Prevalent bone marrow edema-like lesions strongly predict incident subchondral cystlike lesions (SCs) in the same subregion longitudinally, even after adjustment for full-thickness cartilage loss, which supports the bone contusion theory of SC formation.
Abstract
Purpose:
To assess the association of prevalent bone marrow edema–like lesions (BMLs) and full-thickness cartilage loss with incident subchondral cyst–like lesions (SCs) in the knee to evaluate the bone contusion versus synovial fluid intrusion theories of SC formation.
Materials and Methods:
The Multicenter Osteoarthritis study is a longitudinal study of individuals who have or are at risk for knee osteoarthritis. The HIPAA-compliant protocol was approved by the institutional review boards of all participating centers, and written informed consent was obtained from all participants. Magnetic resonance images were acquired at baseline and 30-month follow-up and read semiquantitatively by using the Whole-Organ Magnetic Resonance Imaging Score system. The tibiofemoral and patellofemoral joints were subdivided into 14 subregions. BMLs and SCs were scored from 0 to 3. Cartilage morphology was scored from 0 to 6. The association of prevalent BMLs and full-thickness cartilage loss with incident SCs in the same subregion was assessed by using logistic regression with mutual adjustment for both predictors.
Results:
A total of 1283 knees were included. After adjustment for full-thickness cartilage loss, prevalent BMLs showed a strong and significant association with incident SCs in the same subregion, with an odds ratio of 12.9 (95% confidence interval [CI]: 8.9, 18.6). After adjustment for BMLs, prevalent full-thickness cartilage loss showed a significant but much less important association with incident SCs in the same subregion (odds ratio, 1.4; 95% CI: 1.0, 2.0). There was no apparent relationship between severity of full-thickness cartilage loss at baseline and incident SCs.
Conclusion:
Prevalent BMLs strongly predict incident SCs in the same subregion, even after adjustment for full-thickness cartilage loss, which supports the bone contusion theory of SC formation.
© RSNA, 2010
Introduction
Subchondral cyst–like lesions (SCs) are a common finding in patients with knee osteoarthritis (OA). These lesions have a characteristic appearance on magnetic resonance (MR) images, demonstrating well-defined rounded areas of fluidlike signal intensity on unenhanced images (1,2). No evidence of epithelial lining has been detected in prior histologic studies (2–5). The cause of SCs in subjects with or at risk for knee OA is still unknown. The most likely cause is either synovial fluid intrusion or bone contusions. The synovial fluid intrusion theory posits that elevated intraarticular pressure leads to the intrusion of joint fluid into the subchondral bone through fissured or ulcerated cartilage (3,6), with subsequent development of cystic cavities. If this theory is valid, SCs should develop only in regions of the knee exhibiting full-thickness cartilage loss or fissuring. On the other hand, the bone contusion theory posits that SCs are a consequence of traumatic bone necrosis after impact of two opposing articular surfaces (4,7). Supportive of this theory is the fact that cysts are often observed in areas of the knee exhibiting concomitant bone marrow edema–like lesions (BMLs) that show histologic features of bone trauma, including areas of necrosis. BMLs are defined as noncystic subchondral areas of ill-defined hyperintensity on proton density–weighted, intermediate-weighted, T2-weighted, or short tau inversion-recovery (STIR) MR images and areas of hypointensity on T1-weighted spin-echo MR images (1,8–10). The most common alterations of BMLs found at histologic examination are bone necrosis, fibrosis, and trabecular abnormalities (1,11).
A recent cross-sectional study (12) showed SCs to be highly associated with BMLs in the same subregion of the knee in patients with or at risk for knee OA, which favors the bone contusion theory of SC formation. Furthermore, in the same study, in about half of cases, SCs were found in subregions with no areas of full-thickness cartilage loss. However, the cross-sectional association of SCs and full-thickness cartilage loss was not evaluated, and there was no longitudinal assessment. In a retrospective study of 32 patients who underwent two sequential knee MR imaging examinations, Carrino et al (13) reported that 92% of incident SCs developed in regions with BMLs, which favors the bone contusion theory. However, the association of baseline BMLs and full-thickness cartilage loss with incident SCs was not assessed in that cohort.
The aim of this study was to test the synovial fluid intrusion versus the bone contusion theory of SC formation in subjects with or at risk for knee OA by evaluating the association of prevalent BMLs and full-thickness cartilage loss with incident SCs in the same subregion of the knee by using MR imaging.
Materials and Methods
Two authors (M.D.C. and M.D.M.) are shareholders in and one author (F.W.R.) is vice president of and partner in Boston Imaging Core Lab (BICL) (Boston, Mass), a company that provides radiologic image assessment services. A.G. is president of BICL.
