Abstract
Antibody-mediated rejection (AMR) is a unique, significant, and often severe form of allograft rejection that is not amenable to treatment with standard immunosuppressive medications. Significant advances have occurred in our ability to predict patients at risk for, and to diagnose, AMR. These advances include the development of newer anti-human leukocyte antigen (HLA)-antibody detection techniques and assays for non-HLA antibodies associated with AMR. The pathophysiology of AMR suggests a prime role for antibodies, B cells and plasma cells, but other effector molecules, especially the complement system, point to potential targets that could modify the AMR process. An emerging and potentially larger problem is the development of chronic AMR (CAMR) resulting from de novo donor-specific anti-HLA antibodies (DSA) that emerge more than 100 days posttransplantation. Therapeutic options include: (1) High-dose intravenously administered immunoglobulin (IVIG), which has many potential benefits. (2) The use of IVIG + rituximab (anti-CD20, anti-B cell). (3) The combination of plasmapheresis (PP) + low-dose IVIG with or without rituximab. Data support the efficacy of all of the above approaches. Newer approaches to treating AMR include using the proteosome inhibitor (bortezomib), which induces apoptosis in plasma cells, and eculizumab (anti-C5, anticomplement monoclonal antibody).
Keywords: IVIG, Antibody-mediated rejection, Rituximab, Donor-specific antibodies
Introduction
Renal transplantation is well recognized as the treatment of choice for end-stage renal disease (ESRD), as it offers improved quality of life and survival [1–3]. As a result, the demand for donor kidneys continues to outpace the supply. There are more than 83,153 ESRD patients on the deceased-donor waiting list, and almost 32,000 new patients register annually; yet fewer than 18,000 kidney transplants are performed each year [based on US Department of Health and Human Services Organ Procurement Transplant Network (OPTN) data as of 30 January 2009] [4]. As the demand for organs continues to exceed the supply, the number of days spent waiting for a kidney transplant increases exponentially, particularly for patients who are difficult to match secondary to having broadly reactive human leukocyte antigen (HLA)-specific alloantibodies. Patients sensitized to HLA antigens account for approximately 30% of the transplant list. Recent data obtained from the United Network for Organ Sharing (2001–2008) [5] show that rates of living-donor (LD) and deceased-donor (DD) transplantation by panel-reactive antibody (PRA) status are <16% per year for patients with PRAs 10–80%, and <8% for patients with PRAs >80%. Clearly, any level of sensitization poses a challenge for successful transplantation due to the powerful barrier that preformed anti-HLA antibodies and immunologic memory represent.
Desensitization protocols for highly sensitized (HS) and ABO (blood group)-incompatible patients [6–13] have improved transplant rates and long-term patient and allograft survival in this high-risk population. However, these patients are at higher risk for antibody-mediated rejection (AMR). The incidence of AMR after desensitization treatments is approximately 30% [6, 7, 9–13], thus necessitating development of novel techniques for early diagnosis and approaches to AMR treatment.
AMR is not an uncommon complication of transplantation, especially in centers performing desensitization. The immediate loss of allografts due to hyperacute AMR is rare due to advancements in anti-HLA antibody detection and ABO matching. However, new-onset AMR can occur within hours of transplantation and is usually manifest by a rapid onset of allograft dysfunction associated with high resistivity indices on Doppler renal ultrasound and increases in donor-specific antibodies (DSA). This constellation of findings indicates a true medical emergency for the patient, especially if the allograft is to be salvaged. Treatment approaches have changed little since our last review [14], but there have been many refinements in our understanding of pathogenesis and in monitoring the efficacy of therapies, data on combination therapies, and new drugs that could possibly make a significant impact on AMR in the future. In this review, we discuss advances in the diagnosis and treatment of AMR that have occurred since our last review.
Advancements in detecting anti-HLA antibodies
Solid-phase antibody testing has greatly advanced the ability to characterize the presence and specificity of HLA-specific antibodies. These assays have eliminated many problems encountered with cell-based antibody testing methods, including eliminating the need for viable cells, identifying only HLA-specific antibodies—both complement-fixing and non-complement-fixing antibodies—and clearly identifying class-I versus class-II-specific antibodies. There are two types of solid-phase HLA-specific antibody screening methods: an enzyme-linked immunosorbent (ELISA)-based system and color-coded bead-based fluorometric assays. Both methods use soluble HLA antigens captured onto either a microtiter plate or beads. Fluorometric-based assays include detecting the beads by the flow cytometer or by the Luminex-based method. As this method is more sensitive than the ELISA method, we focused our testing approach to use mainly the Luminex-based method. There are still issues that must be considered in interpreting results generated by these assays. Antigen concentration on the beads may be higher than on cells; antigen density varies among beads; beads of some antigen groups may be overexpressed relative to other groups; antigens on beads may not be in a natural conformation, resulting in cryptic epitopes; and there can be variation between lots and between vendor products. Compared with the cell-based method, the fluorometric bead system is not as susceptible to drug interference, such as antithymocyte globulin, intravenously administered immunoglobulin (IVIG), and rituximab. IVIG may interfere with the bead assay for a few days after administration. Nonetheless, these new techniques allow for greater identification of HLA antibody specificities and a more accurate interpretation of cross-match results.
Despite these limitations, advancements in solid-phase detection of HLA class I/II antibodies and non-HLA-directed antibodies [i.e. major histocompatibility complex class I-related chain A (MICA)] have greatly improved our understanding of sensitization and the impact of desensitization protocols on antibody levels. Most advanced HLA laboratories are evolving practices to determine the best combination of assays that can be used to monitor desensitization efficacy and/or AMR treatment.
The ability to predict the AMR risk using newer solid-phase assays is not yet established. The established pathological criteria for diagnosing AMR (C4d deposition, allograft dysfunction, and pathologic features of inflammation) are still regarded as the cornerstones required for AMR diagnosis [15]. There are several reports indicating a close association between anti-HLA antibody detection and the presences of AMR [16–18]. However, the relationship is far from clear. Most investigators believe that DSA titers are most predictive of AMR risk, but others have seen a less well established relationship between DSA detection and AMR risk [16–18]. This includes data from our group [7]. Despite these limitations, Reinsmoen et al. recently reported on attempts to develop a paradigm to predict AMR risk in patients undergoing desensitization [19].
Critical to the success of desensitization protocols and predicting AMR is the monitoring of antibody levels [15, 20–24]. Achieving acceptable DSA levels that allow for successful transplantation post desensitization and permit long-term graft function with a decreased risk for AMR are critical. Zachary et al. [24] have shown the initial titer and specificity of DSA are critical in determining the likelihood of successful desensitization. Quantitative solid-phase antibody methodologies have allowed for a more defined approach to monitor the feasibility and efficacy of the desensitization protocols and sensitivity to predicting AMR. Our group [15] recently showed that the strength of DSA as detected by a sensitive quantitative DSA tests allowed for successful transplantation after desensitization treatment with high-dose IVIG and rituximab and identification of patients at higher risk for AMR. Posttransplant DSA monitoring using this technique also identified points for therapeutic intervention aimed at improving early AMR diagnosis and potentially improving graft outcomes.
Although these values can be used as guidelines, the relevant cutoff values for each center need to be based on the immunosuppression and desensitization protocols implemented at that particular center and that program’s ability to handle high-risk patients. There are technical as well as immunosuppression protocol-dependent factors that influence the cutoff values, which must be established by each transplant center [20–23]. These results show that successful transplantation can be achieved with acceptable DSA levels. Our results are consistent with those of Zachary et al. [24], who reported that titer and specificity influenced the efficacy of desensitization protocols and transplant outcome. In our study, antibody strength appeared to be critical in determining the level at which successful transplantation could be accomplished. This approach is summarized in Figs. 1 and 2.
