Abstract
Purpose
To study the effects of increasing pelvic bone marrow (BM) radiation dose on acute hematologic toxicity in patients undergoing chemoradiotherapy, using a novel modeling approach to preserve the local spatial dose information.
Methods
The study includes 37 cervical cancer patients treated with concurrent weekly cisplatin and pelvic radiation therapy. The white blood cell count (WBC) nadir during treatment was used as the indicator of acute hematologic toxicity. Pelvic BM radiation dose distributions were standardized across patients by registering the pelvic BM volumes to a common template followed by dose remapping using deformable image registration, resulting in a dose array. Principal component analysis (PCA) was applied to the dose array and the significant eigenvectors were identified by linear regression on the principal components (PCs). The coefficients for PC regression and significant eigenvectors were represented in 3-D to identify critical BM subregions where dose accumulation is associated with hematologic toxicity.
Results
We identified five PCs associated with acute hematologic toxicity. PCA regression modeling explained a high proportion of the variation in acute hematologicity (adjusted R2 0.49). Three-dimensional rendering of a linear combination of the significant eigenvectors revealed patterns consistent with anatomical distributions of hematopoietically active BM.
Conclusions
We have developed a novel approach that preserves spatial dose information to model effects of radiation dose on toxicity, which may be useful in optimizing radiation techniques to avoid critical subregions of normal tissues. Further validation of this approach in a large cohort is ongoing.
Keywords: cervical cancer, hematologic toxicity, principal component analysis, chemoradiotherapy, deformable image registration
INTRODUCTION
Concurrent chemoradiotherapy is standard treatment for patients with locoregionally advanced pelvic cancers, including cervical and anal cancer (1–9). Compared to radiation therapy (RT) alone, chemoradiotherapy improves outcomes in both cervical (4–5) and anal (8–9) cancer. Moreover, randomized trials have found that intensifying chemotherapy regimens improves outcomes as well (6–7,10–11). High grade acute hematologic toxicity, however, is a common problem, occurring typically in 25–33% of patients treated with standard chemoradiotherapy (Table 1), and up to 60% of patients in some studies (6,10,12). This can lead to hospitalizations, treatment breaks, the need for growth factors and antibiotics, and occasionally serious infections and mortality. Importantly, hematologic toxicity limits patients’ tolerance to treatment, preventing optimal chemotherapy delivery, which in turn is associated with inferior clinical outcomes (4,15). Reducing hematologic toxicity is therefore an important strategy to improve the therapeutic ratio of chemoradiotherapy.
Table 1.
Acute hematologic toxicity of pelvic chemoradiotherapy
| Study | Disease Site | Chemotherapy | External Beam Radiation Dose (Gy) | N | Grade ≥ 3 Hematologic Toxicity (%) |
|---|---|---|---|---|---|
| Ajani (2008)6 * | Anal | 5FU, MMC | 45–59 | 324 | 61% |
| 5FU, CDDP | 320 | 42% | |||
| UKCCCR (1996)8 * | Anal | 5FU, MMC | 45 | 292 | 7% |
| None | 295 | 0% | |||
| Flam (1996)7 * | Anal | 5FU, MMC | 45–50.4 | 146 | 18%** |
| 5FU | 145 | 3%** | |||
| Bartelink (1997)9 * | Anal | 5FU, MMC | 45 | 51 | 4%** |
| None | 52 | 0%** | |||
| Salama (2007)12 | Anal | 5FU, MMC | 45–51.5 | 53 | 59% |
| Whitney (1999)2 * | Cervical | HU | 40–60 | 191 | 41% |
| 5FU, CDDP | 177 | 7% | |||
| Peters (2000)4 * | Cervical | CDDP | 49 | 122 | 38% |
| None | 112 | 3% | |||
| Rose (1999)1 * | Cervical | CDDP | 40.8–51.0 | 176 | 23% |
| CDDP, 5FU, HU | 173 | 48% | |||
| HU | 177 | 23% | |||
| Pearcey (2002)13 * | Cervical | CDDP | 45 | 127 | 5% |
| None | 126 | 0% | |||
| Keys (1999)5 * | Cervical | CDDP | 45 | 183 | 21% |
| None | 186 | 2% | |||
| Torres (2008)14 | Cervical | CDDP, 5FU | 45 | 76 | 24% |
| CDDP, 5FU | 45 | 115 | 41% | ||
| CDDP | 45 | 111 | 23% | ||
Randomized controlled trial;
Grade ≥ 4 toxicity reported only; 5FU, 5-Fluorouracil; MMC, mitomycin-C; HU, hydroxyurea; CDDP, cisplatin
Both radiation and chemotherapy are myelosuppressive, but the extent to which radiation contributes to hematologic toxicity in patients undergoing chemoradiotherapy is unknown. Radiation causes apoptosis of bone marrow (BM) stem cells and BM stromal damage, resulting in myelosuppression and characteristic pathologic and radiographic BM changes (16–18). Chemotherapy suppresses compensatory hematopoiesis in unirradiated BM, leading to higher rates of hematologic toxicity than sequential chemotherapy and RT, or either modality given alone (16). Clinical studies have shown that the extent of radiation-induced BM injury depends on both radiation dose and volume of BM irradiated (19). Our previous studies have found that acute hematologic toxicity in patients undergoing chemoradiotherapy depends on the volume of pelvic BM receiving greater than 10–20 Gy (20,21), suggesting that techniques designed to limit BM irradiation could reduce hematologic toxicity.