Study Design and Subjects
Subjects were participants in the Multicenter Osteoarthritis (MOST) study, a prospective epidemiologic study with the goal of identifying risk factors for incident and progressive knee OA in 3026 people aged 50–79 years either with or at high risk of developing OA. They were recruited from two U.S. communities, Birmingham, Alabama, and Iowa City, Iowa, through mass mailing of letters and study brochures, which were supplemented by media and community outreach campaigns. MOST study subjects were recruited and enrolled between June 2003 and March 2005. The Health Insurance Portability and Accountability Act–compliant study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama at Birmingham, University of California at San Francisco, and Boston University School of Medicine. We obtained written informed consent from all patients.
Subjects considered at high risk for knee OA included those who were overweight or obese; those with knee pain, aching or stiffness on most of the past 30 days, a history of knee injury that made it difficult to walk for at least 1 week; or those who underwent previous knee surgery. Subjects were not eligible to participate in the MOST study if they had rheumatoid arthritis (14), ankylosing spondylitis, psoriatic arthritis, Reiter syndrome, renal insufficiency that required hemodialysis or peritoneal dialysis, or a history of cancer (except for nonmelanoma skin cancer); had undergone or planned to undergo bilateral knee replacement surgery; were unable to walk without assistance; or were planning to move out of the area in the next 3 years.
In the present study, we included all participants with available baseline and 30-month follow-up MR imaging results. These knees were previously selected for one or more of three substudies of the MOST study: (a) a cohort study of risk factors for radiographic progression of OA consisting of randomly selected knees with either patellofemoral or tibiofemoral OA; (b) a case-control study of risk factors for incident radiographically depicted OA; and (c) a case-control study of risk factors for onset of consistent frequent knee pain (15).
Radiography
At baseline examination, all subjects underwent weight-bearing posteroanterior fixed-flexion knee radiography by using the protocol of Peterfy et al (16) and a Plexiglas positioning frame (SynaFlexer; Synarc, San Francisco, Calif). A musculoskeletal radiologist and a rheumatologist, who were not authors (both with more than 10 years of experience reading study radiographs) and were blinded to clinical data, independently graded the images according to the Kellgren-Lawrence scale (17). Radiographs were presented sequentially with readers blinded to all clinical data and to MR images. Radiographs were read in approximately 8 months without interruption. Tibiofemoral OA was considered present at radiography if the Kellgren-Lawrence grade was 2 or greater. If readers disagreed on the presence of OA, readings were adjudicated by a panel of three readers (two nonauthors and D.T.F.).
MR Imaging Acquisition
MR images were obtained in both knees at baseline and 30-month follow-up with a 1.0-T dedicated extremity unit (OrthOne; ONI Medical Systems, Wilmington, Mass) with a circumferential extremity coil by using fat-suppressed fast spin-echo proton density–weighted sequences in the sagittal (repetition time msec/echo time msec, 4800/35; 3-mm section thickness; 0-mm intersection gap; 32 sections; 288 × 192 matrix; number of signals acquired, two; 140 × 140-mm field of view; echo train length, eight) and axial (4680/13; 3-mm section thickness; 0-mm intersection gap; 20 sections; 288 × 192 matrix; number of signals acquired, two; 140 × 140-mm field of view; echo train length, eight) planes and a STIR sequence in the coronal plane (6650/15; inversion time, 100 msec; 3-mm section thickness; 0-mm intersection gap; 28 sections; 256 × 192 matrix; number of signals acquired, two; 140-mm2 field of view; echo train length, eight). Examinations were performed at the University of Alabama at Birmingham and at the University of Iowa at Iowa City with the same MR unit.
MR Imaging Interpretation
Two musculoskeletal radiologists (F.W.R. and A.G., with 6 and 8 years of experience, respectively, in standardized semiquantitative MR imaging assessment of knee OA), who were blinded to OA grade at radiography and clinical data, graded BMLs, cartilage status, and SCs according to the Whole-Organ Magnetic Resonance Imaging Score (WORMS) system (18). A recent study (19) showed that WORMS assessment by using a 1.0-T dedicated extremity MR system is possible with a moderate to high degree of agreement and accuracy compared with WORMS assessment by using a 1.5-T large-bore MR imaging unit. Baseline and follow-up MR images were presented paired and sequentially to the readers, with the chronological order known to the readers. Baseline and follow-up MR readings were performed during a period of 2 years.