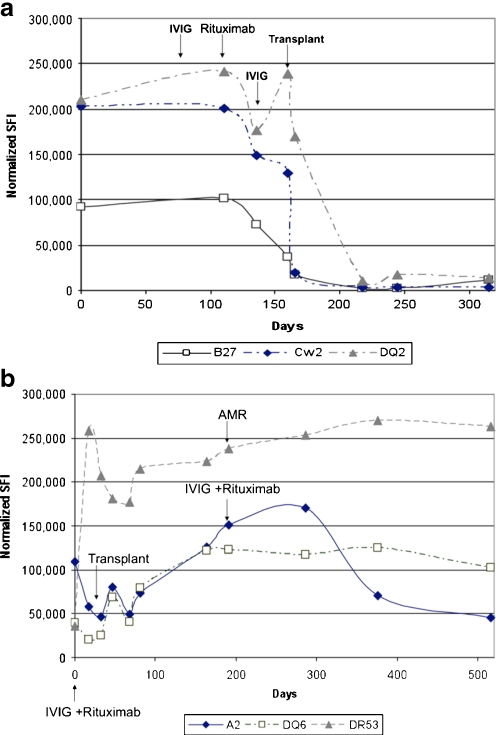
Fig. 1.
a Donor-specific antibody (DSA) pattern for a highly human leukocyte antigen (HLA)-sensitized patient who had awaited deceased-donor (DD) transplantation for >10 years. The patient was treated with intravenously administered immunoglobulin (IVIG) + rituximab as per protocol and received a DD transplantation shortly after completing desensitization. DSA levels rapidly decreased from >200,000 standard fluorescent intensity (SFI) units to unmeasurable. At the time of writing this article, the patient was >1 year posttransplant without antibody-mediated rejection (AMR), with a serum creatinine (Cr) 0.8 mg/dl. b DSA pattern from a patient who received a living-donor (LD) kidney transplant approximately 2 years ago. The patient exhibited DSAs to A2, DR53, and DQ6. After desensitization, good responses were seen, which allowed transplantation. Approximately 3 months posttransplant, the patient experienced AMR, with an increase in DSAs. These responded somewhat to IVIG + rituximab treatment. Serum Cr was stable at 1.4 mg/dl for 2 years, and no proteinuria has been observed
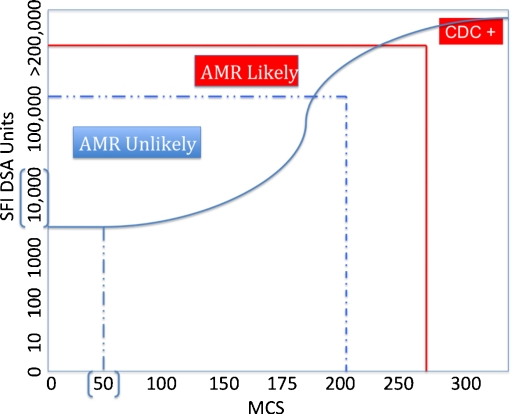
Fig. 2.
Relationship among donor-specific antibody (DSA), flow cytometry cross match (FCMX) results and risk for antibody-mediated rejection (AMR) in patients desensitized with intravenously administered immunoglobulin (IVIG) + rituximab. We show that reduction, but not elimination, of DSA to levels of ~100,000 standard fluorescent intensity (SFI) correlates with a FCMX of ~200–225 mean channel shifts (MCS). This usually allows transplantation of highly human leukocyte antigen (HLA)-sensitized patients with a low risk of AMR. However, patients who demonstrate DSA 100,000–200,000 SFI and FCMX >250 channel shifts (CS) are more likely to experience AMR. Patients who demonstrate complement-dependent cytotoxicity (CDC+) cross matches have FCMXs >300 MCS and DSA levels >200,000. These patients are at an extremely high risk for AMR if transplanted. The line shows the hypothetical relationship between DSA and FCMX results. A normal FCMX is defined as 50 CS or less and an SFI DSA level of 10,000 or less
Further, levels of DSA >105 standard fluorescent intensity (SFI) indicated the need for close posttransplant monitoring of antibody level and specificity. The posttransplant assessment of antibody course and specificity showed that patients without immune complications had similar antibody profiles. Patients with DSA levels <104 SFI units were at low risk for AMR, as were patients with DSA SFI between 104 and 105 with pretransplant cross matches <200 mean channel shifts (MCS). Both DSA and third-party antibody levels continued to decrease, eventually to below the 104 level considered to be flow cross-match negative. These patients’ antibody courses were monitored weekly for the first month posttransplantation and then monthly for 3 months. Patients with DSA >105 and donor-specific cross matches >200 MCS were considered at high risk for AMR and warranted more frequent antibody-level monitoring posttransplantation [15]. Figure 1 a and b shows the DSA course for two highly HLA-sensitized patients. Both were desensitized with IVIG + rituximab. The patient shown in Fig. 1a demonstrates an excellent response to desensitization therapy with elimination of DSA levels. This was associated with a rejection-free course. The patient depicted in Fig. 1b had reductions in DSA levels that allowed LD transplantation, but AMR episode occurred 3 months posttransplant that was associated with an increase in DSA. Retreatment resulted in reduction in DSA to HLA A2 but not DR53. The patient had a good clinical response and was nearly 2 years posttransplant at the time of writing, with serum creatinine 1.4 mg/dl and no proteinuria. Figure 2 summarizes the relationship among flow cytometry cross matches, complement-dependent cytotoxicity cross matches, and DSA. Briefly, we aim to reduce DSA levels to ≤100,000 SFI units to allow transplant to go forward. This is usually associated with a low risk of AMR. Posttransplant DSA monitoring allows us to plan biopsies in patients who show increases >150,000 SFI units, as these are very predictive of AMR episodes.
Lefaucher et al. [18] have shown that DSA at transplantation is a significant risk factor for AMR compared with patients without DSA (34.9% vs 3.1%, p < 0.0001). Eight-year graft survival was also less in the DSA+ vs DSA− group (67.9% vs 77.3%, p = 0.03). However, there was no difference in 8-year graft survival between patients in the DSA+ group who did not develop AMR vs those in the DSA− group (78.5% vs 77.3%, p = NS). These data are intriguing, as DSA presence alone was not a risk factor for long-term graft loss. These investigators also showed that DSA strength was significantly associated with AMR risk. In addition, recent data [17, 18] point to the importance of DSA monitoring during AMR onset as a predictive factor for responses to therapy.
This work has added to our understanding of how a combination of anti-HLA/DSA determinations can help predict posttransplant AMR risk. We use this paradigm in allocating organs to our highly HLA-sensitized patients who have completed desensitization. Of interest in our patient population is that most patients are transplanted with positive cross matches [T-cell flow cytometry cross match (FCMX)/B-cell FCMX] and have detectable DSA. However, only 23% have AMR episodes, suggesting that the desensitization protocols are likely altering other immune effector mechanisms responsible for the AMR process. Thus, DSA and other non-HLA-directed antibodies are a critical marker of sensitization and AMR risk but are not the sole mediators of this process. DSA titer appears to be the best marker for AMR risk, but clearly, other effector pathways, including complement activation and cell-mediated immunity with memory capacity that are not yet clearly defined, likely play an important role in increasing the risk for AMR development.
