Abstract
The present work focused on the histological and ultrastructural studies on haemopoiesis in the kidney of tilapia, Oreochromis niloticus. Haemopoietic tissue was found mainly in the head kidney and a small amount occurred in the mesonephros. The haemopoiesis of tilapia had the following series: erythropoiesis, granulopoiesis, thrombopoiesis, monopoiesis and lymphoplasmopoiesis. Erythropoiesis includes proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts, acidophilic erythroblasts and young and mature erythrocytes. The proerythroblasts were the largest cells in the erythropoietic series. During the maturation process both the nuclear and cellular size decreased gradually due to the chromatin condensation and the progressive substitution of cytoplasmic matrix with a large amount of haemoglobin. Granulopoietic series consisted of cells with variable shape and size at different stages of maturity from myeloblasts to mature granulocytes. The promyelocytes were the largest cells in the series and were characterised by the appearance of primary (azoruphilic) granules. The maturation process involved the appearance of specific granules in the heterophilic, eosinophilic and basophilic series. It is important to mention that eosinophilic granulocytes were the dominant granulopoietic series in the haemopoietic tissue (Ht) of tilapia. Lymphopoietic series consisted of lymphoblasts, large lymphocytes, small lymphocytes and active and inactive plasma cells. Thrombopoietic series consisted of thromboblasts, prothromboblasts and thrombocytes. Thrombocytes of tilapia were nucleated and possessed a spindle shape. Melanomacrophage centres were dominant among the Ht of the head kidney. Also, monocytes were detected and shown to be large cells with an indented nucleus and cytoplasm containing numerous vesicles of different sizes and a few lysosomes.
Keywords: Histology, Ultrastructure, Head kidney, Haemopoietic series, Oreochromis niloticus
Introduction
Although some differences are present, haemopoietic tissue (Ht) is considered to be the origin of blood elements in higher vertebrates and fish (Savage 1983; Meseguer et al. 1990; Zapata et al. 2006). In higher vertebrates the red blood cells, the white blood cells (granular and agranular) and lymphocytes originate from a common progenitor, the stem cell, which gives two components: one of them gives the elements consisting of the erythrocytes and myeloid lineage and megakaryocytes, and the other gives the elements consisting of the lymphoid lineage. Thus, the haemopoietic tissue could consist of the elements of the erythropoietic, myelopoietic, or lymphopoietic series, or all of them depending on the original cells (Majeti et al. 2007).
Head kidney (HK, or pronephros) in fish is a basic organ forming the blood elements (Willett et al. 1999; Rombout et al. 2005). The activity of the blood elements formation differs among teleost fish; it can be organ-forming erythroid lineages only in some fish, or all types of organ-forming blood cells in other fish (Meseguer et al. 1990; Willett et al. 1999; Esteban et al. 2000; Stephens et al. 2004).
Previous histological studies have been concerned with determining the haemopoietic tissue and the series of haemopoiesis in different teleosts. Boomker (1979) mentioned that the kidney (pronephros and mesonephros) and the spleen in Clarias garipinus and Sarothrodon mossambicus are the main organs forming blood, while the peritoneum membrane has a secondary function in this formation. Boomker (1981) also showed that the cells forming the erythroid and granuloid lineage are mostly found in mesonephros, while thrombocytes and monocytes are formed in pronephros and spleen.
Groman (1982) showed that the HK of marine striped bass contains haemopoietic tissue and a few urinary tubules. The haemopoietic tissue contains erythropoietic and granulopoietic series, lymphocytes and phagocytes. Zuasti and Ferrer (1989) were able to determine the erythropoietic series in the HK of Sparus aurata and showed the structural changes that take place during the maturation process, including the increase in the chromatin content of the nucleus and the gradual decrease in the cytoplasmic organelles and haemoglobin formation. The study by Esteban et al. (1989) showed an overlap between the erythropoiesis and thrombopoiesis in marine sea bass Dicentrarchus labrax. The granulopoiesis of D. labrax consists of promyelocytes, myelocytes, metamyelocytes and the mature cells of heterophils, eosinophils and basophils, and there are three types of heterophils (Meseguer et al. 1990).
Romano et al. (2002) studied the histology of the HK in two Antarctic fish and observed a difference in the shape of erythrocytes, growing granular and lymphatic cells, and this is considered an adaptation to the function of the HK at low temperatures, as it is a basic immune organ.
Studies of fish blood cells published to date have presented numerous problems deriving from both the nomenclature and the techniques used. A combination of quantitative and morphological methods is needed and this could be done by flow cytometry and then microscopically. The combined use of flow cytometry and electron microscopy makes it possible to characterise the different cell types and to monitor changes in blood cell populations. Flow cytometry proved to be a rapid and reliable method for monitoring cell population dynamics in the blood of different fish species (Esteban et al. 2000; Morgan et al. 2005).
Owing to the incomplete picture of the haemopoietic series in the Ht of fish, the present work aims to clarify as far as possible the complete series of haemopoiesis in the HK of one of the important economic fish, Nile tilapia (Oreochromis niloticus), using histological and ultrastructural studies.
Materials and methods
Fish samples of adult Nile tilapia, O. niloticus (ranging from 120 to 625 g, n = 30) were collected alive from a commercial fish farm, sacrificed by a sharp blow to the head and then dissected. For optical microscopy samples preparation, kidney were prefixed in situ for 5–10 min in 10% neutral formalin or Bouin’s fluid, then removed from the body and cut into small pieces, refixed for 24 h, dehydrated through a graded series of ethanol, embedded in paraffin and cut into 3- to 5-μm pieces. Histological sections were stained using haematoxylin and eosin. For transmission electron microscopy, the small pieces of HK were excised and immersed in 4% gluteraldhyde buffered at pH 7.2 with sodium cacodylate at 4°C for 3 h and postfixed in 1% osmium tetraoxide buffered at pH 7.4 with cacodylate at 4°C for 2 h and embedded in Epon. Semithin sections (0.5 mm) were stained with toludine blue and examined by binocular microscopy. Ultrathin sections were stained with lead citrate and uranyl acetate (Hayat 1989) and examined with electron microscopy (Jeol Jem-100c × II).
