Abstract
Mild traumatic brain injury (mTBI) refers to the clinical condition of transient alteration of consciousness as a result of traumatic injury to the brain. The priority of emergency care is to identify and facilitate the treatment of rare but potentially life threatening intra-cranial injuries associated with mTBI through the judicious application of appropriate imaging studies and neurosurgical consultation. Although post-mTBI symptoms quickly and completely resolve in the vast majority of cases, a significant number of patients will complain of lasting problems that may cause significant disability. Simple and early interventions such as patient education and appropriate referral can reduce the likelihood of chronic symptoms. Although definitive evidence is lacking, mTBI is likely to be related to significant long-term sequelae such as Alzheimer's disease and other neurodegenerative processes.
Keywords: concussion, trauma, brain, injury
Introduction
The purpose of this review is to provide an overview of mild traumatic brain injury (mTBI) in a form useful for Emergency Physicians. This is a disease with considerable public health impact and is the subject of a vast amount of current research. Our discussion of this disease is necessarily limited both by space constraints and the interests of our target audience. For more detailed discussions of various topics covered in this review the reader is directed to the publications listed in Table 1.
Table 1. Recommended Further Reading.
| Topic | Reference | Notes |
|---|---|---|
| Epidemiology | Summers C.R., lvins B., and Schwab K. A.14 | Brief review of literature pertaining to the epidemiology of both military and civilian TBI. |
| Biomechanics | LaPlaca M.C., Simon C. M., Prado G. R., et al.18 | Thorough review of basic biomechanics relevant to CNS trauma including discussion of experimental models of injury. |
| Mechanisms of LOC | Shaw N. A.33 | Detailed account of the historical development and current theories of altered conciousness resulting from TBI. |
| Acute Clinical Management | Jagoda A. S., Bazarian J. J., Bruns J.J., Jr., et al.42 | American College of Emergency Physicians and Centers for Disease Control clinical policies for the acute management of adult mTBI. |
| Sub-acute and Chronic Clinical Management | VA/DoD Clinical Practice Guideline for Management of Concussion/mTBI 1 | Definitive clinical guidelines for sub-acute and chronic management of adult mTBI (>1 week post injury). |
| Long Term Health Effects | IOM (Institute of Medicine). Gulf War and Health, Volume 7 24 | Comprehensive review of evidence for long term consequences of TBI. |
Definition of Traumatic Brain Injury
Traumatic Brain Injury (TBI) is defined as any traumatically induced structural injury or physiological disruption of brain function as a result of an external force. It is manifested by one or more clinical signs occurring immediately afterwards including a loss, decreased, or altered level of consciousness, amnesia, neurologic deficit, or intracranial lesion 1. External forces may include direct impact of the head with another object, indirect forces from acceleration/deceleration, or a blast injury. The Glasgow Coma Score (GCS) has traditionally been used to classify TBI as mild (GCS 13-15), moderate (GCS 9-12), or severe (GCS 3-8). A more recent classification scheme for TBI uses length of loss of consciousness (LOC), alteration of consciousness (AOC), and post traumatic amnesia (PTA) as well as imaging findings to categorize TBI (Table 2) 1. It is important to stress that mild TBI (mTBI) is clinically defined based solely on self-reported or observed symptoms and often occurs with normal neuroimaging. Indeed, the mTBI definition included in Table 1 classifies TBI with positive neuroimaging as at least moderate in severity. Two older, but still commonly used mTBI definitions by the American Congress of Rehabilitation Medicine 2 and the Centers for Disease Control and Prevention 3 define patients as mild if they have positive neuroimaging findings but meet all other clinical criteria for mTBI. Patients who have abnormal intracranial imaging but otherwise meet a clinical definition of mTBI are referred to as complicated mTBI 4.
Table 2. Clinical Criteria for TBI Severity.
| Criteria | Mild | Moderate | Severe |
|---|---|---|---|
| Structural imaging | Definition dependent* | Normal or abnormal | Normal or abnormal |
| Loss of consciousness (LOC) | 0-30 minutes | > 30 min and < 24 hrs | > 24hrs |
| Alteration of consciousness (AOC)** | A moment up to 24 hours | > 24 hours. Severity based on other criteria | |
| Post Traumatic amnesia (PTA) | 0-1 day | > 1 and < 7 days | > 7 days |
| GCS (best score in first 24 hours) | 13-15 | 9-12 | < 9 |
Patients who otherwise meet the clinical criteria for mTBI but have intracranial imaging abnormalities may be classified as complicated mTBI or moderate TBI depending on the definition used.
Alteration of mental status must be immediately related to the trauma to the head. Typical symptoms may include looking or feeling dazed, confusion, difficulty thinking clearly or responding appropriately to mental status questions, or inability to describe events immediately before or after the traumatic event.
Adapted from: IOM (Institute of Medicine). Gulf War and Health, Volume 7: Long-term Consequences of Traumatic Brain Injury. Washington, D.C.: The National Academies Press; 2009.
Concussion is a common term for mild traumatic brain injury (mTBI) and will be used interchangeably within this article. Our understanding of traumatic brain injury remains rudimentary relative to many other medical problems of similar magnitude. A symptom based classification uses the description of symptoms evident on history and physical exam to classify illness. This method is imprecise, often grouping disparate pathophysiological process together as single clinical entities. This is particularly problematic in moderate and severe TBI where multiple injury processes as evidenced by heterogeneous imaging findings are often present simultaneously. As knowledge of a disease increases and diagnostic tools improve, a more sophisticated classification emerges that may include anatomic, physiologic, metabolic, immunologic, and genetic factors. TBI has been the subject of intensive research in recent years and recommendations for improved classification of this diverse disease are beginning to appear in the literature 5.
Epidemiology
An estimated 1.4 million Americans presented to emergency departments for medical care after TBI each year between 1995 and 2001 6. Over 1 million of these patients had mTBI. The incidence of ED-attended concussion is 444/100,000 in the United States 7 however it is thought that nearly 40% of patients suffering mTBI do not seek hospital based care, and 25% do not report their injuries to health care providers at all 8. An estimated 5-25% of all patients with concussion have post-concussive symptoms or other cognitive deficits that persist beyond one year 9-11. This number is greater than the annual incidence of multiple sclerosis, Parkinson's disease, myasthenia gravis, and Huntington's disease combined 12. TBI is more common in men than women with 60% of TBI occurring in males 6. Young children, adolescents and the elderly suffer the highest rates of TBI 6. The most common mechanisms of TBI are falls, automobile accidents, being struck by or against an object, and assault. The rate of hospitalizations for TBI fell dramatically during the 1980's and 90's 6. Wounded veterans from the wars in Iraq and Afghanistan may represent a non-trivial increase in patients living with mTBI. Approximately 20% of troops returning from combat deployments in Iraq have clinician confirmed mTBI. These patients typically have TBI resulting from a blast 13 and often have comorbid PTSD or depression 14.
Biomechanics
Traumatic injury results from the transfer of energy from the environment to tissue above the amount that can be absorbed without dysfunction. Biomechanics is the study of the interaction of forces and physical responses in biological systems. Traumatic insults generally occur over short periods of time and are referred to as dynamic loading. Dynamic loading includes both direct or impact loading, as well as impulsive loading where no physical contact occurs. The loads absorbed by the brain after trauma generally include linear and rotational components called angular loads. The rate and duration of the insult are important because loads applied at high rates tend to result in more damage 18. For example, the force involved in punching a wall can also be applied by pressing your fist against that same wall for a few minutes: the former instance results in a boxer's fracture whereas the latter does not. Focal injury such as contusion results from direct loading and often occurs in the absence of widespread injury. In contrast, diffuse axonal injury (DAI) often occurs as a result of the rotational acceleration accompanying indirect loading 15. Humans are particularly susceptible given their large cranium connected to the trunk by relatively weak neck musculature. Rotational acceleration produces substantial and widespread strains within the brain resulting from both acceleration and deceleration. These diffuse strains lead to differential movement of the brain relative to the skull which can cause hemorrhage. Shear strain is most prominent after rotational injury, and brain tissue is particularly sensitive to this type of strain 16. In animal models, rotational acceleration is required to produce concussion whereas isolated linear acceleration produced contusions and subdural hematomas but no loss of consciousness 17.
