Abstract
Glioblastoma multiforme is an extremely aggressive and clinically unresponsive form of cancer. Transformed neoplastic neural stem cells, resistant to chemo and radiation therapy, are thought to be responsible for the initial tumor formation and the recurrence of disease following surgical resection. These stem cells express multidrug resistance markers along with CD133. We show, that ectopic overexpression of CD133 in rat C6 glioma cells, leads to significant reluctance to undergo apoptosis from camptothecin and doxorubicin. Although p53 was upregulated in CD133 overexpressing glioma cells treated with DNA damaging agents, apoptosis appears to be p53-independent. At least one ABC transporter, rat P-glycoprotein/ ABCB1, was up regulated by 62% in CD133+ cells with a corresponding increase in activity. Thus, the combination of higher P-glycoprotein mRNA transcription and elevated transporter activity, appears to contribute to the protection from cytotoxic reagents. In conclusion, previous investigators have reported that resilient cancer stem cells co-express CD133 and ABC transporters with increased reluctance toward apoptosis. Our data suggest that CD133 may contribute to the observed resistance to apoptosis of CD133+ cancer stem cells.
Keywords: CD133, C6, Glioma, P-glycoprotein, ABCB1
Introduction
Each year brain tumors occur in 21,810 individuals with astrocyte derived glioma being the most prevalent. Among the four grades of gliomas, glioblastoma multiforme (GBM), WHO classification Grade IV, is the most malignant and the least amenable to therapeutic intervention. GBMs regrow after surgical resection and contain a population of tumor cells resistant to radiation and chemotherapies. Recent advances in neural stem cell research have found that brain tumors have a subpopulation of cancer stem cells, also called tumor initiating cells, that contribute to radiation and chemotherapy resistance (1-23). Ignatova et al. (24) provided the first evidence for the existence of stem cells in human glioblastoma multiforme. These authors isolated clonogenic, neurosphere forming precursor cells which expressed neuronal and astroglial markers upon differentiation. These cells also had altered expression of Delta, Jagged and Survivin relative to normal neural stem cells (NSC) located in subventricular zone and Dentate gyrus of the hippocampus. The NSC rich subventricular zone, is suspected as the most probable origin of gliomas, with tumors arising following exposure to oncogenic viruses or carcinogens (as reviewed by Sanai et al., (12)). As defined by Vescovi et al. (5) to qualify as a brain tumor stem cell the following criteria should be met. 1) Orthotopic implantation results in a phenocopy of the original tumor. 2) Brain tumor stem cells have extensive self-renewal properties. 3) Cells have evidence of genetic or karotypical alterations. 4) Altered differentiation potential. 5) Ability to generate non-tumorigenic end cells. 6) Capacity to, but not necessarily retain the ability of multilineage differentiation. Cancer stem cells share many of the properties of their non-neoplastic counter parts (12). Both are self-renewable, drug resistant, and in culture form spherical colonies. (6, 10, 25-29). Drug resistance is often associated with elevated expression and activity of ABC transporters; identifiable markers for both normal neural and cancer stem cells (5, 30). An additional marker for both types of stem cells is the cell surface protein CD133 (8-10, 14, 31-33). Singh et al. (2004) used the membrane topological location of CD133 to separate CD133+ glioblastoma brain tumor stem cells from CD133− cells (9). On transplant CD133+ cells developed into xenographic tumors, but CD133− cells did not (9) . CD133 positive stem cells have also been found in colorectal cancer, breast cancer, hepatocarcinomas, melanomas, and osteosarcomas (3, 6, 9, 13, 31, 34-37). Thus, CD133 could be one of the most successful biomarkers characterizing cancer stem cells.
CD133/prominin-1 was originally discovered in membrane protrusions on mouse neuroepithelial stem cell. CD133 is a five transmembrane glycoprotein, containing 865 amino acids. The non-glycosylated molecular weight is 97 kDa and the N-terminus is extracellular with two extracellular loops, ending with a 56-59 amino acid cytoplasmic tail (Swiss Prot O43490). There are five potential glycosylation sites on the first extracellular loop and three sites on the second extracellular loop. Membrane topology has been suggested as the functional role for CD133, because it is found in cell membrane protrusions in intestinal microvillus and renal cells as well as protrusions, filopodia and lamellipodia in the apical surface of neural epithelial cells (32, 38-42). CD133 has no known preferential ligands or connections with signaling pathways. In humans CD133 dysfunction is associated with autosomal recessive retinal degeneration. A single nucleotide deletion and resulting frame shift produces a truncated form lacking half the extracellular loop, the entire fifth transmembrane domain, and all the cytoplasmic tail. The resulting mutation leads to impaired photoreceptor disk morphogenesis and a phenotypic retinal degeneration (32, 38, 39, 42, 43). In mice a similar phenotype was observed with loss of CD133/prominin-1(44).
The coincidental finding of CD133+ cancer stem cells being resistant to chemotherapeutic induced apoptosis, led to our efforts to determine whether CD133 expression serves a functional role in triggering multidrug resistance in glioma cells (45). To investigate this potential relationship rat C6 glioma cells ectopically expressing CD133 were employed. These cells normally do not have a major population positive for CD133 or ABC transporters (25, 27). We report that exogenous expression of CD133 promotes a 2-4-fold reduction in apoptosis resulting from therapeutic reagents, camptothecin and doxorubicin. Exogenous expressed CD133 promoted more expression of Bax without apoptosis. Higher induction of p53 upon exposure to camptothecin was observed in CD133+ cells, but the role of p53 in CD133+ cells may serve to promote DNA repair rather than apoptosis. Finally, CD133+ C6 cells have a 62% higher expression of at least one ABC transporter, (known as ABCB1, P-glycoprotein) along with higher transporter activity, that leads to resistance to therapeutic reagents. Our findings support the hypothesis that CD133 plays an anti-apoptotic functional role in protecting cancer cells, i.e. cancer stem cells are protected from chemotherapy. These results support ongoing research to target CD133 as a means to eradicate cancer stem cells.