Results
The kidney of adult tilapia O. niloticus comprised the extra coelomic HK in the pharyngeal region and the trunk kidney (TK, or mesonephros) extending along the trunk region of the body. It was clear from the general histological examination that the HK of adult O. niloticus was mostly formed of haemopoietic tissue (Ht) and adrenal homology occupied the extravascular space and remnant of kidney tubules. In addition to numerous melanomacrophages centres (MMC), there were cells with eccentric nuclei and yellow brown granules in cytoplasm (Fig. 1).
Fig. 1.
Light micrographs of the cross sections of the head kidney in Oreochromis niloticus. a Head kidney: note the haemopoietic tissue (Ht), blood vessel (BV), kidney tubules (T) and sinusoids (s) (H&E, ×160). b Haemopoietic tissue in the head kidney: showing developing blood cells around sinusoids (s) and melanomacrophage centres (MMCs) (H&E, ×250). c Inter-renal tissue around blood vessels (BV) and haemopoietic tissue with scattered numerous mature acidophils (H&E, ×250. d Haemopoiesis in the HK: s sinusoids loaded with RBCs, E erythropoiesis, G granulopoiesis, B blast cell (toludine blue, ×1,000
The trunk kidney consists mainly of excretory tissue with a little scattered lymphoid tissue characterised by eccentric nuclei and with dark yellow granules.
Haemopoiesis
Ultrastructural observation detected the following haemopoietic series, erythropoiesis; granulopoiesis; lymphoplasmapoiesis and thrombopoiesis in the Ht in HK of tilapia.
Erythropoiesis
The erythroid series consisted of proerythroblast (Fig. 2a), basophilic erythroblast (Fig. 2b), polychromatic erythroblast (Fig. 2c), acidophilic erythroblast (Fig. 2d), erythrocytes (young and mature; Fig. 2d) and senile or atretic red blood cells (RBCs). The maturation process mainly involved a gradual decrease in both nuclear and cellular sizes with condensation of the nuclear chromatin, accumulation of ribosomes in rosettes and progressive substitution of cytoplasmic matrix with haemoglobin. A peripheral band of microtubules was almost seen in the stages of erythropoiesis.
Fig. 2.
Electron micrographs of developing erythrocytes (erythropoiesis) illustrating progressive reduction in cell size and cytoplasmic organelles, condensation of nuclear chromatin and cytoplasmic homogeneity with haemoglobin formation. a Proerythroblast has large spherical euchromatic nucleus with finely granulated chromatin and one large nucleolus (Nu) with reticular nucleolema and cytoplasm with numerous free ribosomes (R) with dense matrix and disorganised cristae, Golgi complexes (G) and small vacuoles (V) (×8,000). b Basophilic erythroblast: ovoid cell with cytoplasm loaded with free clustered ribosomes (R), large mitochondria (m), rER cisternae and oval nucleus (N) with marginal chromatin condensation (×8,000). c Polychromatic erythroblast (P): elongated cell with a dense nucleus (N), containing heterochromatin in coarse blocks and a dense ring around the edge, nearly homogeneous cytoplasm with plenty of polyribosomes (arrow) and acidophilic erythroblast (A), scattered ribosomes (R) in rosettes, deformed mitochondria (m) and atrophoid nucleus. G granulocyte (×8,000). d Groups of young and mature erythrocytes. Note acidophilic erythroblast (A), young erythrocytes (YE) with homogeneous cytoplasm and remnant of ribosomal rosettes. Mature erythrocytes (ME) have an oval shape and homogeneous cytoplasm nearly devoid of organelles. Heterochromatic nucleus (N) (×8,000)
Granulopoiesis
The granulopoietic seris of tilapia consisted of cells of variable shapes and sizes at different stages of maturity. These are myeloblasts (Fig. 3a), promyelocytes (Fig. 3b), myelocytes (eosinphilic (Fig. 3c, d), heterophilic (Fig. 4a) and basophilic (Fig. 4c), three types of metamyelocytes (eosinophils; Fig. 3d; basophils; Fig. 4d; and heterophils) and mature granulocytes (Figs. 3, 4).
Fig. 3.
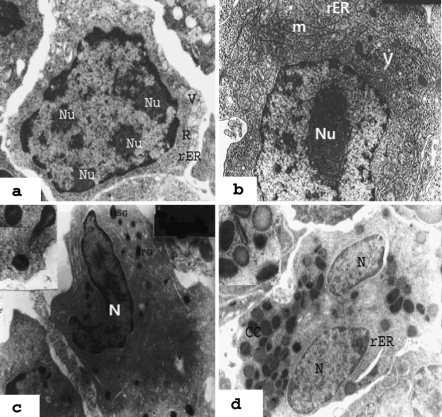
Electron micrographs of granulopoiesis in the haemopoietic tissue of the head kidney of Oreochromis niloticus. a Myeloblast: note the voluminous euchromatic nucleus, which contained 2–4 nucleoli (Nu). The cytoplasm characterised by scattered free ribosomes (R), rER strands and pinocytotic vesicles (V) (×8,000). b Promyelocytes: the largest cells in the granulopoietic series. Large euchromatic nucleus with prominent nucleolus (Nu), cytoplasm with numerous polyribosomes (arrow), mitochondria (m), primary granules (PG), rER and vesicles (V) (×8,000). c Eosinophilic myelocyte: with an oval band nucleus (N), mitochondria (m), polyribosomes, rER, small vesicles (v), primary granules (PG) and specific granules (SG) with light axial crystalloid core (×8,000). d Eosinophilic meta-myelocyte: note the numerous heterogenic granules, some with crystalline core (CC), predominant rER and bilobed nucleus (N) (×8,000). Inset: clarifies heterogeneous granules (×14,000)
Fig. 4.
Electron micrographs of granulopoiesis in the haemopoietic tissue of the head kidney of Oreochromis niloticus. a Heterophil myelocyte: nucleus with prominent nucleolus (Nu), cytoplasm with small primary granules and large dark homogeneous specific granules (G), mitochondria (m), rough endoplasmic granules (rER). M Macrophage (×8,000). b Neutrophil (heterophil): round cell with bilobed (constricted) nucleus (N) and homogeneously dense specific granules (g).m mitochondria, G Golgi (×8,000). c Basophilic myelocyte: with numerous large granules (g), developed cisternae of rER (arrow), numerous free ribosomes (R), large oval nucleus (N) with heterochromatin blocks. m mitochondria (×14,000). d Basophilic metamyelocyte: an elongated cell with an irregular surface with elongated bend nucleus (N) and condensed marginal heterochromatin. The cytoplasm contained few large mitochondria, lamellated cisternae of rER and numerous granules (g) variable in electron density. (×8,000). Inset: showing the fibrillary content of specific granules. (×14,000)
Myeloblast is the first morphologically identifiable granulocyte lineage and is characterised by a voluminous nucleus with numerous nucleoli and basophilic cytoplasm with free ribosomes, a few strands of rER and marginal pinocytotic vesicles (Fig. 3a). Promyelocytes are the largest cells of the granulopoietic series and are characterised by a central euchromatic nucleus with a prominent nucleolus and cytoplasm with plenty of cytoplasmic organelles (polyribosomes, rER, mitochondria and Golgi complex) with the appearance of primary organelles (Fig. 3b).
