity of pathogen-specific CD4+ T cell differentiation in vivo, and how these changes dictate host defense against secondary infection, require re-evaluation.
To address this question, we examined the stability of cytokine production by antigen-specific memory Th cells generated during highly Th1-polarizing intracellular bacterial (Listeria monocytogenes, Lm) or viral (lymphocytic choriomeningitis virus, LCMV) infections. Our results show that CD4+ T cell replication during priming created a context permissive to Th1 commitment, and that perturbations of exogenous type 1 innate immune cytokine signals arising either from mutations affecting the host or the pathogen’s intracellular lifestyle eroded commitment. Expression of Il17a and Il17f by effector CD4+ T cells during priming predicted plasticity for IL-17 production during memory, whereas robust expression of IFN-γ and acquisition of permissive histone modifications at the Ifng promoter during priming predicted the silencing of IL-17 and stable Th1 commitment. Using adoptively transferred cells with specificity against the protective Ag85B protein in M. tuberculosis (MTb) (Tamura et al., 2004), we further demonstrate that CD4+ T cells that retain plasticity also maintain protection against MTb, while stimulation under Th17 polarizing conditions prior to transfer eliminated these protective effects. Together, our findings indicate the events that occur during CD4+ T cell priming, including the stimulation by inflammatory cytokines and other cell intrinsic and stochastic factors, result in a polyclonal memory response in which the majority of cells are heritably committed to the Th1 lineage, while a fraction have acquired the capacity to readily express IFN-γ yet retained the capacity to express IL-17 in the future. As a result, a cadre of committed Th cells able to quickly iterate the effector functions instructed by the priming environment was retained in memory, while the plasticity to adapt to novel environmental conditions that might be encountered in the future was achieved on a population basis.
RESULTS
Innate Immune Cytokines Coordinately Instruct Th1 Commitment
The cytokines IL-12, type I IFNs (IFN-αβ), and IFN-γ are each triggered early after Lm infection (Tripp et al., 1993; Nakane et al., 1984; Leber et al., 2008; Kaufmann et al., 1983). In addition to innate host protection, these cytokines also drive the development of pathogen-specific cytotoxic CD8+ T cells and Th1 helper CD4+ T cells. Consequently, mice with combined defects in both IL-12 and type-I IFN signaling, but not mice lacking just one of these signals, develop Lm-specific CD4+ T cells with a mixed Th1/Th17 phenotype when interrogated directly ex vivo (Orgun et al., 2008). Whether this phenotype is stable to environmental perturbation or more reflects differentiation plasticity is unknown.
To address these questions, we infected mice deficient in type-I IFN signaling and IL-12/23p40 (IFNAR-p40 double knockout [DKO] mice) or type-I IFN and IL-12 signaling (IFNAR-IL12Rβ2 DKO mice) with LmΔactA-Ova. LmΔactA-Ova escapes from the phagosome, invades the cytosol, and induces type 1 immune responses but is attenuated due to its inability to polymerize host cell actin. Unlike wildtype (WT) Lm, LmΔactA-Ova is not lethal in the DKO mice. Furthermore, because bacterial numbers in the liver and spleen are similar in the first days after infection and are cleared soon thereafter in DKO and WT mice, antigen load is equalized (Orgun and Way, 2008). To compare the response induced by WT Lm-OVA and LmΔactA-Ova, we infected WT mice and evaluated memory CD4+ T cells 28 days later. The percentage of cells producing IFN-γ and IL-17 in response to stimulation with cognate LLO189-201 peptide directly ex vivo and after stimulation and culture under various conditions in vitro for 5 days did not differ between mice infected with these two strains of Lm (Figure S1). Given that LmΔactA-Ova induces a similar immune response to WT Lm-Ova, all subsequent experiments were performed using LmΔactA-Ova.
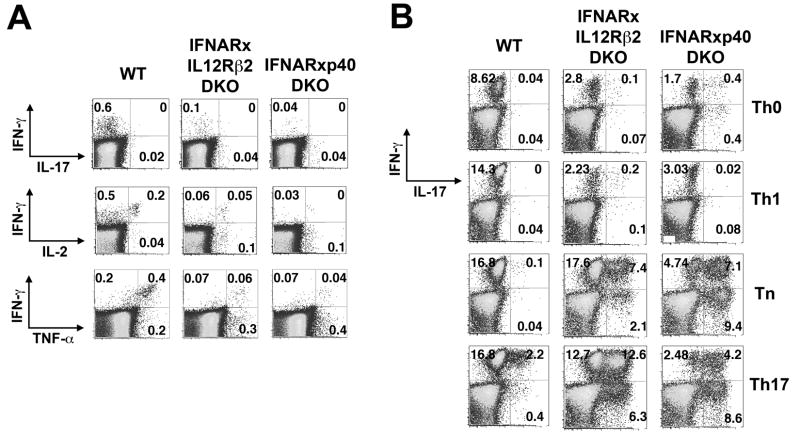
The patterns of cytokine production by splenocytes harvested 28 days following infection and then stimulated with LLO189-201 peptide directly ex vivo were similar to the patterns we previously observed for day 8 effector cells from these mice (Orgun et al., 2008). Most responding memory CD4+ T cells from WT mice produced IFN-γ plus IL-2 and TNF-α but not IL-17 (Figure 1A, Figure S2). This was also true for cells from IFNAR KO, IL-12Rβ2 KO, or p40 KO mice (data not shown). By contrast, memory CD4+ T cells producing IL-17 were detected in IFNAR-IL12Rβ2DKO and IFNAR-p40 DKO mice, indicating that the mixed Th1 plus IL-17 phenotype of effector cells from these mice was propagated into memory.
Figure 1. Type-I IFNs and IL-12 contribute to Th1 stabilization among endogenous pathogen-specific CD4+ T cells.
A. Representative FACS plots demonstrating IFN-γ and IL-17 production by CD4+ T cells from the indicated groups of mice. Splenocytes were harvested 28 days post-infection with LmΔactA-OVA and stimulated for 5 hours with LLO189-201 peptide. B. Representative FACS plots showing percent IFN-γ and IL-17 production by Lm-specific CD4+ T cells after re-stimulation with LLO189-201 following culture in the indicated cell polarizing conditions for 5 days.
To determine the plasticity of these memory responses, ex vivo splenocytes were stimulated in neutralizing (Tn), Th1, or Th17 conditions or media alone (Th0) for 5 days, and cytokine production reassessed after stimulation with LLO189-201 peptide (Figure 1B, Figure S2). The patterns of cytokine production observed directly ex vivo were maintained in Th0 conditions for cells primed by LmΔactA in each type of mice and in Tn conditions for WT mice. However, the percentage of cells producing IL-17 or IL-17 plus IFN-γ increased significantly in Tn compared to Th0 conditions for those recovered from IFNAR-IL12Rβ2 DKO and IFNAR-p40 DKO mice (p<0.01). In Th17 conditions, the fraction of cells from IFNAR-IL12Rβ2 DKO and IFNAR-p40 DKO mice producing IL-17 alone or IL-17 plus IFN-γ increased further (p<0.0001), and such cells, though in substantially smaller percentages, were observed in WT mice (Figure 1B). Conversely, in Th1 conditions, production of IL-17 by CD4+ T cells was abolished and IFN-γ production increased in IFNAR-p40 DKO mice but not in IFNAR-IL12Rβ2 DKO mice, which are refractory to IL-12 signaling. The relative abundance of cytokines in the 10 and 72 hr culture supernatants of re-stimulated cells (Figure S3) generally paralleled the findings obtained by flow cytometry, with 72-hour supernatants demonstrating greater differences between the WT and DKO mice. Cells producing IL-4 were not detected under any of these conditions or under Th2 conditions (data not shown).
