Abstract
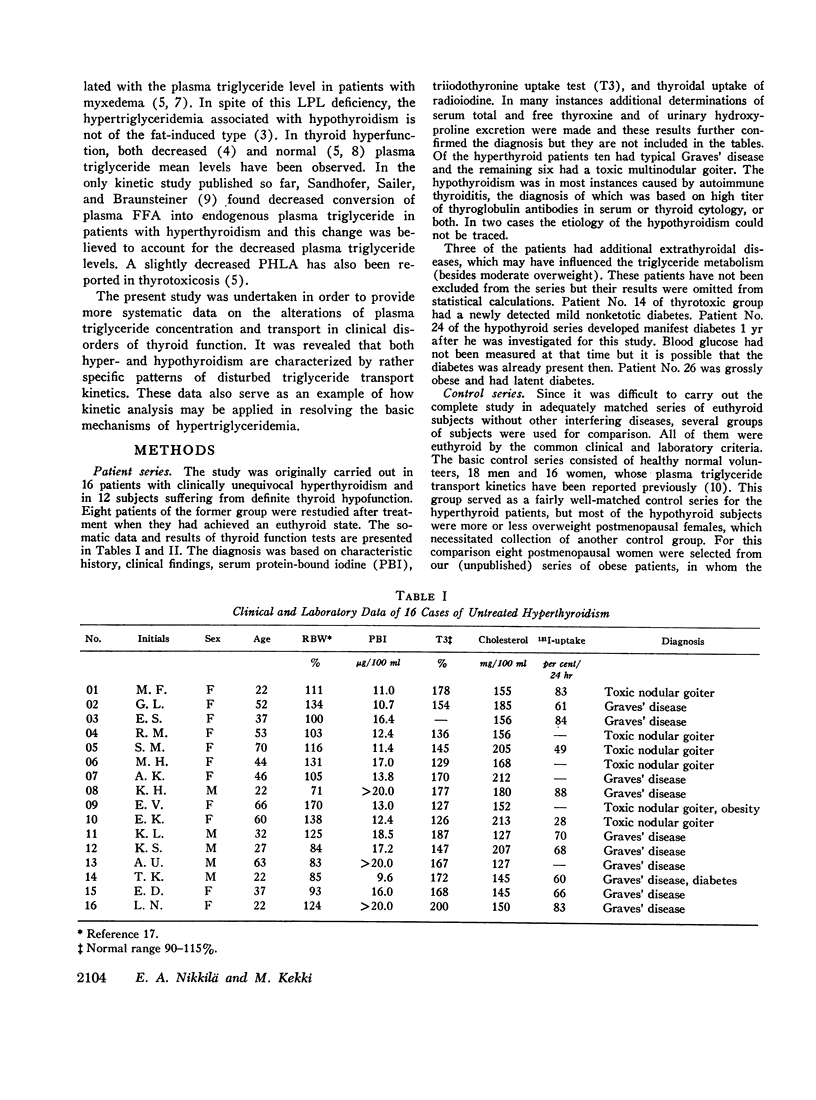
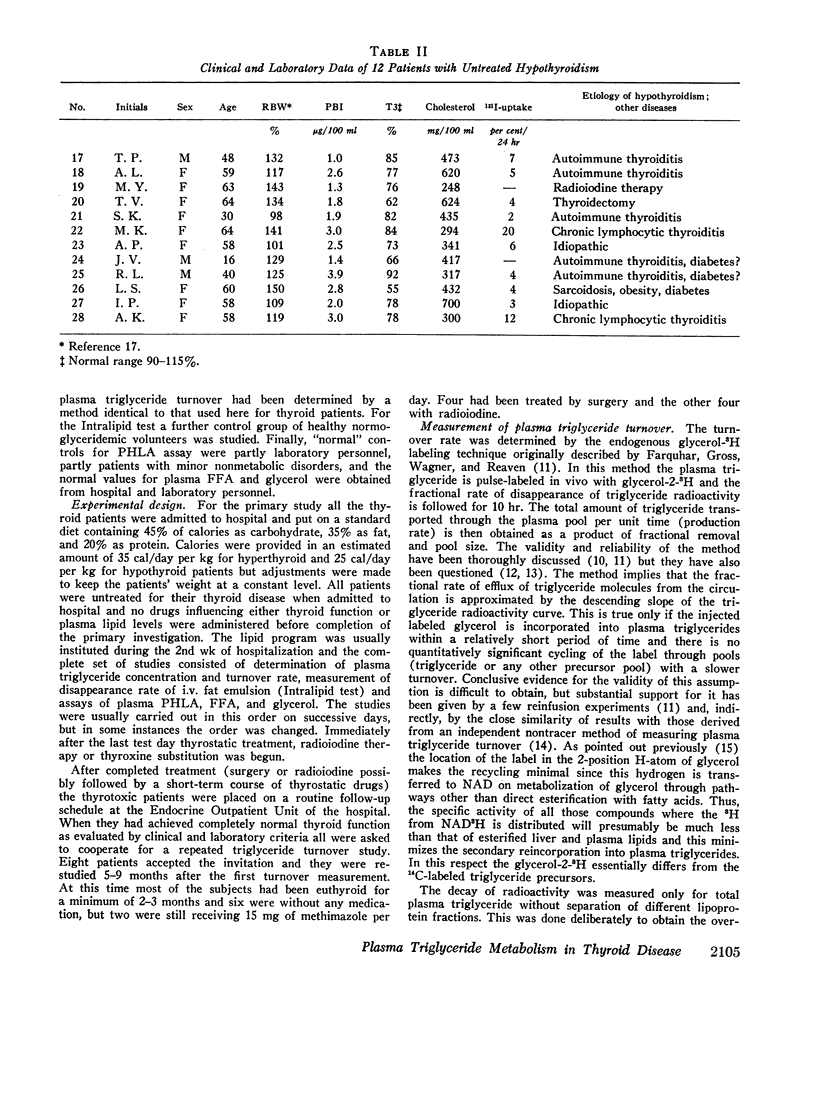
Plasma endogenous triglyceride transport kinetics were determined in 16 hyperthyroid and in 12 hypothyroid patients and the results compared with those of euthyroid control subjects. In addition, the removal of exogenous particulate fat (Intralipid; Vitrum, Sweden) from the circulation and the postheparin plasma lipolytic activity (PHLA) were studied in these patients for further characterization of the alterations of plasma triglyceride metabolism in thyroid disease.
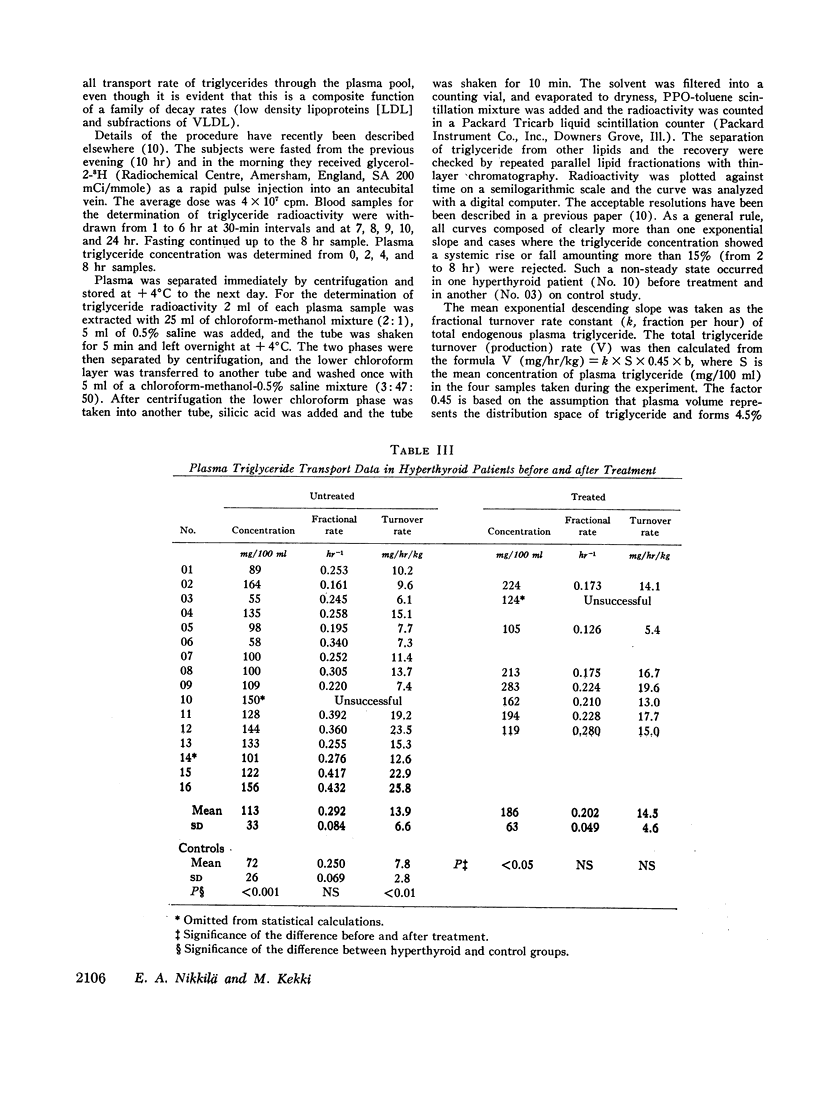
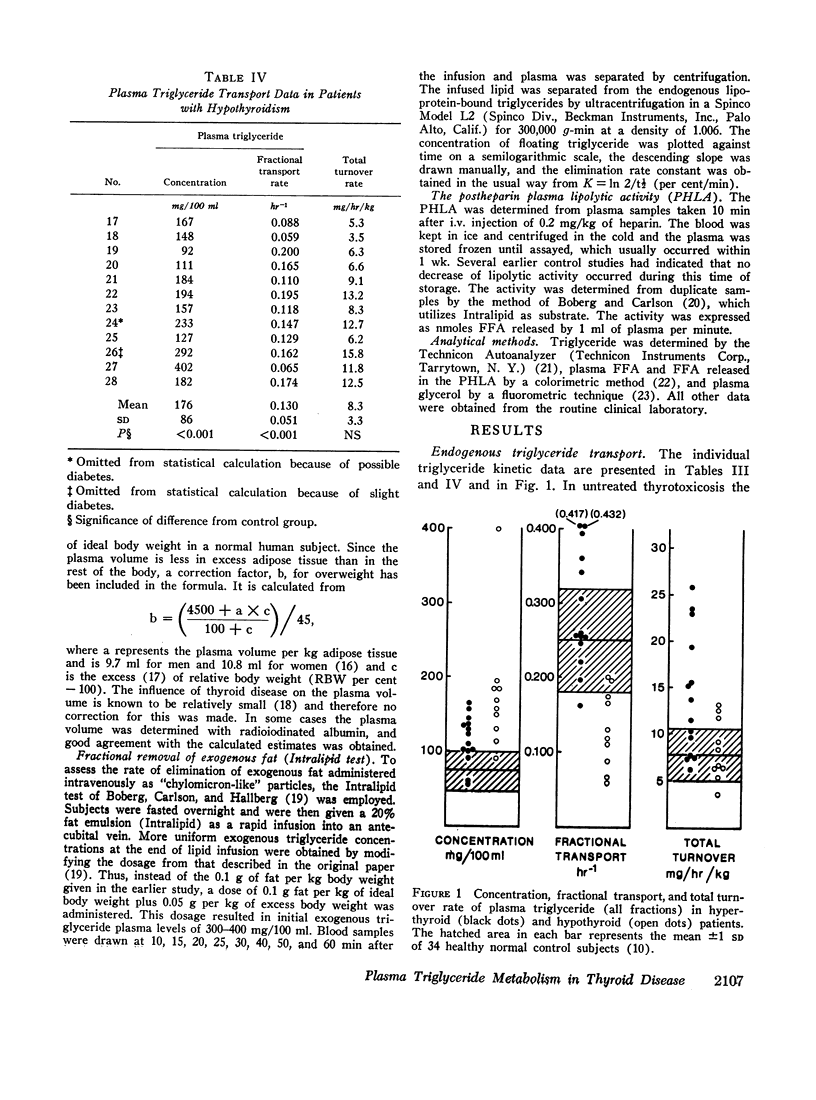
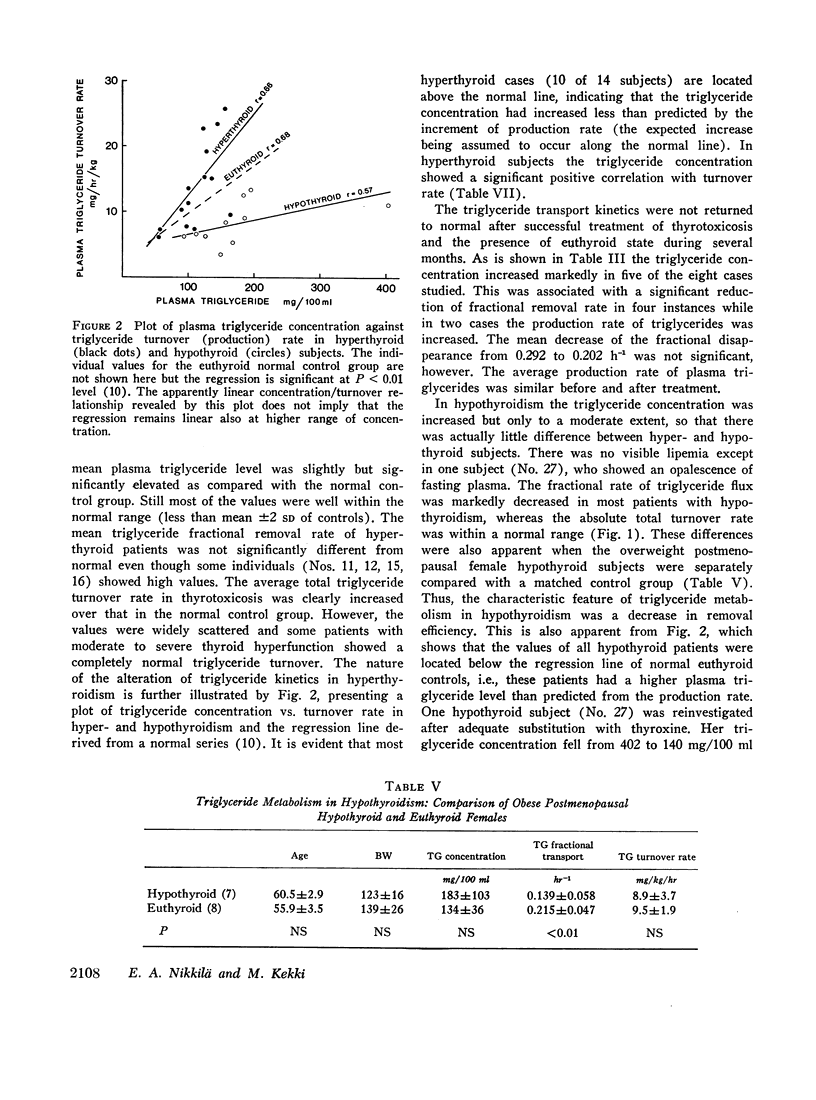
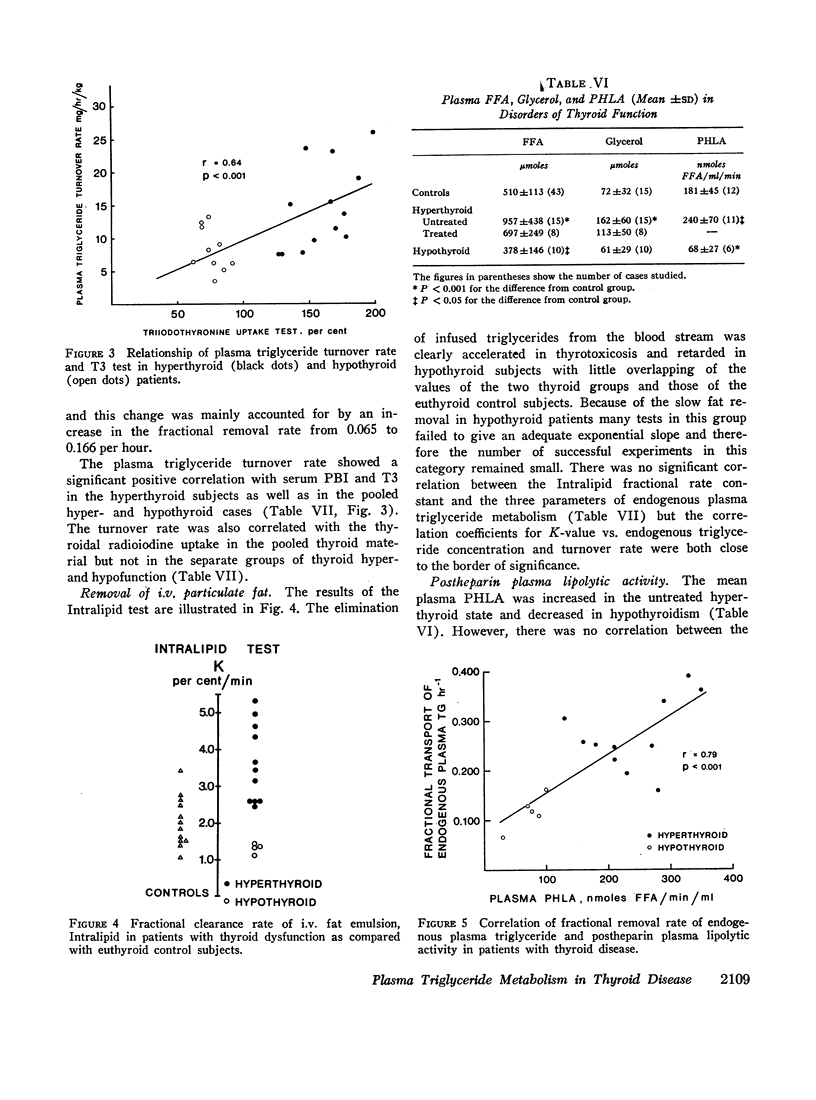
In thyrotoxicosis the average plasma triglyceride level was slightly but significantly increased above that of control subjects. This change was associated with augmented production of triglycerides whereas the mean fractional removal rate was not different from normal. There was a significant linear correlation between the concentration and turnover rate of plasma triglycerides in both hyperthyroid and euthyroid subjects but the concentration/turnover rate ratio was less in the former group suggesting that the efficiency of removal of triglycerides from the circulation was improved in thyroid hyperfunction. The elimination of intravenously administered particulate fat occurred more rapidly in untreated hyperthyroid patients than in euthyroid control subjects. The mean PHLA was also above normal in thyrotoxicosis. Upon adequate treatment of the hyperthyroid state the fasting plasma triglyceride concentration was further increased.