Non-HLA antibodies mediating AMR
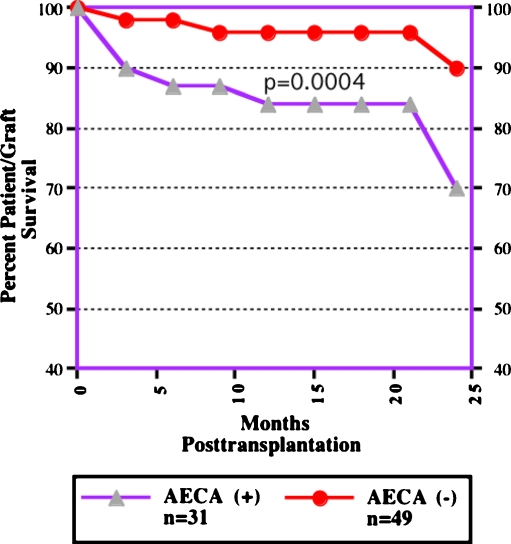
Endothelial cells express a number of antigens not present on lymphocytes, which could be targets of preformed antibodies and result in AMR. Recently, antibodies reactive with non-HLA target antigens expressed on donor endothelium have received closer attention as AMR mediators [25–29]. Our work and that of others have shown that antibodies directed at endothelial cell targets [antiendothelial cell antibody (AECA)] can damage endothelial cells and are closely associated with rejection episodes and poor graft survival in cardiac and kidney transplant recipients [25, 26]. It is also of interest that cytomegalovirus (CMV) infection is a potent inducer of polyclonal AECA responses in cardiac and renal allograft recipients [26]. Figure 3 shows this association and the outcomes of AECA + vs AECA–cardiac allograft recipients at 2 years [25]. Despite these early findings, the precise definition of antigenic targets of AECA was lacking. However, recent data suggest that a major target on endothelium is the angiotensin type 1 receptor (AT1R). Dragun et al. [27] recently identified AT1R antibodies in 16 patients who experienced severe AMR associated with malignant hypertension who did not have detectable anti-HLA antibodies. Indeed, we have patients who had similar presentations and who ultimately lost allografts from AMR without developing anti-HLA antibodies (Reinsmoen et al. unpublished results). Malignant hypertension with rapid onset of allograft dysfunction and thrombosis was also seen in these patients. Standard treatment with antilymphocyte globulin and high-dose steroids was ineffective. The efficacy of other therapies, including plasmapheresis, IVIG, and rituximab is unknown. The incidence and prevalence of AT1R antibodies in patients awaiting kidney transplantation is currently unknown. However, this could be an important assessment for patients, as newer, more rapid, diagnostics will soon be available [28, 29].
Fig. 3.
Outcomes of cardiac transplant recipients followed for >2 years. Patients were divided into those who had persistent demonstration of antiendothelial cell antibody (AECA+) posttransplant vs patients who never demonstrated positivity (AECA−) posttransplant. The importance of non-human leukocyte antigen (non-HLA) antibodies directed at endothelial cell targets not present on lymphocytes can cause antibody-mediated rejection (AMR) and graft loss [25]
Another important polymorphic antigen system expressed on endothelium is MICA. These antigens bind to NKG2D and are important in natural killer cell activation, thus playing and important role in innate immunity [30]. Recent data [31] suggest that kidney transplant recipients, especially retransplant recipients, demonstrating MICA antibodies had inferior allograft survival rates compared with non-MICA-positive patients. These data expand the need to test potential kidney recipients for MICA antibodies to prevent AMR. Of note, MICA antibodies are usually not complement activating; thus, biopsies may be C4d- [30, 31]. Recent reports also describe a novel flow cytometric test that detects antibodies directed against donor-specific endothelial cells [28]. These investigators use beads coated with antibodies to the endothelial cell antigen Tie-2 to isolate circulating endothelial cell precursors from the peripheral blood of potential donors. A simultaneous T-cell, B-cell, and donor-specific endothelial-cell cross match can be performed. Although initial data are interesting, the broader application of this technique will require verification that detection of antibodies to endothelial-specific antigens (i.e. MICA and AT1R antibodies) can be done reliably and will add to AMR prevention.
Treatment options for patients with AMR
Intravenously administered immunoglobulin (IVIG)
IVIG is now recognized as an important “natural” regulator of immunity and inflammation [32–36]. IVIG has become an essential element of all desensitization and AMR treatment protocols, although the exact mechanism(s) of action are not well defined. Recent data suggest that modification of complement activation and cell-mediated immunity are critical components of the anti-inflammatory and immunoregulatory actions of IVIG [37–39].
Modification of complement-mediated injury by IVIG
Immunoglobulin molecules are known for their ability to activate complement as part of the body’s innate defense mechanism against invading pathogens [32–36]. However, the concept that Ig molecules can also inhibit complement activation and “scavenge” anaphylatoxins and active complement components has only recently been identified [32, 37–42]. Preincubation of C3a/C5a with F(ab)’2 fragments of IgG results in binding and inhibition of inflammatory activity [42]. The Fc fragment of IgG molecules also shows significant ability to bind to C3b and C4b. Data from an experimental model of stroke injury shows that animals pretreated with IVIG or treated within 1–2 h post ischemia/reperfusion (I/R) injury had significant reductions in infarct size compared with control animals. It was also demonstrated that the major mediator of I/R injury in the brain was complement and that IVIG was a powerful scavenger of C3b produced in the ischemic brain [32, 37–42]. IVIG treatment has also shown efficacy in limiting antibody-mediated complement activation in several other experimental models [32, 42]. These observations have clear implications for preventing and treating AMR.
Regulation of cell-mediated immunity by IVIG
One of the most important findings regarding immune regulation by IVIG is recent evidence that IVIG actually induces the critical inhibitory receptor FcγRIIb on immune cells [32–36]. Ravetch et al. [43–47] have shown that the beneficial effects of IVIG can actually be recapitulated by recombinant immunoglobulin G (IgG) Fc fragments. These investigators postulate that the anti-inflammatory activity of intravenously administered Ig (IVIG) results from a minor population of the pooled IgG molecules that contains terminal alpha 2,6-sialic acid linkages on their Fc-linked glycans. They have also shown that the anti-inflammatory properties can be recapitulated in mouse models with a fully recombinant preparation of appropriately sialylated IgG Fc fragments. More importantly, the authors have recently demonstrated that these sialylated Fcs bind to a specific C-type lectin, SIGN-R1 (specific ICAM-3 grabbing non-integrin-related 1) expressed on macrophages in the splenic marginal zone in mice. Splenectomy, loss of SIGN-R1(+) cells in the splenic marginal zone, blockade of the carbohydrate recognition domain (CRD) of SIGN-R1, or genetic deletion of SIGN-R1 abrogated the anti-inflammatory activity of IVIG or sialylated Fc fragments. A human ortholog of SIGN-R1, DC-SIGN, displays a similar binding specificity to SIGN-R1 but differs in its cellular distribution, potentially accounting for some of the species differences observed in IVIG efficacy. These studies are important since they are the first to identify a receptor for sialylated Fcs. Recombinant sialylated Fcs or IVIG products enriched for sialylated IgG molecules could represent potential therapeutic agents that could eventually replace IVIG since sialylated Fc population shows equal efficacy to IVIG in significantly reduced dose levels [45–47]. To date, these experiments have only been conducted in animal models and their application to human disease is still questionable [36]. Despite these concerns, this body of work represents a major advancement in our understanding of how IVIG regulates cell-mediated immunity and how IVIG works to regulate alloimmune responses.