Thus, the strong Th1 and mixed Th1 plus IL-17 responses induced by Lm infection in WT mice and the IFNAR-IL12Rβ2 DKO and IFNAR-p40 DKO mice, respectively, were heritable in the absence of alterations in the cytokine milieu. A fraction (10–20%) of memory cells from WT mice retained the ability to express IL-17 under Th17 conditions, though most did not. By contrast, the majority of cells from IFNAR-IL12Rβ2 DKO and IFNAR-p40 DKO mice retained functional plasticity.
Replication plays a permissive role in Th1 lineage commitment
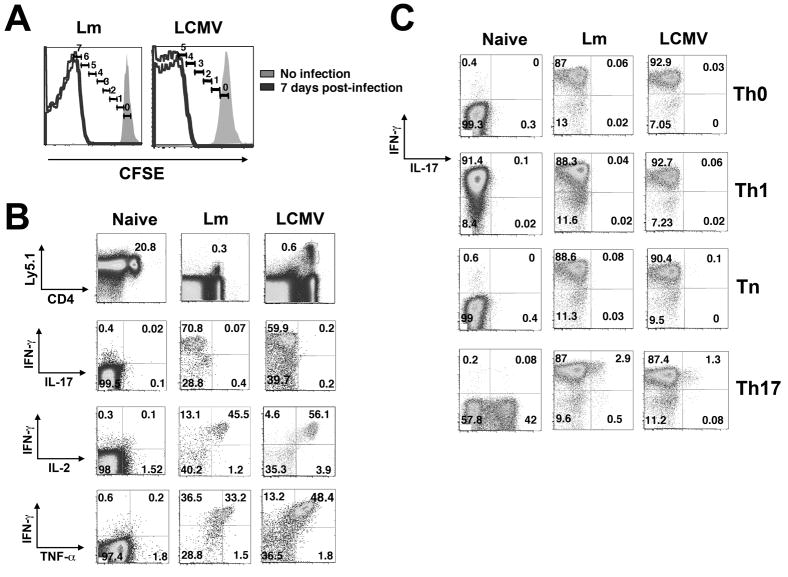
What might account for the inability of 10–20% of memory cells from WT mice to heritably silence IL-17? Heritable silencing of lineage-inappropriate cytokines in Th1 and Th2 cells is not consistently achieved even in strong polarizing conditions in vitro before the fourth cell division (Grogan et al., 2001; Mullen et al., 2001). Therefore, it is possible that the LLO-specific memory cells that had not silenced IL-17 represent Th cells that had not undergone the requisite 4 cell divisions. To address this possibility, CD4+ T cells from P25 T cell receptor (TCR) transgenic mice with specificity for the Ag85B240-254 peptide of MTb were purified from naïve mice, labeled with CFSE and adoptively transferred into WT recipients, which were infected 24 hours later with recombinant Lm engineered to express Ag85B (LmΔactA-Ag85B). All of the transferred cells divided ≥5 times by day 7 (Figure 2A). Donor-derived memory P25 cells harvested at day 28 efficiently produced IFN-γ plus IL-2 and TNF-α but not IL-17 in response to stimulation with cognate peptide directly ex vivo (Figure 2B). To determine the relative stability of this Th1 differentiation program, cells were stimulated in Th0, Tn, Th1 or Th17 conditions for 5 days and cytokine production reassessed after re-stimulation with cognate peptide. We found >85% of the memory T cells produced IFN-γ regardless of the culture conditions (Figure 2C, Figure S4). The only deviation was observed for cells cultured in Th17 conditions, where a small fraction (range 1–3%) of cells also produced IL-17. By contrast to these memory T cells primed by Lm infection in vivo, naïve CD4+ T cells readily adopted the cytokine profile appropriate for the specific culture conditions (Figure 2C). Thus, when naïve CD4+ T cells divided >4 times in vivo in response to Lm infection, they, like cells generated in Th1 cell-polarizing conditions in vitro, became heritably committed to the Th1 lineage. These findings suggest that the dominant factor responsible for the incomplete silencing of IL-17 by 10–20% of the Lm-LLO-specific Th1 cells generated from endogenous, polyclonal naïve progenitors in WT mice (Figure 1) was not their environment or inability to commit, but the absence of sufficient numbers of cell divisions.
Figure 2. Pathogen-specific CD4+ T cells primed by Lm or LCMV are heritably committed to the Th1 lineage.
A. CFSE-labeled CD4+ T cells from P25 TCR or SMARTA TCR transgenic mice were transferred into B6 control recipients that were subsequently infected with LmΔactA-85B or LCMV, respectively. Histograms display number of cell divisions among donor CD4+ T cells 7 days post-infection. B. Representative FACS plots indicating IFN-γ, IL-17, IL-2 and TNF-α production by naive P25, or donor P25 or SMARTA CD4+ T cells recovered day 28 after infection and stimulation with cognate peptide directly ex vivo. C. Representative FACS plots demonstrating IFN-γ and IL-17 production by donor P25 or SMARTA CD4+ T cells following culture in the indicated conditions for 5 days and re-stimulation with cognate peptide.
To determine the generality of these findings, similar experiments tracking the stability of CD4+ T cell differentiation after infection with LCMV were performed. CD4+ T cells from SMARTA TCR Tg mice with specificity for LCMV glycopeptide GP61-80, were transferred into WT recipients and their cytokine production tracked after infection with the Armstrong strain of LCMV. All SMARTA CD4+ T cells divided ≥4 times by day 7 post-infection demonstrated by the absence of detectable CFSE (Figure 2A). When harvested 28 days after infection, memory SMARTA CD4+ T cells produced IFN-γ plus IL-2 and TNF-α but not IL-17 in response to stimulation with cognate peptide directly ex vivo (Figure 2B, Figure S4). When cultured with cognate peptide for 5 days in different polarizing conditions, they responded similarly to T cells primed in response to Lm—more than 85% produced high levels of IFN-γ in all conditions, and only under Th17 conditions did a small fraction (1–3%) also produce IL-17 (Figure 2C, Figure S4). Together, these results indicate that when numbers of cell divisions are not limiting, Lm and LCMV infections both induce heritable Th1 lineage commitment.