Intensity modulated RT (IMRT) is a modern radiation technique that uses multiple beam angles and inverse treatment planning to optimize normal tissue sparing while maintaining target coverage. With IMRT, targets and normal tissues are delineated, then desired dose-volume constraints for each structure are established a priori. Computerized algorithms identify patterns of intensity that optimize conformality of the prescription dose to the target, while sparing normal tissues. IMRT is typically delivered using multileaf collimators, which consist of individual motorized leaves that move in and out of the beam’s path, modulating the beam’s intensity (22). Multiple studies have shown that IMRT plans can reduce dose to normal tissue for any given level of target coverage (23–29). IMRT plans can reduce the volume of BM receiving 20 Gy or more (V20) (24), but the extent of BM sparing is constrained by difficulties in avoiding the large BM volume. Reducing the BM volume required for sparing, by focusing on key subregions, could facilitate IMRT planning optimization.
It is well-known that adult BM is comprised of hematopoietically active, “red” marrow and inactive “yellow” marrow (30). Magnetic resonance imaging (MRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT) have revealed that red BM tends to be concentrated in specific subregions in the pelvis, namely the vertebrae and ilium (31–33). The large volume of active BM irradiated with pelvic RT likely contributes significantly to acute hematologic toxicity. Conventional pelvic RT fields encompass up to 50% of the body’s active BM, which lies within the pelvis and lower spine (34). However, the effect of decreasing active BM radiation dose on hematologic toxicity is presently unknown.
A major factor hampering the development of BM-sparing IMRT is the lack of an adequate model of acute hematologic toxicity as a function of radiation dose, i.e. normal tissue complication probability (NTCP) model. Current NTCP models of radiation effects on pelvic BM (20,21,35) are based on summary metrics derived from BM dose-volume histograms, which fail to account for the spatial radiation dose distribution within BM. We hypothesized that the development of acute hematologic toxicity is correlated with radiation dose received by specific pelvic BM subregions, and sought to develop a model that preserves the spatial BM dose distribution. This approach could identify BM subregions in which dose accumulation is important for predicting hematologic toxicity, better guiding optimization of BM-sparing IMRT techniques.
MATERIALS AND METHODS
Patient and Treatment Characteristics
This study was approved by the University California San Diego (UCSD) Institutional Review Board. Eligible patients had biopsy-proven clinical stage I-IVA or recurrent cervical carcinoma, and no prior history of chemotherapy or pelvic irradiation. Patients treated with extended field (para-aortic) RT were ineligible. All patients underwent weekly concurrent cisplatin (40 mg/m2) and external beam pelvic RT, followed by intracavitary brachytherapy (in patients treated definitively). The sample includes 37 patients treated at UCSD between October 2006 and October 2008 (Table 2).
Table 2.