Use of IVIG for treating AMR
Our group was the first to report on the use of IVIG for treating AMR in kidney and heart allograft recipients with antibody-mediated rejection [48]. Although this experience is now more than a decade old, it was useful in showing that AMR episodes could respond to IVIG and pulse methylprednisolone, although the mechanism(s) of action was not appreciated at that time. The first patient treated with high-dose IVIG for resistant rejection (1994) had failed OKT3 therapy and two courses of pulse methylprednisolone. The features of AMR were not appreciated at that time, but the patient was noted to develop a positive posttransplant complement-dependent cytotoxicity (CDC) cross match with donor cells that showed in vitro inhibition with IVIG. The decision to treat with high-dose IVIG was based on this observation and the patient’s unresponsiveness to other treatments. The patient had a dramatic response to IVIG treatment, with serum creatinine decreasing from 3.0 mg/dl to 1.0 mg/dl over 3–4 days. The patient had no further AMR episodes and has a serum creatinine 1.7 mg/dl 15 years posttransplant. At that time, we felt this represented a major new approach to cross-match-positive rejection episodes, and other patients were treated with high-dose IVIG for severe rejection episodes associated with cross match positivity, as described [14, 48]. Other groups have also described the benefits of high-dose IVIG in treating resistant AMR episodes [18, 49, 50]. IVIG + pulse methylprednisolone with/without plasma exchange was used as our primary treatment for AMR until 2004 [14]. Lefaucher et al. [51] recently reported on a retrospective comparison of high-dose IVIG alone (12 patients) vs IVIG + rituximab + plasma exchange (12 patients) for treating AMR. The IVIG-alone group was treated between January 2000 and December 2003, whereas patients receiving combined therapy were treated from January 2004 to December 2005. The investigators found that the combined therapy was superior to IVIG alone in providing improved graft survival at 36 months (91.7% combined vs 50% IVIG alone, p = 0.02) and providing long-term suppression of DSA levels. Although this is not a randomized study and the number of patients is small, these findings would support our own observations that led us to change our approach to AMR treatment in 2004. What these observations suggests is that combination therapies appear to offer superior outcomes in terms of modification of DSA levels and improving long-term allograft survival.
Rituximab for treating AMR
Rituximab is a chimeric anti-CD20 (anti B-cell) monoclonal antibody that is approved for treating lymphoma. This antibody efficiently eliminates B cells, as the CD20 antigen is expressed early in B-cell ontogeny but is absent on mature plasma cells [52]. Rituximab has also been approved for use in rheumatoid arthritis and has demonstrated significant benefit in a number of autoimmune and inflammatory disorders [53–55]. Of note is the demonstrated benefit in vasculitic disorders that does not always correlate with reduced pathogenic antibody [54, 55]. Recent clinical data suggest that the beneficial effects of rituximab may be due to depriving T cells of antigen-presenting cell (APC) activity provided by antigen-specific B cells, thus altering effector functions and inducing a regulatory profile [56, 57]. These data suggest that the beneficial effects of rituximab on autoimmune disease are more likely related to modification of dysfunctional cellular immunity rather than simply a reduction in antibody.
The variable region of rituximab binds to CD20 and marks the cell for destruction by three different mechanisms: antibody-dependent cell-mediated cytotoxicity (ADCC), CDC, and cell-mediated apoptosis via CD20 cross-linking [52, 54]. ADCC occurs by binding of the Fc portion of rituximab to Fcγ receptors on NK cells, macrophages, and monocytes. These cells then act to destroy the B cell bound by the monoclonal antibody. CDC is mediated by activation of the complement cascade by the Fc portion of anti-CD20, ultimately resulting in the assembly of the membrane attack complex and cell lysis. Finally, cross-linking of bound CD20 proteins causes an influx of calcium, leading to activation of caspases resulting in cell apoptosis.
Rituximab causes a profound and sustained depletion in the number of circulating B cells. It also decreases B-cell populations in lymph nodes and spleen. A recent study by Genberg and colleagues evaluated the pharmacodynamics after a single dose of rituximab (375 mg/m2) in renal transplant recipients [58]. B cell elimination was rapid, occurred in the peripheral blood over 1−3 days, and was prolonged. B-cell populations did not begin to reemerge until after 1 year and remained suppressed for 2 years. This is longer than what is observed in patients with lymphoma or rheumatoid arthritis. Notably, B-cell lymphopenia was present at baseline in the renal transplant population. The delayed recovery of B cells may possibly be related to maintenance immunosuppression. Rituximab also leads to a significant reduction of B cells in lymph nodes, although they were not completely eliminated. It is suggested that the densely populated lymph node is more difficult to penetrate and may require a higher dose of rituximab.
Elimination of some B-cell populations also occurs in the spleen, but not uniformly. Ramos et al. demonstrated the effect of rituximab in the spleens of individuals who underwent desensitization [59]. These investigators quantified B cells in spleens removed from four groups of patients: those who underwent splenectomy for trauma (control group); those who underwent desensitization with plasmapheresis (PP) low-dose IVIG with subsequent splenectomy at the time of transplant (PP/IVIG group); those who underwent desensitization with PP, low-dose IVIG, and rituximab who also had a splenectomy at the time of transplant (PP/IVIG/rituximab group); and those who received rituximab, low-dose IVIG and rabbit antithymocyte globulin (rATG) but not splenectomy (combination group). Splenectomy in the combination group was done in the setting of refractory AMR and inadequate response to PP prior to transplant. Naïve B cells (CD20+) were significantly lower in the spleens of those who received rituximab. There was no difference in CD20+ B-cell depletion between the two rituximab-treated groups, suggesting that the addition of rATG in the combination group did not have an effect on this population of cells. Plasma cells persisted despite treatment with rATG, maintenance immunosuppression, and rituximab. The effect on memory B cells (CD27+) is of interest. In the study by Ramos et al., there was a trend toward a decrease in memory B cells in the combination group compared with the IVIG/PP/rituximab group [59]. From other studies, B-cell depletion with rituximab is often associated with delayed recovery of memory B cells (CD27+) and depletion of pathogenic B cells [60, 61].
Rituximab was initially used for treating refractory rejection based on the demonstration of intrarenal B-cell infiltrates in both cell-mediated rejection (CMR) and AMR [62]. The presence of B-cell infiltrates in acute rejection is a risk factor for steroid resistance and is associated with a worse prognosis. It is unclear whether the B cells present within the allograft function as plasma-cell precursors, antigen-presenting cells, and/or provide costimulatory signals to T cells. B cells also produce inflammatory cytokines that may directly injure the allograft. The effect of rituximab on B-cell infiltration was reported in two studies. Zarkhin et al. prospectively studied pediatric renal transplant recipients who had biopsy proven CMR with B-cell infiltrates [63]. Patients were randomized to receive four weekly doses of rituximab (375 mg/m2) plus standard treatment versus standard treatment alone. The B-cell infiltrate was abolished in all cases after rituximab treatment, with improvement in renal function. Steinmetz et al. identified nine patients with vascular allograft rejection and B-cell clusters present in biopsy specimens [64]. Patients who received one dose of rituximab (375 mg/m2) in addition to conventional treatment had complete resolution of the B-cell infiltrate. Both studies were small, and no differences were seen in clinical outcomes between the groups. It is notable that complete resolution of the B-cell infiltrate was seen whether patients received one dose of rituximab or four.
Many recent reports support the clinical efficacy of rituximab for treating AMR. Becker et al. initially reported the benefit of rituximab in treating refractory rejection [65]. The investigators evaluated 27 patients who received rituximab for refractory AMR diagnosed by the presence of thrombotic microangiopathy (TMA) or endotheliitis in renal allograft biopsies. Patients received treatment with steroids, PP, and/or antithymocyte globulin (ATG) without improvement in creatinine prior to receiving a single dose of rituximab (375 mg/m2). There were three graft losses. The 24 successfully treated patients had good allograft function at the time of discharge after rituximab treatment. Other recent reports also document a beneficial effect of rituximab for refractory AMR [66–69].
Earlier reports did not clearly differentiate between CMR and AMR. Indeed, in the case series reported by Steinmetz et al. [64], seven of the nine patients had positive staining for C4d. There are numerous case reports describing the benefit of rituximab specifically for treating AMR. AMR has classically been diagnosed by allograft dysfunction, the presence of the complement fragment C4d in peritubular capillaries (PTC), and identification of donor-specific or antiendothelial-cell antibodies. The effectiveness of rituximab is reported in combination with IVIG, PP, and/or steroids [66, 68–73]. The ameliorative effects of rituximab on AMR are likely multifactorial. In addition to B-cell depletion and reduced DSA, disruption of T-cell costimulator and APC activities mediated by B cells are likely altered and result in diminished T-cell effector functions. Optimal treatment of AMR probably requires a combination of rituximab with PP and low-dose IVIG or with high-dose IVIG (1–2 gm/kg) due to the inability of rituximab to deplete CD20-negative plasma cells that continue to produce DSA and mediate graft injury.