Pathophysiology
The initial traumatic insult results in mechanical damage including rupture of cellular and vascular membranes with release of intracellular contents, ultrastructural damage of axons, and changes in cerebral blood flow 19-20. Subsequent metabolic derangement includes widespread release of excitatory neurotransmitters such as glutamate, severe dysregulation of calcium homeostasis, energy failure due to ATP depletion, free radical generation, and cell death by necrotic and apoptotic pathways 20-21. More global consequences of the traumatic insult include increased intra-cranial pressure, decreased cerebral blood flow, tissue ischemia, cerebral edema, and functional blood brain barrier dysfunction 22-23. Following the initial damage, repair and recovery processes begin through the removal of cellular debris, glial scar formation, and plastic changes in neural networks 24. Due to the difficulties studying human mTBI, the mechanisms described in this section were derived principally from animal studies and, to a lesser degree, from humans with severe TBI (sTBI). Similar processes are thought to occur in human mTBI
Putative Causes of Altered Consciousness in mTBI
The definitive causes of altered consciousness are not known. Loss of consciousness requires either loss of the function of both cerebral hemispheres or of the reticular activating system. Several plausible hypothetical mechanisms have been proposed for the alteration of consciousness that occurs with mTBI. These include the reticular, pontine-cholinergic system, centripetal, and convulsive hypotheses. The reticular activating system (RAS) resides in the brainstem reticular formation which extends from the top of the spinal column to the rostral midbrain with extensions into the thalamus and hypothalamus. The RAS is excited by input from surrounding sensory tracts and transmits this excitation to the cortex to induce generalized cortical and behavioral arousal. In the absence of input from the RAS, consciousness is impaired.
Under the reticular hypothesis of concussion, loss of consciousness after brain trauma results from a disturbance or depression of the activity of polysynaptic pathways within the RAS 25. It is not completely understood how a traumatic dysfunction of the RAS occurs however it is believed to result from shearing or tensile strains on RAS pathways at the cranio-cervical junction. Neuropathological evidence for this is limited. The hypothesis also fails to address traumatic amnesia. A further difficulty is that EEG findings do not support depression of the RAS in concussion.
The pontine-cholinergic system hypothesis differs from the reticular activating system hypothesis in that RAS dysfunction is thought to occur as a consequence of trauma-induced activation of the inhibitory cholinergic system of the dorsal pontine tegmentum 26. In animal models, injection of cholinergic agonists into the brainstem induces unconsciousness 27-28 whereas similar injections of cholinergic antagonists reduce the duration of traumatic unconsciousness 29. Furthermore, EEG studies show widespread neuronal discharge after concussion and elevated acetylcholine is found in the CSF of patients after TBI. However, it is not clear that activation of this system can produce loss of consciousness due to RAS suppression.
The centripetal hypothesis posits that sudden rotational forces cause shearing strains and stresses that result in functional decoupling of nerve fibers 17. The depth of this functional decoupling is directly related to the extent of rotational acceleration delivered to the brain. Also, with greater rotational acceleration the likelihood of mechanical injury to fibers increases. Lower inertial forces that result in functional decoupling between the subcortex or diencephalon and the cortex may result in amnesia or confusion without LOC. Furthermore, greater forces resulting in decoupling between more superficial structures and the mesenephalon result in LOC. This hypothesis nicely explains post-traumatic amnesia and dazed states, however it also requires very high energy injuries to cause full loss of consciousness. Consequently, patients with LOC would often have accompanying structural brain injury which is simply is not observed.
Patients with concussion have similar symptoms to those who have experienced generalized epileptic seizures or electro convulsive therapy (ECT). This overlap of symptoms has led to speculation that similar pathophysiological events occur in all three conditions. Close observation of human patients and animal models shows that concussive injury generally causes an initial convulsive event followed by a longer and more prominent paralytic phase 30-31. EEG recordings from concussed animals also show initial, transient epileptiform activity. According to the convulsive hypothesis the symptoms associated with concussion are due to direct injury to neurons resulting in hyperexcitability and widespread membrane depolarization followed by neuronal exhaustion 32. These two neuronal states correspond to the convulsive and paralytic phases, respectively.
The convulsive hypothesis is able to reasonably account for a broader range of post-concussive behaviors than its competitors including LOC, amnesia, convulsive movements, autonomic disturbances, and the dazed or “dinged” state 33. While this hypothesis does a better job than the others at providing a unified explanation for the broad range of symptoms observed as an acute result of mTBI, it does not account for the structural abnormalities that occur as a result of mTBI. In summary, none of the individual hypotheses currently available explain all the findings seen with mTBI. Given the often complimentary strengths and weakness of the four hypotheses discussed above, it seems likely that the mechanisms of altered consciousness after TBI may be due to a combination of processes. For a detailed explanation of these hypotheses, the reader is directed to the excellent review by Shaw 33.
Diagnosis of mTBI
Clinical Presentation
A 28-year-old male presents to the ED after a motor vehicle accident. He was the restrained driver in a car that skidded of the road in icy conditions and collided head on with a tree at 50 mph. There was airbag deployment. The paramedic reports that he was unconscious initially but that he was alert and oriented during transport.
Differential Diagnosis
The diagnosis of mTBI is made clinically and relies heavily on the history obtained from the patient and any witnesses. Obtaining a reliable history is often difficult because of post-traumatic amnesia, persistent altered mental status, or intoxication, a frequent comorbid factor in mTBI patients. Diagnoses with similar presentations include seizure, syncope, intoxication, malingering, anxiety, and other psychiatric conditions.
Clinical Criteria
Several clinical criteria for the diagnosis of mTBI exist 1-3. Concussion or mTBI is defined as a loss of consciousness of less than 30 minutes or amnesia lasting less than 24 hours, or any period of altered mental status at the time of injury. In conjunction, patients must also have a GCS of 13-15 and normal structural imaging to meet the criteria for mTBI. Lower GCS scores classify the patient as having moderate (GCS 9-12) or severe (GCS 3-8) TBI.
Imaging
The imaging study of choice in mTBI is non-contrast head computed tomography (CT). This study is preferred over others because it is sensitive for traumatic injuries that require neurosurgical intervention including acute bleeding, increased intra-cranial pressure, and skull fracture. Although as many as 15% of mTBI patients will have an acute injury detected by non-contrast head CT, only 1% of those abnormalities require neurosurgical intervention 34-40. Other imaging modalities are of limited use for the clinical evaluation of mTBI patients and are not recommended. Although magnetic resonance imaging (MRI) is 30% more sensitive than CT for the detection of traumatic abnormalities after mTBI 41, there is no evidence that it identifies more patients requiring neurosurgical intervention 42. More exotic imaging modalities including functional MRI, diffusion tensor imaging MRI, magnetic resonance spectroscopy, single photon emission computed tomography, and positron emission tomography are valuable research tools but do not have proven clinical utility.
Decision Rules
The low rate of clinically important brain injury seen on head CT obtained acutely after mTBI has resulted in efforts to minimize unnecessary studies through the application of rigorously, validated, clinical decision rules. Two major decision rules applying to adult mTBI patients include the Canadian CT Head Rule 43 and the New Orleans Criteria 44. For patients with a GCS of 15, both of these rules have equivalent sensitivities for detecting injuries requiring neurosurgical intervention, however the Canadian CT Head Rule has a higher specificity for some clinical outcomes and its use may reduce imaging rates 45. In pediatric populations, increased concern for radiation exposure and the potential requirement for sedation make minimizing unnecessary CT after mTBI even more compelling than in adult populations. Kupperman and colleagues recently reported a very sensitive decision rule that identifies low risk children who do not require a head CT after mTBI 46. Indications for obtaining head CT after mTBI based on these decision rules are summarized in Figure 1.
Figure 1. Indications for Obtaining Non-contrast Head CT after TBI.
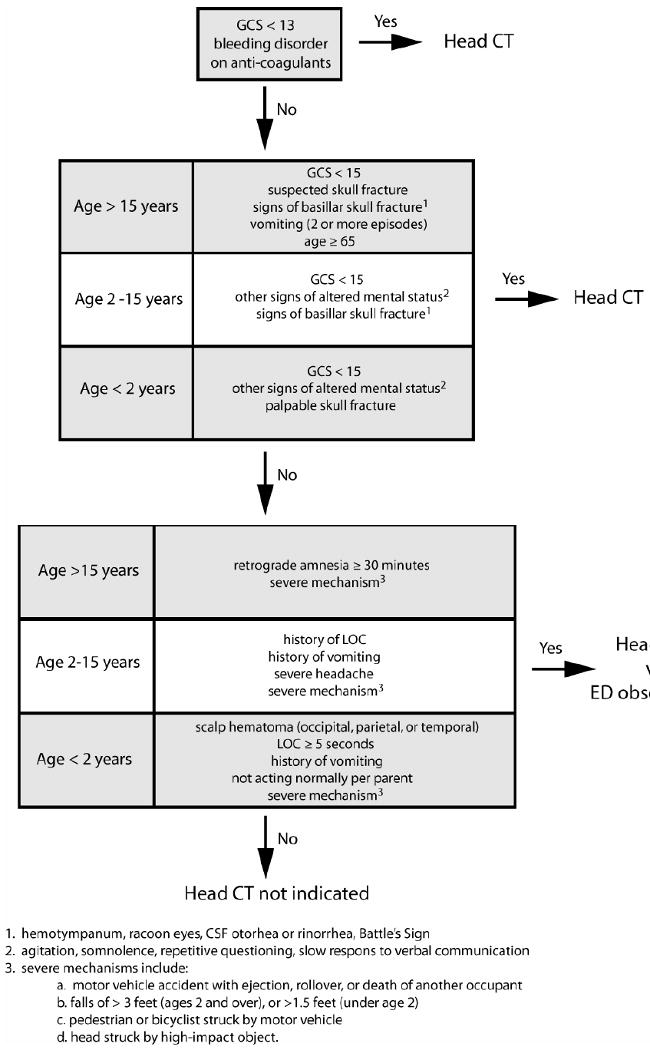
Non-contrast head CT is the study of choice to evaluate TBI patients for clinically important neurotrauma. Clinically important neurotrauma is defined as any traumatically induced intracranial injury that requires neurosurgical intervention or requires hospital admission and neurosurgical follow-up. Clinically important TBI occurs rarely after mTBI, therefore minimizing unnecessary head CT scans is desirable. This figure integrates validated decision rules for both adult45 and pediatric patients46 designed to minimize unnecessary CT scans after mTBI.