Hypothyroid patients showed another pattern of alteration of triglyceride kinetics. The synthesis of plasma triglycerides was normal but the fractional removal of both endogenous and exogenous triglycerides was markedly reduced and this change seems to account for the hypertriglyceridemia associated with thyroid hypofunction. The plasma PHLA was also clearly decreased in the hypothyroid state.
Plasma FFA and glycerol levels were increased in hyperthyroidism and plasma FFA was slightly decreased in hypothyroid patients, but these variables were not significantly correlated with any parameter of triglyceride metabolism.
Endogenous triglyceride turnover rate was significantly correlated with serum protein-bound iodine (PBI) and T3 uptake in thyrotoxicosis but not in hypothyroidism. Removal of exogenous fat was not related to postheparin plasma lipolytic activity but the fractional endogenous triglyceride transport showed a highly significant relationship to this lipase activity in a mixed group of hyper- and hypothyroid patients.
The results suggest that thyroid hormones control both production and removal of plasma triglycerides. Different mechanisms for these interactions are considered.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arons D. L., Schreibman P. H., Downs P., Braverman L. E., Arky R. A. Decreased post-heparin lipases in Graves's disease. N Engl J Med. 1972 Feb 3;286(5):233–237. doi: 10.1056/NEJM197202032860503. [DOI] [PubMed] [Google Scholar]
- BOBERG J., CARLSON L. A. DETERMINATION OF HEPARIN-INDUCED LIPOPROTEIN LIPASE ACTIVITY IN HUMAN PLASMA. Clin Chim Acta. 1964 Nov;10:420–427. doi: 10.1016/0009-8981(64)90171-8. [DOI] [PubMed] [Google Scholar]