Patient and tumor characteristics
| Patients, n | 37 |
| Mean age, years (std) | 49.2 (11.9) |
| Race, n (%) | |
| Hispanic | 23 (62) |
| White | 9 (24) |
| Other | 5 (14) |
| Mean body mass index, kg/m2 (std) | 28.4 (7.2) |
| Clinical Stage, n (%) | |
| IA2 | 1 (3) |
| IB | 2 (5) |
| IB1 | 5 (14) |
| IB2 | 4 (11) |
| IIA | 4 (11) |
| IIB | 15 (41) |
| IIIB | 4 (11) |
| Recurrent | 1 (3) |
| Unknown | 1 (3) |
std: standard deviation
The median external beam RT dose to the planning target volume was 45.0 Gy in 1.8 Gy daily fractions (range:45.0–50.4 Gy). Six patients received 50.4 Gy and 31 received 45.0 Gy. Thirty-two patients were treated exclusively with IMRT, 1 patient was treated exclusively with the four-field box technique, and 4 patients were treated initially with four-field box technique for 2–4 fractions before completing treatment with IMRT. Following external beam RT, brachytherapy was delivered using high dose rate technique in 5 fractions of 5.5–6.0 Gy per fraction.
All patients had complete blood counts (CBC) with differentials weekly during and immediately following chemoradiotherapy. The measure of acute hematologic toxicity was the white blood cell count (WBC) nadir, defined as the lowest value occurring between the start of and 2 weeks following conclusion of external beam RT. In regression modeling, the WBC nadir was natural log–transformed to eliminate skew.
Image and Dose Registration
For each patient, the pelvic bone volume was first defined by delineating the external contour of all pelvic bones (os coxae, lower lumbar vertebrae, sacrum, acetabulae, and proximal femora) on the simulation computed tomography (CT) scan, as described previously (20). In order to identify the important BM subregions, the local radiation doses at each voxel of the pelvic BM was considered a predictor variable in the NTCP model. To compare local dose among patients with different body sizes, prior to modeling, BM radiation doses of the patients had to be standardized, so that they could be combined in the same data set. The standardization was achieved by deformable image registration of the pelvic BM volume of each patient to a template pelvic BM, followed by dose remapping based on the displacement vector field, which are the results of image registration (Figure 1).
Figure 1.
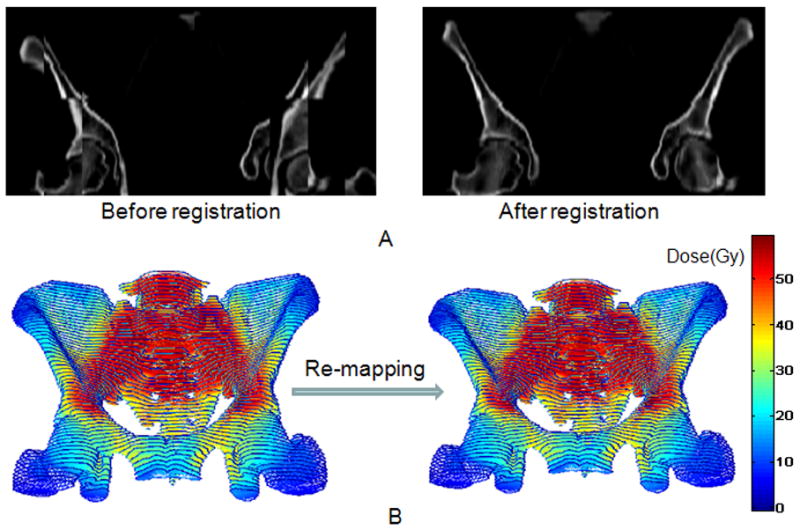
(A) Coronal “checkerboard” image of a patient’s pelvic bone blended with the template before (left) and after (right) registration. (B) Frontal view of the actual radiation dose distribution of the pelvic bone of the same patient (left) and the remapped dose distribution after deformation (right).
A patient with an intermediate sized pelvic volume was chosen as the template, and the remaining patients’ pelvic bone volumes were registered to this template. Considering the registration artifacts caused by internal organ and soft tissue inside the pelvis, the CT images were thresholded during pre-processing. The threshold value was set to 100 HU for all patients, so that both bony structure and textural information within the bone would be preserved, while the information from surrounding soft tissues was blacked out. In some patients, rigid image registration was necessary before deformable registration, because of large shifts in the relative position of their bone volume with respect to the template.