Maturation and differentiation of granulocytes were formed by a reduction in cell and nuclear size, nuclear condensation and lobulation and the appearance of secondary (specific) granules with a gradual increase in their number in mature granulocytes (eosinophils, basophils and heterophils; Figs 3c, d, 4).
It is interesting to mention that the developing and mature eosinophils were the dominant granulopoietic series in the Ht of O. niloticus (Fig. 1c).
Thrombopoiesis
Thrombopoiesis in the HK of O. niloticus and includes thromboblast (Fig. 5a), prothrombocytes (Fig. 5b) and thrombocytes (Fig. 5c).
Fig. 5.
Electron micrographs showing thrombopoiesis in the head kidney of Oreochromis niloticus. a Thromboplast: note the euchromatic nucleus with a prominent nucleolus (Nu), marginal band of microtubules (mt), polyribosomes (R), pseudopodia (P) and vesicles (v) (×10,000). b Prothrombocyte: irregular-shaped cell with surface-connected canalicular system (SCCS), large granules (G) with homogeneous or fibrillary content, rER cisternae (arrow), large and irregularly shaped nucleus (N) and marginal microtubules (mt) (×10,000). c Thrombocyte (B): spindle-shaped cell with large oval heterochromatic nucleus, homogeneous cytoplasm with medium electron density containing scattered mitochondria. Prothrombocyte (A): irregularly shaped cell contains large euchromatic elongated nucleus (N), numerous vesicles through the cytoplasm (arrow), Golgi complex (G) and numerous small mitochondria (m) (×10,000)
The maturation process includes cell and nuclear elongation development of a surface canalicular system (SCCS) and coated granules in prothrombocytes (Fig. 5b, c) and thrombocytes (Fig. 5c).
A marginal bundle of microtubules was clearly detected in thrombopoietic series and adult thrombocytes.
Lymphoplasmopoietic series
In the lymphopoietic series we can distinguish lymphoblasts (Fig. 6a), large lymphocytes (Fig. 6b), small lymphocytes (Fig. 6c) and plasma cells (Fig. 6d).
Fig. 6.
Electron micrographs showing lymphoplasmopoiesis in the head kidney of Oreochromis niloticus. a Lymphoblast: round cells with voluminous nucleus (N) with finely granular chromatin, prominent nucleoli, cytoplasm with a few large mitochondria (m) and free ribosomes (R). L lysosome (×10,000). b Large lymphocyte: the cell had a irregular outline with a large central nucleus (N) containing large blocks of heterochromatin and a moderate amount of cytoplasm filled with numerous ribosomes (R), a few mitochondria (m) and scattered vesicles and vacuoles (×8,000). c Small lymphocyte: irregularly shaped cells with radially arranged chromatin blocks in large nucleus (N) and cytoplasm with scattered free ribosomes (R), vacuoles (V) and numerous microvilli. Plasma membrane exhibits numerous short microvilli (mv) (×10,000). d Plasma cell: note the eccentric nucleus (N) with chromatin blocks and cytoplasm with abundant dilated rER, mitochondria (m) and paranuclear expanded Golgi complex (G) (×10,000)
Lymphoblast is a large round cell with a voluminous nucleus with finely granular chromatin and prominent nucleoli\ with scarce cytoplasm with free ribosomes, vesicles and a few large mitochondria (Fig. 6a). Lymphocytes characterised by nucleus have large blocks of heterochromatin and an ill-defined nucleolus with cytoplasm with numerous microvilli (Fig. 6c).
Macrophage and melanomacrophage centres
Light microscopy observations showed that numerous macrophages could be found as free macrophages or in aggregates as melanomacrophage centres (MMCs) in the head and trunk kidney of tilapia (Fig. 1b).
Ultrastructural examination revealed that MMCs consisted of irregularly shaped cells with pseudopodia-like extension, eccentric nucleoli and cytoplasm with heterogeneous populations (lysosomes, phagosomes, myelin figures, senile RBCs or plenty of melanine granules of variable size and electron density (Fig. 7a–d). These MMC cells were surrounded by a thin capsule of fibroblast (Fig. 7a).
Fig. 7.
Electron micrographs of melanomacrophage centres and macrophages in the haemopoietic tissue (Ht) of the head kidney of Oreochromis niloticus. a Melanomacrophage centre: surrounded by a thin layer of connective tissue capsule (arrow). It contains numerous melanomacrophage cells with melanin granules and heterogeneous cytoplasmic contents (×1,400). b Melanomacrophage cell: note the irregular surface with pseudopodial processes (Pp), nucleus (N), melanin granules (MG), rER (arrow) and vesicles (v) (×10,000). c Erythrophagoytosis: note the engulfed senile or atretic (RBCs) by macrophage. Large eccentric nucleus (N), phagocytic vacuoles (v), residual bodies (r) in the macrophage (×5,000). d Macrophage cell: note the macrophage with euchromatic eccentric nucleus (N) and cytoplasm filled with phagolysosomes (PL), Golgi complex (G), pseudopodial processes (Pp) and ribosomes (R)
Monocytes
Light microscopy observations demonstrate that a small number of monocytes were shown. Monocytes are large rounded cells with an irregular outline of thin microvilli.
Discussion
The teleost HK has been considered a haemopoietic organ similar to the bone marrow of higher vertebrates and has also been described as a primitive system (Tomonaga et al. 1973).