Exogenous type 1 cytokines are critical inducers of Th1 lineage commitment
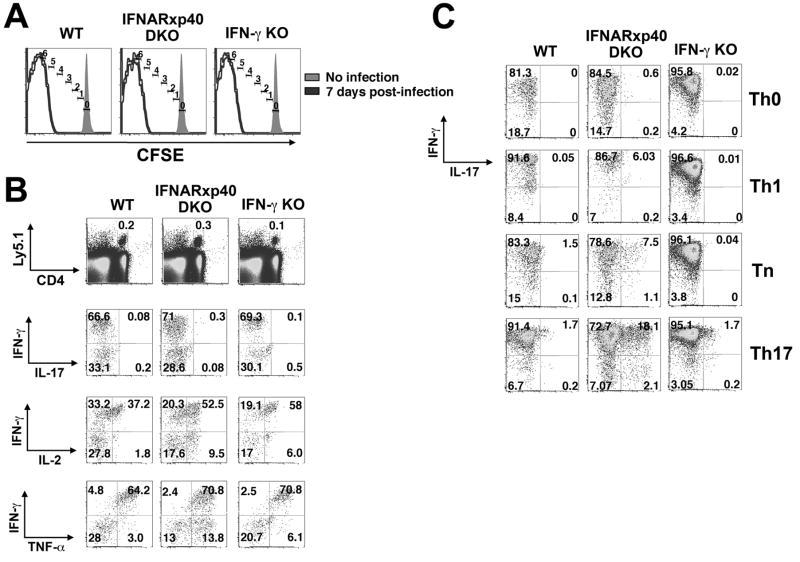
We next sought to determine whether type 1 innate immune cytokines remained essential for heritable commitment when numbers of cell divisions were no longer limiting. CD4+ T cells from P25 TCR Tg mice were transferred into WT and IFNAR-p40 DKO recipients that were then infected with LmΔactA-Ag85B. In each type of recipient, the transferred T cells divided ≥5 times by day 7 (Figure 3A) and accumulated to a similar degree by day 28 (data not shown); more than 60% of memory P25 cells recovered from each recipient mouse produced IFN-γ directly ex vivo, and after they were activated and cultured for 5 days in vitro, regardless of the culture conditions (Figures 3Band C , Figure S5). However, when cells from the IFNAR-p40 DKO recipients were cultured in Tn conditions a population of cells producing IL-17 plus IFN-γ emerged (p<0.003), as did cells producing IL-17 without IFN-γ (p<0.002); these percentages were further amplified under Th17 conditions (p<0.0005). By contrast, <3% of cells from WT recipients produced IL-17 even under Th17 conditions. These results indicate that while heritable IFN-γ production is substantially maintained in the absence of type I IFNs and IL-12/23p40, silencing of IL-17 production and thus Th1 commitment is compromised.
Figure 3. Type-I IFNs and IL-12 promote Th1 commitment among adoptively transferred pathogen-specific CD4+ T cells.
A. CFSE-labeled CD4+ T cells from P25 TCR transgenic mice were transferred into B6 control (WT), IFNAR-p40 DKO, or IFN-γ KO recipients that were subsequently infected with LmΔactA-85B. Histograms display number of cell divisions among adoptive transferred CD4+ T cells 7 days post-infection. B. Representative FACS plots indicating IFN-γ, IL-17, IL-2 and TNF-α production by P25 CD4+ T cells recovered day 28 after infection and stimulation with cognate peptide directly ex vivo. C. Representative FACS plots demonstrating IFN-γ and IL-17 production by P25 CD4+ T cells primed in vivo with LmΔactA-85B following culture in the indicated conditions for 5 days and re-stimulation with cognate peptide.
Type I IFNs and IL-12 induce NK cells to produce IFN-γ during the innate immune response to Lm (Orgun et al., 2008), and NK cell-derived IFN-γ may promote Th1 commitment. However, when P25 cells were transferred into IFN-γ KO recipients, they proliferated during the acute infection and developed into memory T cells whose cytokine production pattern was indistinguishable from WT recipients (Figure 3). These findings indicate that the increase in IL-17 producing cells observed in IFNAR-p40 DKO recipients was not attributable to impaired production of innate IFN-γ.
Entrance of Lm into the cytosol is required for heritable Th1 commitment
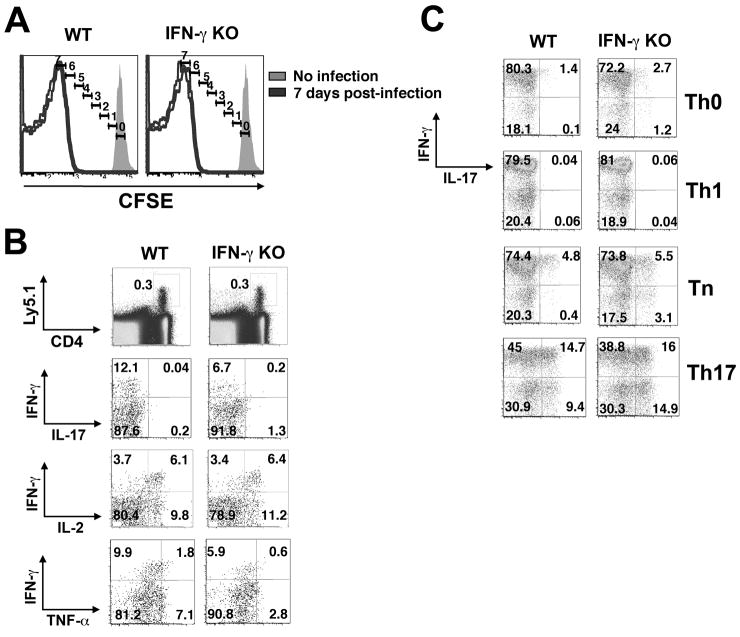
Entry of Lm into the cytosol activates an array of pathogen pattern recognition receptors (Kobayashi et al., 2005; Herskovits et al., 2007; Stetson and Medzhitov, 2006) and is required for the efficient induction of type 1 innate inflammatory cytokines, including type-I IFNs, IL-12, and IFN-γ (O’Riordan et al., 2002; Kang et al., 2008; Hara et al., 2008). Having found that Th1 commitment is impaired in mice with deficits involving these cytokines, we next sought to determine whether an Lm that cannot invade the host cell cytosol—and thus does not alert the innate immune system to the presence of an intracellular pathogen—would have a similar effect. We generated a recombinant Ag85B-expressing Lm in a parental bacterial strain deficient in listeriolysin O (LLO) and phospholipases C (PLC) that does not escape from the phagosome into the cell cytoplasm and therefore does not trigger type I IFN and IL-12 production (O’Riordan et al., 2002; Kang et al., 2008; Hara et al., 2008). Using LmΔLLO/ΔPLC-Ag85B, which we will refer to as vacuolar Lm, and LmΔactA-Ag85B, which we will refer to as cytosolic Lm, we examined how Lm entry into the cell cytoplasm and the distinct inflammatory cytokine environment this process elicits dictates the pathogen-specific CD4+ T cell response to the surrogate Ag85B antigen. Given the higher degree of in vivo attenuation of vacuolar compared with cytosolic Lm, we used a 100-fold higher inoculum of vacuolar Lm, which results in similar numbers of cytosolic and vacuolar Lm in the spleens and livers of mice at 24 hours (Orgun and Way, 2008). In response to infection with vacuolar Lm, adoptively transferred P25 cells readily diluted CFSE, and this occurred with similar kinetics compared with the response following cytosolic Lm infection (compare Figure 4A and Figure 3A). However, when the response of memory Th cells was assessed directly ex vivo 28 days post-infection, 5–10-fold fewer P25 cells primed with vacuolar Lm compared with cytosolic Lm in WT mice produced IFN-γ (p<0.0001) and a larger fraction produced IL-2 but not IFN-γ (p<0.001) (compare Figure 4B and Figure 3B, and Figures S5 and S6). In stark contrast to memory P25 cells from mice primed with cytosolic Lm, memory cells from mice primed with vacuolar Lm produced IL-17 plus IFN-γ after activation in Th0 conditions, and the frequency of these cells increased progressively in Tn and Th17 conditions. But unlike naïve CD4+ T cells cultured in these conditions (Figure 2C), the majority of vacuolar Lm-primed memory cells produced IFN-γ when cultured under Th0, Tn, and Th1 conditions, and under Th17 conditions cells producing IFN-γ only, IL-17 only, or IFN-γ plus IL-17 were evident. These results demonstrate that the majority of memory Th cells in mice infected with vacuolar Lm had become biased towards the production of IFN-γ (producing this cytokine when cultured under any of these conditions), but unlike committed Th1 cells induced by cytosolic Lm, this bias was unstable and could be repressed or amplified, depending on the cytokine milieu. When these experiments were repeated using IFN-γ KO rather than WT mice as recipients, the Th1 bias and commitment were further eroded (Figure 4, Figure S6). Thus, in the absence of cytosolic entry by Lm, exogenous innate IFN-γ was no longer redundant for Th1 commitment, and memory Th cells with considerable plasticity in regard to IFN-γ and IL-17 expression were induced.
