Abstract
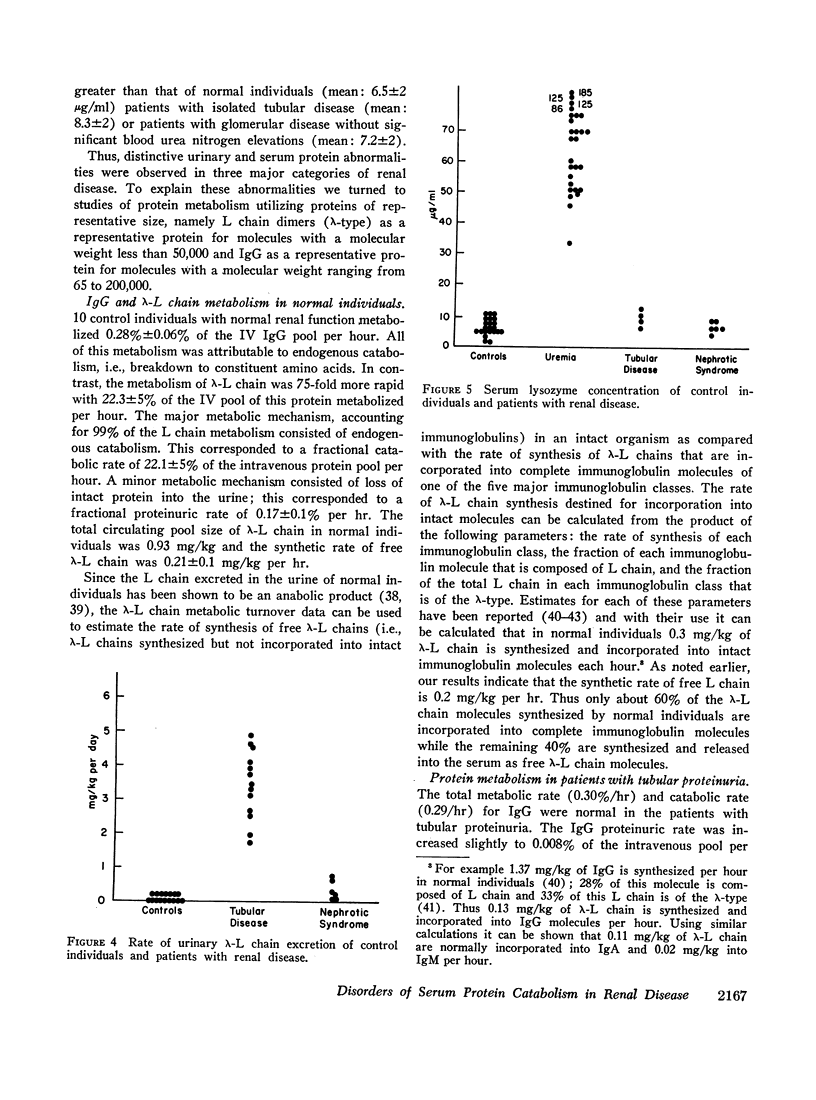
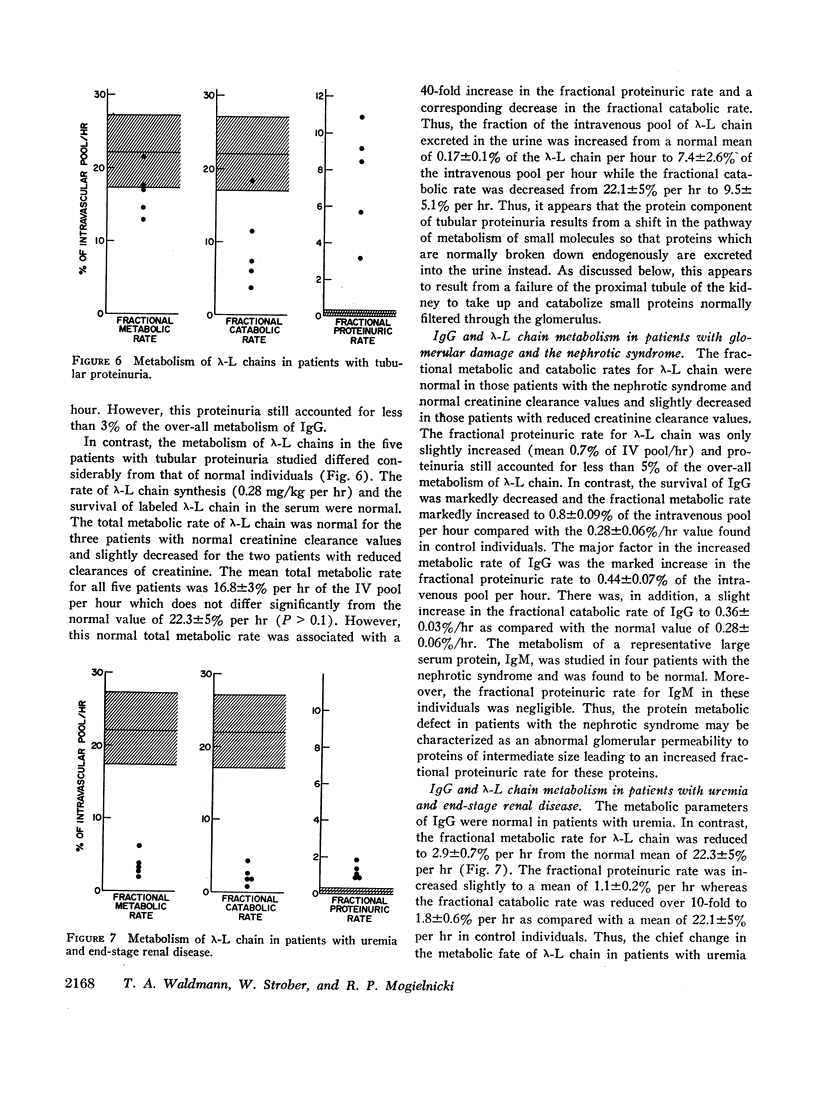
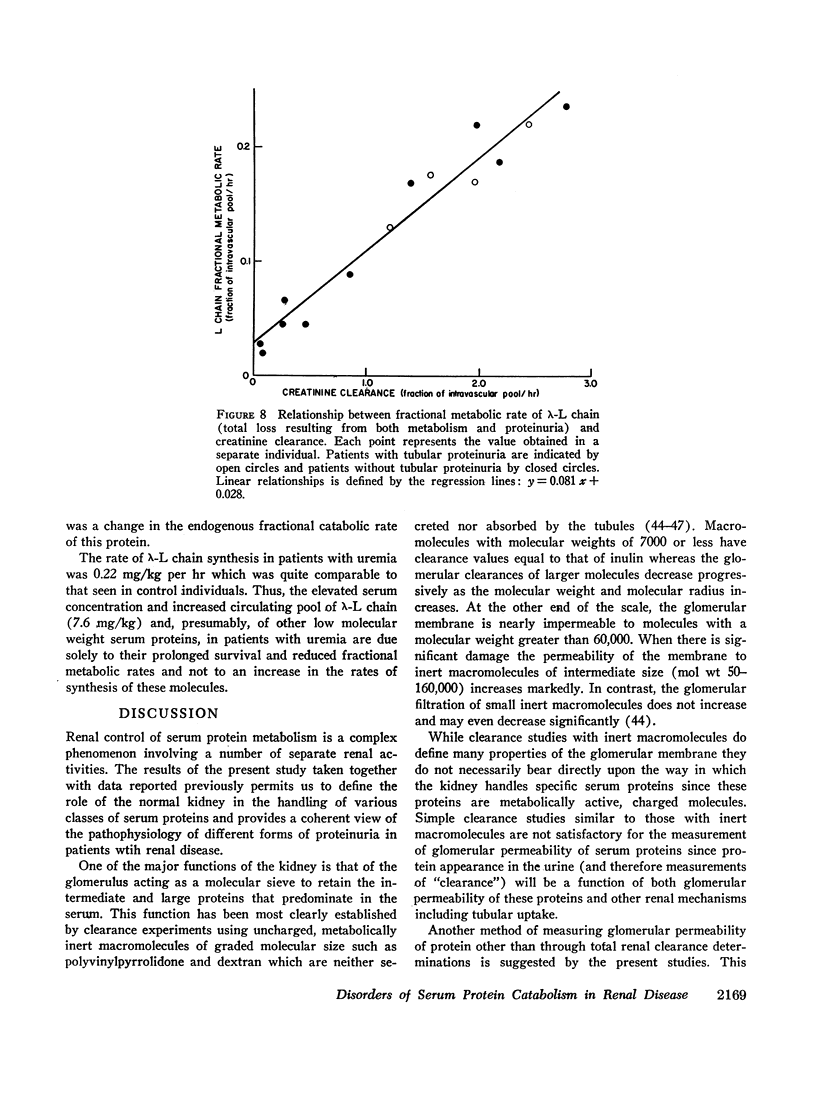
The present study was directed toward determining the role of the kidney in the metabolism of various classes of serum proteins and to define the urinary protein excretion patterns and the pathogenesis of disorders of protein metabolism in patients with proteinuria. To this end, the metabolic fates of a small protein, λ-L chain (mol wt 44,000), and a protein of intermediate size, IgG (mol wt 160,000), were studied in controls and patients with renal disease. Controls metabolized 0.28%/hr of circulating IgG and 22.3%/hr of circulating λ-L chain. All the IgG and 99% of the λ-L chain was catabolized with the remaining λ-L chain lost intact into the