Biomarkers
There is substantial interest in developing protein biomarkers obtained from serum to aid the diagnosis and guide the treatment of TBI of all severities. While several potential biomarkers have been studied 47-53, to date only serum S100B has accepted clinical utility for mTBI. Specifically, elevated S100B has a high negative predictive value for clinically important injury on head CT after mTBI. In a large cohort, elevated S100B was 99% sensitive for the detection of injury on CT scan 54 prompting the use of this test as the clinical standard of care in several European countries. The test has the added advantage of not being affected by concomitant alcohol intoxication 55. Although not yet FDA approved in the US, the American College of Emergency Physicians recently issued a guideline stating that for mTBI patients with serum S100B concentrations of less than 0.1 μg/ml measured within 4 hours of injury, it is reasonable to consider not obtaining a head CT 42. Multi-center studies to evaluate the accuracy of this test in US patient populations are currently underway and may provide the data necessary for FDA approval.
Treatment of mTBI
Clinical Presentation
After receiving the prehospital provider's report, you note that the patient is complaining of headache, back pain and abdominal pain. He has no significant past medical history and takes no medications. Pertinent findings on exam include a slight tachycardia, an abrasion on his forehead, mild tenderness in his upper abdomen, and diffuse tenderness over his lumbar spine. He is alert and oriented but cannot remember any events since the accident. Otherwise, he has a normal neurologic exam.
ED Priorities
Initial assessment of the mTBI patient in the ED is focused on identifying patients who may require medical or neurosurgical intervention for the treatment of increased intracranial pressure or an expanding mass lesion. Patients with “red flag” conditions such as altered mental status, papillary asymmetry, seizures, repeated vomiting, double vision, worsening headache, motor or sensory deficits, or ataxia should have an emergent non-contrast head CT scan performed. See Figure 1 for further imaging recommendations.
Patients with intra-cranial imaging abnormalities or declining mental status require immediate neurosurgical consultation. Worsening mental status is typically due to increasing intracranial pressure (ICP) leading to compromised cerebral blood flow and oxygen delivery. For these patients, airway management with endotracheal intubation to protect against aspiration as well as to control ventilation should be considered. Non-surgical management also includes mitigating ICP increases by raising the head of the bed to 30 degrees and treatment with hyperosmolar agents such as IV mannitol. Finally, brief periods of hyperventilation can also reduce dangerous ICP increases. Mechanistically, hyperventilation causes vasoconstriction and reduces intracranial pressure by decreasing cerebral blood flow. Overaggressive hyperventilation can result in ICP decreases at the expense of adequate tissue perfusion. Therefore, prolonged hyperventilation should be used only when other therapies have failed.
Clinical Presentation
You obtain imaging studies, including computed tomography of head, neck, abdomen and pelvis which reveal no traumatic injuries. After returning from radiology, the patient does not recall meeting you. Although is head CT did not reveal a traumatic injury, he is admitted for observation overnight due to his persistent anterograde amnesia. He has an uneventful night and his amnesia resolves. He receives detailed discharge instructions that include a description of “red flag conditions”, common post-concussive symptoms, and reassurance that the vast majority of patients recover completely from concussion. He is advised to avoid activities that exacerbate his symptoms, to take acetaminophen as needed for headache, and to follow up with a local concussion clinic in one week if he is having any persistent discomfort from his concussion. He is accompanied home by his fiancée who will stay with him over the next 24 hours.
Acute Phase: Within One Week of Injury
After evaluating for “red flag” signs and symptoms (see ED Priorities), a thorough history of symptoms including loss or alteration of consciousness, headache, irritability, unsteadiness, vertigo, photophobia, or phonophobia should be obtained. The physical exam includes a focused neurological exam including assessment of cranial nerves, postural instability, visual function, and mental status. Non-contrast head CT should be obtained when indicated (see Figure 1). Neurosurgical consultation is necessary for patients with imaging abnormalities. These patients are often admitted for 24 hours for ongoing mental status monitoring and repeat head CT prior to discharge. Patients in whom imaging was not indicated or with a normal head CT may be safely discharged 56-58.
Discharge instructions for mTBI patients include two principle elements: symptoms requiring immediate re-evaluation (see ED Priorities) and post-concussive symptom education. Post-concussive symptoms include headache, sleep disturbances, vertigo, nausea, fatigue, sensitivity to light or noise, attention and concentration problems, depression, and emotional lability. The vast majority of adults with post-concussive symptoms recover within 3-12 months 59. Early patient education that includes likely post-concussive symptoms and reassurance about an expected positive recovery has been shown to speed recovery and decrease post-concussive symptoms 60-62. Headache should be managed with acetaminophen. Non-steroidal anti-inflammatory drugs (NSAIDs) may be used in patients with negative neuroimaging but should be deferred until 48 hours after injury if imaging was not obtained. In addition, narcotics should be avoided in the treatment of post-traumatic headache.
Pharmacologic treatment of other post-concussive symptoms is not recommended in the acute phase. Rather, patients with symptoms other than headache should be advised to rest and encouraged to return to normal activity as soon as possible. However, individuals whose normal activity includes a high risk for re-injury should have careful evaluation of their symptoms and exam findings with consideration of their specific activities that result in a high injury risk. Specific limitations on activity may be recommended for these patients to mitigate their individual risk. Patients reporting fatigue may be given a graded return to work or activity. For patients with normal activities involving significant physical activity, exertional testing may be performed. If this results in a return of symptoms, a monitored progressive return to these activities as tolerated should be recommended 1.
Clinical Presentation
Two weeks after the initial injury, the patient continues to suffer from frequent headaches that are only slightly relieved by acetaminophen. He also complains of increased irritability, sleepiness, and difficulty concentrating. During an initial follow up visit, a detailed history and physical examination fails to reveal comorbid psychiatric or physical problems including PTSD, depression, substance abuse, hypertension, cervical spine abnormalities, sinus infections, or visual acuity deficits. However, the patient indicates that he has not been sleeping well due to persistent headache. He is started on an NSAID for his headaches, and provided with education regarding good sleep hygiene and relaxation techniques. He is also advised to begin a regular exercise program. A follow up appointment is scheduled in 4 weeks.
Initial Management of Post-concussive Symptoms
This section provides an overview of treatment for the initial treatment of patients with mTBI and symptoms lasting more than one week after injury. Patients with a delayed initial presentation should also be treated according to these guidelines. Detailed recommendations for the evaluation and treatment of specific symptoms can be found in the VA/DoD Clinical Practice Guideline for Management of Concussion/mTBI 1.
Symptom classification and goals of therapy
Post-concussive symptoms generally fall into three categories: physical, cognitive, and behavioral or emotional. Typical physical symptoms include headache, nausea, vomiting, dizziness, fatigue, blurred vision, sleep disturbances, light or noise sensitivity, balance problems, and transient neurologic abnormalities. Cognitive symptoms may occur with attention, concentration, memory, processing speed, judgment, and executive functioning. Behavioral/emotional symptoms include depression, anxiety, agitation, irritability, and aggression.
Because there is an incomplete understanding of the etiology of symptoms after mTBI, the goal of intervention for post-concussive symptoms is to improve identified problems rather than affect a cure. It is believed that symptoms resulting from mTBI are inter-related and alleviation of one symptom often leads to improvement in others. Post-concussive symptoms are also common to many other psychiatric ailments including depression, anxiety disorders, post-traumatic stress disorder, and substance abuse disorders. Indeed, there is substantial evidence that affective disorders, post traumatic stress disorder (PTSD), and substance abuse disorders are often associated with mTBI 59, 63-64. These disorders are also associated with higher rates of persistent post-concussive symptoms 65-66. Consequently, aggressive treatment of any comorbid psychiatric illness may help to improve post-concussive symptoms.
Patient Evaluation
Patient evaluation should include a thorough history, physical exam, and review of the medical record. A review of sleep habits is particularly important as poor sleep may contribute to symptoms including headache, fatigue, anxiety, irritability, depressive thoughts, poor concentration, memory difficulties, and poor decision making. TBI patients should also be screened for psychiatric conditions including PTSD, depression, and substance abuse disorders. Low yield diagnostic testing should be minimized. There is limited evidence to support the utility of comprehensive neuropsychological/cognitive testing within the first 30 days of mTBI and a focused clinical interview is sufficient to assess for cognitive difficulties 67. Laboratory studies including electrolytes, a complete blood count, and thyroid function testing may be useful, particularly when evaluating behavioral and cognitive symptoms. Imaging studies are of limited use.