Figure 4. Lm restricted from the cell cytoplasm primes pathogen-specific CD4+ T cells with plasticity for IL-17 production.
A. CFSE-labeled P25 CD4+ T cells were transferred into B6 control (WT) or IFN-γ-deficient recipients and subsequently infected with LmΔLLOΔPLC-85B (vacuolar Lm). Histograms display number of cell divisions among donor CD4+ T cells 7 days post-infection. B. Representative FACS plots indicating IFN-γ, IL-17, IL-2 and TNF-α production by P25 CD4+ T cells recovered day 28 after infection and stimulation with cognate peptide directly ex vivo. C. Representative FACS plots demonstrating IFN-γ and IL-17 production by donor P25 CD4+ T cells primed in vivo with LmΔLLOΔPLC-85B following culture in the indicated conditions for 5 days and restimulation with cognate peptide.
P25 CD4+ T cells primed by vacuolar Lm confer protection against MTb
The emergence of cells producing IFN-γ plus IL-17 in response to vacuolar Lm infection suggests that memory CD4+ T cells can emerge in vivo following multiple rounds of cell division that are biased towards the production of IFN-γ yet incompletely committed to the Th1 lineage because they retain the capacity to express IL-17. To identify the potential significance of CD4+ T cell plasticity primed by vacuolar Lm on host defense, memory P25 cells from mice primed 28 days previously with vacuolar Lm were FACS purified and adoptively transferred into naïve B6 mice that were subsequently infected by aerosol with virulent MTb. In comparison with control mice that did not receive cells, recipients of “plastic” P25 cells (vacuolar Lm-primed memory cells cultured under Tn conditions) contained significantly reduced (>70-fold) numbers of recoverable MTb CFUs (Figure 5). By contrast, these protective effects were eliminated for the same P25 cells primed by vacuolar Lm and cultured under Th17 conditions prior to adoptive transfer. Interestingly, co-transfer of Th1 cells (cytosolic Lm-primed memory cells cultured under Tn conditions) (Figure 2C) with Th17-polarized P25 cells (vacuolar Lm-primed memory cells cultured under Th17 conditions) restored the level of protection comparable to that conferred by “plastic” P25 cells (Figure 5). Consistent with the previous demonstration that optimal protection against mucosal MTb infection in immunized mice requires a mixed Th1/Th17 response (Khader et al., 2007), these results confirm that a plastic or mixed Th1/Th17 response confers protection against MTb while a more Th17-polarized response is not protective.
Figure 5. Impacts of differentiation on CD4+ T cell protective potency.
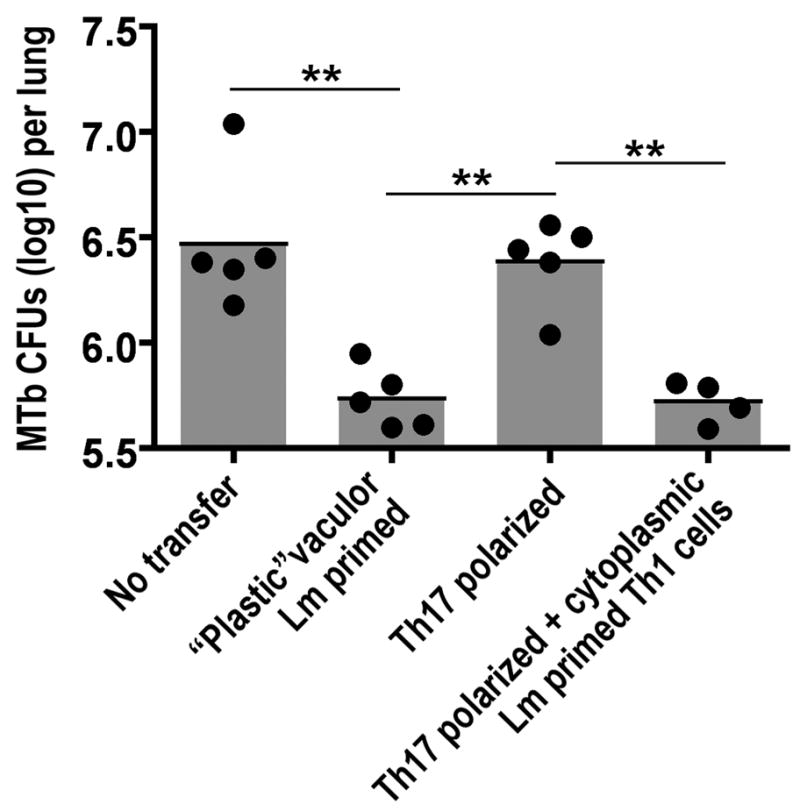
Recoverable pathogen burden in the lung day 28 after aerosol infection with 102 MTb in mice transferred P25 cells (5 × 105) one day prior to infection or no cell transfer control. “Plastic” and Th17 polarized cells were recovered from mice infected with vacuolar LmΔLLOΔPLC-85B and cultured under neutral (Tn) or Th17 conditions, respectively, for five days as described in the Experimental Procedures. Th1 cells were recovered from mice infected with cytoplasmic LmΔactA-85B and cultured under neutral (Tn) conditions. **, p < 0.01.