urine. The kidney was shown to be the major site of catabolism for small serum proteins. Three distinct disorders of protein metabolism were noted in patients with renal tubular disease and tubular proteinuria, glomerular disease (the nephrotic syndrome), and disease involving the entire nephrons (uremia), respectively. Patients with renal tubular disease had a 50-fold increase in the daily urinary excretion of 15-40,000 molecular weight proteins such as lysozyme and λ-L chains. Serum IgG and λ-L chain survivals were normal; however, the fraction of the over-all λ-L chain metabolism accounted for by proteinuria was increased 40-fold whereas endogenous catabolism was correspondingly decreased. Thus, tubular proteinuria results from a failure of proximal tubular uptake and catabolism of small proteins that are normally filtered through the glomerulus. Patients with the nephrotic syndrome had a slight increase in λ-L chain survival whereas IgG survival was decreased and the fraction of IgG lost in the urine was markedly increased. Here, abnormal glomerular permeability to proteins of intermediate size is the basic abnormality. Patients with uremia had a normal IgG survival but a four to 10-fold prolongation of λ-L chain survival due to loss of entire nephrons, the major site of metabolism of these proteins. This results in an increase (up to 10-fold) in the serum concentration of λ-L chain, lysozyme, and other small biologically active proteins, a phenomenon that may be of importance in causing some of the manifestations of the uremic syndrome.