The registration of pelvic BM to the template was performed using the optical flow based deformable image registration method developed by Yang et al. (36). The output of the registration, the deformation field, was used to remap the dose distribution back to the deformed pelvic BM through interpolation. The radiation dose on each voxel of the deformed pelvic BM images was linked to its original dose based on the displacement field, the output of the registration. After deformation, the voxel in the deformed image grid will not exactly lie on the integer grid of the original image. Therefore, the dose was then interpolated in 3D from the involved original voxels.
Principal Component Analysis of the Dose Array
Dose in each pelvic bone voxel represents the underlying data in our statistical model. This is a high dimensional dataset with a large number of spatially correlated variables, and principal component analysis (PCA) was used to summarize the major modes of spatial variation in dose distribution across patients. Given a data set with a large number of correlated variables, PCA is a technique used to reduce the dimensionality of the data set, while retaining the maximum variation in the data. The dimensionality is reduced by constructing a new set of uncorrelated explanatory variables from a linear combination of the original variables (37). PCA has been used previously for NTCP modeling in radiation oncology (38–40). However, in those studies, PCA was applied to dose volume histograms (DVHs). By applying PCA to the original spatially located data, we are able to preserve and summarize the spatial distribution of the radiation dose, which we hypothesized to play a significant role in determining toxicity.
In order to apply PCA, we first align the data into a dose array D (Figure 2). Each patient’s 3-D pelvic BM dose distribution was sampled from left to right, anterior to posterior, and from superior to inferior. The CT slice thickness is 2.5 mm, and the voxel size of the dose array was set as 2.9 × 2.9 × 2.5 mm3, resulting in 44146 variables. Sampled values were concatenated to form a row vector for each patient, corresponding to one row of D. Note that the position of each element in the row vector corresponds to a voxel at a specific 3-D location, preserving spatial information. Thus each column of D represents the dose received in an anatomically registered pelvic BM site. D has dimensions N × K, here 37 × 44146, and is shown in Figure 2, with patients listed in descending order of WBC nadir values, given to the right of the dose array. The variation in dose between the patients at each pelvic BM locus is caused by variation in physician target delineation, prescription dose, planning approach, and patients’ pelvic shape, and not to patients’ propensity to develop hematologic toxicity.
Figure 2.

The dose array with dimensions 37×44146. The standardized patient’s 3-D pelvic BM dose was sampled from left to right, from anterior to posterior, and from superior to inferior; and then sampled voxels were concatenated to form a row vector which corresponds to one row of the 2-D dose array. Each column represents the dose received in an anatomically registered pelvic BM site.
PCA was performed on the dose-array (N × K) using Matlab (R2008b, The MathWorks, Inc., Natick, MA). Since all of the elements in the array are measured on the same scale (Gy), we applied PCA to the covariance matrix (rather than the correlation matrix). This approach is favored (41) and preserves the variation across patients within each voxel, which may contain information regarding regions related to acute hematologic toxicity. From the PCA we obtained a K × N matrix E of standardized eigenvectors corresponding to the non-zero eigenvalues of the sample covariance matrix, arranged in descending order of their eigenvalues e. Note that because the number of subjects N is much less than K, there will be at most N independent eigenvectors. We refer to the jth column E(j) of E as the jth “eigendose.” The principal components (PC’s) are then the columns of the N × N matrix Y= D E, where the ith row of Y corresponds to the ith patient as before, with jth element giving the score of that patient on the jth eigendose. Note that the original data vector for the ith patient is given in terms of the eigendoses and principal components by the linear combination . The variance of the jth PC is the jth eigenvalue ej (37).
Principal Component Regression
The next step was to identify those PC’s with the greatest correlation with log(WBC nadir) using PC regression. In this study, the function of PC regression is two-fold. One is to identify the eigendose(s) significantly correlated with hematologic toxicity, with 3-D rendering to locate critical BM regions related to hematologic toxicity. The other is NTCP modeling, in order to predict the effects of radiation dose on hematologic toxicity. The eigendose(s) significantly related to hematologic toxicity defined the “dose space” for NTCP modeling and were subsequently analyzed to determine the characteristic dose distributions related to hematologic toxicity. We applied linear regression of the log (WBC nadir), using the set of PC’s as the predictor variables, to select the significant PCs to retain in the model. Statistical significance was determined at the 10% level. Note that the PCs with the largest eigenvalues, which account for the greatest patient to patient variation, will not necessarily be those with the greatest correlation with toxicity, as PCs with small eigenvalues may be narrowly targeted to important regions (37). JMP 8 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
RESULTS
Principal Component Analysis on the Dose Array
The output of PCA on the dose array resulted in a set of 36 nonzero eigenvalues with corresponding eigenvectors (eigendoses). Figure 3 is a scree plot representing the variation in the dose array that is carried by each PC. The first three eigendoses, represented in Figure 4, summarize the three largest modes of variation in the dose distribution and intensity across patients. The patterns indicate that the major directions of variation are anterior/posterior (eigendose 1), followed by superior/inferior (eigendose 2), and central/peripheral (eigendose 3).