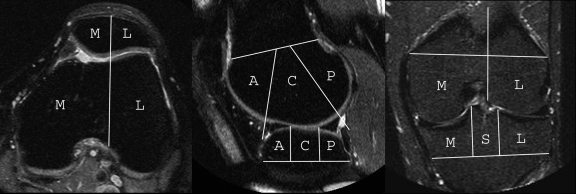
BMLs and SCs were scored in each of the five subregions in the medial and lateral tibiofemoral compartments, as well as in each of the four subregions in the patellofemoral compartment, for a total of 14 subregions per knee (Fig 1). BML and SC sizes were scored from 0 to 3 on the basis of the extent of regional involvement (0, none; 1, <25% of the subregion; 2, 25%–50% of the subregion; 3, >50% of the subregion). BMLs were defined as poorly delineated areas of hyperintensity directly adjacent to the subchondral plate on STIR and proton density–weighted fat-suppressed MR images. SCs were defined as well-delineated areas of hyperintensity directly adjacent to the subchondral plate on STIR and proton density–weighted fat-suppressed MR images. Knees with typical MR imaging signs of traumatic bone contusions, osteonecrosis, fracture, or malignant bone infiltration were excluded from the analysis. However, of all analyzed MR imaging studies, only one knee showed a subacute tibial depression fracture at follow-up and was excluded.
Figure 1:
Axial, sagittal, and coronal MR images show subregional division in the WORMS system. Eleven tibiofemoral subregions are defined: the central (C) and posterior (P) femur medially and laterally, the anterior (A), central, and posterior tibia medially and laterally, and the subspinous (S) region. The subspinous region was not considered in this study because it is not covered by articular cartilage. Four patellofemoral subregions are defined: the medial (M) and lateral (L) patella and the anterior subregions of the femur (trochlea) medially and laterally.
Cartilage morphology and signal intensity were scored semiquantitatively from 0 to 6 in each subregion (0, normal thickness and signal intensity; 1, normal thickness but increased signal intensity on proton density–weighted or STIR images; 2.0, partial-thickness focal defect < 1 cm in greatest width [Fig 2]; 2.5, full-thickness focal defect < 1 cm in greatest width; 3, multiple areas of partial-thickness defects intermixed with areas of normal thickness or a grade 2.0 defect wider than 1 cm but < 75% of the region; 4, diffuse [≥75% of the region] partial-thickness loss; 5, multiple areas of full-thickness loss or a grade 2.5 lesion wider than 1 cm but < 75% of the region; 6, diffuse [≥75% of the region] full-thickness loss). Thus, grades 2.5, 5, and 6 include full-thickness cartilage loss, but the other grades do not. The weighted κ coefficients of interobserver reliability (studies in 30 knees randomly selected and read by both readers) were 0.66 for the readings of BMLs (comparing scores 0–3 in each subregion), 0.57 for SCs (comparing scores 0–3 in each subregion), and 0.78 for cartilage morphology (comparing scores 0–6 in each subregion). The weighted κ coefficients of intrareader observer reliability (studies in 30 knees randomly selected) were 0.8 and 0.94 for the readings of BMLs (comparing scores 0–3 in each subregion), 0.86 and 0.93 for SCs (comparing scores 0–3 in each subregion), and 0.88 for cartilage morphology (comparing scores 0–6 in each subregion).
Figure 2:
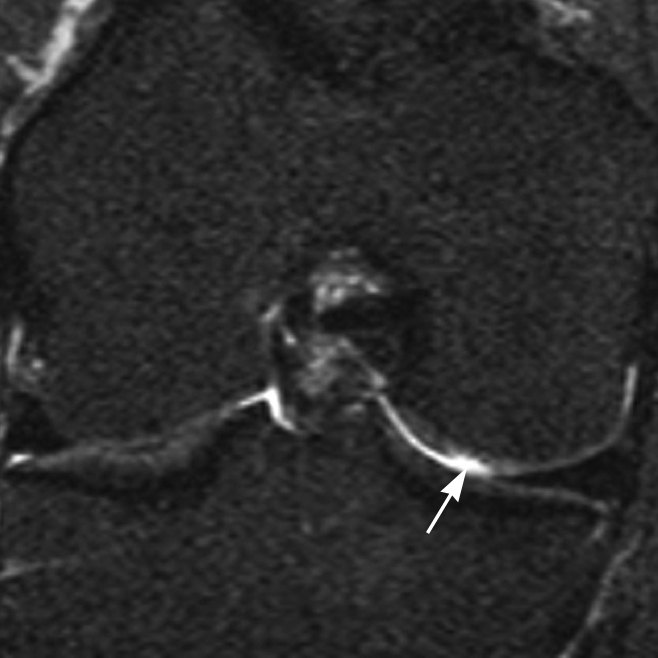
Coronal STIR MR image demonstrates a partial-thickness focal defect of cartilage (grade 2.0) in the central subregion of the medial femoral condyle (arrow).
