Abstract
Although nerve cell membranes are often assumed to be uniform with respect to electrical properties, there is increasing evidence for compartmentalization into subdomains with heterogeneous impacts on the overall cell function. Such microdomains are characterized by specific sets of proteins determining their functional properties. Recently, clustering of large-conductance calcium-activated potassium (BKCa) channels was shown at sites of subsurface membrane cisterns in cerebellar Purkinje cells (PC), where they likely participate in building a subcellular signaling unit, the 'PLasmERosome'. By applying SDS-digested freeze-fracture replica labeling (SDS-FRL) and postembedding immunogold electron microscopy, we have now studied the spatial organization of somatic BKCa channels in neocortical layer 5 pyramidal neurons, principal neurons of the central and basolateral amygdaloid nuclei, hippocampal pyramidal neurons and dentate gyrus (DG) granule cells to establish whether there is a common organizational principle in the distribution of BKCa channels in central principal neurons. In all cell types analyzed, somatic BKCa channels were found to be non-homogenously distributed in the plasma membrane, forming two pools of channels with one pool consisting of clustered channels and the other of scattered channels in the extrasynaptic membrane. Quantitative analysis by means of SDS-FRL revealed that about two-thirds of BKCa channels belong to the scattered pool and about one-third to the clustered pool in principal cell somata. Overall densities of channels in both pools differed in the different cell types analyzed, although being considerably lower compared to cerebellar PC. Postembedding immunogold labeling revealed association of clustered channels with subsurface membrane cisterns and confirmed extrasynaptic localization of scattered channels. This study indicates a common organizational principle for somatic BKCa channels in central principal neurons with the formation of a clustered and a scattered pool of channels, and a cell-type specific density of this channel type.
Key words: BKCa channel, SDS-FRL, postembedding immunogold electron microscopy, plasmerosome, microdomain, subsurface cistern
Abbreviations: BKCa, large-conductance calcium-activated potassium channel; BL, basolateral nucleus of the amygdala; BSA, bovine serum albumin; CaV, voltage-gated calcium channel; CICR, calcium induced calcium release; DG, dentate gyrus; ER, endoplasmic reticulum; fAHP, fast afterhyperpolarization; GC, granule cell; IMP, intramembrane particle; KV, voltage-gated potassium channel; PB, phosphate buffer; PC, Purkinje cell; RT, room temperature; SDS, sodium lauryl sulphate; SDS-FRL, SDS-digested freeze-fracture replica labeling; SSC, subsurface cistern; SSCx, somato-sensory cortex; TBS, tris-buffered saline
Large-conductance Ca2+ activated potassium channels (also called BKCa, KCa1.1, Maxi-K, or Slo1) are widely distributed in the mammalian brain and play diverse physiological roles in neurons ranging from action potential repolarization to control of cell excitability and neurotransmitter release (Storm, 1987a; Lancaster and Nicoll, 1987; Shao et al., 1999; Hu et al., 2001a; Gu et al., 2007; Hou et al., 2009). They are localized primarily to principal neurons where they are targeted to specific subcellular domains (Knaus et al., 1996; Hu et al., 2001a; Sailer et al., 2006; Misonou et al., 2006). Recently we found that BKCa channels are distributed non-homogenously in the somato-dendritic plasma membrane of cerebellar Purkinje cells (PC), forming two distinct pools: one pool consisting of channels that are scattered in the extrasynaptic membrane at low density, and the other consisting of channels that cluster in membrane areas overlying subsurface membrane cisterns (SSC; Kaufmann et al., 2009). These two pools of BKCa channels might differ in function and routes of Ca2+ activation.
BKCa channels (KCa1) form together with the Slack and Slick channels (KCa4) as well as Slo3 channels (KCa5) the structurally related SLO family of high-conductance Ca2+ and Na+ activated potassium channels. Small-conductance (KCa2, or SK) and intermediate-conductance Ca2+ activated potassium channels (KCa3, or IK) are more distantly related to this family (Wei et al., 2005; Salkoff et al., 2006). BKCa channels are homotetramers of principal (alpha) subunits, which are products of the KCNMA1 or Slo1 gene (first cloned in Drosophila melanogaster; Adelman et al., 1992). The principal subunits, constituting the pore of the channel, can co-assemble with 1–3 auxiliary beta subunits (KCNMB1-4; Salkoff et al., 2006), which modify the functional properties of the channel in different tissues (Brenner et al., 2000; Sah and Faber, 2002). Alpha subunits show a relatively short extracellular, amino terminal domain (N-terminus) and a large intracellular, carboxyl terminal domain (C-terminus) where the Ca2+ binding domain (“calcium bowl”) is located (Sah and Faber, 2002; Salkoff et al., 2006). BKCa channels are distinguished from other potassium channels by their unusually high single-channel conductance (averaging over 250 pS) and being gated both by membrane depolarization and changes in cytosolic free [Ca2+] (Salkoff et al., 2006). Increasing [Ca2+]i shifts the activation curve from highly positive potentials into the physiological voltage range (Cui et al., 1997; Latorre and Brauchi, 2006; Fakler and Adelman, 2008). Yet, BKCa channels have a low affinity for Ca2+, typically requiring more than 10 μM [Ca2+]i for activation within physiological membrane potentials (Fakler and Adelman, 2008). The sensitivity of BKCa channels to Ca2+ makes it an important negative-feedback system for Ca2+ entry in many cell types, thus regulating transmitter release and other Ca2+-dependent processes (Storm, 1987a, 1990; Hu et al., 2001a).