Results
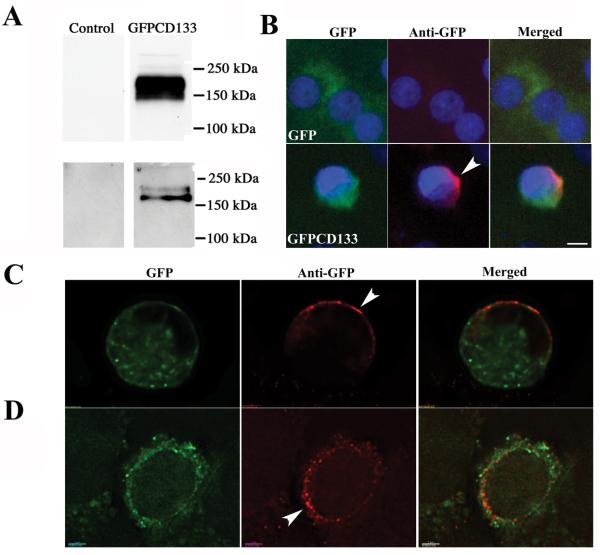
Expression analysis of GFPCD133 shows SDS-PAGE apparent MW of 170-150 kDa
Transient transfection of 293FT cell and stably transfected C6 glioma cells with the GFPCD133 plasmid produced a fusion protein that migrated as a 170-150 kDa polypeptide (Fig. 1A). Cell lysates were used from 293FT cells and immunoprecipitation was used from C6 glioma cells. A band was observed at the predicted molecular weight, corresponding to a fusion protein consisting of GFP (28,973 Da, GFP plus spacer amino acids) and glycosylated CD133. Non-transfected 293T cells and C6 glioma cells stably transfected with GFP were devoid of bands in this region (Fig 1A). C6 glioma cells stably transfected with GFP (C6-GFP) or GFPCD133 (C6-GFPCD133) show green fluorescent cells from GFP in the cytosol, but C6-GFPCD133 also showed green staining at the cell membrane (Fig. 1B). Previous investigators have reported the same locations, cytosolic and membrane, for endogenous CD133 (46, 47). To confirm that GFPCD133 is membrane bound, C6-GFP and C6-GFPCD133 cells were fixed without membrane permeabilization and stained with anti-GFP antibody. The C6-GFP cells revealed minor anti-GFP staining. By contrast, C6-GFPCD133 cells showed significant detection by the anti-GFP antibody at the cell membrane (Fig. 1B). Optical deconvoluted fluorescent microscope sections substantiated our results by showing punctate red anti-GFP labeling at the membrane with overlay of the green GFP intrinsic signal (Fig. 1C and D). Granular formation of the GFP intrinsic signal within the cytoplasm most likely represents GFPCD133 expression in the endoplasmic reticulum (ER) as well as the Golgi, consistent with the ER synthesis and Golgi processing and glycosylation of transmembrane proteins. In addition, the membrane anti-GFP staining illustrated that GFPCD133 expression was polarized in cells (Fig. 1C), but also showed detection throughout the cell membrane with preference on one side of the cell (Fig. 1D). CD133 protein has been shown to be distributed in the uropod of migrating hematopoietic stem and progenitor cells (48) and detected on the apical side of the glandular epithelia in membrane protrusions. Likewise, the apical of embryonic neural epithelia and epidermal cells of the adult brain that line the lateral ventricle also show apical staining of CD133 (49-52). When C6-GFP and C6-GFPCD133 cells were permeabilized by Triton X100 and then stained with anti-GFP, both cell lines showed detection of cytosolic GFP (Fig. 2).
Figure 1. Western immunoblot of the 170-150 kDa GFPCD133 fusion protein in C6 glioma cells.
(A) Upper panel: Cell lysates, 50 μg of protein, from 293FT cells that were control untransfected (Left Lane) and transiently transfected with pcDNA3.1/NT-GFPCD133 fusion (Right Lane); and lower panel anti-GFP immunoprecipitates from 6.508 mg C6-GFP cell lysate (left lane) and 7.859 mg C6-GFPCD133 cell lysate (Right Lane) were separated on SDS-PAGE and then transferred to a PVDF membrane and stained with anti-GFP antibody. GFPCD133 migrates between 150 and 250 kDa. (B, C, D) Epifluorescence microscopy of GFP and GFPCD133 expression in stably transfected C6-GFP (B) and C6-GFPCD133 (B-D) glioma cells, respectively. C6-GFP cells and C6-CD133 cells (not permeabilized) were immunostained with anti-GFP antibody (red), but also show an intrinsic GFP fluorescence (Green). The chromatin was stained with DAPI. (B) C6-GFPCD133 cells exhibit robust polarized membrane staining with anti-GFP (red), but C6-GFP cells show minor detection with anti-GFP staining. (C) Deconvoluted optical section (0.1 μm) with anti-GFP staining (red) further confirms that GFPCD133 occupies the cell membrane in a polarized manner with an intrinsic GFP fluorescent (green) overlay, or (D) uniform anti-GFP staining with more GFPCD133 one side of the cell. Interestingly, GFPCD133 shows punctate membrane anti-GFP staining, while most of the intrinsic GFP signal is in the cytoplasm. Arrowhead shows polarized staining. Scale in (B) is 5 μm and in (C, D) scale is 2 μm. DAPI is 4′, 6′-diamidino-2-phenylindole.
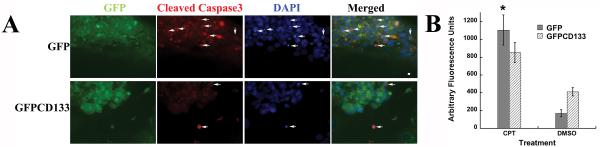
Figure 2. Positive cleaved caspase-3 immunostaining in cells with condensed or fragmented Chromatin validates apoptosis.
(A) Stably transfected C6-GFP and C6-GFPCD133 glioma cells grown on fibronectin-coated coverslips were treated with 10 μM camptothecin (CPT) for 6 days and then were fixed and immunostained with anti-GFP and anti-cleaved caspase-3. Both C6-GFP and C6-GFPCD133 cells are GFP+ (green) and arrows show apoptotic cells that are cleaved caspase-3+ and have condensed and fragmented chromatin. Scale is 5 μm. (B) Cleavage of z-DEVD-R110 to measure caspase 3/7 activity after 2.5 days of 10-μM-campothecin treatment of C6-GFP and C6-GFPCD133 cells. Results are given in arbitrary fluorescent units (one tail Student's t-test showed p=0.045 for *CPT-treated C6-GFP versus CPT-treated C6-GFPCD133 cells.
Expression of GFPCD133 fusion protein promotes resistance to apoptosis resulting from anti-cancer drugs
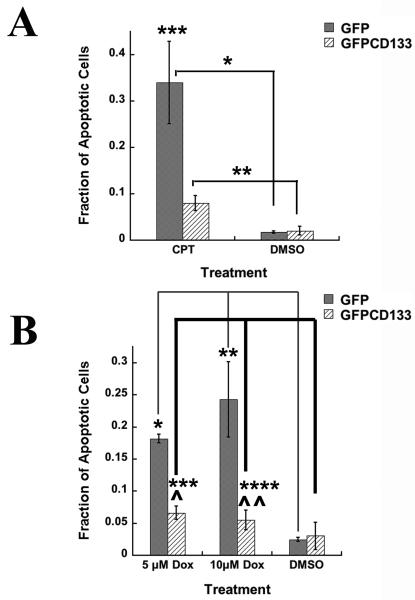
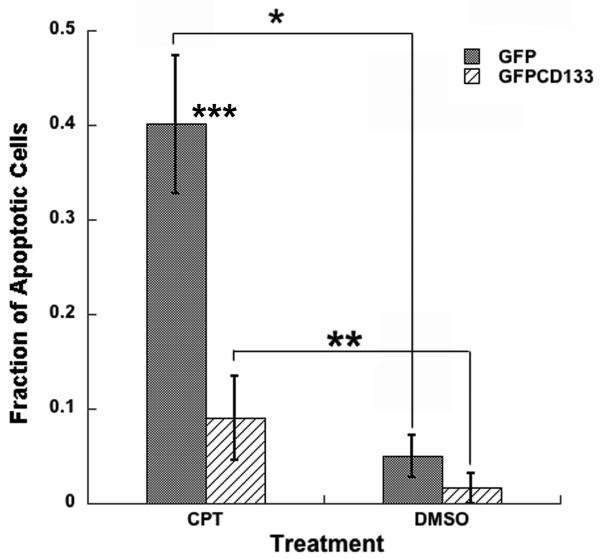
Published findings, showing CD133+ cancer stem cells resistant to anti-cancer drugs, led to our efforts to determine whether expression of CD133 promotes drug resistance. Cells grown in culture treated with topoisomerase I inhibitor (camptothecin, CPT), promotes double strand breaks in the cell's DNA, which results in apoptosis. To examine whether exogenous expression of CD133 leads to protection of C6 glioma cells from camptothecin, C6 cells expressing GFP or GFPCD133 stably transfected, were treated with camptothecin at 10 μM. Cells with condensed or fragmented chromatin were considered apoptotic and were shown to also stain positively for apoptotic marker cleaved caspase-3 (Fig. 2A) along with increased caspase-3/7 activity (Fig. 2B), which further corroborated that breakdown of chromatin serves as a reporter for drug induced apoptosis. Stably transfected C6-GFP glioma cells showed apoptosis in 34% of the cells, whereas only 8% of the stably transfected C6-GFPCD133 cells were apoptotic within 6 days of treatment (Fig. 3A). The DMSO vehicle control showed 1-2% of the C6 cells expressing GFP or GFPCD133 undergoing apoptosis (Fig. 3A). Taken together, the exogenous expression of CD133 evokes resistance to camptothecin-induced apoptosis in C6 glioma cells. Moreover, G418 selection did not lead to drug resistance in stably transfected C6 cells, because stably transfected GFP cells were less drug resistant than stably transfected GFPCD133 cells to camptothecin.