Our center has extensive experience with HS patients who received kidney transplants after desensitization with IVIG and rituximab [7]. Recently, we evaluated 123 HS patients transplanted after desensitization (July 2006 through February 2009). Twenty-two patients developed AMR posttransplant, usually within the first month. All were treated with a combination of steroids (10 mg/kg daily x 3), IVIG 2 g/kg (maximum dose 140 g × 1), and rituximab (375 mg/m2 × 1). Some patients also received PP, and two underwent splenectomy. Six of 22 patients (27%) lost their allograft to severe AMR, usually within 1 month. Thus, a 73% survival rate for severe AMR was seen in this high-risk group [73].
Kaposztas et al. reported 2-year outcomes in their recent retrospective study looking at 54 patients treated for AMR [66]. Group A had 26 patients who underwent treatment with PP and rituximab, and group B had 28 patients who received PP without rituximab. Patients who had low serum IgG levels also received IVIG. Two-year graft survival was significantly better in the group that received rituximab (90% vs 60%), with the difference attributed to rituximab. A trend toward improved graft survival was also seen in those who received IVIG (p = 0.050). This retrospective study has one of the largest cohorts reported to date and supports the use of rituximab for treating AMR, with good short-term allograft survival; however, many patient variables were not consistent between the groups. Mulley et al. [69] recently reported a case series of seven patients with refractory AMR who responded to treatment with a single low dose of rituximab (500 mg). All patients recovered renal function, with patient and graft survivals at a mean 21 months’ follow-up of 100%. Three patients had significant viral infections, but all recovered. Recent data from experimental primate islet transplants show that the addition of B-cell depletion to standard immunosuppression results in significant prolongation of islet allograft survival, with inhibition of alloantibody responses [74]. In addition, Kessler et al. [75] recently reported the complete reversal of an AMR episode of an islet transplant using the combination of IVIG + rituximab. Complete DSA ablation was also noted in association with allograft recovery.
Therapeutic options treating AMR
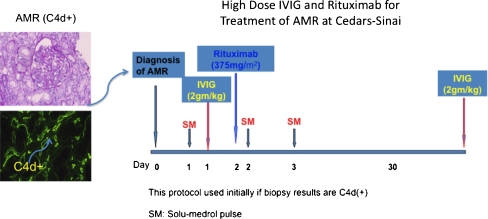
Based on our experience [14, 73] and a review of the literature, we propose the following treatment approaches for AMR management. Patients who develop allograft dysfunction associated with risk factors for acute AMR (DSA positivity, history of desensitization, previous transplants) and show biopsy evidence of antibody-mediated injury (C4d+) with minimal pathologic features [acute tubular necrosis (ATN)-like minimal changes] are treated with a combination of pulse methylprednisolone, high-dose IVIG (2 g/kg), and rituximab (375 mg/m2 × 1) (Fig. 4). This approach is usually sufficient to reverse most AMR episodes with the clinical and pathological features described above.
Fig. 4.
Cedars-Sinai Medical Center protocol for treating antibody-mediated rejection (AMR) that is C4d+. After AMR diagnosis by biopsy, patients are treated with pulse Solumedrol (SM) (10 mg/kg) daily × 3 on day 1 and intravenously administered immunoglobulin (IVIG) 2 g/kg (maximum dose 140 g), followed on day 2 by rituximab 375 mg/m2 . A repeat IVIG dose is usually given at 30–60 days after AMR diagnosis
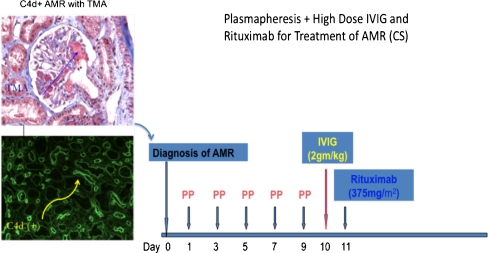
Patients who present with more severe clinical features (rapid cessation of renal function posttransplant associated with diffuse C4d+ staining, DSA positivity, and evidence of capillary and/or glomerular inflammation with thrombosis) are best treated with plasma exchange followed by IVIG and rituximab (Fig. 5). The most important clinical determinant of which protocol to use is the rapidity of onset of graft dysfunction posttransplant. It is important to understand that therapy should be instituted rapidly in the at-risk population, even if DSA levels and renal biopsy results are not available. Delays in treatment initiation can result in irreversible graft loss. The most important preventative measure in desensitized patients is to proceed to transplant only after DSA levels have fallen into what is considered an acceptable range. This is critical, as CMX and DSA levels are rarely negative by traditional standards after desensitization. Thus, proper patient preparation for incompatible transplantation is the best assurance that AMR is not likely to occur (Figs. 1 and 2) [7, 15]. The above protocols are those developed at our institution and by no means are the only way to approach AMR treatment. A more definitive summary of other approaches has been recently published [18].
Fig. 5.
Cedars-Sinai Medical Center protocol for treating more severe antibody-mediated rejection (AMR) associated with glomerular thrombi and graft dysfunction [thrombotic microangiopathy (TMA)]. Here, early plasmapheresis (PP) is essential to remove antibody and other inflammatory mediators before treatment with intravenously administered immunoglobulin (IVIG) and rituximab. In our protocol, we do not use low-dose IVIG after each plasmapheresis but replace it with albumin and fresh-frozen plasma. IVIG 2g/kg (maximum dose 140 g) is given at the completion of plasmapheresis treatment, followed by rituximab 375 mg/m2. Renal function and donor-specific antibodies (DSA) are monitored posttreatment
Chronic antibody-mediated rejection
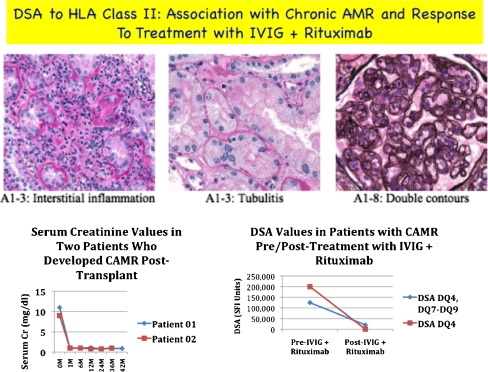
Chronic antibody-mediated rejection (CAMR) is defined by criteria set forward in Banff in 2007 [15]. CAMR has a poor prognosis, with no well-defined treatment protocol. A pilot study, conducted by Billing et al., investigated the use of IVIG with rituximab in six patients (ages 10–26) who had evidence of CAMR [76]. The patients received four weekly doses of IVIG (1 g/kg) followed by one dose of rituximab (375 mg/m2). All patients showed >40% positive C4d staining of PTC. All biopsies had varying degrees of CD20-positive infiltrates. Allograft function stabilized or improved in four patients after 1 year of observation. There was one graft loss at 18 months, and one patient did not respond. Five out of six patients had complete depletion of B cells in the peripheral blood. The two patients who did not respond had the highest degree of transplant glomerulopathy. Fehr et al. [74] recently reported a group of four patients with CAMR who were treated with a combination of IVIG + rituximab. All four patients had significant clinical improvement, although one patient developed an acute rejection episode 12 months later. One patient also developed what was described as rituximab-associated lung toxicity, which resolved. The authors concluded that this approach may represent an important treatment for CAMR. Our experience is similar to the above case reports. Data from two patients with CAMR are shown in Fig. 6. Briefly, both patients underwent desensitization >3 years before proteinuria onset. Neither patient had impaired renal function, but significant biopsy findings were seen, and both had significant elevations in DSA levels associated with the proteinuria. As can be seen from the figure, both patients had significant reductions in DSA levels after treatment with IVIG + rituximab and continue to show normal kidney function with reductions in proteinuria.