Physical symptoms should prompt a search for treatable causes. Screening patients with headaches for preexisting headache conditions, hypertension, cervical spine abnormalities, sinus infections, and visual acuity deficits may provide useful avenues of treatment. Symptoms related to dizziness including poor coordination, unsteadiness, vertigo, or loss of balance, may be due to medication effects, orthostatic hypotension, or peripheral vertigo. Nausea may be caused by medications or gastro-esophageal reflux disease. Nasal polyps, sinus infection, and traumatic injury to the lingual or olfactory nerves may cause appetite changes. Physical injuries to the eye including corneal abrasions, lens dislocation, retinal detachment, and optic nerve injury should be considered in the evaluation of post-concussive visual complaints. Ear abnormalities including infection, tympanic membrane rupture, and auditory nerve injury may lead to phonophobia.
General Treatment Guidelines
Treatment of physical symptoms includes treating the underlying causative or contributory conditions. Interventions targeting specific patient complaints such as sleep hygiene education, physical therapy, relaxation, and modification of the environment should be used. Moreover, medications may be used to relieve pain, enable sleep, and reduce stress 1. Cognitive deficits are often measurable within 30 days of mTBI but generally return to normal within the same period 68-70. Unfortunately, many patients continue to have subjective cognitive complaints 9, 71-75. Educational and cognitive-behavioral interventions consistently improve subjective cognitive complaints 61, 76-79. Behavioral symptoms may improve with psychotherapeutic and pharmacological interventions. Treatment should be based upon severity and nature of the symptom presentation. Patients with atypical symptoms or with significant suspected or confirmed comorbid illnesses may benefit from specialty referral or consultation. Finally, “red flag” conditions indicating an acute neurologic condition requiring urgent neurologic or neurosurgical intervention should prompt emergent transfer to a medical facility with an appropriate level of care.
The primary goal of pharmacological therapies for mTBI is symptomatic improvement. Currently, disease altering therapies are not available. Drug therapy for mTBI symptoms should follow several general principles. Medications that lower the seizure threshold such as buproprion and some anti-psychotic medicines should be avoided. Similarly, medications such as lithium, anti-cholinergic agents, benzodiazepines and others can cause altered mental status and should also be avoided. Starting doses should be as low as possible and titrated to effect under close monitoring. Conversely, maximal tolerated dosing should be trialed before switching to a new agent to avoid under treatment. Patients should be advised to avoid alcohol, caffeine, and herbal supplements. Limited doses of medications with significant toxicity in intentional overdoses such as tri-cyclic antidepressants should also be considered because suicide risk is high in brain injured patients. Finally, patients should be monitored closely for medication interactions and toxicity.
Medication therapy for patients in the first week after injury should be reserved for the treatment of headache only. Acetaminophen is the agent of choice. NSAIDs should not be used until 48 hours after injury unless there is normal neuroimaging data for the patient. Other immediate post-concussive symptoms should not be treated as they typically resolve spontaneously within the first week of injury.
Headache
Headache is the most common symptom after mTBI affecting over 90% of patients. Post-traumatic headaches commonly fall into one of three categories: tension, migraine, or a combination of the two. The evaluation of post-traumatic headache should include assessment for neurologic findings suggestive of serious intra-cranial abnormalities. Focal neurologic deficits should prompt additional urgent investigation with appropriate neuroimaging. A medication review for patients with symptoms lasting more than two weeks is also important as rebound headaches are common in with daily acetaminophen or NSAID use. Similarly, withdrawal from caffeine or nicotine may also result in headache. Patients who state that their headache improves only with opiates should be referred to a pain or headache specialist. Headache symptoms often improve after treatment of comorbid conditions such as sleep disturbances, anxiety, and depression.
Pharmacologic treatment should be selected based on the type of headache suspected. Similar to the opiate dependent patients above, those with symptoms that do not improve within 3 months of initiating therapy should be referred to a headache or pain specialist. Episodic tension type headaches may be treated with aspirin, acetaminophen, or NSAIDs. These medications typically work best when combined with other treatment modalities such as a regular exercise program, relaxation techniques, or biofeedback. Combination medications that include caffeine or a sedative may be more effective but also have a greater likelihood of rebound headache.
Migraine treatment is divided into the prevention and management of acute episodes. Awareness and avoidance of precipitating events should be encouraged. Abortive medications for acute episodes include sumatriptan and zolmatriptan. These medications are most effective when used early in the course of the episode. Some patients with established migraines may require rescue medications to break their headache. Examples of effective rescue medications include ketorolac, butorphanol, opiods, prochlorparazine, and promethazine. Patients with migraine headaches that occur more than once a month should be placed on prophylaxis. First line agents for prophylaxis include metroprolol and toprimate. Prophylactic agents may take as long as three months to become maximally effective. Finally, mixed headache types may require separate agents for treatment of the tension and migraine components.
Disequilibrium and Vertigo
Up to 30% of patients with mTBI complain of disturbed equilibrium or vertigo 87. Despite this, symptoms do not correlate with objective evidence after the first week after injury 88. A thorough medication review should be performed for all mTBI patients complaining of dizziness. Medications such as stimulants, benzodiazepines, tricyclics, monoamine oxidase inhibitors, tetracyclics, neuroleptics, selective serotonin reuptake inhibitors, beta blockers, and cholinesterase inhibitors may cause or exacerbate dizziness. Vestibular suppression may be useful in the acute phase but has not proven effective for persistent symptoms 89. Vestibular suppressants should only be used if the symptoms significantly limit the patient's functional activities as they may result in delayed improvement 90-91. Meclizine is the first agent recommended. Scopolamine and dimenhydrinate may be used if meclizine fails. Benzodiazepines should only be used after careful consideration of their sedating and habit-forming properties. Trials should be limited to two weeks duration.
Fatigue and Sleep Disturbances
Another common symptom after mTBI is fatigue, which may be due to central nervous system dysfunction, sleep disturbances or depression. Proper assessment of this symptom requires a thorough history of pre- and post-injury levels of activity. There are also several validated instruments to objectively measure fatigue 92-93. Physical causes of fatigue may also be assessed with laboratory testing including metabolic panel, a complete blood count, and thyroid function testing. Review of the patient's medication history, alcohol, caffeine, and illicit drug use should also be performed as all of these may result in fatigue. Prior to initiating medications for fatigue conservative measures such as education of the patient, initiating an exercise program, as well as referring the patient for physical or cognitive behavioral therapy should be trialed. There is limited evidence for the efficacy of stimulant treatment for fatigue after mTBI. Commonly used agents include modafanil, methylphenidate, and amantadine. These medications should only be used if symptoms have lasted more than 4 weeks, the patient does not have substance abuse issues, and addressing other factors mentioned in this section have failed to improve symptoms. Trials of these medications should last at least 3 months.
Sleep disturbances are common after mTBI. The goal of therapy is to restore a regular, unbroken night-time sleep pattern and improve the perception of sleep quality. Any drug therapy for sleep disturbances should be accompanied by education regarding good sleep hygiene. Furthermore, concomitant primary sleep disorders such as obstructive sleep apnea, restless legs syndrome, and narcolepsy should be appropriately treated. In the acute phase, short term treatment with non-benzodiazepine sleep medications such as zolpidem may be helpful. Prazosin may be used in patients with nightmares or agitation during sleep.
Clinical Presentation
The patient returns to the concussion clinic 4 weeks after his initial visit and 6 weeks after his accident. He reports that he instituted the sleep hygiene recommendations given to him on the prior visit and that his headaches, sleepiness, irritability, and concentration difficulties subsequently resolved. He has required NSAIDS with decreasing frequency and has not had a headache in the last two weeks. The patient is sent home with instructions to contact the clinic for a future appointment if symptoms return.
Follow Up
All patients require a follow up assessment within 4-6 weeks of initiation of therapy. Patients can be grouped into three categories at this second assessment: those with complete symptom resolution, those with partial resolution, and those with no improvement or worsened symptoms. Patients whose symptoms completely resolve should be given contact information to make a future appointment if symptoms return. Patients with a partial response may benefit from augmentation or adjustment of their current therapy. Those patients whose symptoms are refractory to initial treatment should be considered to have persistent post-concussive symptoms and treated according to the guidelines below (Management of Persistent Post-concussive Symptoms).
Management of Persistent Post-concussive Symptoms
This section is relevant for patients who have had an initial evaluation and failed a trial of treatment for mTBI related symptoms. Patients with delayed presentation for mTBI symptoms should first be treated according to the preceding section (Initial Management of Post-concussive Symptoms) regardless of the interval since injury. The definitive reference is the VA/DoD Clinical Practice Guideline for Management of Concussion/mTBI 1.