To rule out observer bias (since MR images were read in pairs with known chronology), we evaluated BMLs and SCs in a subset of cases (30 cases randomly selected) and were blinded to time point and compared the results with those evaluated without blinding to time point. The weighted κ coefficients of intraobserver reliability were 0.85 for the readings of BMLs and 0.96 for those of SCs, comparing scores 0–3 in each subregion.
Statistical Analysis
Incident SCs, considered the outcome, were defined as grade 0 at baseline and grade 1 or greater at follow-up. Subregions with prevalent SCs (score ≥ 1) were excluded. We assessed the longitudinal association of prevalent BMLs (score ≥ 1) and full-thickness cartilage loss (grades 2.5, 5, and 6) with incident SCs (score ≥ 1) on a per-subregion basis by using logistic regression with generalized estimating equations to account for correlations among the subregions within a knee (using one knee per person). Subregions without BMLs (score = 0) and without full-thickness cartilage loss (scores 0, 1, 2, 3, and 4) were considered the reference group. A longitudinal subanalysis of the individual BML and cartilage morphology grades was performed. Because BMLs are highly associated with cartilage damage in the same subregion of the knee (20,21), we adjusted for full-thickness cartilage loss when testing prevalent BML (bone contusion theory) as the predictor. Similarly, we adjusted for BML when testing full-thickness cartilage loss (synovial fluid intrusion theory) as the predictor. All statistical calculations were performed by using software (SAS, version 9.1 for Windows; SAS Institute, Cary, NC). A P value less than .05 was considered to indicate a significant difference.
Results
One thousand two hundred eighty-three knees (one knee per patient, 16 349 subregions assessable at baseline and follow-up examinations) were included. Mean subject age was 62.3 years ± 7.9 (standard deviation), and mean subject body mass index was 30.1 kg/m2 ± 4.9 (range, 18.0–55.8 kg/m2). Sixty percent were women (n = 776), 86.4% were white (n = 1109), and 44.4% had tibiofemoral radiographically depicted OA (Kellgren-Lawrence grade, ≥2) at baseline (n = 570). Of 19 153 subregions analyzed initially, 663 (3.5%) exhibited SCs at baseline and were excluded. Many subregions were excluded because they were not assessable, mainly because of motion artifacts or field inhomogeneity at baseline and/or at follow-up, which did not allow scoring of the features evaluated in these subregions (cartilage morphology, BMLs, and SCs). A total of 2141 subregions were finally excluded. No statistically significant differences were found for age (P = .53) and sex (P = .87) when considering included versus excluded subregions. Prevalent BMLs were found in 1843 subregions (11.3%), prevalent full-thickness cartilage loss was found in 1624 subregions (9.9%), and incident SCs were found in 216 subregions (1.3%). No statistically significant differences were found for age (P = .97) and sex (P = .68) when considering subregions with incident SCs. Of the incident SCs, 200 (92.6%) were small (grade 1). Prevalent BMLs showed a strong association with incident SCs in the same subregion, with an odds ratio of 15.0 (95% confidence interval [CI]: 10.9, 20.5; P < .0001), compared with subregions without prevalent BMLs (Figs 3 and 4). The association did not change materially after adjusting for full-thickness cartilage loss, with an odds ratio of 12.9 (95% CI: 8.9, 18.6; P < .0001). Larger prevalent BMLs were associated with an increased incidence of SCs (Table 1). Prevalent full-thickness cartilage loss showed a significant association with incident SCs in the same subregion, with an odds ratio of 5.4 (95% CI: 4.1, 7.2; P < .0001), compared with subregions without baseline full-thickness cartilage loss. However, the effect was significantly attenuated after adjustment for BMLs, with an odds ratio of 1.4 (95% CI: 1.0, 2.0; P = .036). Furthermore, there was no apparent relationship between severity of full-thickness cartilage loss at baseline and incident SCs (Table 2).
Figure 3a:

(a) Coronal STIR MR image at baseline shows a grade 1 BML at the central subregion of the medial tibia (arrowheads). (b) Coronal STIR MR image at 30-month follow-up demonstrates an incident SC developed in the same location (arrow). No adjacent full-thickness cartilage loss is seen.