As in most teleosts, the HK of tilapia O. niloticus lies outside the peritoneal cavity close to the pericardium in a location similar to the embryonic pronephros and is not merely connected to the trunk kidney. The parenchyma of the HK is composed mainly of haemopoietic tissue in addition to adrenal homology (inter-renal tissue and chromaffin cells) and a few remnants of tubular segments with no glomeruli. On the other hand, in Clarias gariepinus the HK contained only lymphoid tissue and inter-renal tissue localised in paired organs, situated retroperitoneally anterior to the kidney (Vermeulen et al. 1995).
Ultrastructural examination revealed that haemopoietic tissue in the kidney of O. niloticus includes erythropoietic, myelopoietic, lympho-plasmopoietic and thrombopoietic series like that of other species of teleosts (Zuasti and Ferrer 1988; Belosevic et al. 2006). The presence of MMCs can also be observed (Oguri 1976; Agius 1980; Zuasti et al. 1986, 1989). The blood cells develop extravascularly and intermingled, unlike in mammals in which erythropoiesis occurs in clusters near the sinusoids while granulopoiesis takes place in other areas (Weiss and Chen 1975; Weiss 1988).
In the present study, erythropoiesis is produced continuously in the HK of O. niloticus and comprises proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts and erythrocytes. The nomenclature that we have adopted for the different stages is recommended by Klontz (1972) and accepted by Savage (1983). The basic cytological changes that occur during maturation of the erythropoietic series are reduction of cell size, heterochromatinisation of the nucleus and reduction of the cytoplasmic organelles with the progressive substitution of hemoglobin. These changes are similar to those described in other species of teleosts (Zapata 1980; Zuasti and Ferrer 1988, 1989; Esteban et al. 1989) and the maturation process terminates in the formation of oval nucleated erythrocytes in the blood vessels, as was reported by Romestand and Trilles (1984) and Esteban et al. (1989).
The ribosome content of vertebrate erythroid cells provides a good index of the degree of cellular maturity (Tooze and Davies 1976; Yamamoto and Iuchi 1976) and the occurrence of the polyribosomes is considered to be directly related to the synthetic activity of haemoglobin (Majeti et al. 2007). A progressive decrease in ribosomes and their association in polyribosomes was found from proerythroblasts to the young erythrocyte stage in the erythropoiesis of the species studied, which suggests a high synthetic activity from the early erythropoietic stages, but this is no longer retained in the late maturation stages.
As reported in the present study, previous studies revealed the presence of peripheral bands of microtubules in the immature stages of the erythropoiesis in other species such as Myxine glutinosa (Mattisson and Fange 1977); sea bass (Esteban et al. 1989); Oncorhynchus mykiss (Yamamoto and Iuchi 1976) that are comparable to the marginal band of microtubules characteristic of erythrocytes (Esteban et al. 1989), which is probably involved in the maintenance of the cellular shape and in intracellular transport, as has been suggested for other vertebrates (Majeti et al. 2007).
It is interesting to mention that deformed erythrocytes, which take the form of large spheroid cells with karyolytic nuclei (spherocytosis) were observed engulfed by macrophages in the MMCs.
The thrombocytes of fish should not be considered as the platelets of the higher vertebrates because they are true cells (Cannon et al. 1980; Savage 1983; Hightower et al. 1984). Thrombocytes have been described in goldfish by Weinreb (1963) as cells with dense chromatin and morphologically similar to lymphocytes. In tilapia, O. niloticus the thrombocytes are clearly differentiated from lymphocytes by their spindle shape, clear vacuoles, marginal microtubules and the electron-dense granules in the cytoplasm. These cells are similar to those described in other species of fish (Ferguson 1976; Daimon et al. 1979; Morrow and Pulsford 1980; Barber and Westerman 1981; Zuasti and Ferrer 1988). Following the erythrocytes, the thrombocytes are the most numerous cell type in fish blood (Murray 1984). However, its morphology and function in the teleosts have been studied little compared with erythrocytes and leukocytes (Zapata 1980). The structure and the origin of the teleost thrombocytes and mammalian blood platelets are quite different and thus may reflect evolutionary differences (Daimon et al. 1979).
Only one maturation stage has been described from blast cells to adult thrombocytes and has been termed the prothrombocyte (Romestand and Trilles 1984). Esteban et al. (1989) found that thrombopoiesis in the HK of sea bass consists of immature prothrombocytes, mature prothrombocytes and thrombocytes, and that the maturation process is associated with an increase in the nuclear and cytoplasmic electron density, a progressive and marked development of the SCCS, peripheral vesicles and coated granules, as reported in the present study.
Ultrastructural descriptions of granulopoiesis in fish are scarce, their nomenclature and characterisation being unclear (Zapata 1979; Savage 1983; Zuasti and Ferrer 1988). Granulopoietic stages have been characterised by the size, structure and staining properties of the cells in some teleost species (Bielek 1981), whilst some morphological similarities between mature and immature cells have been considered in others (Savage 1983; Zuasti and Ferrer 1988). Meseguer et al. (1990) name promyelocytes the first cell type with granules, whereas a new granule population characterises the myelocytes. Metamyelocytes and mature granulocytes are established according to granule density, the number of nuclear lobes and the presence of specific granules, as was reported in the present study and for other vertebrates (Curtis et al. 1979; Brederoo et al. 1986).
In the present study, the granulopoiesis in the HK of tilapia includes heterophilic (neutrophilic), eosinophilic and basophilic series, as described in other teleosts (Zapata 1979; Meseguer et al. 1990), although more than three granulocyte types have been described (Morrow and Pulsford 1980; Mainwaring and Rowley 1985; Parish et al. 1985, 1986) and one or two granulopoietic series have frequently been found (Barber and Westerman 1981; Hyder et al. 1983; Savage 1983; Hofte et al. 1984; Bayne 1986).
The ultrastructural feature of heterophilic myelocytes and metamyelocytes have only been reported in a few teleost species (Zapata 1979; Bielek 1981; Savage 1983; Zuasti and Ferrer 1988) being noticeable by rER and the electron-dense granules with fibrillary or crystalline inclusions (Zapata 1979; Bielek 1980, 1981; Cannon et al. 1980; Zuasti and Ferrer 1988). On the contrary, heterophilic myelocytes and metamyelocytes show indented nuclei and electron-dense homogeneous granules of variable size.
The O. niloticus heterophils display a similar ultrastructure to that formed in heterophilic granulocytes in other teleosts (Cannon et al. 1980; Savage 1983; Meseguer et al. 1990). In salmonids and sea bass the heterochromatic nucleus shows two or three lobes (Bielek 1980; Meseguer et al. 1990), whilst a round or indented nucleus was present in cyprinids (Bielek 1981), sea bream (Zuasti and Ferrer 1988) and in the tilapia of the present study.