Patients with persistent post-concussive symptoms often have concomitant behavioral health, psychosocial support, or compensation and litigation issues. Attention should be given to addressing these issues as this may help mitigate symptoms refractory to initial treatment. The evaluation of the patient with persistent post-concussive symptoms should include an assessment of available support systems, a mental health history including pre-morbid conditions, co-occurring symptoms such as chronic pain or personality disorders, substance abuse disorders, secondary gain issues, job status, and other financial or legal difficulties 1. Finally, all patients presenting with persistent post-concussive symptoms should be assessed for any potential danger to themselves or others.
Less than 5% of patients have persistent symptoms one year or more after injury 11. Patients typically have more physical complaints within 4 weeks of injury after which emotional complaints predominate 80. Once a thorough assessment has been obtained, the principle goal is to identify appropriate referrals for management of the persistent symptoms. Patients with behavioral symptoms and possible co-morbid psychiatric conditions may benefit from referral to mental health professionals. Persistent physical symptoms should be evaluated by appropriate specialists. Persistent cognitive symptoms are rare and are frequently accompanied by comorbid conditions such as mood disorders, poor physical health, poor psychosocial support, or chronic pain. In addition to addressing these comorbid conditions, these patients should be referred for neuropsychiatric evaluation to determine appropriate treatment options. Cognitive rehabilitation may be helpful for patients with persistent difficulties in memory, executive function, or attention 81-83. A social work referral is appropriate for patients with poor psychosocial support, legal difficulties, or financial problems. While there is consistent evidence of an association between mTBI related compensation or litigation and increased symptom reporting and poor outcome 59, 84-86, there is no evidence to support a therapeutic benefit of attributing persistent symptoms to these secondary gain issues 1. Consequently, clinicians should not allow symptom exaggeration by patients seeking compensation to alter their care plans.
Given the diverse group of health professionals involved in the treatment of persistent post-concussive symptoms, a multi-disciplinary, team approach with the referring provider as the coordinator of care is required. A designated case manager can be very helpful for coordinating care. Typical tasks benefiting from case management includes coordination of referrals, ensuring appropriate patient and family education, participation in short and long-term goal setting, ensuring that appropriate social service and mental health screening is performed, and coordination with the multi-disciplinary team. Ongoing follow up visits should occur regularly with goals of monitoring symptom severity, reviewing symptom impact on activities, and the effectiveness of treatments.
Return to Play after Sports Injury
Guidelines for returning to play in an athlete differ from general instructions for return to normal activities after mTBI in that they are designed to prevent a repeat mTBI while the patient is recovering from the initial injury. In general, the risks of suffering a second and third TBI are 3 fold and 8 to 9 fold greater than the risk of a first TBI, respectively 94. Furthermore, case reports suggest that athletes are at increased risk for concussion in the period immediately after their initial injury 95. Therefore, consideration of the concussion risk in a sports-specific manner is important 96. Return to play guidelines are consensus rather than evidence based. The most commonly used guidelines include those by R.C. Cantu and the American Academy of Neurology (AAN) and of Dr. Cantu 97-98. Both sets of guidelines use severity of the concussion and presence of post-concussive symptoms as the criteria for return to play decision making. The Cantu guidelines allow a player to return once he/she is asymptomatic for one week if post traumatic amnesia lasted less than 24 hours and the initial loss of unconsciousness was less than five minutes. Players with more severe symptoms at the time of their concussion should not play for one month and then can return after an additional one week without symptoms. In players with a history of multiple concussions, consideration should be given to sitting out for the remainder of the season.
While the consensus based back to play guidelines referenced in this section are clinically accepted as the standard of care they are not infallible. A recent example of a second injury despite scrupulous application of these guidelines is seen with Brian Westbrook, a professional football player in the National Football League (NFL). Mr. Westbrook suffered the first concussion of his 8 year NFL career on October 26th 2009, suffering a brief loss of consciousness with associated retrograde amnesia after being tackled in a game. He was held from play for three weeks due to lingering headache and then suffered a second concussion in his first game back from injury. While there was widespread speculation that he might not return that season or even that his football career was over, he did play the final two games of the season without further injury after being out for a total of 5 weeks. While no guideline can prevent all adverse events, it is possible that improved guidelines could result in fewer repeat injuries. Prospectively validated, evidence based return to play guidelines are needed.
Sequelae of mTBI
Post Concussive Symptoms and Cognitive Deficits
Common post-concussive symptoms include headache, dizziness, fatigue, sleep disturbances, memory problems, balance problems, sensitivity to sound or tinnitus, concentration difficulties, and irritability. These symptoms are notably non-specific and are associated with many other diseases. Nonetheless, several studies have reported higher rates of these symptoms in patients after mTBI than in patients with no injury or extra-cranial trauma without TBI 64, 99-100. The percentage of patients who suffer from persistent post-concussive symptoms diminishes with time after injury. Less than 25% of patients are likely to have problems lasting more than 12 months after injury 11. Although cognitive complaints are fairly common after mTBI, measurable cognitive deficits are generally only present after severe or moderate TBI 101-102. There is little evidence of objective cognitive deficits after mTBI 103.
Motor, Balance, and Cranial Nerve Abnormalities
In general, objective findings after mTBI are absent. Balance problems are emerging as a promising exception to this rule. In one study of 37 mTBI patients, testing of saccades, oculomotor smooth pursuit, upper-limb visuomotor function and neuropsychologic domains was performed and the results compared to uninjured control patients. At one year after injury, eye and upper limb movement, but not cognitive function remained impaired in the mTBI patients 104. In a more recent study, the same group found that eye movement impairment was significantly worse in mTBI patients suffering from post-concussive syndrome relative to mTBI patients with good recovery 105.
Psychiatric Diagnoses
Many studies have found an association between TBI of all severities and major depressive disorder 63, 106-107. This observed association was not likely to be explained by depression prior to injury however prior mood disorder may be an increased risk for TBI 108-109. While there are few studies of relationship between mania or bipolar disorder and TBI, the existing evidence suggests that there is not a strong relationship between them 110-112. There is limited evidence supporting an association between mTBI and PTSD in military populations. In a study of 2,525 soldiers returning after a one year deployment to Iraq, researchers identified a clear association between PTSD and mild TBI with LOC (OR, 2.98; 95% CI, 1.70–5.24) 64. A second cross-sectional study of 2,235 Afghanistan and Iraq war veterans also found an association between PTSD and mTBI 113. However, two studies of civilian populations found no relationship between mTBI and PTSD 114-115.
Second Impact Syndrome
Second impact syndrome (SIS) is a dreaded, rare complication of mTBI that occurs after a patient suffers a second mTBI while remaining symptomatic from the first. Typically, a patient will suffer a head injury during play resulting in post-concussive symptoms. After returning to play while still suffering symptoms they sustain a second, apparently minor head trauma, and rapidly suffer depressed mental status resulting in death or a persistent vegetative state. It is postulated that this disorder is caused by disordered cerebral autoregulation resulting from the initial TBI. The condition has mainly been reported in young men who play contact sports. The term SIS was first coined by Saunders and Harbaugh 116, however a similar syndrome was previously described by Schneider 117.
While SIS has become firmly fixed in the minds of clinicians as an important complication of mTBI, there is some question regarding whether it is a true clinical entity 118. A critical review of reported cases of SIS found that most did not meet a reasonable clinical definition of SIS. Cases often lacked a neuropathologic evidence of unexplained cerebral swelling 119. Even more problematic, most of the reported cases of precipitous neurologic collapse after a seemingly minor trauma occurred in the absence of any documented “first impact”. Of the seventeen cases reviewed, only five where classified as “probable SIS”. Given this analysis it is reasonable to conclude that the term SIS is inaccurate. Diffuse cerebral swelling can very rarely occur after mTBI, principally in children and adolescents, however a second mTBI is not required.
Seizures
While there is sufficient evidence to support a causal relationship between moderate or severe TBI and the development of unprovoked seizures, the evidence is limited for an association between seizures and mTBI 24. In non-military TBI populations, there is a 3.6 fold increase in the incidence of seizures relative to non-injured patients after TBI of all severities. After severe TBI, there was a 17 fold increase in seizure incidence which declined to 2.9 fold in moderate TBI patients. For mTBI patients with loss of consciousness or post traumatic amnesia, the incidence of seizures was 1.5 times that of controls (95% CI 1.0 – 2.2) 94, 120. These studies were limited in that pediatric patients, who have a higher baseline incidence of seizures than adults, were not analyzed separately from adults. Post-traumatic seizure risk is greatest in the first year after injury. After 4 years, TBI patients are no longer at increased risk relative to uninjured subjects 121.
Dementia and Neurodegeneration
Alzheimer's disease is the most common neurodegenerative disease and results in progressive dementia and eventual death. Familial or early onset Alzheimer's disease is caused by specific mutations and comprises approximately 10% cases. The remaining 90% of cases are referred to as sporadic. Although the mechanisms of disease progression in sporadic Alzheimer's disease are not known, it likely results from a combination of genetic and environmental factors. TBI is the strongest known environmental exposure associated with subsequent development of sporadic Alzheimer's disease. A retrospective cohort study of World War II veterans with documented closed head injury demonstrated an increase risk of Alzheimer's type dementia relative to non-head injured controls (Hazard ratio 2.00, 95% CI 1.03-3.90) 122. A meta-analysis of seven case control studies revealed similar results 123.