Figure 3.

Scree plot indicating the percentage of the variation in the dose array explained by each principal component.
Figure 4.

First three eigenvectors of the covariance matrix rendered in 3-D, showing the major modes of variation in the dose array.
Principal Component Regression
Linear regression identified 5 PC’s (12th,23rd,24th,25th, and 31st) that were significantly correlated with log(WBC nadir) (Table 3). The percentages of total variation in the original data carried by these 5 PC’s were 1.9%, 0.7%, 0.6%, 0.6%, 0.4%, respectively (total:4.2%). Multivariate regression of log(WBC nadir) on these 5 PC’s had an R2 of 0.56, indicating that collectively these account for 56% of the variation in WBC nadir. The adjusted R2 of the model is 0.49.
Table 3.
Results of principal components regression
| β | e | 95% CI | p | |
|---|---|---|---|---|
| Intercept | 0.766 | 0.680, 0.851 | <0.0001 | |
| PC 12 | 7.46e-4 | 0.019 | 2.3-4, 12.62e-4 | 0.007 |
| PC 23 | 11.79e-4 | 0.007 | 3.65e-4, 19.93e-4 | 0.007 |
| PC 24 | −10.38e-4 | 0.006 | −19.16e-4, −1.60e-4 | 0.025 |
| PC 25 | 14.12e-4 | 0.006 | 5.20e-4, 23.04e-4 | 0.003 |
| PC 31 | −13.82-4 | 0.004 | −24.54e-4, −3.1e-4 | 0.015 |
CI: confidence interval; PC: principal component; e: eigenvalue (variance of PC)
Correlation between Spatial Variation in Dose and Hematologic Toxicity
The coefficient estimates corresponding to each eigendose served as weighting factors of the radiation dose at each pelvic BM subregion. To predict a new patient’s toxicity outcome, we apply the estimates from the regression model to the patient’s dose vector, expressed as:
| (1) |
where y is the predicted log(WBC nadir), b̂v̂ and β̂ are the intercept and the vector of regression coefficients estimated from PC regression (Table 3), is the matrix of significant eigendoses, d is the patient’s dose vector (after registration to the template and dose remapping), and v̂ represents the weighted (by the coefficients) sum of the significant eigendoses.
In order to see how well the newly found “dose space” predicts the propensity of a patient to develop acute hematologic toxicity, we divided patients into two groups: no acute hematologic toxicity, defined as WBC nadir ≥ 2000/μL (n=23), and acute hematologic toxicity, defined as WBC nadir < 2000/μL (n=14). We compared the difference in BM radiation dose between the two groups with and without acute hematologic toxicity, by directly subtracting the average dose of the latter from the former (Figure 5A). To visualize key BM subregions related to hematologic toxicity, as revealed by PC regression, we plotted v̂ (Fig 5B). The pattern appears consistent with the dose difference pattern shown in Figure 5A, indicating that dose accumulation in the posterolateral sacrum, medial ilium, and iliac crest is associated with a higher likelihood of developing acute hematologic toxicity. These regions are known to be rich in active BM (30,34), supporting the hypothesis that increased radiation dose to active BM increases hematologic toxicity.
Figure 5.