Fig. 6.
Outcomes of two patients who developed class II donor-specific antibodies (DSA) 3–4 years posttransplant associated with the onset of proteinuria with minimal changes in allograft function. The biopsy for one patient is shown, but both patients had similar findings of transplant glomerulopathy. Both patients showed a significant reduction in class II DSAs, with stabilization of renal function and some reduction in proteinuria after treatment with intravenously administered immunoglobulin (IVIG) + rituximab
In summary, reports have demonstrated the efficacy of rituximab for treating refractory rejections, rejection that contains B-cell infiltrates, AMR, and CAMR. The available evidence suggests improved creatinine and allograft survival in the short- and medium term. The combination of IVIG + rituximab appears to be a promising approach to reduce DSA and treat AMR and CAMR [76, 77]. Plasmapheresis has an important role in managing rapid-onset AMR associated with TMA or other significant pathologic features of vasculitis. Clearly, the combination of these therapies appears to have benefits over the use of any one agent alone. As more data are accumulated, a better understanding of how to construct treatment paradigms will emerge.
Evolving approaches treating AMR
Bortezomib
Bortezomib is a modified dipeptidyl boronic acid analog that binds selectively and reversibly to the 26S proteasome. Proteasomes are abundant in both the cytoplasm and nucleus of cells and act to degrade ubiquitinated proteins and activate cytokine signaling pathways through inhibitor of Kappa B (ikB) degradation, subsequently allowing nuclear factor Kappa B (NF-κB) signal transduction in the nucleus. This important signaling pathway is critical for transducing nuclear signals of most cytokines. Inhibition of the 26S proteasome prevents degradation of key proteins and affects multiple signaling cascades within the cell, which ultimately lead to cell death via apoptosis. Bortezomib acts in the bone marrow microenvironment by inhibiting the binding of myeloma cells to bone marrow stromal cells. Importantly, bortezomib causes plasma-cell apoptosis in bone marrow, which can inhibit antibody production [78]. Bortezomib also has an anabolic effect on bones, inhibiting human osteoclast activity and stimulating osteoblast function. Bortezomib is primarily metabolized by cytochrome P450 (CYP) enzymes CYP3A4, CYP2C19, and CYP1A2, with a minor amount of metabolism occurring via CYP2D6 and CYP2C9. The most frequently reported adverse events (incidence ≥30%) associated with the use of bortezomib are asthenic conditions (fatigue, weakness, malaise), gastrointestinal events (nausea, diarrhea, constipation, vomiting), peripheral neuropathy, pyrexia, thrombocytopenia, neutropenia, psychiatric disorders, and anorexia/decreased appetite. Drug-related adverse events could generally be managed by dosage modifications and supportive therapy [78].
A few reports have emerged regarding the use of bortezomib for modifying anti-HLA antibodies and treating cell-mediated and AMR episodes [79, 80]. Although the reports are of very small numbers of patients, the investigators found that bortezomib was effective in reducing anti-HLA antibody levels and reversing both cell-mediated and AMR episodes in all patients treated. Long-term follow-up was not available on these patients, but some patients had recurrence of anti-HLA antibody titers after treatment. These two studies are also complicated by the use of more standard therapies (plasmapheresis, ATG, rituximab, and IVIG) for these patients, as well. The authors also reported no significant adverse events with bortezomib therapy, contrasting with the relatively high AE profile reported in treating myeloma patients [78]. Clearly, more studies at multiple centers are required before this therapy can be recommended for AMR desensitization or treatment, but these reports cautiously suggest that bortezomib may have an important role in treating AMR in the future.
Eculizumab: an inhibitor of complement activation
Eculizumab (Alexion, Cheshire, CT, USA) is a humanized monoclonal antibody that binds to and subsequently prevents activation of C5 by the amplified C3 convertase molecules [81]. As a consequence of this inhibition, the generation of the phlogistic C5a anaphylatoxin and formation of the C5b-C9 membrane attack complex [C5b-C9 membrane attack complex (MAC)] is prevented. Eculizumab is approved by the US Food and Drug Administration for treating paroxysmal nocturnal hemoglobinemia (PNH) only, but there is a great deal of interest in investigating this molecule in other immunologic disorders in which complement activation plays an important role in pathogenesis.
One of the principal goals of desensitization is to reduce DSA levels to a point where accommodation will occur rather than AMR with graft loss. This is often a delicate balance that is not well understood. Accommodation refers to a process where DSA interacts with targets on vascular endothelium of the allograft but does not cause injury. Eventually, the allograft “accommodates” to the antibody and is able to maintain good function without evidence of allograft injury. Williams et al. [82] have shown that the difference between accommodation and AMR is the level of complement activation. In experimental models of xenotransplantation, the grafts that underwent AMR and loss showed deposition of all complement components, including C4d and—more importantly—C5b-C9 MAC. However, xenografts that demonstrated accommodation showed C4d deposits only. The authors concluded that incomplete complement activation by antibody is critical for accommodation development. Complement activation plays a critical role in mediating AMR after kidney transplantation. As eculizumab has the ability to inhibit C5b-C9 MAC and C5a generation, it should act as a strong accommodation promoter and prevent AMR. Fortunately, recent data presented by Stegall et al. [83] supports this contention. These investigators treated ten patients who underwent desensitization with plasmapheresis + IVIG with eculizumab after transplantation. Traditionally, the expected rate of AMR in this group of patients was 31–39%. After nearly 12 months of follow-up for all patients, none developed AMR. Several protocol biopsies showed C4d deposits but no evidence of AMR. This finding is suggestive of incomplete complement activation, which is permissive for accommodation. There were no significant adverse events associated with eculizumab use. Overall, this study is very encouraging, and this unique monoclonal antibody may add significantly to our armamentarium of agents to prevent and treat AMR. As current therapies for AMR desensitization and treatment are aimed at reducing pathogenic antibodies, it is not unreasonable to assume that the addition of a monoclonal antibody such as eculizumab, which inhibits terminal effectors, would act in concert with our established therapies. For example, a combination of high-dose IVIG + eculizumab could act to modify elements of cellular immunity, humoral immunity, and complement effectors. Confirmation of these ideas awaits clinical trials.
Acknowledgements
This work was supported by The Rebecca Sakai Memorial Fund and The Joyce Jillson Fund for Transplant Research. We also express our gratitude to the entire staff of the Transplant Immunotherapy Program, HLA Laboratory, and Transplant Immunology Laboratory at Cedars-Sinai Medical Center for their hard work and dedication.
Questions:
(Answers appear following the reference list)
Which of the following is NOT a characteristic of antibody-mediated rejection (AMR)?
C4d deposition
Thrombotic microangiopathy
DSA detection
T-cell infiltrates
Rapid onset of allograft dysfunction
- The most up to date way for detecting DSA is the use of CDC assays.
- True
- False
- When dealing with suspected AMR in a highly-HLA sensitized patient, the most important aspect is to wait for biopsy results before initiation of antirejection therapy for AMR.
- True
- False
- Which of the following would NOT be considered a primary therapy for treatment of AMR?
- Plasmapheresis + low-dose IVIG
- Thymoglobulin
- Rituximab
- High-dose IVIG
- High-dose IVIG + rituximab
- Chronic allograft nephropathy (CAN) is not mediated by immunologic mechanisms.
- True
- False
- Newer approaches to preventing and treating AMR include bortezomib and anticomplement therapies.