Figure 3. (A) Camptothecin (CPT) treatment at 10 μM of C6 glioma cells.
Fraction of apoptotic cells from stably transfected C6-GFP or C6-GFPCD133 from treatment with 10 μM camptothecin or DMSO. Values represent the mean ± SEM for three cultures in which at least 100-200 transfected cells were evaluated per culture. (Student's t-test; *CPT-treated GFP versus DMSO-treated GFP cells, p= 0.011; **CPT-treated GFPCD133 cells versus DMSO-treated GFPCD133, p= 0.019; ***CPT-treated GFP cells versus CPT-treated GFPCD133 cells, p= 0.025).
(B) Doxorubicin (DOX) treatment at 5 μM or 10 μM of stably transfected C6-GFP and C6-GFPCD133 glioma cells. Fraction of apoptotic cells from stably transfected C6-GFP or C6-GFPCD133 from treatment with 10-μM dox, 5-μM dox, or DMSO. Doxorubicin treatment was for 5 days. Values represent the mean ± SEM for three cultures in which at least 100-200 transfected cells were evaluated per culture. (Student's t-test; Dox-treated GFP versus DMSO-treated GFP cells at 5 μM, *p= 1.57 × 10−5, at 10 μM, **p= 0.011; Dox-treated GFPCD133 cells versus DMSO-treated GFPCD133, ***p= 0.14, at 5 μM, ****p= 0.32, at 10 μM; Dox-treated GFP cells versus Dox-treated GFPCD133 cells at 5 μM, ^p=0.0003, at 10 μM, ^^p=0.020.
The DNA synthesis inhibitor, doxorubicin, was next tested to determine whether exogenous expression of CD133 promotes drug resistance to more than one anti-cancer drug. For doxorubicin, 5 μM and 10 μM concentrations were used. The stably transfected GFP C6 cells revealed 18% and 24% of the cells were apoptotic for 5 μM and 10 μM doxorubicin, respectively. By contrast, stably transfected GFPCD133 C6 cells revealed only 5.5% and 6.6% were apoptotic for 5 μM and 10 μM doxorubicin, respectively (Fig. 3B). The DMSO vehicle had only 2-3% apoptotic cells (Fig. 3B). Collectively, CD133 provided significant protection from apoptosis evoked by both camptothecin and doxorubicin.
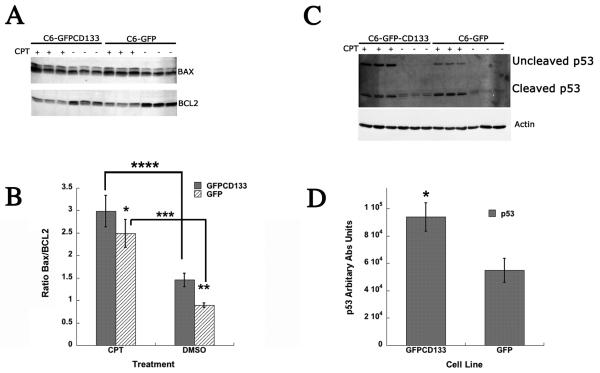
CD133 Leads to higher tolerance for Bax prior to Camptothecin Treatment
To assess the mechanism of the CD133 induced protective action, the expression of pro-apoptotic Bax and anti-apoptotic BCL2 were examined in C6-GFP and C6-GFPCD133 lines before and after camptothecin treatment. Oligomerization of Bax results in the formation of pores in the outer mitochondria membrane, promoting the release of cytochrome c, while BCL2 binds to Bax and inhibits the formation of Bax oligomers. Thus, increasing the level of BCL2 to Bax, i.e. lower Bax/BCL2 ratio, promotes the mitochondrial retention of cytochrome c. The western immunoblot shows an apparent reduction of BCL2 after 6 days of camptothecin treatment both in C6-GFP and C6-GFPCD133 cells (Fig. 4A). In contrast, Bax protein levels are shown to be increased with camptothecin (Fig. 4A). The intensity of each protein band was digitally quantitated and the data is expressed as a Bax/BCL2 ratio (Fig. 4B). The Bax/BCL2 protein ratio is shown to be statistically significant between untreated C6-GFP cells and untreated C6-GFPCD133 cells, with C6-GFPCD133 cells having a higher Bax/BCL2 ratio (Fig. 4B). By contrast, the camptothecin treated C6-GFPCD133 and C6-GFP cells have statistically insignificant ratios (Fig. 4B). The apparent higher Bax protein level along with less BCL2 expressed in the untreated C6-GFPCD133 cells appears to have led to the higher ratio shown in Fig. 4B, but without detectable increases in apoptosis compared to untreated C6-GFP cells (Fig. 3A). Taken together, the higher Bax expression along with caspase-3/7 activity (Fig. 2B) suggests that CD133 promotes reluctance for cells to undergo apoptosis.
Figure 4. Bax, BCL2, and p53 ratio from camptothecin-treated stably transfected C6-GFP and C6-GFPCD133 glioma cells at 10 μM.
(A) Western immunoblot probed with anti-Bax (upper band) and then with anti-BCL2 (lower band) for 3 individual C6-GFPCD133 cultures treated with CPT (Lane 1-3) or with DMSO vehicle (Lane 4-6); and 3 individual C6-GFP cultures treated with CPT (Lanes 7-9) or with DMSO vehicle (Lane 10-12). 50 μg of cell lysate was added for each lane for SDS-PAGE-Western Blot. (B) Western blots were scanned and quantitated with densitometry. Bax/BCL2 values represent the mean ± SD for three cultures (Student's t-test; *CPT-treated Bax/BCL2 GFP vs CPT-treated Bax/BCL2 GFPCD133, p= 0.14; **Bax/BCL2 GFP vs Bax/BCL2 GFPCD133, p= 0.004; *** CPT-treated Bax/BCL2 GFP vs Bax/BCL2 GFP, p= 0.0019; **** CPT-treated Bax/BCL2 CD133GFP vs Bax/BCL2 GFPCD133, p= 0.0011) (C) Camptothecin (CPT) Induces Greater Expression of P53 Protein in C6-GFPCD133 Cells Than C6-GFP Cells. Western immunoblot probed with Anti-p53 (upper blot) and then with anti-β-actin (lower blot) to show normalized sample loading for 3 individual C6-GFPCD133 cultures treated with CPT (Lane 1-3) or with DMSO vehicle (Lane 4-6); and 3 individual C6-GFP cultures treated with CPT (Lanes 7-9) or with DMSO vehicle (Lane 10-12). 50 μg of cell lysate was added for each lane for SDS-PAGE. (D) The western Blot was scanned and quantitated by densitometry and p53 values normalized against β-actin represent the mean ± SD for three cultures (Student's t-test; *CPT-treated GFPCD133 p53 vs. CPT-treated-GFP p53, p= 0.011). Anti-p53 reveals full-length p53 and p21-22 p53 cleaved fragments.