Dementia pugilistica, also known as chronic traumatic encephalopathy, is a neurodegenerative condition that affects athletes in sports that involve repeated head trauma such as boxing and mixed martial arts 124. Characteristic neuropathologic changes include cerebellar damage, cortical damage, and other scarring of the brain; substantia nigral degeneration; neurofibrillary tangles in the cerebral cortex and temporal horn areas; and abnormalities of the septum pellucidum. Autopsy of professional football players who died in their forties after developing dementia also showed neurodegenerative changes consistent with chronic traumatic encephalopathy 125-127. Neuropsychologic deficits associated with dementia pugilistica have been found in some studies 128-129 but not others 130-131.
Parkinsonism is a constellation of symptoms including tremor, rigidity, and bradikinesia, and postural instability and is caused by loss of central dopamine. Very little has been reported regarding association between TBI and parkinsonism, however, several case-control studies have shown an increased risk after mTBI with LOC or post-traumatic amnesia 132-133. The risk for the development of parkinsonism appears to increase with severity of TBI 132, 134.
Summary
Mild traumatic brain injury is a widespread problem. Because of our limited understanding of the injury pathophysiology, the diagnosis of mTBI is based entirely on clinical symptoms, and often occurs in the absence of objective findings. The central feature of mTBI is a transiently altered state of consciousness after a traumatic injury to the head. The priority of emergency care is to identify potentially life threatening intra-cranial injuries through the judicious application of appropriate imaging studies and neurosurgical consultation. Although post-mTBI symptoms quickly and completely resolve in the vast majority of cases, a significant number of patients will complain of lasting problems. Post-concussive complaints tend to be inter-related and relief of one may have beneficial effects on others. Although the evidence is not definitive, longer term sequelae of mTBI may include seizure disorders and neurodegeneration. Recognizing the potentially life changing aspects of mTBI should be an important priority for the emergency physician because simple, early interventions such as education regarding the expected positive outcome from the injury and prompt treatment can prevent chronic symptoms from occurring.
Acknowledgments
This work was supported by Grant No. 5R01HD051865-03 from the National Institutes of Health (Drs. Bazarian and Blyth) and a Jahnigen Career Development Scholars Award (Dr. Blyth).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Concussion/mTBI Working Group. VA/DoD Clinical Practice Guideline for Management of Concussion/mTBI. [October, 2009]; Available at: http://www.dvbic.org/images/pdfs/Providers/VADoD-CPG---Concussion-mTBI.aspx.
- 2.ACRM. American Congress of Rehabilitation Medicine Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group. Definition of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1993;8(3):86–87. [Google Scholar]
- 3.National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 4.Lange RT, Iverson GL, Franzen MD. Neuropsychological functioning following complicated vs. uncomplicated mild traumatic brain injury. Brain Inj. 2009;23(2):83–91. doi: 10.1080/02699050802635281. [DOI] [PubMed] [Google Scholar]
- 5.Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, GA: Centers for Disease Control and Prevention National Center for Injury Prevention and Control; 2006. [Google Scholar]
- 7.Jager TE, Weiss HB, Coben JH, et al. Traumatic brain injuries evaluated in U.S. emergency departments, 1992-1994. Acad Emerg Med. 2000;7(2):134–40. doi: 10.1111/j.1553-2712.2000.tb00515.x. [DOI] [PubMed] [Google Scholar]
- 8.Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10(1):47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 9.Alves WM, Macciocchi SN, Barth JT. Postconcussive symptoms after uncomplicated mild head injury. The Journal of Head Trauma Rehabilitation. 1993;8(3):48–59. [Google Scholar]
- 10.Middleboe T, Andersen HS, Birket-Smith M, et al. Minor head injury: impact on general health after 1 year. A prospective follow-up study. Acta Neurol Scand. 1992;85(1):5–9. [PubMed] [Google Scholar]
- 11.Iverson GL. Post-concussive disorder. New York, NY: Demos Medical Publishing LLC; 2007. [Google Scholar]
- 12.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45(7):1253–60. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 13.Gondusky JS, Reiter MP. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Mil Med. 2005;170(6):546–49. doi: 10.7205/milmed.170.6.546. [DOI] [PubMed] [Google Scholar]
- 14.Summers CR, Ivins B, Schwab KA. Traumatic brain injury in the United States: an epidemiologic overview. Mt Sinai J Med. 2009;76(2):105–10. doi: 10.1002/msj.20100. [DOI] [PubMed] [Google Scholar]
- 15.Gennarelli TA, Thibault LE, Adams JH, et al. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12(6):564–74. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 16.Holbourn AH. Mechanics of Head Injuries. Lancet. 1943;242(6267):438–41. [Google Scholar]
- 17.Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain. 1974;97(4):633–54. doi: 10.1093/brain/97.1.633. [DOI] [PubMed] [Google Scholar]
- 18.LaPlaca MC, Simon CM, Prado GR, et al. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh TK. Neurochemical sequelae of traumatic brain injury: therapeutic implications. Cerebrovasc Brain Metab Rev. 1994;6(2):109–62. [PubMed] [Google Scholar]
- 20.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 21.Thompson HJ, Lifshitz J, Marklund N, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22(1):42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 22.Statler KD, Jenkins LW, Dixon CE, et al. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J Neurotrauma. 2001;18(11):1195–206. doi: 10.1089/089771501317095232. [DOI] [PubMed] [Google Scholar]
- 23.Marklund N, Bakshi A, Castelbuono DJ, et al. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr Pharm Des. 2006;12(13):1645–80. doi: 10.2174/138161206776843340. [DOI] [PubMed] [Google Scholar]
- 24.IOM (Institute of Medicine) Gulf War and Health, Volume 7: Long-term Consequences of Traumatic Brain Injury. Washington, D.C.: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 25.Adams RD, Victor M, Ropper AH. Principles of Neurology. 6th. New York: McGraw-Hill; 1997. [Google Scholar]
- 26.Hayes RL, Lyeth BG, Jenkins LW. Neurochemical mechanisms of mild and moderate head injury: implications for treatment. In: Levin HS, Eisenberg HM, Benton AL, editors. Mild Head Injury. Oxford: Oxford University Press; 1989. pp. 54–79. [Google Scholar]
- 27.Katayama Y, Watkins LR, Becker DP, et al. Evidence for involvement of cholinoceptive cells of the parabrachial region in environmentally induced nociceptive suppression in the cat. Brain Res. 1984;299(2):348–53. doi: 10.1016/0006-8993(84)90717-0. [DOI] [PubMed] [Google Scholar]
- 28.Hayes RL, Pechura CM, Katayama Y, et al. Activation of pontine cholinergic sites implicated in unconsciousness following cerebral concussion in the cat. Science. 1984;223(4633):301–03. doi: 10.1126/science.6701514. [DOI] [PubMed] [Google Scholar]
- 29.Lyeth BG, Dixon CE, Hamm RJ, et al. Effects of anticholinergic treatment on transient behavioral suppression and physiological responses following concussive brain injury to the rat. Brain Res. 1988;448(1):88–97. doi: 10.1016/0006-8993(88)91104-3. [DOI] [PubMed] [Google Scholar]
- 30.Ishige N, Pitts LH, Hashimoto T, et al. Effect of hypoxia on traumatic brain injury in rats: Part 1. Changes in neurological function, electroencephalograms, and histopathology. Neurosurgery. 1987;20(6):848–53. doi: 10.1227/00006123-198706000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Marmarou A, Foda MA, van den Brink W, et al. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80(2):291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 32.Walker AE, Kollros JJ, Case TJ. The Physiological Basis of Concussion*. Journal of Neurosurgery. 1944;1(2):103–16. [Google Scholar]
- 33.Shaw NA. The neurophysiology of concussion. Prog Neurobiol. 2002;67(4):281–344. doi: 10.1016/s0301-0082(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 34.Stein SC, Ross SE. The value of computed tomographic scans in patients with low-risk head injuries. Neurosurgery. 1990;26(4):638–40. doi: 10.1097/00006123-199004000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Nagurney JT, Borczuk P, Thomas SH. Elder patients with closed head trauma: a comparison with nonelder patients. Acad Emerg Med. 1998;5(7):678–84. doi: 10.1111/j.1553-2712.1998.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 36.Miller EC, Holmes JF, Derlet RW. Utilizing clinical factors to reduce head CT scan ordering for minor head trauma patients. J Emerg Med. 1997;15(4):453–57. doi: 10.1016/s0736-4679(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 37.Jeret JS, Mandell M, Anziska B, et al. Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery. 1993;32(1):9–15. doi: 10.1227/00006123-199301000-00002. discussion 15-16. [DOI] [PubMed] [Google Scholar]