- True
- False
Footnotes
Answers
1. d
2. b
3. b
4. b
5. b
6. a
References
- 1.Evans RW, Manninen DL, Garrison LP, Jr, Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG. The quality of life of patients with end-stage renal disease. N Engl J Med. 1985;312:553–559. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 2.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–1343. doi: 10.1001/jama.270.11.1339. [DOI] [PubMed] [Google Scholar]
- 3.Russell JD, Beecroft ML, Ludwin D, Churchill DN. The quality of life in renal transplantation–a prospective study. Transplantation. 1992;54:656–660. doi: 10.1097/00007890-199210000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Organ Procurement Transplantation Network/Scientific Registry of Transplant Recipients: OPTN. (2009); http://www.optn.org/data/
- 5.United Network for Organ Sharing Data Base as of 5/30/2008. http://www.unos.org
- 6.Jordan SC, Tyan D, Stablein D, McIntosh M, Rose S, Vo A, Toyoda M, Davis C, Shapiro R, Adey D, Milliner D, Graff R, Steiner R, Ciancio G, Sahney S, Light J. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 7.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA, Melancon JK, Ratner L, Zachary AA, Haas M. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 9.Gloor JM, DeGoey SR, Pineda AA, Moore SB, Prieto M, Nyberg SL, Larson TS, Griffin MD, Textor SC, Velosa JA, Schwab TR, Fix LA, Stegall MD. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003;3:1017–1023. doi: 10.1034/j.1600-6143.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RA, Cooper M, Kraus E, Rabb H, Samaniego M, Simpkins CE, Sonnenday CJ, Ugarte RM, Warren DS, Zachary AA (2003) Renal transplantation at the Johns Hopkins Comprehensive Transplant Center. Clin Transpl: 199–213 [PubMed]
- 11.Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1:421–432. doi: 10.2215/CJN.01651105. [DOI] [PubMed] [Google Scholar]
- 12.Glotz D, Antoine C, Julia P, Pegaz-Fiornet B, Duboust A, Boudjeltia S, Fraoui R, Combes M, Bariety J. Intravenous immunoglobulins and transplantation for patients with anti-HLA antibodies. Transpl Int. 2004;17:1–8. doi: 10.1111/j.1432-2277.2004.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 13.Schweitzer EJ, Wilson JS, Fernandez-Vina M, Fox M, Gutierrez M, Wiland A, Hunter J, Farney A, Philosophe B, Colonna J, Jarrell BE, Bartlett ST. A high panel-reactive antibody rescue protocol for cross-match-positive live donor kidney transplants. Transplantation. 2000;70:1531–1536. doi: 10.1097/00007890-200011270-00023. [DOI] [PubMed] [Google Scholar]
- 14.Jordan SC, Vo AA, Tyan D, Nast CC, Toyoda M. Current approaches to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:408–415. doi: 10.1111/j.1399-3046.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 15.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 16.Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant. 2009;9:1063–1071. doi: 10.1111/j.1600-6143.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando) 2009;23:34–46. doi: 10.1016/j.trre.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C, Gautreau C, Charron D, Glotz D. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8:324–331. doi: 10.1111/j.1600-6143.2008.02342.x. [DOI] [PubMed] [Google Scholar]
- 19.Reinsmoen NL, Lai CH, Vo A, Cao K, Ong G, Naim M, Wang Q, Jordan SC. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization therapy. Transplantation. 2008;86:820–825. doi: 10.1097/TP.0b013e3181856f98. [DOI] [PubMed] [Google Scholar]
- 20.Bray RA, Nickerson PW, Kerman RH, Gebel HM. Evolution of HLA antibody detection: technology emulating biology. Immunol Res. 2004;29:41–54. doi: 10.1385/IR:29:1-3:041. [DOI] [PubMed] [Google Scholar]
- 21.Scornik JC, Clapp W, Patton PR, Werf WJ, Hemming AW, Reed AI, Howard RJ. Outcome of kidney transplants in patients known to be flow cytometry crossmatch positive. Transplantation. 2001;71:1098–1102. doi: 10.1097/00007890-200104270-00015. [DOI] [PubMed] [Google Scholar]
- 22.Wen R, Wu V, Dmitrienko S, Yu A, Balshaw R, Keown PA. Biomarkers in transplantation: Prospective, blinded measurement of predictive value for the flow cytometry crossmatch after negative antiglobulin crossmatch in kidney transplantation. Kidney Int. 2006;70:1474–1481. doi: 10.1038/sj.ki.5001785. [DOI] [PubMed] [Google Scholar]
- 23.Vasilescu ER, Ho EK, Colovai AI, Vlad G, Foca-Rodi A, Markowitz GS, D'Agati V, Hardy MA, Ratner LE, Suciu-Foca N. Alloantibodies and the outcome of cadaver kidney allografts. Hum Immunol. 2006;67:597–604. doi: 10.1016/j.humimm.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 2005;66:364–370. doi: 10.1016/j.humimm.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Fredrich R, Toyoda M, Czer LS, Galfayan K, Galera O, Trento A, Freimark D, Young S, Jordan SC. The clinical significance of antibodies to human vascular endothelial cells after cardiac transplantation. Transplantation. 1999;67:385–391. doi: 10.1097/00007890-199902150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda M, Petrosian A, Jordan SC. Immunological characterization of anti-endothelial cell antibodies induced by cytomegalovirus infection. Transplantation. 1999;68:1311–1318. doi: 10.1097/00007890-199911150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Dragun D, Hegner B. Non-HLA antibodies post-transplantation: clinical relevance and treatment in solid organ transplantation. Contrib Nephrol. 2009;162:129–139. doi: 10.1159/000170845. [DOI] [PubMed] [Google Scholar]
- 28.Breimer ME, Rydberg L, Jackson AM, Lucas DP, Zachary AA, Melancon JK, Visger J, Pelletier R, Saidman SL, Williams WW, Jr, Holgersson J, Tyden G, Klintmalm GK, Coultrup S, Sumitran-Holgersson S, Grufman P. Multicenter evaluation of a novel endothelial cell crossmatch test in kidney transplantation. Transplantation. 2009;87:549–556. doi: 10.1097/TP.0b013e3181949d4e. [DOI] [PubMed] [Google Scholar]
- 29.Grandtnerova B, Mackova N, Hovoricova B, Jahnova E. Hyperacute rejection of living related kidney grafts caused by endothelial cell-specific antibodies: case reports. Transplant Proc. 2008;40:2422–2424. doi: 10.1016/j.transproceed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Stastny P, Zou Y, Fan Y, Qin Z, Lavingia B. The emerging issue of MICA antibodies: antibodies to MICA and other antigens of endothelial cells. Contrib Nephrol. 2009;162:99–106. doi: 10.1159/000170842. [DOI] [PubMed] [Google Scholar]
- 31.Zou Y, Stastny P, Susal C, Dohler B, Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–1300. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 32.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88:1–6. doi: 10.1097/TP.0b013e3181a9e89a. [DOI] [PubMed] [Google Scholar]
- 33.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 34.Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Clynes R. Protective mechanisms of IVIG. Curr Opin Immunol. 2007;19:646–651. doi: 10.1016/j.coi.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Kaveri SV, Lacroix-Desmazes S, Bayry J. The antiinflammatory IgG. N Engl J Med. 2008;359:307–309. doi: 10.1056/NEJMcibr0803649. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, Magnus T, Chan SL, Jo DG, Ouyang X, Fairlie DP, Granger DN, Vortmeyer A, Basta M, Mattson MP. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci USA. 2007;104:14104–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arumugam TV, Selvaraj PK, Woodruff TM, Mattson MP. Targeting ischemic brain injury with intravenous immunoglobulin. Expert Opin Ther Targets. 2008;12:19–29. doi: 10.1517/14728222.12.1.19. [DOI] [PubMed] [Google Scholar]
- 39.Basta M, Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, Szebeni J, Alving CR, Carroll MC, Berkower I, Stojilkovic SS, Metcalfe DD. F(ab)'2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. 2003;9:431–438. doi: 10.1038/nm836. [DOI] [PubMed] [Google Scholar]