Previous expression of Ifng predicts Th1 commitment by memory cells
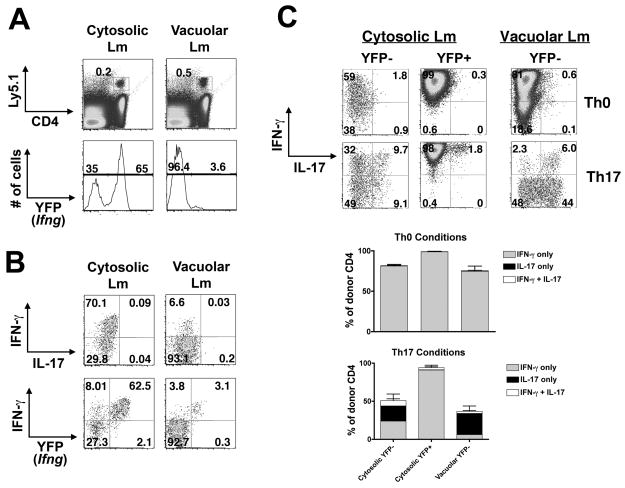
Although IL-17 producing Th17 CD4+ T cells represent a defined cell lineage distinct from IFN-γ producing Th1 CD4+ T cells, Th17 cells retain the capacity to express IFN-γ even after 4 weeks in polarizing cultures unless constantly exposed to TGF-β, whereas no such flexibility was detected in Th1 cells generated in parallel (Lee et al., 2009; McGeachy and Cua, 2008). Interestingly, memory P25 cells producing IFN-γ plus IL-17 were consistently much more frequent than cells producing only IL-17 in WT and IFN-γ KO mice infected with vacuolar Lm and in IFNAR-p40 DKO mice infected with cytosolic Lm. This finding suggested that the capacity to produce both of these cytokines either arose concomitantly or that some IFN-γ-producing memory cells had not silenced and later expressed IL-17. To address these possibilities, we inter-crossed P25 TCR transgenic mice to IFN-γ-YFP reporter (Yeti) mice (Stetson et al., 2003), and used cells from these mice to trace the origin of cells capable of making both cytokines. In Yeti mice, cells that have previously produced IFN-γ continue to produce YFP protein, which is translated from an internal ribosomal entry site, even after the production of IFN-γ protein has ceased. Thus YFP expression is a durable marker that denotes previous production of IFN-γ by these cells.
P25 x Yeti CD4+ T cells were transferred into WT recipients, which were infected with cytosolic or vacuolar Lm 24 hours later. When assessed on day 28, the majority (65–70%) of memory cells that had been primed with cytosolic Lm expressed YFP, whereas only 3–4% of memory cells primed with vacuolar Lm expressed YFP (Figure 6A). Following stimulation with cognate peptide directly ex vivo, nearly all IFN-γ-producing cells from mice primed with cytosolic Lm were YFP+, but only ~50% of IFN-γ-producing cells from mice primed with vacuolar Lm were YFP+ (Figure 6A,B), suggesting reduced or more labile Th1 commitment in this context. To address this possibility, YFP+ and YFP− cells were purified by FACS to more than 95% purity, stimulated under Th0 or Th17 conditions, then restimulated with cognate peptide; due to the low frequency of YFP+ cells primed during vacuolar Lm infection, we were unable to sort this population in sufficient purity and numbers to accurately assess Th1 commitment. In Th0 conditions, the vast majority of cells from each of these populations produced IFN-γ (Figure 6C). When cells from the YFP+ population of mice infected with cytosolic Lm were cultured under Th17 conditions, strong IFN-γ production was maintained and IL-17 was silenced in all but a very small fraction (<3%) of cells, representing either the progeny of rare, truly YFP+ cells or an occasional YFP− cell not removed from this population by cell sorting. In contrast, IFN-γ production was markedly repressed and cells producing IL-17 plus IFN-γ or IL-17 alone were readily induced when the YFP− populations were cultured in Th17 conditions, with this shift in cytokine production being more marked for the YFP− population from mice infected with vacuolar Lm compared to cytosolic Lm. Thus, expression of Ifng during priming predicted heritable IL-17 silencing and Th1 commitment by memory CD4+ T cells.
Figure 6. Previous Ifng expression marks Th1 committed cells.
A. P25 x Yeti CD4+ T cells were transferred into control B6 recipients 24 hours prior to infection with cytosolic or vacuolar Lm-Ag85B. Histograms represent percent donor cells that express Ifng (YFP+) directly ex vivo day 28 after infection. B. Representative FACS plots demonstrating IFN-γ and IL-17 production or YFP expression by cytosolic Lm or vacuolar Lm-primed CD4+ T cells after stimulation with cognate peptide directly ex vivo. C. Representative FACS plots (top) and composite bar graphs (bottom) demonstrating IFN-γ and IL-17 production by YFP− or YFP+ donor memory cells after culture in the indicated conditions for 5 days and restimulation with cognate peptide.
Ifng and Il17 expression and chromatin modifications in effector cells predict future Th1 heritability vs. plasticity
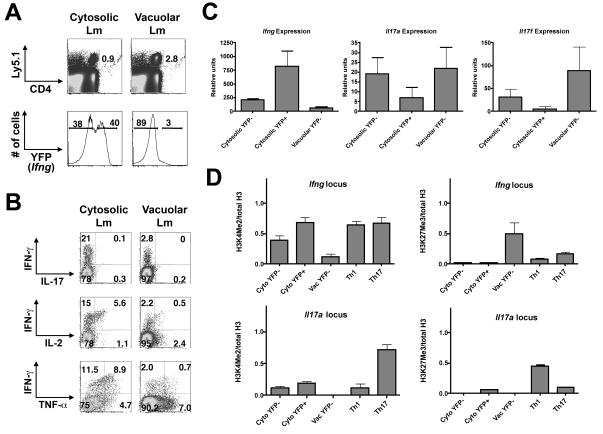
To further explore cell-intrinsic epigenetic changes during priming that predicted future T helper cell heritability or plasticity, we analyzed P25 cells at the peak of T cell expansion (day 7) after infection with either cytosolic or vacuolar Lm. Similar to what was observed with memory cells, cytosolic Lm induced a much greater fraction of P25 cells that were YFP+ directly ex vivo and produced IFN-γ following re-stimulation compared with effector cells from mice infected with vacuolar Lm (Figure 7A, B). By contrast, a greater percentage of effector cells from mice infected with vacuolar Lm produced IL-2 or TNF-α in the absence of IFN-γ (Figure 7B). Consistent with these findings, when we purified cells from these mice based on YFP expression and used RT-PCR to enumerate relative mRNA abundance for the transcripts that encode these cytokines, we found that the YFP− cell populations from mice infected with cytosolic or vacuolar Lm had significantly reduced expression of Ifng mRNA (cytosolic: p<0.01; vacuolar: p<0.006) compared to the YFP+ population from mice infected with cytosolic Lm (Figure 7C). Moreover, the YFP− cell populations expressed more Il17a and Il17f mRNA directly ex vivo, whereas YFP+ cells from mice infected with cytosolic Lm did not express Il17f above background and had lower amounts of Il17a than the YFP− cell populations. None expressed detectable amounts of Il4 mRNA.