- Boberg J., Carlson L. A., Hallberg D. Application of a new intravenous fat tolerance test in the study of hypertriglyceridaemia in man. J Atheroscler Res. 1969 Mar-Apr;9(2):159–169. doi: 10.1016/s0368-1319(69)80051-7. [DOI] [PubMed] [Google Scholar]
- DAYTON S., DAYTON J., DRIMMER F., KENDALL F. E. Rates of acetate turnover and lipid synthesis in normal, hypothyroid and hyperthyroid rats. Am J Physiol. 1960 Jul;199:71–76. doi: 10.1152/ajplegacy.1960.199.1.71. [DOI] [PubMed] [Google Scholar]
- DEYKIN D., VAUGHAN M. RELEASE OF FREE FATTY ACIDS BY ADIPOSE TISSUE FROM RATS TREATED WITH TRIIODOTHYRONINE OR PROPYLTHIOURACIL. J Lipid Res. 1963 Apr;4:200–203. [PubMed] [Google Scholar]
- Doar J. W., Stamp T. C., Wynn V., Audhya T. K. Effects of oral and intravenous glucose loading in thyrotoxicosis. Studies of plasma glucose, free fatty acid, plasma insulin and blood pyruvate levels. Diabetes. 1969 Sep;18(9):633–639. doi: 10.2337/diab.18.9.633. [DOI] [PubMed] [Google Scholar]
- EATON R. P., STEINBERG D., THOMPSON R. H. RELATIONSHIP BETWEEN FREE FATTY ACID TURNOVER AND TOTAL BODY OXYGEN CONSUMPTION IN THE EUTHYROID AND HYPERTHYROID STATES. J Clin Invest. 1965 Feb;44:247–260. doi: 10.1172/JCI105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELT V., SCHOVANEC B., BENES P., PLZAK F., VRBENSKY V. The effect of thyroid state, adrenaline and glucose on the release of free fatty acids form adipose tissue. Experientia. 1962 Aug 15;18:379–379. doi: 10.1007/BF02172262. [DOI] [PubMed] [Google Scholar]