Camptothecin Induces Greater Expression of p53 Protein in C6-GFPCD133 Cells Than C6-GFP Cells
Camptothecin has been shown to up regulate p53 protein. Western immunoblotting shows that 6 days of camptothecin exposure led to higher levels of p53 protein in stably transfected C6-GFPCD133 cells compared to stably transfected C6-GFP-glioma cells. In addition to the p53 protein, p21-22 bands were observed for camptothecin treated and untreated cells, but were less intense for untreated cells (Fig. 4C). The p21-22 bands have been determined to be p53 cleavage products that have affinity for mitochondria and aid initiating cytochrome c release (53). Digitally quantitated p53 western blots revealed at least that C6-GFPCD133 cells have twice the expression of p53 compared to C6-GFP cells during camptothecin treatment (Fig. 4D). The higher level of p53 in the camptothecin treated C6-GFPCD133 cells would suggest a greater induction for apoptosis. Our surprising reciprocal effect of high p53 levels with reduced apoptosis in C6-GFPCD133 cells compared to C6-GFP cells, suggests that p53 may serve to stop cell cycle for DNA repair rather than trigger apoptosis. Transfection of dominant negative p53 into C6-GFP and C6-GFPCD133 cells, revealed that the typical camptothecin induced-apoptosis was at least 4 times more prevalent in the C6-GFP cells than the C6-GFPCD133 cells (Fig. 5). Thus, induction of apoptosis and CD133 protection are both independent of p53.
Figure 5. Camptothecin (CPT, 10 μM) treatment of C6-GFP and C6-GFPCD133 glioma cells transfected with pCIN-d/n-p53 and pQCx-I-DsRED (2:1 ratio).
Fraction of apoptotic cells from stably transfected C6-GFP or C6-GFPCD133 from treatment with 10-μM camptothecin or DMSO. CPT treatment was for 6 days. Values represent the mean ± SEM for three cultures in which at least 100-200 transfected cells were evaluated per culture. (Student's t-test; *CPT-treated GFP versus DMSO-treated GFP cells, p= 0.005; **CPT-treated GFPCD133 cells versus DMSO-treated GFPCD133, p= 0.13; ***CPT-treated GFP cells versus CPT-treated GFPCD133 cells, p= 0.011).
C6-GFPCD133 cells have a higher initial level of ABCB1 (P-glycoprotein) mRNA transcripts and ABC Transport Activity than C6-GFP cells
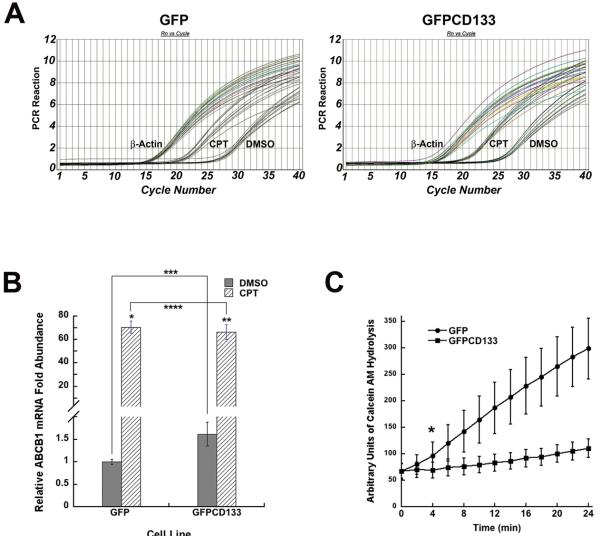
Because multiple drug resistant (MDR) proteins have been shown to be expressed in CD133+ cancer stem cells, this is thought to lead to protection from anti-cancer drugs. The levels of MRD mRNAs were next examined in C6-GFP and C6-GFPCD133 cells. Rat Mrp2, ABCB1 (Fig. 6A), and ABCg2 MDR mRNA transcript levels were quantitated by real-time PCR from both C6-GFP and C6-GFPCD133 cells. Mrp2 and ABCg2 were expressed significantly less than ABCB1, and did not show any significant difference between C6-GFP and C6-GFPCD133 cells. ABCB1 was present at a statistically significant, 1.62 fold higher level in C6-GFPCD133 cells than C6-GFP cells (untreated-GFP vs untreated-GFPCD133; p= 0.0469) (Fig. 6B). Anti-cancer drugs have been known to induce the expression of MDR mRNA transcripts and as shown by the 6-day camptothecin treatment, the expression of ABCB1 was induced 71-fold in C6-GFP and 41 fold in C6-GFPCD133 cells GFP: GFPCD133 (71:41; p= 0.027) (Fig. 6B). A plausible explanation for lower fold change induction in C6-GFPCD133 derives from the fact that C6-GFPCD133 had a higher initial expression of ABCB1. In fact, the ratio of the ABCB1-fold change between C6-GFP and C6-GFPCD133 cell lines was 1.7, which is essentially the same as the initial fold difference between both cell lines prior to treatment (Fig. 6B). In addition, ABC transporter activity was measured by the influx of calcein AM into the C6 glioma cells. In the cytoplasm, esterases hydrolyze the calcein AM from its hydrophobic chain yielding a product that fluorescence at 517 nm. The greater the ABC transporter activity, the less entry of calcein AM into the cytoplasm with less fluorescence. GFPCD133 C6 glioma cells have less fluorescence compared to GFP C6 glioma cells (Fig. 6C). Thus, CD133 promotes up-regulation of ABCB1 and higher ABC transporter activity.
Figure 6. Relative p-Glycoprotein mRNA prior and after CPT treatment, and activity measurements of p-Glycoprotein in C6 cells permanently transfected with GFP or GFP-CD133.
(A) Quantitative real-time PCR curves of C6-GFP(left) and C6-GFPCD133 (right) treated with DMSO vehicle or 10 μM CPT. Treatment was achieved in 3 trials and 3 PCR reactions were accomplished for each trial. Corresponding β-actin PCR curves were used to normalize input cDNAs by subtracting actin Ct values from p-Glycoprotein Ct values. (B) Relative p-Glycoprotein mRNA abundance based on DMSO treated C6-GFP with the lowest level, which is compared to the abundance of mRNA with CPT-treated C6-GFP and C6-GFPCD133 cells as well as DMSO treated C6-GFPCD133 cells (Student's t-test; *CPT-treated GFP versus DMSO-treated GFP cells, p= 7.33 × 10−5; **CPT-treated GFPCD133 cells versus DMSO-treated GFPCD133, p= 0.000237; ***DMSO-treated GFP cells versus DMSO-treated GFPCD133 cells, p= 0.0469; ****CPT-treated GFP cells versus CPT-treated GFPCD133 cells, p= 0.552) (C) Hydrolysis of calcein AM from 0 to 24 min. (Values represent the mean ± SD for twelve wells. SD shown as calculated average percentage of SD/mean for each line. *Student's t-test revealed statistical significance starting at 4 min with a p= 0.0046. Duplicate wells of C6-GFP and C6-GFPCD133 without calcein AM were measured to determine background emission from green fluorescent protein. Background fluorescence from C6 cells lines did not change during the 24 min assay. Therefore GFP did not contribute to rising calcein signal detection.