Figure 4a:
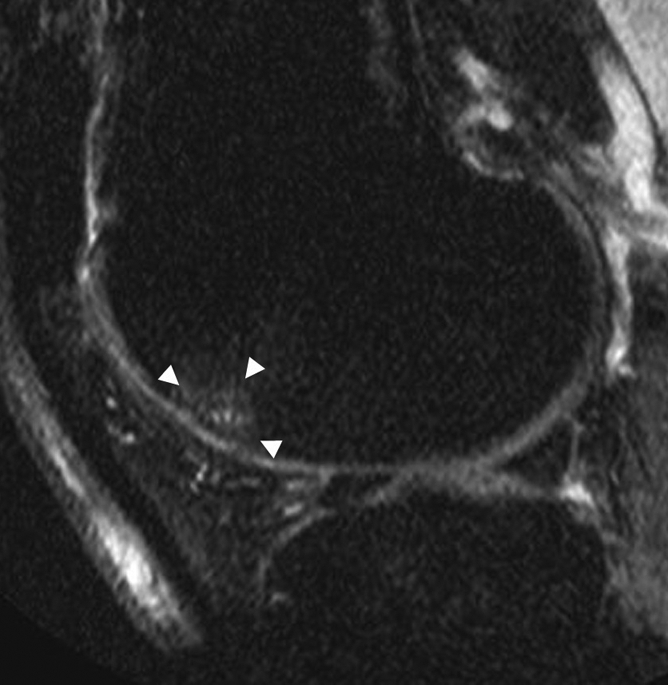
(a) Sagittal proton density–weighted fat-suppressed MR image at baseline shows a grade 1 BML at the anterior (trochlear) subregion of the lateral femur (arrowheads). No baseline full-thickness cartilage loss was detected in this subregion. (b) Sagittal proton density–weighted fat-suppressed MR image at 30-month follow-up shows an incident SC (arrow) in the middle of the BML depicted at baseline. Full-thickness cartilage loss is seen in this subregion at follow-up.
Table 1.
Longitudinal Association between Prevalent BMLs and Incident SCs in the Same Subregion of the Knee
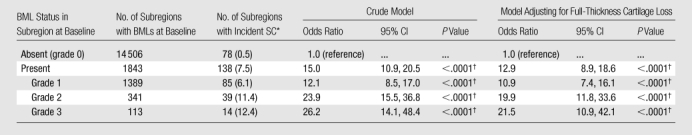
Data in parentheses are percentages.
Statistically significant differences were defined as having P <.05.
Table 2.
Longitudinal Association between Prevalent Full-Thickness Cartilage Loss and Incident SCs in the Same Subregion of the Knee
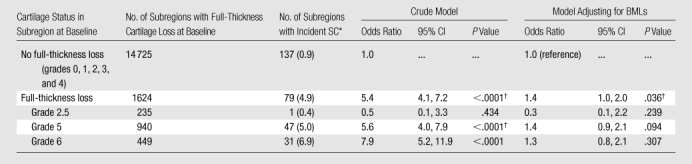
Data in parentheses are percentages.
Statistically significant differences were defined as having P <.05.
Figure 3b:
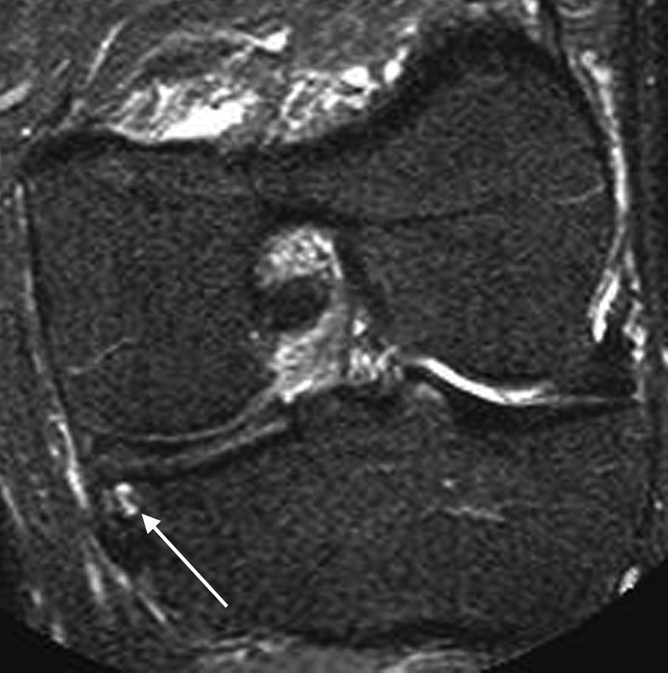
(a) Coronal STIR MR image at baseline shows a grade 1 BML at the central subregion of the medial tibia (arrowheads). (b) Coronal STIR MR image at 30-month follow-up demonstrates an incident SC developed in the same location (arrow). No adjacent full-thickness cartilage loss is seen.
