Abstract
The mechanism underlying the reversible depression of erythropoiesis by chloramphenicol has been investigated in rabbits in which hemolytic anemia had been induced by phenylhydrazine so that the compensatory erythroid hyperplasia would provide a situation where abnormalities in the bone marrow cells reflected predominantly those of erythroid precursors.
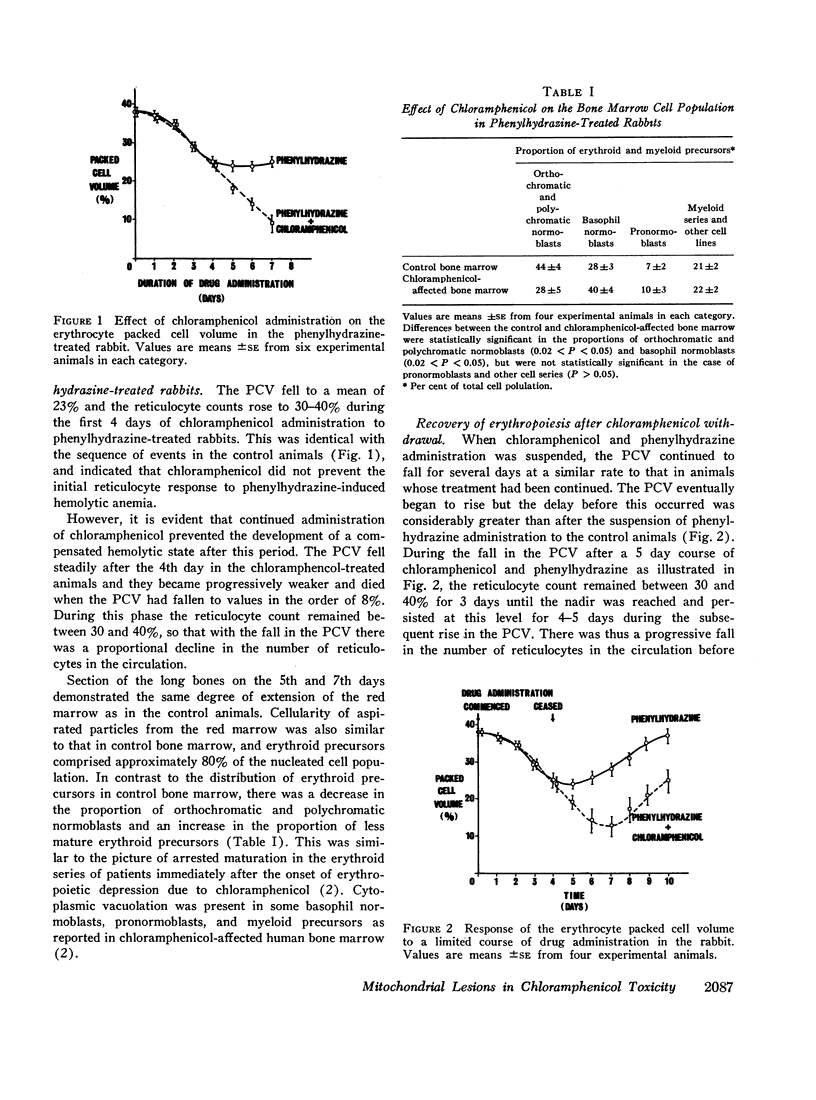
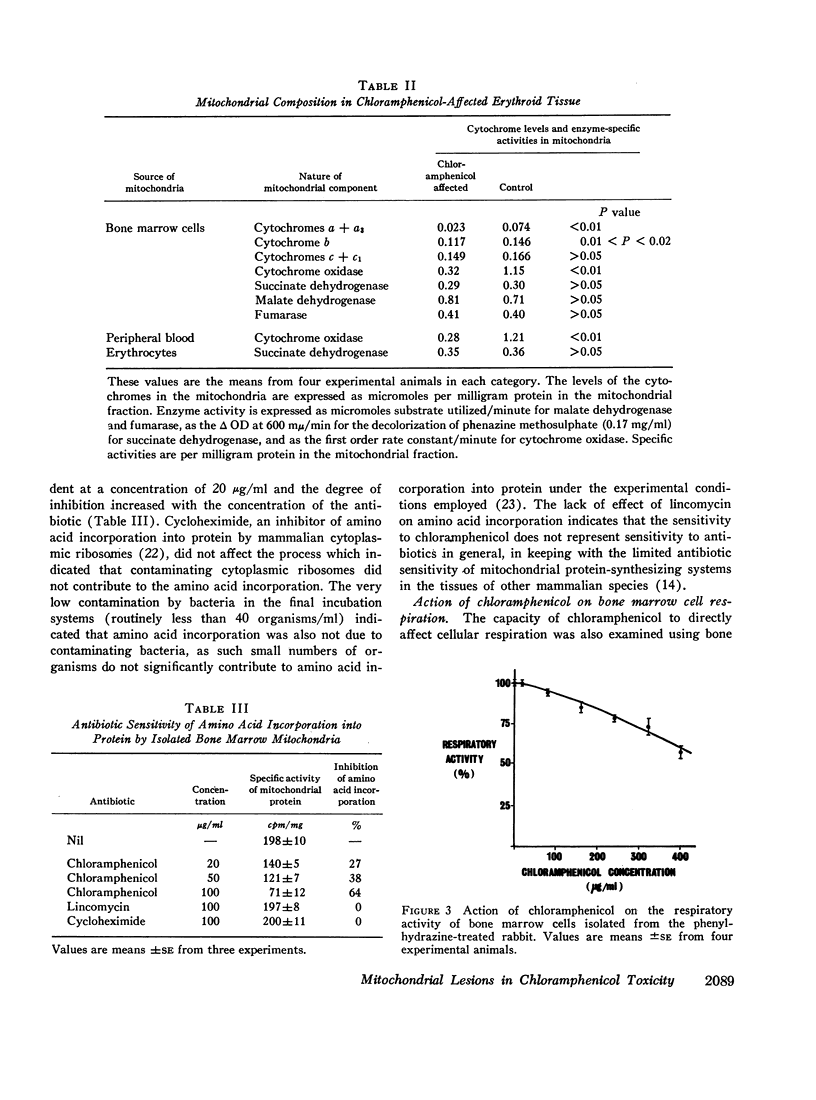
Maintenance of chloramphenicol in the serum of these animals at concentrations in the order of 15 μg/ml resulted in erythropoietic depression after several days. The onset of this depression corresponded to the development of a cellular respiratory defect in the erythroid precursors which was associated with an abnormality in the composition of the mitochondrial respiratory pathway. The abnormality took the form of a selective depletion of cytochromes a + a3 and b which can be explained by an inhibitory effect of the antibiotic on their formation by the mitochondrial protein-synthesizing system. The relationship between the mitochondrial lesion and the depression of proliferative activity was further indicated by the correlation between the restoration of the cytochrome deficit and the recovery of erythropoiesis after chloramphenicol administration was ceased.
The features of the reversible depression of erythropoiesis corresponded closely to those in man, so that a specific action of chloramphenicol on mitochondrial formation provides a reasonable explanation for this important manifestation of chloramphenicol toxicity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- BORSOOK H., GRAYBIEL A., KEIGHLEY G., WINDSOR E. Polycythemic response in normal adult rats to a nonprotein plasma extract from anemic rabbits. Blood. 1954 Jul;9(7):734–742. [PubMed] [Google Scholar]
- BURNS L. E., HODGMAN J. E., CASS A. B. Fatal circulatory collapse in premature infants receiving chloramphenicol. N Engl J Med. 1959 Dec 24;261:1318–1321. doi: 10.1056/NEJM195912242612604. [DOI] [PubMed] [Google Scholar]
- Beattie D. S. Studies on the biogenesis of mitochondrial protein components in rat liver slices. J Biol Chem. 1968 Aug 10;243(15):4027–4033. [PubMed] [Google Scholar]