Discussion
N-nitrosomethylurea derived rat glial tumor C6 cells have been used as a model for both astrocytoma tumors and cancer stem cells (25, 54). The majority of cultured C6 cells are considered to be cancer stem cells; based on self-renewal properties, limited in vitro differentiation, and their ability to form tumors in nude mice (25). These cells also contain minor subpopulations that are CD133 positive and/or resistant to the cytotoxicity of Hoechst 33342. Cells that are resistant to Hoechst 33342 are often called side population cells, and ABCG2 transporter effluxes Hoechst 33342 (45). By contrast, our work suggests that ABCB1 is the main efflux transport for camptothecin. However, the vast majority of the C6 cells are sensitive to Hoechst 33342, lack multidrug resistance and are not CD133+ (27). Thus, the ectopic expression of CD133 in all C6 cells supports the role of this membrane protein as the key element for the development of xenobiotic resistance (25, 27). The higher expression of CD133 in transfected cells resulted in elevated MDR pump activity, supportive of a working relationship between these proteins. The possibility of CD133 accentuating the activity of MDR pumps is strengthened by their spatial relationship; MDR-1 like CD133 is polarized to the apical membrane surface (55). We are presently in the process of determining the proximity of this relationship.
The results of the present study demonstrating that expression of CD133 elevates the resistance of C6 glioma cells to chemotherapeutic reagents, strongly suggest the necessity for the implementation of therapeutic regimes that consider CD133 as a priority target. In fact, one investigation has used anti-CD133 monoclonal antibodies tagged with therapeutic drug to destroy cancer stem cells. This approach may have had two consequences, one being the anticipated specific delivery of the therapeutic agent, and second the unexpected enhancement of the agents efficacy by interfering with CD133 through antibody-antigen binding (34).
A CD133 knockout mouse has been reported, which verified its role in photoreceptor disk formation (44). However, a functional role for CD133 with respect to cytotoxic resistance has not yet been established in this knockout mouse. Biological roles for CD133 could be inferred from its physical properties. CD133 is found in cholesterol-rich lipid rafts within membrane protrusions of epithelium cells that could allow recruitment of ABC transporters to the raft for efflux transport of toxic compounds. Protrusions also allow greater surface area for more effluxing transporters to occupy. In contrast to this scenario, one investigation revealed that in leukemia cells, expression and activity of MDR (P-glycoprotein) was unaffected with or without protrusions and large folding of the cell membrane (56).
Lipid rafts have been shown to generate plasma membrane topology for signal transduction. Thus, CD133 may not carry out ligand binding itself, but it is involved in lipid raft formation for attracting ligand-binding receptors that integrate intracellular signal transduction pathways. Evidence for cell signaling is shown by 62% induction in the elevation of MDR-ABCB1 (P-glycoprotein) mRNA in C6-GFPCD133 cells prior to addition of camptothecin. This induction could be explained by the discovery that exogenous CD133 triggered a signaling pathway for mRNA transcription. Alternately, the plasma membrane topology, formulated by CD133, could allow more redistribution of ABCB1 to the membrane and/or provide a better environment for efficient pump activity. Nonetheless, direct or indirect signaling has been shown to be carried out by CD133. In support of cell signaling, C6-GFPCD133 cells were shown to have a higher Bax and lower BCL2 expression compared to C6-GFP cells without cytotoxic treatment. Exogenous CD133 expression in C6 cells appears to have contributed to the above, and the cells were shown to be more reluctant to undergo apoptosis with more Bax and less BCL2. Bax bound to BCL2 in the presence of camptothecin prevents initiation of apoptosis. Taken together, exogenously expressed CD133 increased ABCB1 mRNA with more Bax and reduced BCL2 protein. These data support the inference that CD133-directed regulation promotes increased multidrug resistance and greater reluctance to undergo apoptosis.
The increase of ABCB1 with higher ABC transporter activity in C6-GFPCD133 does not necessarily mean that all the camptothecin is prevented appreciable access to the cell since access is probably necessary to affect the increased production of p53. High levels of p53 have been shown to lead to apoptosis, but it also promotes cell cycle arrest necessary for DNA repair, which could translate into the apoptotic resistance observed by C6-GFPCD133 cells. However, use of the p53 dominate negative in the CD133 + cells did not result in the cell death upon drug challenge; therefore p53 production doesn't explain drug resistance. Finally, p53 protein has been shown to be deleted or mutated in 70% of glioblastomas (57). Consequently, these data provide evidence that CD133+ cancer stem cells would be resistant to chemotherapy even with a disrupted p53 pathway.
In summary, our findings show that CD133 has a functional role in regulating cytotoxic resistance in C6 glioma cells. Such regulation appears to be in part due to increasing the level of ABCB1 with ABC transporter activity, and by a p53-independent reluctance to enter into apoptosis. Therapeutic intervention to target CD133 combined with traditional chemotherapy reagents could formulate a treatment to ubiquitously eradicate cancer stem cells along with their tumor progeny.
Methods
Materials
Cell growth media DMEM with 4 mM Glutamine and 1mM pyruvate, fetal bovine serum, LiptofectAMINE 2000, TRIzol reagent, Superscript III, PureLink Quick Plasmid Miniprep kit, and pcDNA3.1/NT-GFP-TOPO vector kit were from Invitrogen. Expand High FidelityPlus PCR system was from Roche. BCA protein assay reagent was from Thermo-Scientific. ECL Plus Western blotting detection system was from GE Healthcare. Camptothecin and doxorubicin anticancer drugs were from Sigma-Aldrich. Each was dissolved in DMSO at 25 mM and 10 mM, respectively.