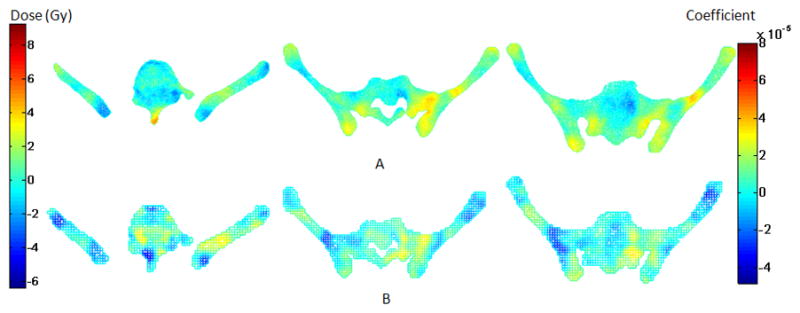
Renderings of pelvic BM dose related information in the axial plane at 3 levels. Row (A) displays the average dose difference between patients with and without acute HT (WBC nadir < 2000/μL). Row (B) displays the coefficients based on the regression model that are significantly correlated with acute HT.
DISCUSSION
Acute hematologic toxicity is a common problem with pelvic chemoradiotherapy that limits treatment intensity (4,14,15,20,42). Evidence is growing that increased pelvic BM radiation dose exacerbates toxicity (20,21,43), suggesting that techniques designed to limit BM irradiation could permit more intensive treatment and improve outcomes. Currently, development of effective BM-sparing pelvic RT techniques is limited by the lack of two key pieces of knowledge: (1) the spatial location of critical BM subregions to be spared, and (2) the degree of sparing necessary to achieve clinically significant reductions in toxicity. Previous attempts at NTCP modeling have addressed the latter question by considering summary metrics from dose-volume histograms, an approach that discards the spatial dose information. Here we have described a novel approach to NTCP modeling that preserves spatial dose information, lending insight into both the effects of radiation on BM and the location of critical BM subregions common among patients.
In order to demonstrate that specific BM subregions are “active”, ideally one would ablate (or spare) selected subregions and observe the effects on toxicity. Conformal radiation techniques like IMRT offer unprecedented ability to alter and optimize dose distributions in patients. For most diseases, however, what constitutes an “optimal” dose distribution, in the sense of redistributing dose to achieve a specific set of aims, remains to be defined. The increasing use of multi-field IMRT techniques has resulted in natural variations in BM radiation dose distributions, due to differences in treatment planning and patient anatomy. By correlating toxicity with variations in dose distributions in these patients, we are better able to identify critical subregions and rationally design BM-sparing pelvic RT techniques, prior to testing them in clinical trials.
Current efforts to optimize BM-sparing pelvic RT are additionally focusing on the role of quantitative functional imaging in identifying hematopoietically active BM subregions. Previously, Roeske et al. explored the feasibility of delineating active BM using SPECT BM imaging (33) or qualitative T1-weighted MRI imaging (31) during IMRT planning. Recently, we have applied a novel MRI technique called Iterative Decomposition of water and fat Echo Asymmetry and Least-square Estimation (44) to quantify BM fat fraction, as a way to differentiate less active, fat-rich subregions from more active, fat-poor ones (45). In this study, we observed a resemblance between the patterns seen on subtraction images and eigendoses identified as significantly correlated with WBC nadir. Both appear consistent with anatomical distributions of hematopoietically active BM from imaging studies described above (31,33,45). Combining novel analytic approaches and quantitative imaging technologies such as PET, fat fraction MRI, and SPECT will hopefully provide greater understanding into how to optimally design pelvic IMRT treatments. Ideally, this strategy could be applied to predict toxicity and optimize conformal radiation techniques in other disease sites as well.
The current study has some limitations. We selected significant principal components from the regression model, which was developed to fit the data. Future work to optimize and validate this approach in an independent cohort is needed. Furthermore, how to determine optimal thresholds to define “critical” BM subregions, using either quantitative imaging or regression coefficients, is unclear. Although these quantities are given on a continuous scale, a binary decision as to what constitutes the avoidance volume is ultimately necessary in currently available techniques for IMRT planning. The utility of this modeling approach and its ultimate impact on patient outcomes, therefore, needs to be studied further.
Nevertheless, our findings indicate significant potential in this approach for identifying critical subregions of a heterogeneously functioning organ system. This method could be useful in examining radiation effects in other organs such as brain, lung, or liver. Evaluating the benefits of conformal radiation techniques requires detailed understanding of effects on normal tissue complications. NTCP models that harness the information embedded within the spatial dose distribution represent an exciting and potentially useful innovation to guide RT planning.