- DAVIES D. D., KUN E. Isolation and properties of malic dehydrogenase from ox-heart mitochondria. Biochem J. 1957 Jun;66(2):307–316. doi: 10.1042/bj0660307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W., HOLOWINSKY A. Studies on the content and organization of the respiratory enzymes of mitochondria. J Biophys Biochem Cytol. 1961 Jan;9:19–28. doi: 10.1083/jcb.9.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Biogenesis of mitochondria. 8. The effect of chloramphenicol on regenerating rat liver. Exp Cell Res. 1969 Apr;55(1):68–76. doi: 10.1016/0014-4827(69)90457-1. [DOI] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Differential effects of chloramphenicol on the growth and respiration of mammalian cells. Biochem Biophys Res Commun. 1968 Aug 13;32(3):398–402. doi: 10.1016/0006-291x(68)90674-8. [DOI] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Phylogenetic differences in the sensitivity of mitochondrial protein synthesising systems to antibiotics. FEBS Lett. 1969 Mar;2(5):330–332. doi: 10.1016/0014-5793(69)80056-6. [DOI] [PubMed] [Google Scholar]
- Freeman K. B., Haldar D. The inhibition of mammalian mitochondrial NADH oxidation by chloramphenicol and its isomers and analogues. Can J Biochem. 1968 Sep;46(9):1003–1008. doi: 10.1139/o68-151. [DOI] [PubMed] [Google Scholar]
- Haldar D., Freeman K. B. The inhibition of protein synthesis and respiration in mouse ascites tumor cells by chloramphenicol and its isomers and analogues. Can J Biochem. 1968 Sep;46(9):1009–1017. doi: 10.1139/o68-152. [DOI] [PubMed] [Google Scholar]
- KILLMANN S. A., CRONKITE E. P., FLIEDNER T. M., BOND V. P. MITOTIC INDICES OF HUMAN BONE MARROW CELLS. 3. DURATION OF SOME PHASES OF ERYTHROCYTIC AND GRANULOCYTIC PROLIFERATION COMPUTED FROM MITOTIC INDICES. Blood. 1964 Sep;24:267–280. [PubMed] [Google Scholar]
- KROON A. M. Amino acid incorporation into the protein of mitochondria and mitochondrial fragments from beef heart. Biochim Biophys Acta. 1963 Jan 1;69:184–185. doi: 10.1016/0006-3002(63)91245-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manyan D. R., Yunis A. A. The effect of chloramphenicol treatment on ferrochelatase activity in dogs. Biochem Biophys Res Commun. 1970 Nov 25;41(4):926–931. doi: 10.1016/0006-291x(70)90172-5. [DOI] [PubMed] [Google Scholar]