- 38.Jennett B, Teasdale G, Galbraith S, et al. Severe head injuries in three countries. J Neurol Neurosurg Psychiatry. 1977;40(3):291–98. doi: 10.1136/jnnp.40.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harad FT, Kerstein MD. Inadequacy of bedside clinical indicators in identifying significant intracranial injury in trauma patients. J Trauma. 1992;32(3):359–61. doi: 10.1097/00005373-199203000-00014. discussion 61-63. [DOI] [PubMed] [Google Scholar]
- 40.Borczuk P. Predictors of intracranial injury in patients with mild head trauma. Ann Emerg Med. 1995;25(6):731–36. doi: 10.1016/s0196-0644(95)70199-0. [DOI] [PubMed] [Google Scholar]
- 41.Mittl RL, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol. 1994;15(8):1583–89. [PMC free article] [PubMed] [Google Scholar]
- 42.Jagoda AS, Bazarian JJ, Bruns JJ, Jr, et al. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52(6):714–48. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357(9266):1391–96. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- 44.Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343(2):100–05. doi: 10.1056/NEJM200007133430204. [DOI] [PubMed] [Google Scholar]
- 45.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511–18. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 46.Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160–70. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 47.Tasci A, Okay O, Gezici AR, et al. Prognostic value of interleukin-1 beta levels after acute brain injury. Neurol Res. 2003;25(8):871–74. doi: 10.1179/016164103771953998. [DOI] [PubMed] [Google Scholar]
- 48.Ringger NC, O'Steen BE, Brabham JG, et al. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J Neurotrauma. 2004;21(10):1443–56. doi: 10.1089/neu.2004.21.1443. [DOI] [PubMed] [Google Scholar]
- 49.Pelinka LE, Kroepfl A, Schmidhammer R, et al. Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma. 2004;57(5):1006–12. doi: 10.1097/01.ta.0000108998.48026.c3. [DOI] [PubMed] [Google Scholar]
- 50.Olsson A, Csajbok L, Ost M, et al. Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251(7):870–76. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 51.Missler U, Wiesmann M, Wittmann G, et al. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem. 1999;45(1):138–41. [PubMed] [Google Scholar]
- 52.Berger RP, Heyes MP, Wisniewski SR, et al. Assessment of the macrophage marker quinolinic acid in cerebrospinal fluid after pediatric traumatic brain injury: insight into the timing and severity of injury in child abuse. J Neurotrauma. 2004;21(9):1123–30. doi: 10.1089/neu.2004.21.1123. [DOI] [PubMed] [Google Scholar]
- 53.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury*. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biberthaler P, Linsenmeier U, Pfeifer KJ, et al. Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock. 2006;25(5):446–153. doi: 10.1097/01.shk.0000209534.61058.35. [DOI] [PubMed] [Google Scholar]
- 55.Biberthaler P, Mussack T, Wiedemann E, et al. Elevated serum levels of S-100B reflect the extent of brain injury in alcohol intoxicated patients after mild head trauma. Shock. 2001;16(2):97–101. doi: 10.1097/00024382-200116020-00002. [DOI] [PubMed] [Google Scholar]
- 56.Nagy KK, Joseph KT, Krosner SM, et al. The utility of head computed tomography after minimal head injury. J Trauma. 1999;46(2):268–70. doi: 10.1097/00005373-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Livingston DH, Loder PA, Hunt CD. Minimal head injury: is admission necessary? Am Surg. 1991;57(1):14–17. [PubMed] [Google Scholar]
- 58.Dunham CM, Coates S, Cooper C. Compelling evidence for discretionary brain computed tomographic imaging in those patients with mild cognitive impairment after blunt trauma. J Trauma. 1996;41(4):679–86. doi: 10.1097/00005373-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 59.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 60.Holm L, Cassidy JD, Carroll LJ, et al. Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2005;37(3):137–41. doi: 10.1080/16501970510027321. [DOI] [PubMed] [Google Scholar]
- 61.Comper P, Bisschop SM, Carnide N, et al. A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005;19(11):863–80. doi: 10.1080/02699050400025042. [DOI] [PubMed] [Google Scholar]
- 62.Borg J, Holm L, Peloso PM, et al. Non-surgical intervention and cost for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):76–83. doi: 10.1080/16501960410023840. [DOI] [PubMed] [Google Scholar]
- 63.Fann JR, Burington B, Leonetti A, et al. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry. 2004;61(1):53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- 64.Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 65.Mooney G, Speed J. The association between mild traumatic brain injury and psychiatric conditions. Brain Inj. 2001;15(10):865–77. doi: 10.1080/02699050110065286. [DOI] [PubMed] [Google Scholar]
- 66.Rapoport MJ, Kiss A, Feinstein A. The impact of major depression on outcome following mild-to-moderate traumatic brain injury in older adults. J Affect Disord. 2006;92(2-3):273–76. doi: 10.1016/j.jad.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 67.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 68.Belanger HG, Curtiss G, Demery JA, et al. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc. 2005;11(3):215–27. doi: 10.1017/S1355617705050277. [DOI] [PubMed] [Google Scholar]
- 69.Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: a meta-analysis. J Int Neuropsychol Soc. 2005;11(4):345–57. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- 70.Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15(4):341–49. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- 71.Deb S, Lyons I, Koutzoukis C. Neurobehavioural symptoms one year after a head injury. Br J Psychiatry. 1999;174:360–65. doi: 10.1192/bjp.174.4.360. [DOI] [PubMed] [Google Scholar]
- 72.Dikmen S, McLean A, Temkin N. Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry. 1986;49(11):1227–32. doi: 10.1136/jnnp.49.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartlage LC, Durant-Wilson D, Patch PC. Persistent neurobehavioral problems following mild traumatic brain injury. Arch Clin Neuropsychol. 2001;16(6):561–70. [PubMed] [Google Scholar]
- 74.Luis CA, Vanderploeg RD, Curtiss G. Predictors of postconcussion symptom complex in community dwelling male veterans. J Int Neuropsychol Soc. 2003;9(7):1001–15. doi: 10.1017/S1355617703970044. [DOI] [PubMed] [Google Scholar]
- 75.Powell TJ, Collin C, Sutton K. A follow-up study of patients hospitalized after minor head injury. Disabil Rehabil. 1996;18(5):231–37. doi: 10.3109/09638289609166306. [DOI] [PubMed] [Google Scholar]
- 76.Anson K, Ponsford J. Evaluation of a coping skills group following traumatic brain injury. Brain Inj. 2006;20(2):167–78. doi: 10.1080/02699050500442956. [DOI] [PubMed] [Google Scholar]
- 77.Bedard M, Felteau M, Mazmanian D, et al. Pilot evaluation of a mindfulness-based intervention to improve quality of life among individuals who sustained traumatic brain injuries. Disabil Rehabil. 2003;25(13):722–31. doi: 10.1080/0963828031000090489. [DOI] [PubMed] [Google Scholar]
- 78.Hinkle JL, Alves WM, Rimell RW, et al. Restoring social competence in minor head-injury patients. J Neurosci Nurs. 1986;18(5):268–71. doi: 10.1097/01376517-198610000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Mittenberg W, Tremont G, Zielinski RE, et al. Cognitive-behavioral prevention of postconcussion syndrome. Arch Clin Neuropsychol. 1996;11(2):139–45. [PubMed] [Google Scholar]
- 80.Yang CC, Tu YK, Hua MS, et al. The association between the postconcussion symptoms and clinical outcomes for patients with mild traumatic brain injury. J Trauma. 2007;62(3):657–63. doi: 10.1097/01.ta.0000203577.68764.b8. [DOI] [PubMed] [Google Scholar]
- 81.Cicerone KD. Remediation of “working attention” in mild traumatic brain injury. Brain Inj. 2002;16(3):185–95. doi: 10.1080/02699050110103959. [DOI] [PubMed] [Google Scholar]
- 82.Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86(8):1681–92. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 83.Tiersky LA, Anselmi V, Johnston MV, et al. A trial of neuropsychologic rehabilitation in mild-spectrum traumatic brain injury. Arch Phys Med Rehabil. 2005;86(8):1565–74. doi: 10.1016/j.apmr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Binder LM, Rohling ML. Money matters: a meta-analytic review of the effects of financial incentives on recovery after closed-head injury. Am J Psychiatry. 1996;153(1):7–10. doi: 10.1176/ajp.153.1.7. [DOI] [PubMed] [Google Scholar]
- 85.Kashluba S, Paniak C, Casey JE. Persistent symptoms associated with factors identified by the WHO Task Force on Mild Traumatic Brain Injury. Clin Neuropsychol. 2008;22(2):195–208. doi: 10.1080/13854040701263655. [DOI] [PubMed] [Google Scholar]