Figure 7. Histone modifications at the Ifng loci coincide with Th1 differentiation stability.
A. P25 CD4+ T cells were transferred into B6 control recipients that were infected with cytosolic or vacuolar Lm. Donor CD4+ T cells were harvested at day 7 and sorted based on YFP expression. Histograms indicate gates used to sort YFP+ and YFP− donor CD4+ T cells. B. Representative plots indicating IFN-γ, IL-17, IL-2 and TNF-α production by cytosolic or vacuolar Lm-primed effector CD4+ T cells after stimulation with cognate peptide directly ex vivo. C. Bar graphs indicate expression of Ifng, Il17a and Il17f by cytosolic or vacuolar Lm-primed CD4+ T cells purified based on YFP expression. D. Bar graphs display enrichment of H3K4Me2 and H3K27Me3 normalized to total histone H3 at the Ifng and Il17a promoters. Day 7 YFP+ and YFP− P25 cells from mice infected with cytosolic (Cyto) or vacuolar (Vac) Lm are plotted for comparison with Th1 and Th17 cells generated by culturing naïve P25 cells under the respective polarizing conditions for 5 days in vitro. Representative graphs from one of three independent experiments are shown depicting the average and standard deviation of three technical replicates.
Epigenetic processes, including post-translational modifications of histones, help to assure the heritability of cell fate decisions, including Th cell lineage commitment (Wilson et al., 2009). To determine if epigenetic modifications during priming foretold differences in Th1 commitment vs. plasticity in memory CD4+ T cells, we performed chromatin immunoprecipitation (ChIP) on these purified cell populations using antibodies to H3K4me2, a mark of poised and active chromatin, or H3K27me3, a mark of repressed chromatin (Figure 7D). YFP+ cells from mice infected with cytosolic Lm exhibited a pattern of histone modifications at the Ifng promoter most similar to that seen in Th1 cells generated in vitro—substantial H3K4me2 enrichment without enrichment for K3K27me3. Conversely, YFP− cells from mice infected with vacuolar Lm had minimal H3K4me2 enrichment and greater enrichment for H3K27me3 than seen in the other cell populations; findings in YFP− cells from mice infected with cytosolic Lm were intermediate between those seen in the YFP+ cells from these mice and the YFP− cells from mice infected with vacuolar Lm. The Il17a promoter was marked by permissive H3K4me2 in Th17 cells and by repressive H3K27me3 in Th1 cells generated in vitro, but neither of these marks was substantially or consistently above background in YFP+ or YFP− cells from Lm-infected mice. Thus, the ability of memory CD4+ T cells generated in vivo to heritably iterate strong IFN-γ production and to silence IL-17 even under Th17 conditions was foretold by strength of Ifng expression and of permissive histone modifications acquired at the Ifng promoter and the paucity of Il17a and Il17f expression during priming. Cells that expressed Il17a and IL17f but little Ifng during priming–but nonetheless acquired permissive histone modifications at the Ifng promoter—were primed to produce IFN-γ in the future, yet retained the plasticity to produce IL-17.
DISCUSSION
The degree whereby Th CD4+ T cells undergo committed differentiation into each defined lineage after in vivo priming, and the functional consequences of lineage stability versus plasticity on the protective potency of these cells against infection remains largely undefined. Our studies on the polyclonal T cell response to cytosolic Lm in WT mice demonstrated that while the majority of antigen-specific memory CD4+ T cells were heritably committed to the Th1 lineage, 10–20% remained plastic and produced IL-17 (almost always in concert with IFN-γ) when exposed to Th17 conditions. The major factor accounting for this residual population-based plasticity appeared to be heterogeneity in the numbers of times cells had divided during the primary infection, because only 1–3% of adoptively transferred Lm-specific cells, all of which had divided >4 times during priming, exhibited such plasticity. Thus, proliferation was an important antecedent of Th1 commitment in vivo as it was shown previously to be in vitro (Grogan et al., 2001; Mullen et al., 2001). Commitment at the single cell level was conditioned during priming by the extrinsic cytokine milieu and by cell intrinsic cytokine production and chromatin modifications.
Alterations to the inflammatory cytokine milieu, induced either by engineered mutations to the host or to Lm, affected the differentiation of naïve CD4+ T cells and compromised the degree of Th1 commitment even when cell division was not limiting. In hosts deficient in either type-I IFN or IL-12 signaling, Th1 commitment was not compromised. However, when both signals were missing, the memory response shifted from a heritable Th1 response to a more plastic and mixed response in which cells produced IFN-γ, IL-17 or both these cytokines. The endogenous memory Th cell response in IFNAR-p40 DKO mice exhibited this shift (Figure 1), as did adoptively transferred IFNAR wildtype (Figure 3) or IFNAR deficient (unpublished observations) memory Th cells in IFNAR-p40 DKO mice. These results show that the essential functions of type-I IFNs in Th1 commitment were extrinsic to the responding T cells.
A similar shift from a Th1 to a plastic and mixed Th1 and IL-17 response was observed when mice were infected with mutant Lm that did not invade the cytosol. Absent entry into the cytosol, vacuolar Lm resides within the phagosome and no longer efficiently induces the type I innate immune cytokines that promote protection against intracellular pathogens. Accordingly, our results demonstrate that CD4+ T cells primed by vacuolar Lm confer significant reductions in MTb pathogen burden (Figure 5) and are consistent with the primary residence of MTb within the phagosome of macrophage cells during in vivo infection. Following aerosolized MTb infection, Th17 cells have been suggested to traffic to the site of infection first where they may acquire the capacity to produce IFN-γ and help to recruit Th1 cells from the blood to control infection (Khader et al., 2007). Interestingly, protection against MTb primed by Bacille Calmette-Guerin (BCG) is significantly enhanced when engineered to express Listeriolysin-O (LLO) that allows entry into the cytoplasm of host cells (Grode et al., 2005). Based on our results, we predict that cytosolic LLO-expressing BCG, compared with regular BCG, would prime a more stable Th1 response. Whether this change mechanistically dictates or contributes the increased protective efficacy of this strain represents an important area for future investigation.
To gain a better understanding of the events occurring in the responding T cells during the primary response that are later manifest in memory, we assessed cytokine expression and chromatin modifications in effector cells. Heritable and robust IFN-γ production and loss of the plasticity to express IL-17 even under future Th17 conditions was strongly correlated with cell intrinsic expression of IFN-γ during priming. This correlation is consistent with the observation that the IFN-γ receptor is recruited to the immunological synapse upon T cell activation (Maldonado et al., 2008), which places this receptor where it can respond to autocrine IFN-γ and thereby foster Th1 differentiation and heritable repression of IL-17. Stable Th1 commitment was also associated with the acquisition of permissive histone modifications at the Ifng promoter and lack of Il17a and Il17f expression during priming. It is perhaps notable that IL-17 production was silenced in such cells even though the Il17a promoter was not marked by repressive H3K27me3, unlike Th1 cells generated in vitro, in which this modification was readily detected; silencing of IL-17 during Th1 commitment in vivo may result from acquisition of H3K27me3 in amounts below those we could reproducibly detect, at other regulatory elements or by other mechanisms. Conversely, cells that expressed I17a and IL17f during priming retained the plasticity to produce IL-17 in the future, suggesting that locus activation in primary effector cells promotes the potential for future expression within those that persist as memory cells.