Full text
PDF

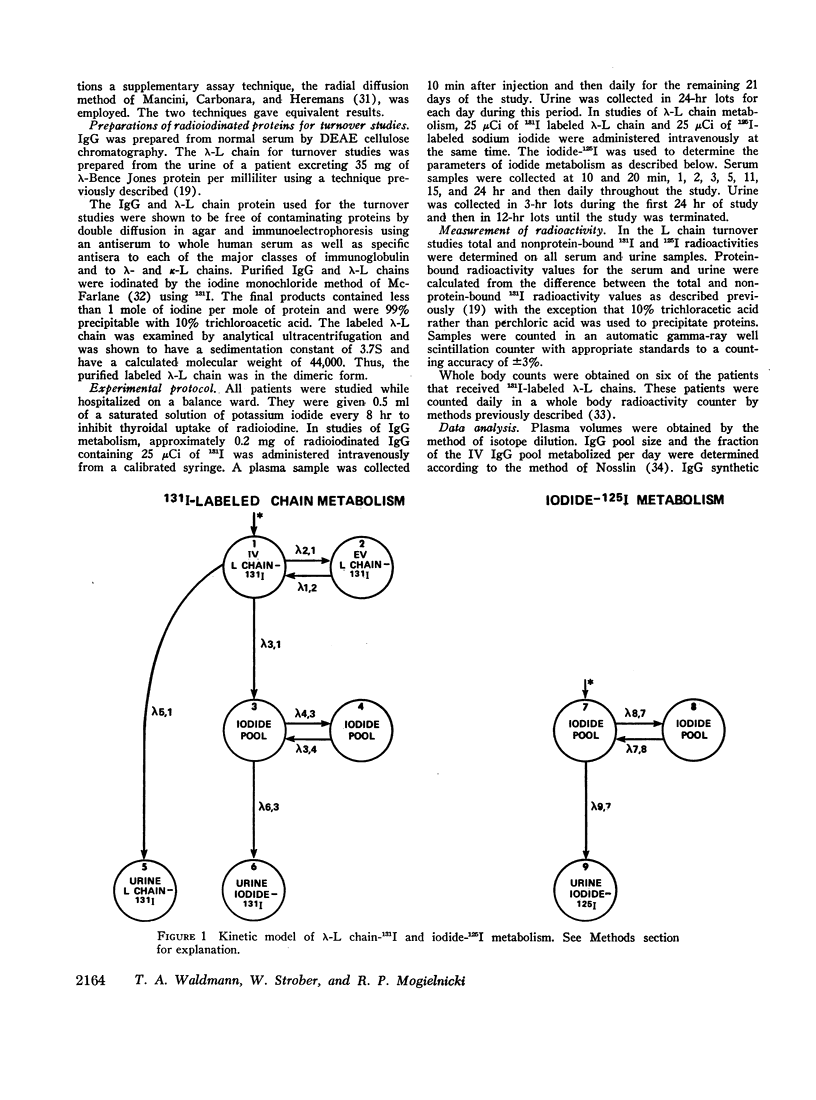

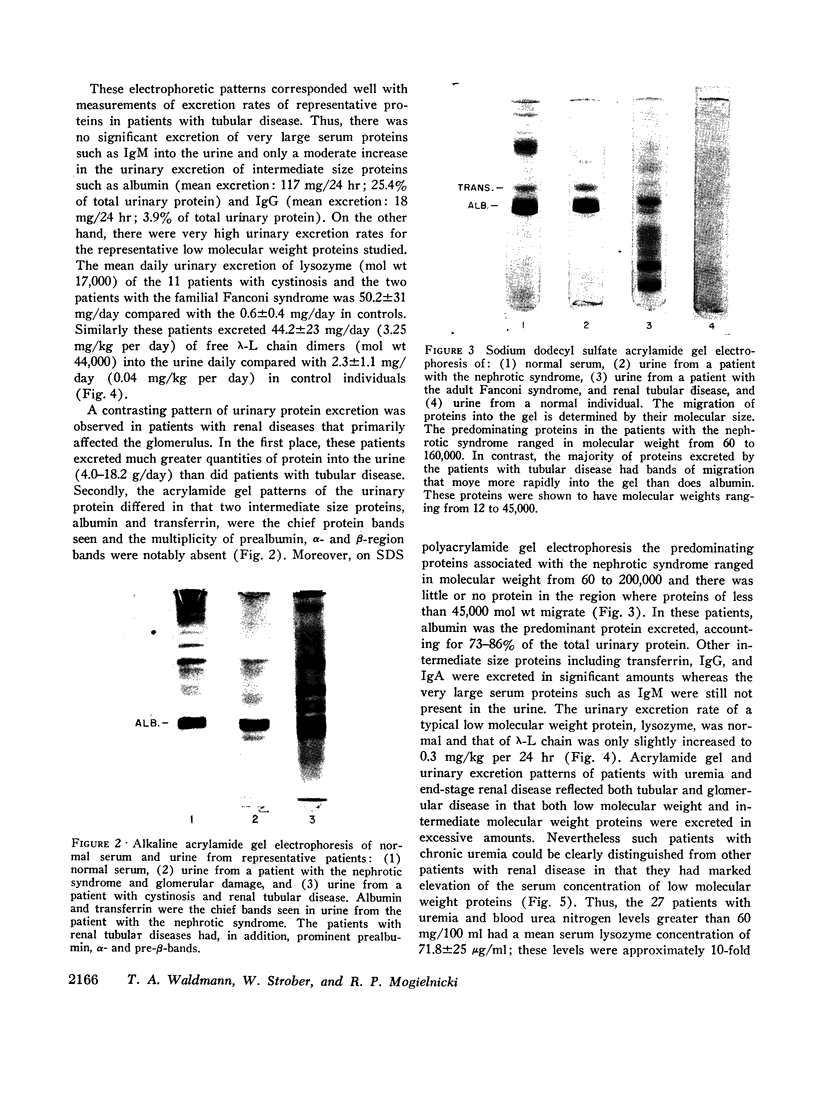





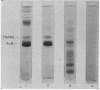
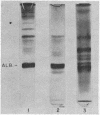
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN S. B. METABOLISM OF GAMMA-SS GLOBULIN IN SECONDARY HYPOGAMMAGLOBULINEMIA. Am J Med. 1963 Nov;35:708–714. doi: 10.1016/0002-9343(63)90141-4. [DOI] [PubMed] [Google Scholar]
- ARTURSON G., WALLENIUS G. THE RENAL CLEARANCE OF DEXTRAN OF DIFFERENT MOLECULAR SIZES IN NORMAL HUMANS. Scand J Clin Lab Invest. 1964;16:81–86. doi: 10.3109/00365516409060486. [DOI] [PubMed] [Google Scholar]
- BARTH W. F., WOCHNER R. D., WALDMANN T. A., FAHEY J. L. METABOLISM OF HUMAN GAMMA MACROGLOBULINS. J Clin Invest. 1964 Jun;43:1036–1048. doi: 10.1172/JCI104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKE G., LILJEDAHL S. O., OLHAGEN B., PLANTIN L. O., AHLINDER S. Catabolism and distribution of gamma-globulin. A preliminary study with 131 I-labelled gammaglobulin. Acta Med Scand. 1963 May;173:589–603. [PubMed] [Google Scholar]
- BLAINEY J. D., BREWER D. B., HARDWICKE J., SOOTHILL J. F. The nephrotic syndrome. Diagnosis by renal biopsy and biochemical and immunological analyses related to the response to steroid therapy. Q J Med. 1960 Apr;29:235–256. [PubMed] [Google Scholar]
- BREWER D. B. Renal clearances of dextrans of varying molecular weights. Proc R Soc Med. 1951 Jul;44(7):561–563. doi: 10.1177/003591575104400709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER E. A., FLYNN F. V., HARRIS H., ROBSON E. B. A study of urine proteins by two-dimensional electrophoresis with special reference to the proteinuria of renal tubular disorders. Clin Chim Acta. 1962 Jan;7:34–41. doi: 10.1016/0009-8981(62)90113-4. [DOI] [PubMed] [Google Scholar]
- BUTLER E. A., FLYNN F. V. The occurrence of post-gamma protein in urine: a new protein abnormality. J Clin Pathol. 1961 Mar;14:172–178. doi: 10.1136/jcp.14.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER E. A., FLYNN F. V. The proteinuria of renal tubular disorders. Lancet. 1958 Nov 8;2(7054):978–980. doi: 10.1016/s0140-6736(58)90473-2. [DOI] [PubMed] [Google Scholar]