Three types of granule are recorded in some teleosts such as in mature neutrophils (Meseguer et al. 1991) in sea bass, whilst one or two granular types have been found in other teleosts (Cannon et al. 1980; Bielek 1980, 1981; Cenini 1984; Zuasti and Ferrer 1988), similar to what has been recorded in this study.
Eosinophils or acidophils may be present or absent in fish peripheral blood (Watson et al. 1963; Sherburne 1974; Ellis 1977), the mature acidophils being the least frequent (Lester and Desser 1975; Zapata 1979; Zuasti and Ferrer 1988) and the only granulocyte type in the circulating blood of Fundulus heteroclitus, Fundulus mayalis and Cyprinodon variegatus (Gardner and Yevich 1969). Furthermore, numerous mature acidophils are present in inflammatory response (Gardner and Yevich 1969; Lester and Desser 1975). Also, two types of acidophilic granulocytes have been observed in some fish (Morrow and Pulsford 1980). In this study it was mentioned that developing and mature eosinophils were the dominant granulocytes in the Ht and sinusoids in the HK of O. niloticus.
Two types of granules with granular or fibrillary content were found in acidophilic myelocytes of Sparus aurata (Zuasti and Ferrer 1988). Crystalloids have been observed in the eosinophilic granules of the goldfish (Weinreb 1963), cry fish (Smith et al. 1970) and nurse shark (Hyder et al. 1983), and granules with homogeneously dense content and those possessing a dense core were mentioned in tench (Kelenyi and Nemeth 1969) and Sparus aurata (Zuasti and Ferrer 1988). Eosinophilic granules without inclusions have been described in goldfish (Davies and Haynes 1975), paddle fish (Clawson et al. 1966) and Catostomus commersonii (Lester and Desser 1975). In O. niloticus three granular types were clearly demarcated, granules with homogeneous electron-dense appearance, granules with light electron density dense core and granules with axial light crystalline core.
On the other hand, basophilic leukocytes have not been described for some fish species (Weinberg et al. 1972; Sherburne 1974; Murray 1984), although they have been observed in others (Romestand and Trilles 1984; Zuasti and Ferrer 1988). In the HK of the O. niloticus we identified some developing cells of the basophilic series (myelocyte and metamyelocyte) characterised by prominent rER and large, rounded cytoplasmic granules with fibrillary content as mentioned in Sparus aurata (Zuasti and Ferrer 1988). Large granules with an electron-dense content were observed in Catostomus commersoni (Lester and Desser 1975; Zapata 1979) and Sparus aurata (Meseguer et al. 1990).
In the development of lymphocytes, three stages have been described in the present study, lymphoblasts, large lymphocytes and small lymphocytes, as reported by Mulcahy et al. 1983 in Esox lucius, while Zuasti and Ferrer (1988) found only two stages, immature and mature, in Sparus aurata, similar to those described in Esox lucius (Savage 1983). The lymphocytes are morphologically similar to those described in other species of teleost fish (Ferguson 1976; Cannon et al. 1980; Hightower et al. 1984) and characterised by the existence of pinocytotic vesicles, small granules, numerous microvilli and a large nucleus with heterochromatin blocks.
There are only few plasmatic cells in the HK of O. niloticus in the present study. They are morphologically similar to those described in other teleosts (Smith et al. 1970; Boomker 1981; Pulsford et al. 1982; Zuasti and Ferrer 1988).
Previous studies have mentioned that the structure of the developing blood cells and their cell populations found in the haemopoietic organs of different fish species differ only in the relative numbers of the various types of cells and this may be due to the physiological condition of the fish, their immediate environment and the season of the year (Ezzat et al. 1974; Smith et al. 1976; Safer and El-Sayed 1986). Also, teleost blood cells possess a common stem cell (Boomker 1980; Romestand and Trilles 1984), which seems to derive from the fixed reticular cells and in the process of rounding up, to become a multipotential stem cell (Savage 1983).
It is important to mention that phagocytes were present alone or in groups among haemopoietic tissue in the kidney of O. niloticus and those cells were characterised by eccentric nuclei and cytoplasm filled with brownish yellow granules. Those granules are formed of haemosiderin (Agius 1980, 1981; Pulsford et al. 1982; Zuasti et al. 1989; Abdel-Rahman 1997; Bin-Dohish 2001, 2003; Sarmento et al. 2004). Agius (1985) called the groups of phagocytes a centre of melanin phagocytes, as its job is to store iron resulting from the breakdown of erythrocytes (Agius and Agbede 1984; Zuasti et al. 1989), breaking down the destroyed tissue, catching the free radical in an immune response (Agius 1985) and blood purification from suspended harmful substances (Pulsford et al. 1982). It is also important to mention that in tilapia, the monocytes are very scarce. These cells have been described in very few species of teleost (Mccumber et al. 1982; Romestand and Trilles 1984). These monocytes are large, rounded cells with numerous slender microvilli, the nucleus being eccentric with a large amount of agranular endoplasmic reticulum cisterns and also a few lysosomes in the cytoplasm (Cannon et al. 1980).