Cloning of Rat CD133
The kidneys from a pentobarbital euthanized male Sprague Dawley rat (200 g) (Charles Rivers) was used as a source for CD133 as described by Weigmann A. et al. (49). The tissue was first freeze clamped and subsequently reduced to a fine frozen tissue dust with a liquid nitrogen cooled mortar and pestle. Aliquots of 200 mg were then dissociated with TRIzol reagent (cat # 15596-018 Invitrogen) according to manufacturer's instructions. The upper layer containing the RNA was diluted with an equal volume of 70% ethanol and the total RNA further purified using RNeasy Midi kit (cat # 75142 Qiagen). The mRNA was obtained from the previous total RNA using an Oligotex mRNA midi Kit (Cat # 70042 Qiagen). To avoid degradation upon storage the mRNA was immediately converted to cDNA using random hexamers and SuperScript III First-Strand Synthesis system (Cat # 18080-051, Invitrogen). CD133 was amplified from the cDNA by using Expand High FidelityPlus PCR system (Roche) according to manufacturer's specifications using the following PAGE purified Forward primer ‘5- ATGGCTCTCGTATTCAGTGTCCTGCT-3’ (Tm = 61.4°C) and Reverse primer ‘5-TCAGTATCGAGACGGGCTTGTCATAACAG-3’(Tm = 61.0°C) (Integrated DNA Technologies, Coralville IA). The amplification product was separated on a 1.0% SeaKem GTG Agarose gel (Lonza) in TAE buffer and the band extracted from the gel using a QIAquick Gel Extraction Kit (cat # 28704, Qiagen). The product was inserted in-frame with GFP using the vector pcDNA3.1/NT-GFP-TOPO (cat # 45-0247). The plasmid was introduced into TOP10 E. coli and plated on LB agar containing 100 μg Carbenicillin/ml. Colonies were screened for the correct plasmid insert size and orientation by PCR. Positive clones were expanded by growth in LB media (100 μg/ ml carbenicillin) and the plasmid extracted for sequencing (Davis Sequencing, Davis CA) using PureLink Quick Plasmid Miniprep kit (Cat # K2100-10, Invitrogen). The sequence was identical to the NCBI data base NM_021751.2 for Rattus norvegicus prominin 1, except for wobble at position 2036 where G was substituted for A without alteration in amino acid identity.
Plasmid pcDNA3.1/NT-GFP without the CD133 insert, but still expressing GFP, was employed as the transfected control (Invitrogen TOPO kit). To check for a fusion translated product, 293FT cells grown to near confluence in a 60 mm dish were transfected with pcDNA3.1/NT-GFPCD133 fusion and Lipofectamine 2000 reagent (cat # 11668-019 Invitrogen). This was performed with a ratio of plasmid (8 μg) to 20μL of reagent according to manufacturer's suggestion. After 48 hr, cells were gently washed with ice cold PBS and lysed in 50 mM Tris buffer (pH 7.4), 150 mM NaCl, 1% NP40, 0.25 % sodium deoxycholic acid, 0.1% SDS, 1X protease inhibitor cocktail Set I (cat # 539131, Calbiochem), 1 mM NaF and 1 mM activated sodium orthovanadate (RIPA buffer). The reaction was allowed to progress on ice for 1 hour and insoluble material was removed by centrifugation at 16,000 x g for 30 minutes. Protein was determined using the BCA protein assay reagent. Protein was separated on an 11%T: 2.75%C SDS PAGE gel and subsequently blotted using the tank method and Towbin buffer onto PVDF membrane (0.2 μm, BIO-RAD). Membranes were subsequently blocked with 5% milk in 50 mM Tris (pH 7.4) and 150mM NaCl for an hour at room temperature and then exposed to GFP rabbit Antiserum (1/2000, Invitrogen) overnight at 4°C in blocking buffer. Blots were washed 3 x for 5 min each with TBS and then exposed to donkey anti-rabbit conjugated to HRP (1/5000, cat # NA934V, GE Healthcare) for 1 hr (room temp) in blocking buffer. Blots were subsequently washed with 0.05% Tween-20 in TBS (5 min) and washed 4 x with TBS. Positive material was detected using the ECL Plus Western blotting detection system (cat # RPN2132 Amersham – GE Healthcare) A positive fusion product was detected (Fig. 1) corresponding to a mass indicating a GFP fusion product and glycosylated CD133.
Transfection of C6-glioma cells
C6-glioma cells were dissociated by 0.05% trypsin 1 mM EDTA and plated onto 24 well plates in DMEM with 4mM glutamine, 1mM pyruvate, 4.5 g/ml and 10% fetal bovine serum medium. The next day, cells were transfected with 2 μg of pcDNA3.1/NT-GFP or pcDNA3.1/NT-GFPCD133 plasmid per well and LiptofectAMINE 2000 as recommended by the supplier (Invitrogen). Dominant negative (d/n)-p53, 1 μg pCIN-d/n-p53 (from Wei Gu, Columbia University, New York), and 0.5μg pQCxI-dsRed and 5μl LiptofectAMINE 2000 were added per well to transfect C6-GFP and C6-GFPCD133 cells in a 24 well plate. The C6 glioma cells were treated with the anticancer drugs 24 hours later.
Establishment of stably transfected C6-GFP control cells and C6-GFP-CD133 fusion protein cells
Permanent rat C6 glioma cell lines, overexpressing rat CD133GFP fusion protein and GFP, were created by transfecting 100mm plates of confluent C6 glioma cells with 20 μg of pcDNA3.1/NT-GFPCD133 or pcDNA3.1/NT-GFP plasmid and LiptofectAMINE 2000 as recommended by Invitrogen. After 2 days, cells stably expressing GFPCD133 or GFP were selected using 4 mg of G418 (Sigma) per ml of DMEM with 4mM glutamine, 1mM pyruvate, 4.5g/mL glucose and 10% fetal bovine serum. After several weeks, the surviving green fluorescencing C6 cells expressing different levels of CD133GFP or GFP were expanded into 60 mm wells. Stably transfected C6 glioma cells were further expanded and passaged onto 100 mm tissue culture plates by 0.05% trypsin 1mM EDTA dissociation with medium (as described above) with 2 mg/ml G418 to maintain expression levels of GFPCD133 fusion or GFP. For cytotoxic drug experiments, the stably transfected C6 glioma cells were plated onto 24 well plates without G418 and treated with drugs 24 hours later. To confirm translation of GFPCD133 in stably transfected C6 cells, GFP control and GFPCD133 cells were grown to confluency in75 cm2 flasks. The cells in each flask were scraped into PBS, sedimented, washed in PBS and subsequently lysed in 2 mls of 1% Triton-X-100 in 150 mM NaCl and 50 mM Tris buffer (pH 7.4) with 1 x Calbiochem Protease inhibitor cocktail set I., 1 mM NaF and1 mM Na3VO4 on ice for 1 hr. The lysates were centrifuged at 16,000 x g for 1 hour, and the supernatant protein was measured by BCA assays. For immunoprecipitation, 6.508 mg of protein from C6-GFP cells and 7.859 mg of protein from C6-GFPCD133 cells were diluted to 12 mls with PBS in a conical centrifuge tube and allowed to rock for 4.5 hours at room temperature in the presence of Vector Fusion-Aid-GFP resin (50 mg). The resin was allowed to settle and was subsequently washed 2 x with PBS (12 ml).
Resin was resuspended in 2X Laemmli sample buffer (100 μl) and boiled for 2 minutes. The entire recovered supernatant for both the GFP and the GFP-CD133 were separated by SDS-PAGE and blotted as previously described. The blot was blocked with 5% milk for 1 hour, and then developed with 1 μg/ml Anti-GFP mouse monoclonal clone N86/8 cat 75-131 (non profit UC Davis/NINDS/NIMH NeuroMab Facility) dissolved in 5% milk TBS overnight at 4°C. The blot was washed 3 x with TBS and then developed in 1/2000 dilution of Anti mouse HRP from Sheep in 5% milk for 1 hour. Washed 1X with 0.05% Tween 20 TBS and 3 x with TBS and developed with ECL plus reagent for 20 minutes. For cytotoxic drugs experiments, the stably transfected C6 glioma cells were plated onto 24 or 96 well plates without G418 and treated with agents 24 hours later.