Acknowledgments
This research is supported by the American Society of Clinical Oncology and grants L30-CA135746-01 and T32-RR023254 from the National Institute of Health. We also would like to thank Dr. Deshan Yang from the Department of Radiation Oncology at Washington University, St. Louis for allowing us to use his deformable image registration software.
Footnotes
CONFLICT OF INTEREST NOTIFICATION
No authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group Study. Journal of Clinical Oncology. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 3.Morris M, Eifel PJ, Lu JD, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. New England Journal of Medicine. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 4.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of Clinical Oncology. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 5.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal - A randomized controlled trial. Jama-Journal of the American Medical Association. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 7.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. Journal of Clinical Oncology. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 8.Epidermoid anal cancer: Results from the UKCCCR randomized trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 9.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 10.Dueñas-Gonzalez A, Cetina-Perez L, Lopez-Graniel C, et al. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer: A randomized phase II study. International Journal of Radiation Oncology Biology Physics. 2005;61:817–823. doi: 10.1016/j.ijrobp.2004.07.676. [DOI] [PubMed] [Google Scholar]
- 11.Dueñas-González A, Zarba JJ, Alcedo JC, et al. A phase III study comparing concurrent gemcitabine (Gem) plus cisplatin (Cis) and radiation followed by adjuvant Gem plus Cis versus concurrent Cis and radiation in patients with stage IIB to IVA carcinoma of the cervix. Journal of Clinical Oncology. 2009:CRA5507. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 12.Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25:4581–6. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 13.Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966–72. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]
- 14.Torres MA, Jhingran A, Thames HD, et al. Comparison of treatment tolerance and outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy in a prospective randomized trial or with standard treatment. International Journal of Radiation Oncology Biology Physics. 2008;70:118–125. doi: 10.1016/j.ijrobp.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Parker K, Gallop-Evans E, Hanna L, et al. Five years’ experience treating locally advanced cervicle cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: results from a single institution. International Journal of Radiation Oncology Biology Physics. 2009;74:140–146. doi: 10.1016/j.ijrobp.2008.06.1920. [DOI] [PubMed] [Google Scholar]
- 16.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Pergamon-Elsevier Science Ltd; 1995. pp. 1319–1339. [DOI] [PubMed] [Google Scholar]
- 17.Blomlie V, Rofstad EK, Skjonsberg A, et al. Female pelvic bone marrow: serial MR Imaging before, during, and after radiation therapy. Radiology. 1995;194:537–543. doi: 10.1148/radiology.194.2.7824737. [DOI] [PubMed] [Google Scholar]
- 18.Fajardo LF, Berthrong M, Anderson RE. Hematopoietic tissue. In: FLF, Anderson MBRE, editors. Radiation pathology. Oxford: Oxford University; 2001. pp. 379–388. [Google Scholar]
- 19.Rubin P, Landman S, Mayer E, et al. Bone marrow regeneration and extension after extended field irradiation in Hodgkins disease. Cancer. 1973;32:699–711. doi: 10.1002/1097-0142(197309)32:3<699::aid-cncr2820320324>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. International Journal of Radiation Oncology Biology Physics. 2006:1356–1365. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. International Journal of Radiation Oncology Biology Physics. 2008:1431–1437. doi: 10.1016/j.ijrobp.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 22.Xia P, Amols HI, Ling CC. Three-dimensional conformal radiotherapy and intensity-modulated radiotherapy. In: Leibel SA, Phillips TL, editors. Textbook of Radiation Oncology. Philadelphia: Elsevier; 2004. [Google Scholar]