Figure 4b:
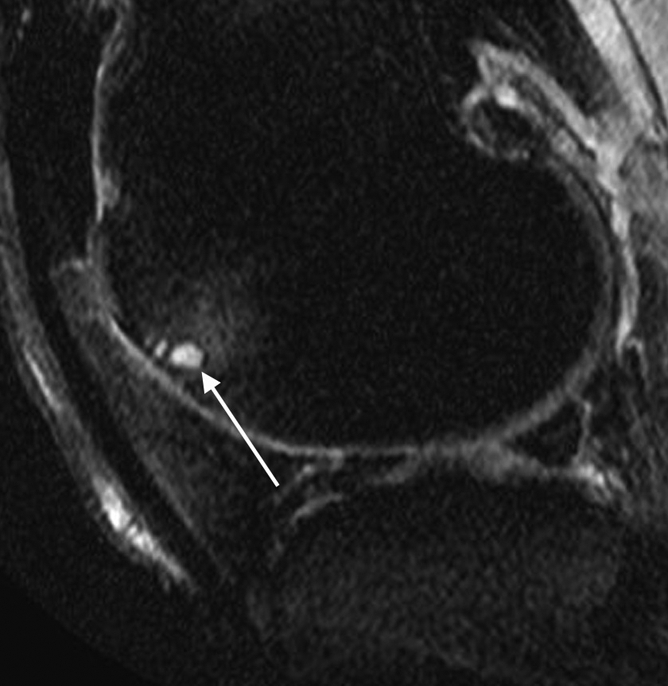
(a) Sagittal proton density–weighted fat-suppressed MR image at baseline shows a grade 1 BML at the anterior (trochlear) subregion of the lateral femur (arrowheads). No baseline full-thickness cartilage loss was detected in this subregion. (b) Sagittal proton density–weighted fat-suppressed MR image at 30-month follow-up shows an incident SC (arrow) in the middle of the BML depicted at baseline. Full-thickness cartilage loss is seen in this subregion at follow-up.
Discussion
To our knowledge, this is the largest prospective and longitudinal study to assess the temporal relationship between MR imaging–detected BMLs and full-thickness cartilage loss and SCs in the same subregion of the knee for the evaluation of the pathogenesis of SCs in light of the synovial fluid intrusion and bone contusion theories. We showed that both prevalent BMLs and full-thickness cartilage loss predict SC formation longitudinally, with the association much stronger for prevalent BMLs.
SCs have a characteristic appearance on MR images, demonstrating well-defined rounded areas of fluidlike signal intensity on unenhanced images (1,2). We used the MR imaging definition to assess SCs, because radiographic assessment may not depict the small incident SCs in this study. Detection of SCs on radiographs (sometimes referred in radiography as geodes) is usually possible when they are big enough to produce areas of hyperlucency in the subchondral bone, which usually occurs in advanced disease. In our study, most (92.6%) incident SCs detected were small (grade 1).
According to the synovial fluid intrusion theory (3,6), SCs should develop only in subregions with full-thickness cartilage loss, where breaches of the articular surface could allow synovial fluid and/or synovial tissue to intrude into the subchondral bone. In a recent cross-sectional study evaluating the distribution of SCs in subregions of the knee with normal cartilage, partial-thickness loss, or full-thickness loss of cartilage, Crema et al (12) found that 46.5% of MR imaging–detected SCs were present in subregions with no full-thickness cartilage loss, which speaks against the synovial fluid intrusion theory.
According to the WORMS system (18), grades 2.5, 5, and 6 for cartilage morphology indicate subregions with full-thickness cartilage loss, with different amounts of the cartilage surface involved. Per the synovial fluid intrusion theory, one would expect those scores to indicate increased risk for incident SCs in the same subregion at follow-up. Indeed, we found that, without any adjustment, grades 5 and 6 were significantly associated with incident SCs compared with subregions with no full-thickness cartilage loss at baseline. Grade 2.5 cartilage morphology, which indicates a small (<1 cm) focal area of full-thickness cartilage loss, showed no such association. Because there is a high prevalence of BMLs in subregions exhibiting cartilage damage (20,21), we adjusted these results for the presence of concomitant BMLs when considering full-thickness cartilage loss as the predictor. To our surprise, the effects of grades 5 and 6 of cartilage morphology were completely diluted after this adjustment, and no association was found between prevalent full-thickness cartilage loss and incident SCs when each grade was assessed separately, which speaks against the synovial fluid intrusion theory of SC formation. However, we did find a weak but significant association when all grades were combined after adjusting for prevalent BMLs.