- FURMAN R. H., HOWARD R. P., LAKSHMI K., NORCIA L. N. The serum lipids and lipoproteins in normal and hyperlipidemic subjects as determined by preparative ultracentrifugation. Effects of dietary and therapeutic measures. Changes induced by in vitro exposure of serum to sonic forces. Am J Clin Nutr. 1961 Jan-Feb;9:73–102. doi: 10.1093/ajcn/9.1.73. [DOI] [PubMed] [Google Scholar]
- Gibson J. G., Harris A. W. CLINICAL STUDIES OF THE BLOOD VOLUME. V. HYPERTHYROIDISM AND MYXEDEMA. J Clin Invest. 1939 Jan;18(1):59–65. doi: 10.1172/JCI101026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompertz D., Greenbaum A. L. The effects of thyroxine on the pattern of fatty acid synthesis in rat liver. Biochim Biophys Acta. 1966 Jun 1;116(3):441–459. doi: 10.1016/0005-2760(66)90114-7. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Bray G. A. Role of thyroid hormones in lipolysis. Am J Physiol. 1966 May;210(5):1053–1058. doi: 10.1152/ajplegacy.1966.210.5.1053. [DOI] [PubMed] [Google Scholar]
- HALES C. N., HYAMS D. E. PLASMA CONCENTRATIONS OF GLUCOSE, NON-ESTERIFIED FATTY ACID, AND INSULIN DURING ORAL GLUCOSE-TOLERANCE TESTS IN THYROTOXICOSIS. Lancet. 1964 Jul 11;2(7350):69–71. doi: 10.1016/s0140-6736(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekki M., Nikkilä E. A. Plasma triglyceride turnover during use of oral contraceptives. Metabolism. 1971 Sep;20(9):878–889. doi: 10.1016/0026-0495(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Kirkeby K. Post heparin plasma lipoprotein lipase activity in thyroid disease. Acta Endocrinol (Copenh) 1968 Dec;59(4):555–563. doi: 10.1530/acta.0.0590555. [DOI] [PubMed] [Google Scholar]