These results show that infection with an intracellular pathogen such as Lm induces a highly skewed yet balanced polyclonal Th1 response in which the preponderance of CD4+ T cells heritably commit to the Th1 lineage, while a fraction retain the plasticity to produce IL-17 in response to future alterations in their environment. Why retain a measure of functional plasticity at the population level? In addition to the protective roles described for MTb, it may be advantageous for the immune system to retain the flexibility to recognize an identical antigen but respond in accordance to shifts in the pathogen’s lifestyle. Candida albicans represents an example where this plasticity appears to be of benefit—in its yeast form, it initiates a Th1 response but in its hyphal form, it induces a Th17 response (Acosta-Rodriguez et al., 2007). Furthermore, memory CD4+ T cells with the capacity to produce multiple cytokines have also been implicated in providing enhanced protection against pathogens like Leishmania major (Darrah et al., 2007).
In conclusion, our results show that Th1 commitment in vivo is dependent on cells proliferating sufficiently in an environment where type 1 innate immune cytokines are not limiting and is strongly promoted by T cell intrinsic production of IFN-γ during priming. Plasticity reflects limitations in one or more of these events, stochastic factors and perhaps other determinants, such as asymmetric cell division (Reiner et al., 2007). These findings illuminate factors that vaccines will need to emulate if they are to induce stable, long-lasting memory Th cell responses that are simultaneously resistant to deviation by pathogen evasion tactics yet sufficiently adaptable to allow some memory cells to produce additional or alternative cytokines when useful.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6, IL-12Rβ2 KO, IL-12/23p40 KO and IFN-γ KO mice on a B6 background were purchased from The Jackson Laboratory. Type-I IFN receptor-deficient (IFNAR KO) mice backcrossed to B6 mice for 12 generations. IFNAR KO mice were intercrossed to IL12Rβ2 KO or IL-12/23p40 KO mice to generate IFNAR-IL12Rβ2 DKO or IFNAR-p40 DKO mice, respectively. P25 TCR-transgenic (Tg) mice specific for the I-Ab MTb epitope Ag85B240-254 (Tamura et al., 2004) and congenic for Ly5a were originally provided by Dr. K. Takatsu (University of Tokyo). SMARTA TCR-Tg mice (Oxenius et al., 1998) specific for the I-Ab LCMV epitope GP61–80 and congenic for Thy1.1 were obtained from Dr. C. Surh (The Scripps Research Institute). IFN-γ reporter Yeti mice (Stetson et al., 2003) were obtained from Dr. R.M. Locksley (University of California, San Francisco) and intercrossed to the P25 TCR-Tg mice. Mice were housed in a specific pathogen free facility at the University of Washington. All experiments were performed under IACUC approved protocols.
Expression constructs and infections
Recombinant Lm expressing MTb Ag85b was constructed by amplifying the full-length coding sequence from MTb genomic DNA using the primers 5′-ggtaccgacgtgagccgaaagattcgag-3′ and 5′-gcatgcgccggcgcctaacgaactct-3′, which was TA cloned into the topo2.1 vector, sequenced, and then cloned in-frame behind the promoter and signal sequence for Lm-hly (N-terminus) and HA tag (C-term) in the gram-positive expression vector pAM401; plasmids were verified by sequencing and used to electroporate Lm strains DPL1942 (ΔactA) and DPL2319 (ΔLLO/ΔPLC) using previously described methods (Orr et al., 2007; Park et al., 1990). Each of these strains was confirmed to express Ag85B by Western blotting (not shown). For infection, Lm was grown and subcultured in BHI containing chloramphenicol (10 μg/mL) to early log-phase (OD600 0.1), washed, diluted in PBS to a final concentration of 1×106 CFUs/200μl (LmΔactA-85B) or 1×108 CFUs/200μl (LmΔLLO/ΔPLC-85B) and inoculated i.v into mice 24 hours after transfer of P25 Tg+ CD4+ T cells. Lm-Ova and LmΔactA-Ova were prepared for infection using methods described (Orgun et al., 2008). For LCMV infections, mice were infected i.p. with 2×105 PFU of LCMV (Armstrong strain) 24 hours after transfer of SMARTA Tg+ CD4+ T cells as described (Kolumam et al., 2005). For MTb infection, the WT H37Rv strain was infected in an aerosol infection chamber with 100 CFU deposited in the lungs of each mouse as described (Scott-Browne et al., 2007).
Adoptive cell transfer and CFSE labeling
Naïve splenocytes harvested from TCR transgenic mice were stained with a cocktail of PE-conjugated antibodies for B220, CD8, CD11b, CD11c, I-Ab, and NK1.1 (BD Pharmingen) and purified by negative selection on a FACSAria (BD) to a purity of >85%. 5×104 purified CD4+ T cells were transferred i.v. into recipient hosts. To track cell divisions, purified CD4+ T cells were labeled with 2 μM CFSE. Splenocytes were harvested at 48 hours or 7 days after infection, and dilution of CFSE was analyzed by flow cytometry.
Cell culture, activation and functional analysis in vitro
I-Ab-restricted peptides LLO-2 (WNEKYAQAYPNVS), LCMV-GP P13 (GLNGPDIYKGVYQFKSVEFD) and MTb-Ag85B P25 (FQDAYNAAGGHNAVF) were purchased from United Biochemical Research, Inc. (Seattle, WA). For in vitro polarization, splenocytes were stimulated with peptide in Iscove’s modified Dulbecco’s medium supplemented with 10% FBS, penicillin, streptomycin, gentamycin, and 50 nM 2-mercaptoethanol. Tn culture conditions included 10 μg/mL anti-IFN-γ (AMC4834; BioSource), 10 μg/mL anti-IL-12 (AMC0122; Biosource), and 10 μg/mL anti-IL-4 (11B.11; NCI Biological Resources Branch); Th1 culture conditions included 5 ng/mL recombinant IL-12 (R&D Systems) and 10 μg/mL anti-IL-4; Th2 culture conditions included 50 ng/mL recombinant IL-4 (R&D Systems), 50 μg/mL anti-IL-12 and 50 μg/mL anti-IFN-γ; Th17 culture conditions included 10 ng/mL recombinant IL-23 (eBioscience), 5 ng/mL rhTGF-β1 (R&D Systems), 20 ng/mL recombinant IL-6 (PeproTech), 10 μg/mL anti-IFN-γ, and 10 μg/mL anti-IL-4. Cell populations were split on day 3 in fresh cIMDM plus cytokines. After 5 days in culture, cells were washed twice in cIMDM, plated in the presence of naïve splenocyte feeder cells and stimulated for 5 hours in the presence of Brefeldin A (Golgi Plug, BD Biosciences) for intracellular cytokine staining, or for 10 or 72 hours for supernatant analysis. Direct ex vivo analysis of cytokine secretion was done in a similar manner, with the exception that feeders were not added. Antibodies for flow cytometry were purchased from BD Pharmingen or eBioscience. IL-17, IFN-γ, IL-4 and TNF-α concentrations were analyzed using Luminex Beadlyte Mouse Multi-Cytokine Flex Kit (Upstate) on the Bio-Plex System (Bio-Rad).