Western analysis of Bax, Bcl-2 and p53 from rat C6-Glioma cells permanently transfected with GFP or GFP-CD133 in the presence and absence of Camptothecin
Rat C6- Glioma cells were cultured in DMEM media (cat # 11995, GIBCO) with 10% FBS in 75 cm2 flasks. Permanently transfected control cells containing GFP or GFP-CD133 were exposed to vehicle DMSO or 10 μM camptothecin for 6 days. Cells were scraped into the media present in the flask at time of harvest to avoid the loss of potentially non-adherent or apoptotic cells. They were centrifuged in 15 ml conical tubes and washed with 12 mls of ice cold PBS, sedimented by centrifugation, resuspended in PBS, transferred to 1.5 ml tubes and lysed (RIPA buffer). SDS-PAGE (50 μg protein/lane), blotting, and exposures to primary and secondary antibodies were conducted as previously detailed. The following mouse monoclonal antibodies and concentrations were employed Bcl2 (1/200, Cat # sc-7382 , Santa Cruz), Bax (1/200, Cat # sc-7480, Santa Cruz), p53 (1/1000, cat # 1C12, Cell Signaling technologies) β-actin (1/5000, cat # A-5441, Sigma). The HRP conjugated anti-mouse HRP conjugated secondary was from GE Healthcare (1/5000 cat # NXA931). The same blot was used for BCL2 and subsequently for Bax after stripping for 10 minutes (Restore Western Blot stripping buffer) and rinsing with TBS and re-blocking.
Relative Quantitation of multi-drug resistance transporters (MDR) in rat C6-Glioma cells
Cells were grown (25 cm2 flasks), treated and harvested as described in the previous section. Isolation of RNA and conversion to c-DNA was as described above except material was not subjected to m-RNA isolation. For real time PCR, primers for rat ABCB1 (P-glycoprotein), ABCG2, Mrp2 and beta-actin were designed using IDT free software (Table 1). Reactions were composed of 250 nM of forward and reverse primers, 50 ng of total RNA (260/280 ratio above 1.9) and SYBR Green PCR Master Mix (cat # 4309155, Applied Biosystems), total volume 25 μL. Samples, in triplicate, were developed using a 7300 Real Time PCR system (Applied Biosystems) with default setting at 40 cycles and a dissociation stage with automatically set baselines and Ct.
Table 1.
List of Quantitative Real-Time PCR templates with forward and reverse primers.
| Template | Forward Primer | Reverse Primer |
|---|---|---|
| ABCB1 | 5′-GCTTATGCGAAAGCTGGAGCAGTT-3′ | 5′-TGGCCGTGATGGCTTTCTTTATGC-3′ |
| ABCg2 | 5′-AAAGGATGTCTAAACAGGGCCGGA-3′ | 5′-GTGCTGGGCCATGAAACATGAGTT-3′ |
| Mrp2 | 5′-AACCGGGAAGGTCAAGTTCTCCAT-3′ | 5′-TTGTCAGAGTCACTGGTCCAAGCA-3′ |
| β-actin | 5′-TGAGCGCAAGTACTCTGTGTGGAT-3′ | 5′-TAGAAGCATTTGCGGTGCACGATG-3′ |
Fluorescent Microscopy
C6-GFPCD133 and C6-GFP glioma cells with and without camptothecin 6 Day treatment were fixed in 4% paraformaldehyde in PBS for 45 min at room temperature. After three washes with PBS, the cells were blocked in 10 % goat serum with 1% Triton X100 for anti-cleaved caspase-3/anti-GFP or without Triton X100 for single labeling with anti-GFP for 2 hours), and were immunolabeled overnight. The sections subsequently were incubated for 1 hr with goat Alexa 488-conjugated anti-mouse/goat Alexa 568-conjugated anti-rabbit antibodies for anti-cleaved caspase-3/anti-GFP or goat Alexa 568-conjugated anti-mouse for single labeling with anti-GFP in 10% nonimmune goat serum.
Microscopy was performed on either a Zeiss Axiovert 200 microscope (cleaved caspase-3/GFP) or a Delta Vision Deconvolution microscope (Anti-GFP with intrinsic GFP single) at 0.1 μm optical sections enhanced by Huygens Deconvolution Software. Images of xy and yz planes confirmed co localization in cell sections.
Measurement of cell survival
Transfected or stably transfected C6-glioma cells were treated with 10 μM final concentration of camptothecin, 10-5 μM of concentration of doxorubicin, or DMSO vehicle control with one 1 ml of media). Between 4-6 days, the growth medium was removed from each well, and replaced with phosphate buffered saline containing 1μg/ml DAPI and 0.1% Triton X-100, and exchanged with PBS after 10 min. GFP+ cells possessing condensed nuclei and fragmented chromatin were scored as apoptotic using Zeiss Axiovert 200 microscope. Data were presented as the proportion of GFP+ cells with apoptotic nuclei (58). Measurement of caspase-3/7 was preformed using Apo-ONE®Homogeneous Caspase-3/7 Assay kit according to manufacture's directions (Promega). Initial plating of cells was 50,000 per well of a 96 well plated with camptothecin at 10 μM or DMSO as the vehicle control. Assay was performed 2.5 days after initial treatment. Before the assay, cells were equilibrated for 30 min in EBM media (Lonza, cat # CC-3129) devoid of phenol red and containing EGM-2MV SingleQuots (Lonza, CC-4147), the assay subsequently preformed in this same media.
Measurement of ABC transporter activity
Each of the stably transfected GFPCD133 and GFP C6 glioma cell lines were plated in 12 wells at 1.7 × 105 cells per well. Calcein AM hydrolysis was measured from 0 to 24 min at 37° C in Molecular Devices SpectraMax M2 microplate counter. The calcein AM was added to the cells according to the manufacturer (Invitrogen, Vybrant® Multidrug Resistance Assay Kit, SKU# V13180). Before the assay, cells were equilibrated for 30 min in EBM media (Lonza, cat # CC-3129) devoid of phenol red and containing EGM-2MV SingleQuots (Lonza, CC-4147), the assay subsequently preformed in this same media.
Statistical analyses
Student's t test was used.
Acknowledgments
Grant Support: National Institutes of Health–National Cancer Institute (J. M. A.)