- Bernier G. M., Conrad M. E. Catabolsm of human beta-2-microglobulin by the rat kidney. Am J Physiol. 1969 Nov;217(5):1359–1362. doi: 10.1152/ajplegacy.1969.217.5.1359. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Poortmans J. Renal clearance of 15 plasma proteins in renal disease. J Lab Clin Med. 1970 Feb;75(2):297–306. [PubMed] [Google Scholar]
- CREETH J. M., KEKWICK R. A., FLYNN F. V., HARRIS H., ROBSON E. B. An ultracentrifuge study of urine proteins with particular reference to the proteinuria of renal tubular disorders. Clin Chim Acta. 1963 May;8:406–414. doi: 10.1016/0009-8981(63)90078-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain M. J., Stimmler L. The renal handling of insulin. J Clin Invest. 1967 Jun;46(6):911–919. doi: 10.1172/JCI105597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Debray-Sachs M., Sachs C. Proteinuria in patients with homotransplanted kidneys. Nature. 1966 Oct 8;212(5058):209–210. doi: 10.1038/212209a0. [DOI] [PubMed] [Google Scholar]
- Dillard M. G., Pesce A. J., Pollak V. E., Boreisha I. Proteinuria and renal protein clearances in patients with renal tubular disorders. J Lab Clin Med. 1971 Aug;78(2):203–215. [PubMed] [Google Scholar]
- Flynn F. V., Platt H. S. The origin of the proteins excreted in tubular proteinuria. Clin Chim Acta. 1968 Sep;21(3):377–399. doi: 10.1016/0009-8981(68)90067-3. [DOI] [PubMed] [Google Scholar]
- GITLIN D., JANEWAY C. A., FARR L. E. Studies on the metabolism of plasma proteins in the nephrotic syndrome. I. Albumin, gamma-globulin and iron-binding globulin. J Clin Invest. 1956 Jan;35(1):44–56. doi: 10.1172/JCI103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDWICKE J., SQUIRE J. R. The relationship between plasma albumin concentration and protein excretion in patients with proteinuria. Clin Sci. 1955 Aug;14(3):509–530. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hardwicke J. Proteinuria. Sci Basis Med Annu Rev. 1970:211–229. [PubMed] [Google Scholar]
- Harrison J. F., Blainey J. D. Low molecular weight proteinuria in chronic renal disease. Clin Sci. 1967 Oct;33(2):381–390. [PubMed] [Google Scholar]
- Harrison J. F., Blainey J. D. Low molecular weight proteinuria in chronic renal disease. Clin Sci. 1967 Oct;33(2):381–390. [PubMed] [Google Scholar]