RT-PCR and ChIP
Effector P25 CD4+ T cells were generated by adoptive transfer as described above, harvested on day 7, and sorted by YFP expression on a FACSAria (BD). 5×104 of each population were purified, stimulated with PMA/ionomycin for 4.5 hours, and total cellular RNA was extracted (Qiagen). SuperScript II RNase H Reverse Transcriptase (Invitrogen) was used to create cDNA and transcript abundance was analyzed by RT-PCR using TaqMan probes (Applied Biosystems) and the following primer sets: Ifng (Mm00801778_m1); Il17a (Mm00439619_m1); Il17f (Mm00521423_m1); Il4 (Mm00445259_m1); Il22 (Mm00444241_m1); eukaryotic 18s ribosomal RNA. Alternatively, donor P25 cells were purified based on YFP expression and microChIP was performed as previously described (Dahl and Collas, 2008). Samples were analyzed by a SybrGreen-based quantitative PCR reaction. Data are expressed as (specific IP- rIgG)/input normalized to total histone H3 to account for differences in underlying histone density.
Statistics
The differences in percentages of cytokine producing cells and cytokine concentrations in culture supernatants between groups of mice were evaluated by using the unpaired Student’s t test (Graph Pad, Prism software).
HIGHLIGHTS.
Cytokines IL-12 or type I IFNs are required for CD4+ Th1 differentiation stability
Listeria cytoplasmic entry dictates CD4+ Th1 lineage differentiation stability
Listeria restricted to the vacuole prime a “plastic” mixed Th1/Th17 response
“Plastic” memory CD4+ T cells confer protection to Mycobacterium tuberculosis
Supplementary Material
Acknowledgments
We thank Richard Locksley and Dan Stetson for providing Yeti mice, Dan Portnoy for providing the parental ΔactA and ΔLLO/PLC Lm strains, Hao Shen for providing the recombinant Lm-OVA strain, and Kiyoshi Takatsu for providing P25 TCR transgenic mice. This work was supported in part by National Institutes of Health Grants T32 CA009537 and T32 GM07270 (MMC), T32-AI07411(ER), R01 HD18184 (CBW), K08HD51584 (SSW), and a Puget Sound Partners in Global Health Pilot Grant (SSW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez E, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing Th memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Curtis MM, Way SS. Interleukin-17 in host defense against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JA, Collas P. μChIP—a rapid micro chromatin immunoprecipitation assay for small cell samples and biopsies. Nucleic Acids Res. 2008;36:e15. doi: 10.1093/nar/gkm1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, DeLuca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- Grode L, Seller P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan JL, Mohrs M, Harmon B, Lacy DA, Sedat JW, Locksley RM. Early transcription and silencing of cytokine genes underlie polarization of Th cell subsets. Immunity. 2001;14:205–15. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- Hara H, Tsuchiya K, Nomura T, Kawamura I, Shoma S, Mitsuyama M. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J Immunol. 2008;180:7859–68. doi: 10.4049/jimmunol.180.12.7859. [DOI] [PubMed] [Google Scholar]
- Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:431–43. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–33. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Hahn H, Berger R, Kircher H. Interferon-gamma production by Listeria monocytogenes-specific T cells active in cellular antibacterial immunity. Eur J Immunol. 1983;13:265–68. doi: 10.1002/eji.1830130318. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–34. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk CM, Shen H, Pearce EJ. Functional plasticity in memory Th cell responses. J Immunol. 2007;178:4080–88. doi: 10.4049/jimmunol.178.7.4080. [DOI] [PubMed] [Google Scholar]
- Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the Th 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhning M, Hegazy AN, Pinschewer DD, Busse D, Lang KS, Höfer T, Radbruch A, Zinkernagel RM, Hengartner H. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting Th type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, Soriano MA, Perdomo LC, Sigrist K, Irvine DJ, Decker T, Glimcher LH. Control of Th cell differentiation through cytokine receptor inclusion in the immunological synapse. J Exp Med. 2009;206:877–92. doi: 10.1084/jem.20082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–53. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human Th1 and Th2 lymphocytes. Nat Immunol. 2002;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- Mullen AC, Hutchins AS, Villarino AV, Lee HW, High FA, Cereb N, Yang SY, Hua X, Reiner SL. Cell cycle controlling the silencing and functioning of mammalian activators. Curr Biol. 2001;11:1695–99. doi: 10.1016/s0960-9822(01)00533-4. [DOI] [PubMed] [Google Scholar]
- Nakane A, Minagawa T. The significance of alpha/beta interferon’s and gamma interferon produced in mice infected with Listeria monocytogenes. Cell Immunol. 1984;88:29–40. doi: 10.1016/0008-8749(84)90049-2. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of Th 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: In vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. 2009;182:2565–68. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci. 2002;99:13861–66. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Hunter CA, Germain RN. T cell heterogeneity: firmly fixed, predominantly plastic or merely malleable? Nat Immunol. 2008;9:450–3. doi: 10.1038/ni0508-450. [DOI] [PubMed] [Google Scholar]
- Orgun NN, Mathis MA, Wilson CB, Way SS. Deviation from a strong Th1-dominated to a modest Th17-dominated CD4+ T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells. J Immunol. 2008;180:4109–15. doi: 10.4049/jimmunol.180.6.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgun NN, Way SS. A critical role for phospholipase C in protective immunity conferred by listeriolysin O-deficient Listeria monocytogenes. Microb Pathog. 2008;44:159–63. doi: 10.1016/j.micpath.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr MT, Orgun NN, Wilson CB, Way SS. Cutting Edge: Recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol. 2007;178:4731–35. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Park SF, Stewart GS. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene. 1990;94:129–32. doi: 10.1016/0378-1119(90)90479-b. [DOI] [PubMed] [Google Scholar]
- Park AY, Hondowicz BD, Scott P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J Immunol. 2000;165:896–902. doi: 10.4049/jimmunol.165.2.896. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–5. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JS, Hunter CA. With a little help from their friends: interleukin-21, T cells and B cells. Immunity. 2008;29:7–9. doi: 10.1016/j.immuni.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Stetson D, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobie L, Gurunathan S, Prussin C, Sacks DL, Glaichenhaus N, Wu CY, Seder RA. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc Natl Acad Sci. 2000;97:8427–32. doi: 10.1073/pnas.160197797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Ariga H, Kinashi T, Uehara S, Kikuchi T, Nakada M, Tokunaga T, Xu W, Kariyone A, Saito T, et al. The role of antigenic peptide in Th phenotype development in a T cell receptor transgenic model. Int Immunol. 2004;16:1691–99. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci. 1993;90:3725–29. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–37. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–31. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4+ T cells: fates, functions, and faults. Blood. 2008;112:1557–69. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.