References
- 1.Zhang QB, Ji XY, Huang Q, Dong J, Zhu YD, Lan Q. Differentiation profile of brain tumor stem cells: a comparative study with neural stem cells. Cell Res. 2006;16(12):909–15. doi: 10.1038/sj.cr.7310104. [DOI] [PubMed] [Google Scholar]
- 2.Zeppernick F, Ahmadi R, Campos B, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14(1):123–9. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 3.Yi JM, Tsai HC, Glockner SC, et al. Abnormal DNA methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res. 2008;68(19):8094–103. doi: 10.1158/0008-5472.CAN-07-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122(4):761–8. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 5.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6(6):425–36. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 6.Tirino V, Desiderio V, d'Aquino R, et al. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS ONE. 2008;3(10):e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thon N, Damianoff K, Hegermann J, et al. Presence of pluripotent CD133(+) cells correlates with malignancy of gliomas. Mol Cell Neurosci. 2008 doi: 10.1016/j.mcn.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- 9.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg Clin N Am. 2007;18(1):31–8. doi: 10.1016/j.nec.2006.10.014. viii. [DOI] [PubMed] [Google Scholar]
- 11.Schrot RJ, Ma JH, Greco CM, Arias AD, Angelastro JM. Organotypic distribution of stem cell markers in formalin-fixed brain harboring glioblastoma multiforme. J Neurooncol. 2007;85(2):149–57. doi: 10.1007/s11060-007-9401-8. [DOI] [PubMed] [Google Scholar]
- 12.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 13.Salnikov AV, Kusumawidjaja G, Rausch V, et al. Cancer stem cell marker expression in hepatocellular carcinoma and liver metastases is not sufficient as single prognostic parameter. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Perez Castillo A, Aguilar-Morante D, Morales-Garcia JA, Dorado J. Cancer stem cells and brain tumors. Clin Transl Oncol. 2008;10(5):262–7. doi: 10.1007/s12094-008-0195-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Shen G, Shi Z, et al. Brain tumour stem cells and neural stem cells: still explored by the same approach? J Int Med Res. 2008;36(5):890–5. doi: 10.1177/147323000803600504. [DOI] [PubMed] [Google Scholar]
- 16.Kondo T. Stem cell-like cancer cells in cancer cell lines. Cancer Biomark. 2007;3(4-5):245–50. doi: 10.3233/cbm-2007-34-508. [DOI] [PubMed] [Google Scholar]
- 17.Kang MK, Hur BI, Ko MH, Kim CH, Cha SH, Kang SK. Potential identity of multi-potential cancer stem-like subpopulation after radiation of cultured brain glioma. BMC Neurosci. 2008;9:15. doi: 10.1186/1471-2202-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joo KM, Kim SY, Jin X, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88(8):808–15. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 19.Hassan HT, Zhai X, Goodacre JA. CD133 stem cells in adult human brain. J Neurooncol. 2008;89(2):247–8. doi: 10.1007/s11060-008-9620-7. author reply 9. [DOI] [PubMed] [Google Scholar]
- 20.Gunther HS, Schmidt NO, Phillips HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 21.Dell'Albani P. Stem cell markers in gliomas. Neurochem Res. 2008;33(12):2407–15. doi: 10.1007/s11064-008-9723-8. [DOI] [PubMed] [Google Scholar]
- 22.Beier D, Wischhusen J, Dietmaier W, et al. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 2008;18(3):370–7. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altaner C. Glioblastoma and stem cells. Neoplasma. 2008;55(5):369–74. [PubMed] [Google Scholar]
- 24.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Shen G, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67(8):3691–7. doi: 10.1158/0008-5472.CAN-06-3912. [DOI] [PubMed] [Google Scholar]
- 26.Shervington A, Lu C. Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Invest. 2008;26(5):535–42. doi: 10.1080/07357900801904140. [DOI] [PubMed] [Google Scholar]
- 27.Shen G, Shen F, Shi Z, et al. Identification of cancer stem-like cells in the C6 glioma cell line and the limitation of current identification methods. In Vitro Cell Dev Biol Anim. 2008;44(7):280–9. doi: 10.1007/s11626-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 28.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10(6):454–6. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 30.Borst P, Jonkers J, Rottenberg S. What makes tumors multidrug resistant? Cell Cycle. 2007;6(22):2782–7. doi: 10.4161/cc.6.22.4936. [DOI] [PubMed] [Google Scholar]
- 31.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23(43):7267–73. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 32.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214(1):3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 33.Fargeas CA, Huttner WB, Corbeil D. Nomenclature of prominin-1 (CD133) splice variants - an update. Tissue Antigens. 2007;69(6):602–6. doi: 10.1111/j.1399-0039.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith LM, Nesterova A, Ryan MC, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99(1):100–9. doi: 10.1038/sj.bjc.6604437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodward WA, Sulman EP. Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev. 2008;27(3):459–70. doi: 10.1007/s10555-008-9130-2. [DOI] [PubMed] [Google Scholar]
- 36.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10(1):R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaBarge MA, Bissell MJ. Is CD133 a marker of metastatic colon cancer stem cells? J Clin Invest. 2008;118(6):2021–4. doi: 10.1172/JCI36046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaszai J, Fargeas CA, Florek M, Huttner WB, Corbeil D. Focus on molecules: prominin-1 (CD133) Exp Eye Res. 2007;85(5):585–6. doi: 10.1016/j.exer.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Corbeil D, Roper K, Weigmann A, Huttner WB. AC133 hematopoietic stem cell antigen: human homologue of mouse kidney prominin or distinct member of a novel protein family? Blood. 1998;91(7):2625–6. [PubMed] [Google Scholar]
- 40.Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2001;2(2):82–91. doi: 10.1034/j.1600-0854.2001.020202.x. [DOI] [PubMed] [Google Scholar]
- 41.Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2(9):582–92. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 42.Shmelkov SV, Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37(4):715–9. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Maw MA, Corbeil D, Koch J, et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9(1):27–34. doi: 10.1093/hmg/9.1.27. [DOI] [PubMed] [Google Scholar]
- 44.Zacchigna S, Oh H, Wilsch-Brauninger M, et al. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29(7):2297–308. doi: 10.1523/JNEUROSCI.2034-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 46.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saigusa S, Tanaka K, Toiyama Y, et al. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16(12):3488–98. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 48.Giebel B, Corbeil D, Beckmann J, et al. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104(8):2332–8. doi: 10.1182/blood-2004-02-0511. [DOI] [PubMed] [Google Scholar]
- 49.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 1997;94(23):12425–30. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfenninger CV, Roschupkina T, Hertwig F, et al. CD133 is not present on neurogenic astrocytes in the adult subventricular zone, but on embryonic neural stem cells, ependymal cells, and glioblastoma cells. Cancer Res. 2007;67(12):5727–36. doi: 10.1158/0008-5472.CAN-07-0183. [DOI] [PubMed] [Google Scholar]
- 51.Karbanova J, Missol-Kolka E, Fonseca AV, et al. The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J Histochem Cytochem. 2008;56(11):977–93. doi: 10.1369/jhc.2008.951897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coskun V, Wu H, Blanchi B, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105(3):1026–31. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sayan BS, Sayan AE, Knight RA, Melino G, Cohen GM. p53 is cleaved by caspases generating fragments localizing to mitochondria. J Biol Chem. 2006;281(19):13566–73. doi: 10.1074/jbc.M512467200. [DOI] [PubMed] [Google Scholar]
- 54.Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161(839):370–1. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 55.Scoazec JY, Bringuier AF, Medina JF, et al. The plasma membrane polarity of human biliary epithelial cells: in situ immunohistochemical analysis and functional implications. J Hepatol. 1997;26(3):543–53. doi: 10.1016/s0168-8278(97)80419-9. [DOI] [PubMed] [Google Scholar]
- 56.Radel S, Fredericks W, Mayhew E, Baker R. P-glycoprotein expression and modulation of cell-membrane morphology in adriamycin-resistant P388 leukemia cells. Cancer Chemother Pharmacol. 1990;25(4):241–6. doi: 10.1007/BF00684879. [DOI] [PubMed] [Google Scholar]
- 57.Collins VP. Brain tumours: classification and genes. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 2):ii2–11. doi: 10.1136/jnnp.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelastro JM, Canoll PD, Kuo J, et al. Selective destruction of glioblastoma cells by interference with the activity or expression of ATF5. Oncogene. 2006;25(6):907–16. doi: 10.1038/sj.onc.1209116. [DOI] [PubMed] [Google Scholar]