- 23.Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiotherapy and Oncology. 2003;69:201–207. doi: 10.1016/j.radonc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Mell LK, Tiryaki H, Ahn KH, et al. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. International Journal of Radiation Oncology Biology Physics. 2008;71:1504–1510. doi: 10.1016/j.ijrobp.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Kestin LL, Sharpe MB, Frazier RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: Initial clinical experience. International Journal of Radiation Oncology Biology Physics. 2000;48:1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 26.Burman C, Chui CS, Kutcher G, et al. Planning, delivery, and quality assurance of intensity-modulated radiotherapy using dynamic multileaf collimator: A strategy for large-scale implementation for the treatment of carcinoma of the prostate. International Journal of Radiation Oncology Biology Physics. 1997;39:863–873. doi: 10.1016/s0360-3016(97)00458-6. [DOI] [PubMed] [Google Scholar]
- 27.Ling CC, Burman C, Chui CS, et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. International Journal of Radiation Oncology Biology Physics. 1996;35:721–730. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 28.Sultanem K, Shu HK, Xia P, et al. The University of California-San Francisco experience. Elsevier Science Inc; 2000. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma; pp. 711–722. [DOI] [PubMed] [Google Scholar]
- 29.Lujan AE, Mundt AJ, Yamada SD, et al. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Elsevier Science Inc; 2003. pp. 516–521. [DOI] [PubMed] [Google Scholar]
- 30.Vogler JB, Murphy WA. Bone marrow imaging. Radiology. 1988;168:679–693. doi: 10.1148/radiology.168.3.3043546. [DOI] [PubMed] [Google Scholar]
- 31.Roeske JC, Mundt AJ. Incorporation of magnetic resonance imaging into intensity modulated whole-pelvic radiation therapy treatment planning to reduce the volume of pelvic bone marrow irradiated. International Congress Series. 2004;1268:307–312. [Google Scholar]
- 32.Basu S, Houseni M, Bural G, et al. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography: A novel application with significant implications for combined structure-function approach. Molecular Imaging and Biology. 2007;9:361–365. doi: 10.1007/s11307-007-0112-5. [DOI] [PubMed] [Google Scholar]
- 33.Roeske JC, Lujan A, Reba RC, et al. Incorporation of SPECT bone marrow imaging into intensity modulated whole-pelvic radiation therapy treatment planning for gynecologic malignancies. Radiotherapy and Oncology. 2005;77:11–17. doi: 10.1016/j.radonc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Ellis RE. Distribution of active bone marrow in adult. Physics in Medicine and Biology. 1961;5:255. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 35.Mutyala S, Thawani N, Vainshtein JM, et al. Dose constraint recommendations and a predictive nomogram of incidence of hematological toxicity for cervix cancer patients treated with concurrent cisplatin and intensity modulated radiation therapy (IMRT) Elsevier Science Inc; 2008. pp. S359–S360. [Google Scholar]
- 36.Yang D, Li H, Low DA, et al. A fast inverse consistent deformable image registration method based on symmetric optical flow computation. Physics in Medicine and Biology. 2008;53:6143–6165. doi: 10.1088/0031-9155/53/21/017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolliffe IT. Principal Component Analysis. 2. Springer; 2002. [Google Scholar]
- 38.Skala M, Rosewall T, Dawson L, et al. Patient-assessed late toxicity rates and principal component analysis after image-guided radiation therapy for prostate cancer. Elsevier Science Inc; 2007. pp. 690–698. [DOI] [PubMed] [Google Scholar]
- 39.Sohn M, Alber M, Yan D. Principal component analysis-based pattern analysis of dose-volume histograms and influence on rectal toxicity. Elsevier Science Inc; 2007. pp. 230–239. [DOI] [PubMed] [Google Scholar]
- 40.Dawson LA, Biersack M, Lockwood G, et al. Use of principal component analysis to evaluate the partial organ tolerance of normal tissues to radiation. International Journal of Radiation Oncology Biology Physics. 2005:829–837. doi: 10.1016/j.ijrobp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Borgognonea MG, Bussia J, Hough G. Principal component analysis in sensory analysis: covariance or correlation matrix? Food Quality and Preference. 2001;12:323–6. [Google Scholar]
- 42.Green J, Kirwan J, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005:CD002225. doi: 10.1002/14651858.CD002225.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose BS, Aydogan B, Yeginer M. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. International Journal of Radiation Oncology Biology Physics. 2009 doi: 10.1016/j.ijrobp.2009.11.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeder SB, Pineda AR, Wen Z, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636–44. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 45.Mell LK, Liang Y, Bydder M, et al. Functional MRI-guided bone marrow-sparing intensity modulated radiotherapy for pelvic malignancies (abstr) International Journal of Radiation Oncology Biology Physics. 2009;75:S121. doi: 10.1016/j.ijrobp.2012.04.044. [DOI] [PubMed] [Google Scholar]