- 40.Arumugam TV, Woodruff TM, Lathia JD, Selvaraj PK, Mattson MP, Taylor SM. Neuroprotection in stroke by complement inhibition and immunoglobulin therapy. Neuroscience. 2009;158:1074–1089. doi: 10.1016/j.neuroscience.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basta M. Ambivalent effect of immunoglobulins on the complement system: activation versus inhibition. Mol Immunol. 2008;45:4073–4079. doi: 10.1016/j.molimm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sameulsson A, Towers TL, Ravetch JV. Anti-inflamatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;29:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 47.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan SC, Quartel AW, Czer LS, Admon D, Chen G, Fishbein MC, Schwieger J, Steiner RW, Davis C, Tyan DB. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation. 1998;66:800–805. doi: 10.1097/00007890-199809270-00017. [DOI] [PubMed] [Google Scholar]
- 49.Casadei DH, del CRM, Opelz G, Golberg JC, Argento JA, Greco G, Guardia OE, Haas E, Raimondi EH. A randomized and prospective study comparing treatment with high-dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid-resistant rejection. Transplantation. 2001;71:53–58. doi: 10.1097/00007890-200101150-00009. [DOI] [PubMed] [Google Scholar]
- 50.Luke PP, Scantlebury VP, Jordan ML, Vivas CA, Hakala TR, Jain A, Somani A, Fedorek S, Randhawa P, Shapiro R. Reversal of steroid- and anti-lymphocyte antibody-resistant rejection using intravenous immunoglobulin (IVIG) in renal transplant recipients. Transplantation. 2001;72:419–422. doi: 10.1097/00007890-200108150-00010. [DOI] [PubMed] [Google Scholar]
- 51.Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C. Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099–1107. doi: 10.1111/j.1600-6143.2009.02591.x. [DOI] [PubMed] [Google Scholar]
- 52.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 53.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 54.Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol. 2006;2:221–230. doi: 10.1038/ncpneph0133. [DOI] [PubMed] [Google Scholar]
- 55.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 56.Stasi R, Cooper N, Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, Amadori S. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112:1147–1150. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 57.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008;127:280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007;84:S33–36. doi: 10.1097/01.tp.0000296122.19026.0f. [DOI] [PubMed] [Google Scholar]
- 59.Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7:402–407. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 60.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 61.Levesque MC, St Clair EW. B cell-directed therapies for autoimmune disease and correlates of disease response and relapse. J Allergy Clin Immunol. 2008;121:13–21. doi: 10.1016/j.jaci.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O., Jr Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 63.Zarkhin V, Li L, Kambham N, Sigdel T, Salvatierra O, Sarwal MM. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant. 2008;8:2607–2617. doi: 10.1111/j.1600-6143.2008.02411.x. [DOI] [PubMed] [Google Scholar]
- 64.Steinmetz OM, Lange-Husken F, Turner JE, Vernauer A, Helmchen U, Stahl RA, Thaiss F, Panzer U. Rituximab removes intrarenal B cell clusters in patients with renal vascular allograft rejection. Transplantation. 2007;84:842–850. doi: 10.1097/01.tp.0000282786.58754.2b. [DOI] [PubMed] [Google Scholar]
- 65.Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996–1001. doi: 10.1111/j.1600-6143.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 66.Kaposztas Z, Podder H, Mauiyyedi S, Illoh O, Kerman R, Reyes M, Pollard V, Kahan BD. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 67.Faguer S, Kamar N, Guilbeaud-Frugier C, Fort M, Modesto A, Mari A, Ribes D, Cointault O, Lavayssiere L, Guitard J, Durand D, Rostaing L. Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation. 2007;83:1277–1280. doi: 10.1097/01.tp.0000261113.30757.d1. [DOI] [PubMed] [Google Scholar]
- 68.Celik A, Saglam F, Cavdar C, Sifil A, Atila K, Sarioglu S, Bora S, Gulay H, Camsari T. Successful therapy with rituximab of refractory acute humoral renal transplant rejection: a case report. Transplant Proc. 2008;40:302–304. doi: 10.1016/j.transproceed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Mulley WR, Hudson FJ, Tait BD, Skene AM, Dowling JP, Kerr PG, Kanellis J. A single low-fixed dose of rituximab to salvage renal transplants from refractory antibody-mediated rejection. Transplantation. 2009;87:286–289. doi: 10.1097/TP.0b013e31819389cc. [DOI] [PubMed] [Google Scholar]
- 70.Moscoso-Solorzano GT, Baltar JM, Seco M, Lopez-Larrea C, Mastroianni-Kirsztajn G, Ortega F. Single dose of Rituximab plus plasmapheresis in an HIV patient with acute humoral kidney transplant rejection: a case report. Transplant Proc. 2007;39:3460–3462. doi: 10.1016/j.transproceed.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 71.Wade E, Goral S, Kearns J, Pierce E, Trofe J, Bloom R, Kamoun M (2006) Experience with antibody-mediated rejection in kidney allograft recipients. Clin Transpl:439–446 [PubMed]
- 72.Yang YW, Lin WC, Wu MS, Lee PH, Tsai MK. Early diagnosis and successful treatment of acute antibody-mediated rejection of a renal transplant. Exp Clin Transplant. 2008;6:211–214. [PubMed] [Google Scholar]
- 73.Vo A, Cao K, Lai C-H, Reinsmoen N, Toyoda M, Ge S, Peng A, Villicana R, Jordan SC. Characteristics of patients who develop antibody-mediated rejection (AMR) post-transplant after desensitization with IVIG + rituximab: analysis of risk factors and outcomes, (Abstract #494) Am J Transplantation. 2009;9:334. [Google Scholar]
- 74.Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B, Wang Z, Paessler ME, Velidedeoglu E, Rostami SY, Yu M, Barker CF, Naji A. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13:1295–1298. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 75.Kessler L, Parissiadis A, Bayle F, Moreau F, Pinget M, Froelich N, Cazenave JP, Berney T, Benhamou PY, Hanau D. Evidence for humoral rejection of a pancreatic islet graft and rescue with rituximab and IV immunoglobulin therapy. Am J Transplant. 2009;9:1961–1966. doi: 10.1111/j.1600-6143.2009.02711.x. [DOI] [PubMed] [Google Scholar]
- 76.Billing H, Rieger S, Ovens J, Susal C, Melk A, Waldherr R, Opelz G, Tonshoff B. Successful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation. 2008;86:1214–1221. doi: 10.1097/TP.0b013e3181880b35. [DOI] [PubMed] [Google Scholar]
- 77.Fehr T, Rusi B, Fischer A, Hopfer H, Wuthrich RP, Gaspert A. Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation. 2009;87:1837–1841. doi: 10.1097/TP.0b013e3181a6bac5. [DOI] [PubMed] [Google Scholar]
- 78.Curran MP, McKeage K. Bortezomib: a review of its use in patients with multiple myeloma. Drugs. 2009;69:859–888. doi: 10.2165/00003495-200969070-00006. [DOI] [PubMed] [Google Scholar]
- 79.Trivedi HL, Terasaki PI, Feroz A, Everly MJ, Vanikar AV, Shankar V, Trivedi VB, Kaneku H, Idica AK, Modi PR, Khemchandani SI, Dave SD. Abrogation of anti-HLA antibodies via proteasome inhibition. Transplantation. 2009;87:1555–1561. doi: 10.1097/TP.0b013e3181a4b91b. [DOI] [PubMed] [Google Scholar]
- 80.Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, Roy-Chaudhury P, Govil A, Mogilishetty G, Rike AH, Cardi M, Wadih G, Tevar A, Woodle ES. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 81.Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet. 2009;373:759–767. doi: 10.1016/S0140-6736(09)60001-5. [DOI] [PubMed] [Google Scholar]
- 82.Williams JM, Holzknecht ZE, Plummer TB, Lin SS, Brunn GJ, Platt JL. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78:1471–1478. doi: 10.1097/01.TP.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]
- 83.Stegall M, Diwan T, Burns J, Dean P. Prevention of acute humoral rejection with C5 inhibition, (Abstract #174) Am J Transplantation. 2009;9:241. [Google Scholar]