- Krishna G., Hynie S., Brodie B. B. Effects of thyroid hormones on adenyl cyclase in adipose tissue and on free fatty acid mobilization. Proc Natl Acad Sci U S A. 1968 Mar;59(3):884–889. doi: 10.1073/pnas.59.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberg B. A. Glucose metabolism in thyroid disease. Acta Med Scand. 1965 Sep;178(3):351–362. doi: 10.1111/j.0954-6820.1965.tb04279.x. [DOI] [PubMed] [Google Scholar]
- Laurell S., Tibbling G. An enzymatic fluorometric micromethod for the determination of glycerol. Clin Chim Acta. 1966 Mar;13(3):317–322. doi: 10.1016/0009-8981(66)90210-5. [DOI] [PubMed] [Google Scholar]
- MALMROS H., SWAHN B. Lipid metabolism in myxedema. Acta Med Scand. 1953;145(5):361–369. doi: 10.1111/j.0954-6820.1953.tb07030.x. [DOI] [PubMed] [Google Scholar]
- MARKS B. H., KIEM I., HILLS A. G. Endocrine influences on fat and carbohydrate metabolism in man. 1. Effect of hyperthyroidism on fasting serum nonesterified fatty acid concentration and on its response to glucose ingestion. Metabolism. 1960 Dec;9:1133–1138. [PubMed] [Google Scholar]
- MYERS J. D., BRANNON E. S., HOLLAND B. C. A correlative study of the cardiac output and the hepatic circulation in hyperthyroidism. J Clin Invest. 1950 Aug;29(8):1069–1077. doi: 10.1172/JCI102338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKKILA E. A., KONTTINEN A. Effect of physical activity on postprandial levels of fats in serum. Lancet. 1962 Jun 2;1(7240):1151–1154. doi: 10.1016/s0140-6736(62)92196-7. [DOI] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- Nikkilä E. A. Control of plasma and liver triglyceride kinetics by carbohydrate metabolism and insulin. Adv Lipid Res. 1969;7:63–134. doi: 10.1016/b978-0-12-024907-7.50009-9. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M. Measurement of plasma triglyceride turnover in the study of hyperglyceridemia. Scand J Clin Lab Invest. 1971 Apr;27(2):97–104. doi: 10.3109/00365517109080194. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A., Kekki M. Polymorphism of plasma triglyceride kinetics in normal human adult subjects. Acta Med Scand. 1971 Jul-Aug;190(1-2):49–59. doi: 10.1111/j.0954-6820.1971.tb07395.x. [DOI] [PubMed] [Google Scholar]
- O'Hara D. D., Porte D., Jr, Williams R. H. The effect of diet and thyroxin on plasma lipids in myxedema. Metabolism. 1966 Feb;15(2):123–134. doi: 10.1016/0026-0495(66)90033-3. [DOI] [PubMed] [Google Scholar]
- PETERS J. P., MAN E. B. The significance of serum cholesterol in thyroid disease. J Clin Invest. 1950 Jan;29(1):1–11. doi: 10.1172/JCI102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D., Jr, Bierman E. L. The effect of heparin infusion on plasma triglyceride in vivo and in vitro with a method for calculating triglyceride turnover. J Lab Clin Med. 1969 Apr;73(4):631–648. [PubMed] [Google Scholar]
- Porte D., Jr, O'Hara D. D., Williams R. H. The relation between postheparin lipolytic activity and plasma triglyceride in myxedema. Metabolism. 1966 Feb;15(2):107–113. doi: 10.1016/0026-0495(66)90031-x. [DOI] [PubMed] [Google Scholar]
- RICH C., BIERMAN E. L., SCHWARTZ I. L. Plasma nonesterified fatty acids in hyperthyroid states. J Clin Invest. 1959 Feb;38(2):275–278. doi: 10.1172/JCI103799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHSCHILD M. A., BAUMAN A., YALOW R. S., BERSON S. A. The effect of large doses of desiccated thyroid on the distribution and metabolism of albumin-I 131 in euthyroid subjects. J Clin Invest. 