- Martelo O. J., Manyan D. R., Smith U. S., Yunis A. A. Chloramphenicol and bone marrow mitochondria. J Lab Clin Med. 1969 Dec;74(6):927–940. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- RENDI R. The effect of chloramphenicol on the incorporation of labeled amino acids into proteins by isolated subcellular fractions from rat liver. Exp Cell Res. 1959 Aug;18:187–189. doi: 10.1016/0014-4827(59)90307-6. [DOI] [PubMed] [Google Scholar]
- SAIDI P., WALLERSTEIN R. O., AGGELER P. M. Effect of chloramphenicol on erythropoiesis. J Lab Clin Med. 1961 Feb;57:247–256. [PubMed] [Google Scholar]
- SCOTT J. L., FINEGOLD S. M., BELKIN G. A., LAWRENCE J. S. A CONTROLLED DOUBLE-BLIND STUDY OF THE HEMATOLOGIC TOXICITY OF CHLORAMPHENICOL. N Engl J Med. 1965 Jun 3;272:1137–1142. doi: 10.1056/NEJM196506032722201. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. D., Moore R. D., Weisberger A. S. Differentiation and functional expression of potential antibody-producing cells in the presence of chloramphenicol. J Cell Biol. 1967 Feb;32(2):401–414. doi: 10.1083/jcb.32.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Joslyn D. A., Gruhzit O. M., McLean I. W., Penner M. A., Ehrlich J. Chloromycetin: Biological Studies. J Bacteriol. 1948 Mar;55(3):425–448. [PMC free article] [PubMed] [Google Scholar]
- Suhrland L. G., Weisberger A. S. Delayed clearance of chloramphenicol from serum in patients with hematologic toxicity. Blood. 1969 Oct;34(4):466–471. [PubMed] [Google Scholar]
- WEISBERGER A. S., WOLFE S. EFFECT OF CHLORAMPHENICOL ON PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:976–983. [PubMed] [Google Scholar]
- Ward H. P. The effect of chloramphenicol on RNA and heme synthesis in bone marrow cultures. J Lab Clin Med. 1966 Sep;68(3):400–410. [PubMed] [Google Scholar]
- Wheeldon L. The problem of bacterial contamination in studies of protein synthesis by isolated mitochondria. Biochem Biophys Res Commun. 1966 Aug 12;24(3):407–411. doi: 10.1016/0006-291x(66)90174-4. [DOI] [PubMed] [Google Scholar]
- Zelkowitz L., Arimura G. K., Yunis A. A. Chloramphenicol and protein synthesis in mammalian cells. J Lab Clin Med. 1968 Apr;71(4):596–609. [PubMed] [Google Scholar]