In addition to the previously mentioned morphological and ultrastructural studies, considerable progress has been made in understanding the molecular basis of blood cells and their development through the use of cell line models and the membrane markers detected by fluorescence (Blaxhall 2006; Onnebo et al. 2004). A combination of quantitative and morphological methods is needed if the classification of fish blood cells is to advance from its present provisional state. This could be done by isolating the blood cell populations by flow cytometry and by characterising them microscopically. Blood cell populations must be isolated according to their FSC (size) and SSC (granularity) properties by flow cytometry. The isolated populations are then processed for light and transmission and scanning electron microscopic characterisation. The combined use of flow cytometry and electron microscopy makes it possible to characterise the different cell types present in the fish blood with a high degree of certainty. The combined use of flow cytometry and electron microscopy makes it possible to characterise the different cell types present in the fish blood with a high degree of certainty (Esteban et al. 2000; Morgan et al. 2005).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abdel-Rahman MA (1997) Toxicological studies on heavy metals on Siganus rivulatus. M. Sc. Thesis. Department of Oceanography, Faculty of Science, Alexandria University, Egypt, p 188
- Agius C. Phylogenetic development of melano-macrophages centers in fish. J Zool. 1980;191:11–31. doi: 10.1111/j.1469-7998.1980.tb01446.x. [DOI] [Google Scholar]
- Agius C. Preliminary studies on the ontogeny of the melano-macrophages of teleost haemopoietic tissues and age-related changes. Dev Comp Immunol. 1981;5:597–606. doi: 10.1016/s0145-305x(81)80034-1. [DOI] [PubMed] [Google Scholar]
- Agius C. The melano-macrophages centers of fish: a review. In: Maning MJ, Taner MF, editors. Fish immunology. London: Academic Press; 1985. pp. 85–105. [Google Scholar]
- Agius C, Agbede SA. An electron microscopical study of the genesis of lipofucsin, melanin and hemosiderin in haemopoietic tissues of fish. J Fish Biol. 1984;24:471–488. doi: 10.1111/j.1095-8649.1984.tb04818.x. [DOI] [Google Scholar]
- Barber DL, Westerman JE. The blood cells of the Antarctic icefish Chaenocephalus aceratus Lonnberg. Light and electron microscopic observations. J Fish Biol. 1981;19:11–28. doi: 10.1111/j.1095-8649.1981.tb05807.x. [DOI] [Google Scholar]
- Bayne CJ. Pronephric leucocytes of Cyprinus carpio: isolation, separation and characterization. Vet Immunol Immunopathol. 1986;12:141–151. doi: 10.1016/0165-2427(86)90118-2. [DOI] [PubMed] [Google Scholar]
- Belosevic M, Hanington PC, Barreda DR. Development of goldfish macrophages in vitro. Fish Shellfish Immunol. 2006;20:152–171. doi: 10.1016/j.fsi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Bielek E. Elektronen mikroskopische Untersuchungen der Blutzellen der Teleostier. III. Granulocyten. Zool Jahrb Anat. 1980;103:105–121. [Google Scholar]
- Bielek E. Development stages and localization of peroxidatic activity in the leucocytes of three teleost species (Cyprinus carpio L; Tinca tinca L.; Salmo gairdneri Richardson) Cell Tissue Res. 1981;220:163–180. doi: 10.1007/BF00209975. [DOI] [PubMed] [Google Scholar]
- Bin-Dohish GA (2001) Effect of environmental pollutions on histological and functional aspects of Siganus rivulatus in some coastal regions on the Red Sea of kingdom of Saudi Arabia. Ph.D. Thesis. Girls College of Jeddah, Saudi Arabia, p 350
- Bin-Dohish GA. Effect of water pollution of the Red Sea coastal zone of Jeddah, Saudi Arabia on the histological character of some body organs of the Red Sea spot. Lethrinus lentjan. J Egypt Ger Soc Zool. 2003;42:21–42. [Google Scholar]
- Blaxhall PC. Electron microscope studies of fish lymphocytes and thrombocytes. J Fish Biol. 2006;22(2):223–229. doi: 10.1111/j.1095-8649.1983.tb04742.x. [DOI] [Google Scholar]
- Boomker J. The haemocytology and histology of the haemopoietic organs of Clarias gariepinus and Sarotherodon mossambicus. J Vet Res. 1979;46:217–222. [PubMed] [Google Scholar]
- Boomker J. The haemocytology and histology of the haemopoietic organs of South African freshwater fish. II. Erythrocytes and thrombocytes of Clarias gariepinus and Sarotherodon mossambicus. J Vet Res. 1980;47:95–100. [PubMed] [Google Scholar]
- Boomker J. The haemocytology and histology of the haemopoietic organs of South African freshwater fish. III. The leucocytes, plasma cells and macrophages of Clarias gariepinus and Sarotherodon mossambicus. J Vet Res. 1981;48:185–193. [PubMed] [Google Scholar]
- Brederoo P, Van Der Meulen J, Deams WT. Ultrastructural localization of peroxidase activity in developing neutrophil granulocytes from human bone marrow. Histochemistry. 1986;84:445–453. doi: 10.1007/BF00482977. [DOI] [PubMed] [Google Scholar]
- Cannon MS, Mollenhauer HH, Eurrell TE, Lewis DH, Cannon AM, Tompkins C. An ultrastructural study of the leukocytes of the channel cat fish Ictalurus punctatus. J Morphol. 1980;164:1–23. doi: 10.1002/jmor.1051640102. [DOI] [PubMed] [Google Scholar]
- Cenini P. The ultrastructure of leukocytes in carp Cyprinus carpio. J Zool. 1984;204:509–520. doi: 10.1111/j.1469-7998.1984.tb02383.x. [DOI] [Google Scholar]
- Clawson CC, Finstan J, Good RA. Evolution of the immune response. II. Electron microscopy of plasma cells and lymphoid tissue of the paddle fish. Lab Invest. 1966;15:1830–1847. [PubMed] [Google Scholar]
- Curtis SK, Cowden RR, Nagel JW. Ultrastructure of the bone marrow of the salamander Plethodon glutinosus (Caudata: Plethodontidae) J Morphol. 1979;159:151–184. doi: 10.1002/jmor.1051590202. [DOI] [PubMed] [Google Scholar]