- Harrison J. F., Lunt G. S., Scott P., Blainey J. D. Urinary lysozyme, ribonuclease, and low-molecular-weight protein in renal disease. Lancet. 1968 Feb 24;1(7539):371–375. doi: 10.1016/s0140-6736(68)91350-0. [DOI] [PubMed] [Google Scholar]
- JOACHIM G. R., CAMERON J. S., SCHWARTZ M., BECKER E. L. SELECTIVITY OF PROTEIN EXCRETION IN PATIENTS WITH THE NEPHROTIC SYNDROME. J Clin Invest. 1964 Dec;43:2332–2346. doi: 10.1172/JCI105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. Metabolism of Bence Jones proteins in multiple myeloma patients and in patients with renal disease. Scand J Clin Lab Invest. 1970 Aug;26(1):13–21. doi: 10.3109/00365517009049207. [DOI] [PubMed] [Google Scholar]
- Jensen K. Metabolism of bence jones proteins in non-myeloma patients with normal renal function. Scand J Clin Lab Invest. 1970 May;25(3):281–289. doi: 10.3109/00365517009046207. [DOI] [PubMed] [Google Scholar]
- KATZ J., ROSENFELD S., SELLERS A. L. Role of the kidney in plasma albumin catabolism. Am J Physiol. 1960 Apr;198:814–818. doi: 10.1152/ajplegacy.1960.198.4.814. [DOI] [PubMed] [Google Scholar]
- LATHEM W., DAVIS B. B., ZWEIG P. H., DEW R. The demonstration and localization of renal tubular reabosorption of hemoglobin by stop flow analysis. J Clin Invest. 1960 Jun;39:840–845. doi: 10.1172/JCI104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER F., PALADE G. E. LYTIC ACTIVITIES IN RENAL PROTEIN ABSORPTION DROPLETS. AN ELECTRON MICROSCOPICAL CYTOCHEMICAL STUDY. J Cell Biol. 1964 Dec;23:519–552. doi: 10.1083/jcb.23.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mann D., Granger H., Fahey J. L. Use of insoluble antibody for quantitative determination of small amounts of immunoglobulin. J Immunol. 1969 Mar;102(3):618–624. [PubMed] [Google Scholar]
- Maunsbach A. B. Absorption of I-125-labeled homologous albumin by rat kidney proximal tubule cells. A study of microperfused single proximal tubules by electron microscopic autoradiography and histochemistry. J Ultrastruct Res. 1966 Jun;15(3):197–241. doi: 10.1016/s0022-5320(66)80108-9. [DOI] [PubMed] [Google Scholar]