- 86.Suhr JA, Gunstad J. Postconcussive symptom report: the relative influence of head injury and depression. J Clin Exp Neuropsychol. 2002;24(8):981–93. doi: 10.1076/jcen.24.8.981.8372. [DOI] [PubMed] [Google Scholar]
- 87.Cicerone KD, Kalmar K. Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1995;10(3):1–17. [Google Scholar]
- 88.Gottshall KR, Gray NL, Drake AI, et al. To investigate the influence of acute vestibular impairment following mild traumatic brain injury on subsequent ability to remain on activity duty 12 months later. Mil Med. 2007;172(8):852–57. doi: 10.7205/milmed.172.8.852. [DOI] [PubMed] [Google Scholar]
- 89.Zee DS. Perspectives on the pharmacotherapy of vertigo. Arch Otolaryngol. 1985;111(9):609–12. doi: 10.1001/archotol.1985.00800110087009. [DOI] [PubMed] [Google Scholar]
- 90.Pyykko I, Magnusson M, Schalen L, et al. Pharmacological treatment of vertigo. Acta Otolaryngol Suppl. 1988;455:77–81. doi: 10.3109/00016488809125063. [DOI] [PubMed] [Google Scholar]
- 91.Hain TC, Yacovino D. Pharmacologic treatment of persons with dizziness. Neurol Clin. 2005;23(3):831–53. vii. doi: 10.1016/j.ncl.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 93.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 94.Annegers JF, Grabow JD, Kurland LT, et al. The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935-1974. Neurology. 1980;30(9):912–19. doi: 10.1212/wnl.30.9.912. [DOI] [PubMed] [Google Scholar]
- 95.Kelly JP, Nichols JS, Filley CM, et al. Concussion in sports. Guidelines for the prevention of catastrophic outcome. JAMA. 1991;266(20):2867–69. doi: 10.1001/jama.266.20.2867. [DOI] [PubMed] [Google Scholar]
- 96.Kissick J, Johnston KM. Return to play after concussion: principles and practice. Clin J Sport Med. 2005;15(6):426–31. doi: 10.1097/01.jsm.0000186683.59158.8b. [DOI] [PubMed] [Google Scholar]
- 97.Anonymous. Practice Parameter: The management of concussion in sports (summary statement) Neurology. 1997;48(3):581–85. doi: 10.1212/wnl.48.3.581. [DOI] [PubMed] [Google Scholar]
- 98.Cantu RC. Return to play guidelines after a head injury. Clin Sports Med. 1998;17(1):45–60. doi: 10.1016/s0278-5919(05)70060-0. [DOI] [PubMed] [Google Scholar]
- 99.Gerber DJ, Schraa JC. Mild traumatic brain injury: Searching for the syndrome. The Journal of Head Trauma Rehabilitation. 1995;10(4):28–40. [Google Scholar]
- 100.Masson F, Maurette P, Salmi LR, et al. Prevalence of impairments 5 years after a head injury, and their relationship with disabilities and outcome. Brain Inj. 1996;10(7):487–97. doi: 10.1080/026990596124205. [DOI] [PubMed] [Google Scholar]
- 101.Incoccia C, Formisano R, Muscato P, et al. Reaction and movement times in individuals with chronic traumatic brain injury with good motor recovery. Cortex. 2004;40(1):111–15. doi: 10.1016/s0010-9452(08)70924-9. [DOI] [PubMed] [Google Scholar]
- 102.Ruff RM, Evans R, Marshall LF. Impaired verbal and figural fluency after head injury. Arch Clin Neuropsychol. 1986;1(2):87–101. [PubMed] [Google Scholar]
- 103.Vanderploeg RD, Curtiss G, Belanger HG. Long-term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc. 2005;11(3):228–36. doi: 10.1017/S1355617705050289. [DOI] [PubMed] [Google Scholar]
- 104.Heitger MH, Jones RD, Dalrymple-Alford JC, et al. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj. 2006;20(8):807–24. doi: 10.1080/02699050600676354. [DOI] [PubMed] [Google Scholar]
- 105.Heitger MH, Jones RD, Macleod AD, et al. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132(Pt 10):2850–70. doi: 10.1093/brain/awp181. [DOI] [PubMed] [Google Scholar]
- 106.Jorge RE, Robinson RG, Moser D, et al. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61(1):42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- 107.Vanderploeg RD, Curtiss G, Luis CA, et al. Long-term morbidities following self-reported mild traumatic brain injury. J Clin Exp Neuropsychol. 2007;29(6):585–98. doi: 10.1080/13803390600826587. [DOI] [PubMed] [Google Scholar]
- 108.Fann JR, Leonetti A, Jaffe K, et al. Psychiatric illness and subsequent traumatic brain injury: a case control study. J Neurol Neurosurg Psychiatry. 2002;72(5):615–20. doi: 10.1136/jnnp.72.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vassallo JL, Proctor-Weber Z, Lebowitz BK, et al. Psychiatric risk factors for traumatic brain injury. Brain Inj. 2007;21(6):567–73. doi: 10.1080/02699050701426832. [DOI] [PubMed] [Google Scholar]
- 110.Koponen S, Taiminen T, Portin R, et al. Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am J Psychiatry. 2002;159(8):1315–21. doi: 10.1176/appi.ajp.159.8.1315. [DOI] [PubMed] [Google Scholar]
- 111.Sagduyu K. Association of mild traumatic brain injury with bipolar disorder. J Clin Psychiatry. 2002;63(7):594. doi: 10.4088/jcp.v63n0710a. [DOI] [PubMed] [Google Scholar]
- 112.Silver JM, Kramer R, Greenwald S, et al. The association between head injuries and psychiatric disorders: findings from the New Haven NIMH Epidemiologic Catchment Area Study. Brain Inj. 2001;15(11):935–45. doi: 10.1080/02699050110065295. [DOI] [PubMed] [Google Scholar]
- 113.Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. 2008;167(12):1446–52. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- 114.Bryant RA, Harvey AG. The influence of traumatic brain injury on acute stress disorder and post-traumatic stress disorder following motor vehicle accidents. Brain Inj. 1999;13(1):15–22. doi: 10.1080/026990599121836. [DOI] [PubMed] [Google Scholar]
- 115.Creamer M, O'Donnell ML, Pattison P. Amnesia, traumatic brain injury, and posttraumatic stress disorder: a methodological inquiry. Behav Res Ther. 2005;43(10):1383–89. doi: 10.1016/j.brat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Saunders RL, Harbaugh RE. The second impact in catastrophic contact-sports head trauma. JAMA. 1984;252(4):538–39. [PubMed] [Google Scholar]
- 117.Schneider RC. Head and neck injuries in football: mehanisms, treatment and prevention. Baltimore: Williams and Wilkins; 1973. pp. 35–43. [Google Scholar]
- 118.McCrory PR, Berkovic SF. Second impact syndrome. Neurology. 1998;50(3):677–83. doi: 10.1212/wnl.50.3.677. [DOI] [PubMed] [Google Scholar]
- 119.McCrory P. Does second impact syndrome exist? Clin J Sport Med. 2001;11(3):144–49. doi: 10.1097/00042752-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 120.Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338(1):20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 121.Singer RB. Incidence of seizures after traumatic brain injury--a 50-year population survey. J Insur Med. 2001;33(1):42–45. [PubMed] [Google Scholar]
- 122.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55(8):1158–66. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 123.Van Duijn CM, Clayton DG, Chandra V, et al. Interaction between genetic and environmental risk factors for Alzheimer's disease: a reanalysis of case-control studies. EURODEM Risk Factors Research Group. Genet Epidemiol. 1994;11(6):539–51. doi: 10.1002/gepi.1370110609. [DOI] [PubMed] [Google Scholar]
- 124.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270–303. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 125.Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–34. doi: 10.1227/01.neu.0000163407.92769.ed. discussion 28-34. [DOI] [PubMed] [Google Scholar]
- 126.Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a National Football League player: part II. Neurosurgery. 2006;59(5):1086–92. doi: 10.1227/01.NEU.0000245601.69451.27. discussion 92-93. [DOI] [PubMed] [Google Scholar]
- 127.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drew RH, Templer DI, Schuyler BA, et al. Neuropsychological deficits in active licensed professional boxers. J Clin Psychol. 1986;42(3):520–25. doi: 10.1002/1097-4679(198605)42:3<520::aid-jclp2270420319>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 129.Roberts AJ. Brain Damage in Boxers. London: Pitman Medical Scientific Publications; 1969. [Google Scholar]
- 130.Porter MD. A 9-year controlled prospective neuropsychologic assessment of amateur boxing. Clin J Sport Med. 2003;13(6):339–52. doi: 10.1097/00042752-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 131.Porter MD, Fricker PA. Controlled prospective neuropsychological assessment of active experienced amateur boxers. Clin J Sport Med. 1996;6(2):90–96. doi: 10.1097/00042752-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 132.Bower JH, Maraganore DM, Peterson BJ, et al. Head trauma preceding PD: a case-control study. Neurology. 2003;60(10):1610–15. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 133.Goldman SM, Tanner CM, Oakes D, et al. Head injury and Parkinson's disease risk in twins. Ann Neurol. 2006;60(1):65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 134.Taylor CA, Saint-Hilaire MH, Cupples LA, et al. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am J Med Genet. 1999;88(6):742–49. [PubMed] [Google Scholar]