1957 Mar;36(3):422–428. doi: 10.1172/JCI103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKOLOFF L., FRANCIS C. M., CAMPBELL P. L. THYROXINE STIMULATION OF AMINO ACID INCORPORATION INTO PROTEIN INDEPENDENT OF ANY ACTION ON MESSENGER RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:728–736. doi: 10.1073/pnas.52.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhofer F., Sailer S., Braunsteiner H. Fettsäure- und Triglyceridumsatz bei Schilddrüsenüberfunktion. Klin Wochenschr. 1966 Dec 15;44(24):1389–1393. doi: 10.1007/BF01752479. [DOI] [PubMed] [Google Scholar]
- Sandhofer F., Sailer S., Braunsteiner H. Plasmalipide bei Störungen der Schilddrüsenfunktion des Menschen. Klin Wochenschr. 1966 Apr 15;44(8):433–436. doi: 10.1007/BF01727456. [DOI] [PubMed] [Google Scholar]
- Tibbling G. Glycerol turnover in hyperthyroidism. Clin Chim Acta. 1969 Apr;24(1):121–130. doi: 10.1016/0009-8981(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Walton K. W., Scott P. J., Dykes P. W., Davies J. W. The significance of alterations in serum lipids in thyroid dysfunction. II. Alterations of the metabolism and turnover of 131-I-low-density lipoproteins in hypothyroidism and thyrotoxicosis. Clin Sci. 1965 Oct;29(2):217–238. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. De novo synthesis of fatty acid in perfused rat liver as a determinant of plasma lipoprotein production. Arch Biochem Biophys. 1967 Nov;122(2):362–369. doi: 10.1016/0003-9861(67)90206-8. [DOI] [PubMed] [Google Scholar]