- Mogielnicki R. P., Waldmann T. A., Strober W. Renal handling of low molecular weight proteins. I. L-Chain metabolism in experimental renal disease. J Clin Invest. 1971 Apr;50(4):901–909. doi: 10.1172/JCI106562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVER J., MACDOWELL M., LEE Y. C. Cellular mechanisms of protein metabolism in the nephron. I. The structural aspects of proteinuria; tubular absorption, droplet formation, and the disposal of proteins. J Exp Med. 1954 Jun 1;99(6):589–604. doi: 10.1084/jem.99.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscator M. Proteinuria in chronic cadmium poisoning. 3. Electrophoretic and immunoelectrophoretic studies on urinary proteins from cadmium workers, with special reference to the excretion of low molecular weight proteins. Arch Environ Health. 1966 Mar;12(3):335–344. doi: 10.1080/00039896.1966.10664380. [DOI] [PubMed] [Google Scholar]
- ROSENFELD S., KATZ J., SELLERS A. L. Effect of nephrectomy on I-131 albumin turnover in the dog. J Lab Clin Med. 1962 Mar;59:381–386. [PubMed] [Google Scholar]
- SELLERS A. L., KATZ J., ROSENFELD S. Plasma albumin catabolism in experimental nephrosis. Nature. 1961 Nov 11;192:562–563. doi: 10.1038/192562a0. [DOI] [PubMed] [Google Scholar]
- SOLOMON A., WALDMANN T. A., FAHEY J. L., MCFARLANE A. S. METABOLISM OF BENCE JONES PROTEINS. J Clin Invest. 1964 Jan;43:103–117. doi: 10.1172/JCI104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Terry W. D., Fahley J. L., Steinberg A. G. GM and INV factors in subclasses of human IgG. J Exp Med. 1965 Dec 1;122(6):1087–1102. doi: 10.1084/jem.122.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F., Laurell C. B. Distribution of alpha-chain subclasses in normal and pathological IgA-globulins. Immunology. 1968 Mar;14(3):425–432. [PMC free article] [PubMed] [Google Scholar]
- WALLENIUS G. [Renal clearance of dextran as a measure of glomerular permeability]. Acta Soc Med Ups Suppl. 1954 Apr 8;59(4):1–91. [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wochner R. D., Strober W., Waldmann T. A. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967 Aug 1;126(2):207–221. doi: 10.1084/jem.126.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]