According to the bone contusion theory (4,7), a subchondral cyst forms independently of the condition of adjacent cartilage. Rhaney and Lamb (4) have demonstrated the histologic similarity of subchondral cysts and the surrounding subchondral bone marrow, which suggests that subchondral cyst formation is secondary to subchondral bone marrow necrosis due to increased loading. When comparing the MR imaging features of the tibial plateau in 16 patients with severe knee OA with histologic specimens prior to joint replacement, Zanetti et al (1) found that abnormal tissue appeared in only about half of the regions with MR imaging–detected BMLs. The most common abnormalities were bone marrow necrosis, fibrosis, and trabecular abnormalities (1). In a study comparing MR imaging features with histologic findings in 19 patients after hip replacement, Taljanovic et al (11) found microfractures in different stages of healing and bone marrow necrosis in 100% of patients, and 85% had bone marrow fibrosis. These were the most common abnormalities found at histologic examination, whereas only 40% of patients had small amounts of edema. Thus, according to the bone contusion theory, MR imaging–detected BMLs should represent the source of SCs in subjects with or at risk for knee OA.
A recent study (12) demonstrated that BMLs are highly associated with SCs in the same subregion of the knee. However, that was a cross-sectional study, and no temporal relationship between these two features could be assessed. In the present study, we showed that prevalent BMLs are strongly associated with incident SCs in the same subregion of the knee compared with those subregions without BMLs at baseline, which supports the bone contusion theory of SC formation. Even after adjustment for prevalent full-thickness cartilage loss, prevalent BMLs showed a strong association with incident SCs. Furthermore, a larger size of BMLs at baseline was associated with an increased risk for the development of SCs at follow-up, which also favors the bone contusion theory. For subregions demonstrating incident SCs without prevalent BMLs at baseline, it is still possible that an incident BML developed after the baseline visit that turned into a subchondral cyst, which was then observed at follow-up. The underlying BML may have vanished (20). However, we may only hypothesize this in the present study, because several visits within short time intervals including MR imaging were not available in the MOST study to detect such causality.
BMLs, which represent focal bone remodeling due to overloading, are predictors of pain and progression of cartilage damage in OA (15,20) and are potential treatment targets. Therapeutic approaches targeting BMLs, including unloading or pharmacologic intervention, may delay or prevent cyst development, but this is unknown. The clinical relevance of subchondral cysts in regard to pain or structural progression of OA is not well understood as of to date.
Some limitations to the current study need mentioning. First and probably most important is that no arthroscopic or histologic correlation was performed. The resolution of the MR images obtained in the MOST study might be below the threshold for detection of small full-thickness fissuring of cartilage, which may be responsible for synovial fluid intrusion. However, spin-echo MR imaging is able to depict and help differentiate BMLs and SCs, in accordance with previous studies correlating the MR imaging appearance of these abnormalities and histologic findings (1,2,11). Second, even though spin-echo MR imaging sequences are widely accepted as an accurate technique to evaluate articular cartilage (22–24), we do not have arthroscopic or histologic proof of cartilage status. Finally, a reading bias toward SC when a BML or an area of full-thickness cartilage loss is present cannot be ruled out completely, although the reading experience of both our experts makes this less likely. Readers cannot be blinded to features of relevance because those are depicted on the paired images and are seen simultaneously. However, readers were not aware of the aim of the study at the time of MR imaging assessment.
In conclusion, both prevalent BMLs and full-thickness cartilage loss predicted incident SCs in the same subregion. However, the effect of full-thickness cartilage loss was diluted after adjustment for BMLs, and no significant association was found when evaluating different grades separately, which does not support the synovial fluid intrusion theory of SC formation. Prevalent BMLs strongly predicted incident SCs in the same subregion longitudinally, even after adjustment for full-thickness cartilage loss, which supports the bone contusion theory of SC formation.
Advance in Knowledge.
Subchondral bone marrow edema-like lesions represent a predictor of subchondral cyst–like lesions (SCs), which supports the bone contusion theory of SC formation.
Implication for Patient Care.
Patients may develop subchondral cysts in areas of marrow edema; the clinical relevance of subchondral cysts concerning symptomatic osteoarthritis needs to be explored.
Received August 8, 2009; revision requested September 15; revision received November 30; accepted January 7, 2010; final version accepted March 3.
From the 2009 RSNA Annual Meeting.
Funding: This work was supported by National Institute on Aging (grants U01-AG-18947, U01-AG-18832, U01-AG-19069, and U01-AG-18820).
See Materials and Methods for pertinent disclosures.