- Daimon T, Mizuhira V, Uchida K. Fine structural distribution of the surface-connected canalicular system in frog thrombocytes. Cell Tissue Res. 1979;201(3):431–439. doi: 10.1007/BF00237001. [DOI] [PubMed] [Google Scholar]
- Davies HG, Haynes ME. Light and electron microscope observations on certain leukocytes in a teleost fish and a comparison of the envelope limited monolayers and chromatin structural units in different species. J Cell Sci. 1975;17:263–285. doi: 10.1242/jcs.17.3.263. [DOI] [PubMed] [Google Scholar]
- Ellis AE. The leucocytes of fish: a review. Fish Biol. 1977;11:453–491. doi: 10.1111/j.1095-8649.1977.tb04140.x. [DOI] [Google Scholar]
- Esteban MA, Meseguer J, Garcia Ayala A, Agullerio B. Erythropoiesis and thrombopoiesis in the head kidney of the sea bass Dicentrarchus labrax L. An ultrastructural study. Arch Histol Cytol. 1989;52:407–419. doi: 10.1679/aohc.52.407. [DOI] [PubMed] [Google Scholar]
- Esteban MÁ, Muñoz J, Meseguer J. Blood cells of sea bass Dicentrarchus labrax L. flow cytometric and microscopic studies. Anat Rec. 2000;258:80–89. doi: 10.1002/(SICI)1097-0185(20000101)258:1<80::AID-AR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ezzat AA, Shabana MB, Farghaly AM. Studies on the blood characteristics of Tilapia zillii (Gervais). I. Blood cells. J Fish Biol. 1974;6:1–12. doi: 10.1111/j.1095-8649.1974.tb04516.x. [DOI] [Google Scholar]
- Ferguson HV. The ultrastructure of plaice Pleuronectes platessa leukocytes. J Fish Biol. 1976;8:139–143. doi: 10.1111/j.1095-8649.1976.tb03927.x. [DOI] [Google Scholar]
- Gardner GR, Yevich PP. Studies on the blood morphology of three estuarine Cyprinodontiform fishes. J Fish Res Board Can. 1969;26:433–437. [Google Scholar]
- Groman DB (1982) Histology of striped bass. American Fish Society Monograph, no.3. American Fish Society, Maryland
- Hayat CM. Principle and techniques of electron microscopy. 3. London: McMillan; 1989. [Google Scholar]
- Hightower JA, McCumber LJ, Welsh MG, Whatley DS, Hartvigsen RE, Sigel MM. Blood cells of Fundulus heteroclitus L. J Fish Biol. 1984;24:587–598. doi: 10.1111/j.1095-8649.1984.tb04829.x. [DOI] [Google Scholar]
- Hofte BT, Lehmann J, Storenberg FJ. Untersuchungen zum Blutbild gesunder und an der “Infektiosen Bauchwassersucht” erkrankter Karpfen (Cyprinus carpio L.) Fisch Umwelt. 1984;13:71–87. [Google Scholar]
- Hyder SL, Cayer KL, Pettey CL. Cell types in peripheral blood of the nurse shark: an approach to structure and function. Tissue Cell. 1983;15:437–455. doi: 10.1016/0040-8166(83)90075-7. [DOI] [PubMed] [Google Scholar]
- Kelenyi G, Nemeth A. Comparative histochemistry and electron microscopy of the eosinophil leucocytes of vertebrates. I. A study of avian, reptile, amphibian and fish leucocytes. Acta Biol Acad Sci Hung. 1969;20:405–422. [PubMed] [Google Scholar]
- Klontz GW. Haematological techniques and the immune response in rainbow trout. Symp Zool Soc Lond. 1972;30:89–99. [Google Scholar]
- Lester RJ, Desser SS. Ultrastructural observations on the granulocytic leucocytes of the teleost Catostomus commersoni. Can J Zool. 1975;53:1648–1657. doi: 10.1139/z75-198. [DOI] [PubMed] [Google Scholar]
- Mainwaring G, Rowley AF. Studies on granulocyte heterogeneity in elasmobranches. In: Manning MJ, Tatner MF, editors. Fish immunology. London: Academic Press; 1985. pp. 57–69. [Google Scholar]
- Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattisson AGM, Fange R. Light- and electronmicroscopic observations on the blood cells of the Atlantic hagfish, Myxine glutinosa (L.) Acta Zool Stockh. 1977;58:205–221. doi: 10.1111/j.1463-6395.1977.tb00257.x. [DOI] [Google Scholar]
- McCumber LJ, Sigel MM, Trauger RJ, Cuchens MA. RES structure and function of the fishes. In: Cohen N, Sigel MM, editors. The reticuloendothelial system, vol 3. Phylogeny and ontogeny. Cambridge: Cambridge University Press; 1982. pp. 393–422. [Google Scholar]
- Meseguer J, Esteban MA, Garcia Ayala A, Lopez Ruiz A, Agulleiro B. Granulopoiesis in the head kidney of the sea bass Dicentrarchus labrax L. An ultrastructural study. Arch Histol Cytol. 1990;53:287–296. doi: 10.1679/aohc.53.287. [DOI] [PubMed] [Google Scholar]
- Meseguer J, Esteban MA, Agulliro B. Stromal cells, macrophages and lymphoid cells in the head kidney of sea bass Dicentrarchus labrax L. Arch Histol Cytol. 1991;54:229–309. doi: 10.1679/aohc.54.299. [DOI] [PubMed] [Google Scholar]
- Morgan JAW, Pottinger TG, Rippon P. Evaluation of flow cytometry as a method for quantification of circulating blood cell populations in salmonid fish. J Fish Biol. 2005;42(1):131–141. doi: 10.1111/j.1095-8649.1993.tb00311.x. [DOI] [Google Scholar]
- Morrow WJ, Pulsford A. Identification of peripheral blood leukocytes of the dogfish Scyliorhinus canicula L. by electron microscopy. J Fish Biol. 1980;17:461–475. doi: 10.1111/j.1095-8649.1980.tb02779.x. [DOI] [Google Scholar]
- Mulcahy MF, Savage AG, Casey N (1983) The leukocytes of the pike Esox lucius L. Advances in fish biology in Ireland. Irish Fish Invest (A):24
- Murray SA. Haematological study of the bluegill Lepomis macrochirus RAF. Comp Biochem Physiol. 1984;78(4):787–791. doi: 10.1016/0300-9629(84)90635-2. [DOI] [Google Scholar]
- Oguri M. Histochemical observations on the dark brown pigment granules found in the kidney tissue of rainbow trout. Bull Jap Soc Sci Fish. 1976;42:1223–1227. [Google Scholar]
- Onnebo S, Yoong S, Ward A. Harnessing zebrafish for the study of white blood cell development and its perturbation. Exp Hematol. 2004;32(9):789–796. doi: 10.1016/j.exphem.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Parish N, Wrathmell A, Harris JE. Phagocytic cells in the dogfish Scyliorhinus canicula L. In: Manning MJ, Tatner MF, editors. Fish immunology. London: Academic Press; 1985. pp. 71–83. [Google Scholar]