Abbreviations:
- BML
- bone marrow edema–like lesion
- CI
- confidence interval
- MOST
- Multicenter Osteoarthritis
- OA
- osteoarthritis
- SC
- subchondral cyst–like lesion
- STIR
- short tau inversion recovery
- WORMS
- Whole-Organ Magnetic Resonance Imaging Score
References
- 1.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology 2000;215(3):835–840 [DOI] [PubMed] [Google Scholar]
- 2.Pouders C, De Maeseneer M, Van Roy P, Gielen J, Goossens A, Shahabpour M. Prevalence and MRI-anatomic correlation of bone cysts in osteoarthritic knees. AJR Am J Roentgenol 2008;190(1):17–21 [DOI] [PubMed] [Google Scholar]
- 3.Landells JW. The bone cysts of osteoarthritis. J Bone Joint Surg Br 1953;35-B(4):643–649 [DOI] [PubMed] [Google Scholar]
- 4.Rhaney K, Lamb DW. The cysts of osteoarthritis of the hip: a radiological and pathological study. J Bone Joint Surg Br 1955;37-B(4):663–675 [DOI] [PubMed] [Google Scholar]
- 5.Resnick D, Niwayama G, Coutts RD. Subchondral cysts (geodes) in arthritic disorders: pathologic and radiographic appearance of the hip joint. AJR Am J Roentgenol 1977;128(5):799–806 [DOI] [PubMed] [Google Scholar]
- 6.Freund E. The pathological significance of intra-articular pressure. Edinb Med J 1940;47:192–203 [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson AB., Jr The pathological changes in degenerative arthritis of the hip and treatment by rotational osteotomy. J Bone Joint Surg Am 1964;46:1337–1352 [PubMed] [Google Scholar]
- 8.Bergman AG, Willén HK, Lindstrand AL, Pettersson HT. Osteoarthritis of the knee: correlation of subchondral MR signal abnormalities with histopathologic and radiographic features. Skeletal Radiol 1994;23(6):445–448 [DOI] [PubMed] [Google Scholar]
- 9.Yu JS, Cook PA. Magnetic resonance imaging (MRI) of the knee: a pattern approach for evaluating bone marrow edema. Crit Rev Diagn Imaging 1996;37(4):261–303 [PubMed] [Google Scholar]
- 10.Roemer FW, Hunter DJ, Guermazi A. MRI-based semiquantitative assessment of subchondral bone marrow lesions in osteoarthritis research. Osteoarthritis Cartilage 2009;17(3):414–415; author reply 416–417 [DOI] [PubMed] [Google Scholar]
- 11.Taljanovic MS, Graham AR, Benjamin JB, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol 2008;37(5):423–431 [DOI] [PubMed] [Google Scholar]
- 12.Crema MD, Roemer FW, Marra MD, et al. MRI-detected bone marrow edema-like lesions are strongly associated with subchondral cysts in patients with or at risk for knee osteoarthritis: the MOST study [abstr]. Osteoarthritis Cartilage 2008;16(suppl 4):S160 [Google Scholar]
- 13.Carrino JA, Blum J, Parellada JA, Schweitzer ME, Morrison WB. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage 2006;14(10):1081–1085 [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol 1995;5(4):297–302 [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 2007;56(9):2986–2992 [DOI] [PubMed] [Google Scholar]
- 16.Peterfy CG, Lynch JA, Miaux Y, et al. Non-fluoroscopic method for flexed radiography of the knee that allows reproducible joint-space width measurement [abstr]. Arthritis Rheum 1998;41(suppl):S361 [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16(4):494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12(3):177–190 [DOI] [PubMed] [Google Scholar]
- 19.Roemer FW, Lynch JA, Niu J, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage 2010;18(2):168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study—a longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis 2009;68(9):1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guymer E, Baranyay F, Wluka AE, et al. A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage 2007;15(12):1437–1442 [DOI] [PubMed] [Google Scholar]
- 22.Bredella MA, Tirman PF, Peterfy CG, et al. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol 1999;172(4):1073–1080 [DOI] [PubMed] [Google Scholar]
- 23.Sonin AH, Pensy RA, Mulligan ME, Hatem S. Grading articular cartilage of the knee using fast spin-echo proton density-weighted MR imaging without fat suppression. AJR Am J Roentgenol 2002;179(5):1159–1166 [DOI] [PubMed] [Google Scholar]
- 24.Jungius KP, Schmid MR, Zanetti M, Hodler J, Koch P, Pfirrmann CW. Cartilaginous defects of the femorotibial joint: accuracy of coronal short inversion time inversion-recovery MR sequence. Radiology 2006;240(2):482–488 [DOI] [PubMed] [Google Scholar]