- Parish N, Wrathmell A, Hart S, Harris JE. The leucocytes of the elasmobranch Scyliorhinus canicula L. A morphological study. J Fish Biol. 1986;28:545–561. doi: 10.1111/j.1095-8649.1986.tb05192.x. [DOI] [Google Scholar]
- Pulsford AR, Fange R, Morrow WJ. Cell types and interactions in the spleen of the dogfish Scyliorhinus canicula L.: an electron microscopic study. J Fish Biol. 1982;21:649–662. doi: 10.1111/j.1095-8649.1982.tb02869.x. [DOI] [Google Scholar]
- Romano N, Ceccariglia S, Mastrolia L, Mazzini M. Cytology of lympho-myeloid head kidney of Antarctic fishes Trematomus bernacchii (Nototheniidae) and Chionodraco hamatus (Channicthyidae) Cell Tissue. 2002;34(2):63–72. doi: 10.1016/S0040-8166(02)00005-8. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, Huttenhuis HBT, Picchietti S, Scapigliati G. Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol. 2005;19:441–455. doi: 10.1016/j.fsi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Romestand B, Trilles JP. Nomenclature et cytologie descriptive des éléments figurés du sang et des organes hématopoïétiques du bar Dicentrarchus labrax. Rec Med Vet. 1984;160:833–840. [Google Scholar]
- Safer A, El-Sayed N. The fine structure of the nephronic tubule of the mudskipper Periophthalmus koelreuteri Pallas. J Morphol. 1986;187:109–121. doi: 10.1002/jmor.1051870109. [DOI] [PubMed] [Google Scholar]
- Sarmento A, Guilhermino L, Afonso A. Mercury chloride effects on the function and cellular integrity of sea bass Dicentrarchus labrax head kidney macrophages. Fish Shellfish Immunol. 2004;5:489–498. doi: 10.1016/j.fsi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Savage AG. The ultrastructure of the blood cells of the pike Esox lucius L. J Morphol. 1983;178:187–206. doi: 10.1002/jmor.1051780209. [DOI] [PubMed] [Google Scholar]
- Sherburne SW. Occurrence of both heterophils and neutrophils in the blood of the spiny dogfish Squalus acanthias. Copeia. 1974;74:259–261. doi: 10.2307/1443037. [DOI] [Google Scholar]
- Smith AM, Wivel NA, Potter M. Plasmacytopoiesis in the pronephros of the carp Cyprinus carpio L. Anat Rec. 1970;167:351–370. doi: 10.1002/ar.1091670308. [DOI] [PubMed] [Google Scholar]
- Smith AM, Potter M, Merchant EB. Antibody-forming cells in the pronephros of the teleost Lepomis. J Immunol. 1976;99:876–882. [PubMed] [Google Scholar]
- Stephens FJ, Raidal SR, Jones B. Haematopoietic necrosis in a goldfish Carassius auratus associated with an agent morphologically similar to herpesvirus. J Aust Vet. 2004;82(3):167–169. doi: 10.1111/j.1751-0813.2004.tb12650.x. [DOI] [PubMed] [Google Scholar]
- Tomonaga S, Hirokane T, Awaka K. Lymphoid cells in the hagfish. Zool Mag. 1973;82:133–135. [Google Scholar]
- Tooze J, Davies HG. Light and electron microscope studies on the spleen of the newt Triturus cristalus: the fine structure of erythropoietic cells. J Cell Sci. 1976;2:617–640. doi: 10.1242/jcs.2.4.617. [DOI] [PubMed] [Google Scholar]
- Vermeulen GJ, Lambert JG, Teitsma CA, Zandbergen MA, Goose HJ. Adrenal tissue in the male African catfish Clarias gariepinus: localization and steroid hormone secretion. Cell Tissue Res. 1995;280:653–657. [Google Scholar]
- Watson LJ, Shechmeister IL, Jackson LL. The hematology of goldfish Carassius auratus. Cytologia (Tokyo) 1963;28:228–240. [Google Scholar]
- Weinberg SR, Siegel CD, Gordon AS. Studies on the peripheral blood cell parameters and morphology of the red paradise fish Macropodus opercularis effect of blood deprivation on erythropoiesis. Anat Rec. 1972;175:7–14. doi: 10.1002/ar.1091750103. [DOI] [PubMed] [Google Scholar]
- Weinreb EL. Studies on the fine structure of teleost blood cells. Anat Rec. 1963;147:219–238. doi: 10.1002/ar.1091470206. [DOI] [PubMed] [Google Scholar]
- Weiss L. Bone marrow. In: Mitchell CW, editor. Cell and tissue biology. Baltimore: Urban and Schwarzenberg; 1988. pp. 469–478. [Google Scholar]
- Weiss L, Chen LT. The organization of hemopoietic cords and vascular sinuses in bone marrow. Blood Cells. 1975;1:617–638. [Google Scholar]
- Willett C, Cortes A, Zuasti A, Zapata A. Early hematopoiesis and developing lymphoid organs in zebrafish. Dev Dyn. 1999;214:323–336. doi: 10.1002/(SICI)1097-0177(199904)214:4<323::AID-AJA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Iuchi I. Electron microscopic study of erythrocytes in developing rainbow trout Salmo gairdneri with particular reference to changes in the cell line. J Exp Zool. 1976;191:407–426. doi: 10.1002/jez.1401910311. [DOI] [PubMed] [Google Scholar]
- Zapata A. Estudio ultraestructural de la mielopoiesis en peces teleosteos. Morfol Norm Patol Sec A. 1979;3:737–747. [Google Scholar]
- Zapata A. Estudio ultraestructural de la eritropoyesis de peces teleosteos. Morfol Norm Patol Sec A. 1980;4:159–178. [Google Scholar]
- Zapata A, Diez B, Cejalvo T, Gutiérrez-de Frías C, Cortés A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006;20(2):126–136. doi: 10.1016/j.fsi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zuasti A, Ferrer C. Granulopoiesis in the head kidney of Sparus auratus. Arch Histol Cytol. 1988;51:425–431. doi: 10.1679/aohc.51.425. [DOI] [PubMed] [Google Scholar]
- Zuasti A, Ferrer C. Haemopoiesis in the head kidney of Sparus auratus. Arch Histol Cytol. 1989;52(3):249–255. doi: 10.1679/aohc.52.249. [DOI] [PubMed] [Google Scholar]
- Zuasti A, Ferrer C, Elbal MT. Histoquimicay morfologia de los acumulos pigmentarios del rinon de Sparus auratus. Acta Microsc. 1986;9:49–53. [Google Scholar]
- Zuasti A, Jara JR, Ferrer C, Solano F. Occurrence of melanin granules and melanosynthesis in the kidney of Sparus auratus. Pigment Cell Res. 1989;2:93–100. doi: 10.1111/j.1600-0749.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]