Activation of BKCa channels in neurons generates a macroscopic current, which has been called IC or ICT. This current contributes to action potential repolarization (Lancaster and Nicoll, 1987; Storm, 1987a; Robitaille et al., 1993; Faber and Sah, 2002), mediates an early component of the fast afterhyperpolarization (fAHP; Storm, 1987a; Faber and Sah, 2003), shapes dendritic Ca2+ spikes (Golding et al., 1999) and regulates neurotransmitter release at least under certain conditions (Lingle et al., 1996; Hu et al., 2001a; Raffaelli et al., 2004). The diverse functional properties mediated by BKCa channels (Hou et al., 2009) might be correlated with their differential localization to various subcellar sites and non-homogenous distribution within specific subdomains, as observed in cerebellar PC (Kaufmann et al., 2009). In the present study, we aimed at establishing whether the dual distribution pattern of BKCa channels found in PC is a general organizational principle for somatic BKCa channels, common to different types of neurons within different brain areas, rather than being specific to a certain cell type. Hence, we examined BKCa channel distribution in hippocampal CA3 pyramidal neurons, neocortical layer 5 pyramidal neurons, principal neurons of the central and basolateral amygdaloid nuclei, as well as granule cells of the hippocampal dentate gyrus (DG). All these cell types were shown to express functional BKCa channels (Brenner et al., 2005; Shao et al., 1999; Benhassine and Berger, 2005; Sausbier et al., 2006; Meis and Pape, 1997) yet their precise subcellular localization has remained unidentified. By means of SDS-digested freeze-fracture replica labeling (SDS-FRL), we performed detailed quantitative analysis of BKCa channels in the somatic plasma membrane of these cell types (except for principal neurons of the central amygdaloid nucleus) for comparison with respective data in PC (Kaufmann et al., 2009). We also analyzed the subcellular localization of BKCa channels in DG granule cells by means of thin-section electron microscopy for comparison with PC, since these two cell types show similar membrane specializations like SSC (Le Beux, 1972), but are highly different with respect to the sizes and characteristics of their somata and dendritic trees (Paxinos, 1995), function (excitatory versus inhibitory), and BKCa channel-related physiological properties (Edgerton and Reinhart, 2003; Sausbier et al., 2004; Brenner et al., 2005).
Experimental procedures
Materials
Paraformaldehyde, osmium tetroxide, uranyl acetate and pioloform were obtained from Agar Scientific Ltd. (Stansted, UK). EM grade glutaraldehyde was purchased from Polysciences Inc. (Warrington, PA, USA). Thiopental was from Sandoz (Kundl, Austria). Lead (II) citrate was from Merck KGaA (Darmstadt, Germany), picric acid from Fluka GmbH (Buchs, Switzerland). Glycerin and sodium lauryl sulfate (SDS) were from Carl Roth GmbH (Karlsruhe, Germany). Normal goat serum was from Bender (Vienna, Austria), bovine serum albumin (BSA) from Serva (Heidelberg, Germany). Gold-conjugated immunoglobulins were from British BioCell Int. (Cardiff, UK). Eukitt was from Kindler (Freiburg, Germany). Platinum and carbon rods were from Bal-Tec (Balzers, Liechtenstein). Lowicryl HM20 resin and all remaining chemicals were from Sigma-Aldrich (Vienna, Austria).
Antibody production and specification
BKCa channel antibodies were produced and kindly provided by Hans-Guenther Knaus (Department Medical Genetics, Molecular and Clinical Pharmacology, Innsbruck Medical University, Austria). In brief, antibodies were affinity purified from immune sera raised in rabbit using immunogenic peptides designed from the mouse Slo1 sequence, containing residues 913–926 [anti-BKα (913–926)] (Knaus et al., 1995) and residues 1118–1132 [anti-BKα (1118–1132)] (Wanner et al., 1999). Both sequences show 100% homology between rat and mouse. Antibodies were characterized by enzyme-linked immunosorbent assay, immunoprecipitation and immunoblotting. The antibodies recognized single bands of approximately 125 kDa in immunoblot analysis of rat and mouse whole-brain membranes (Knaus et al., 1996; Sausbier et al., 2004). Specificity of these antibodies was tested and confirmed by immunocytochemistry in light microscopy (Grunnet and Kaufmann, 2004; Sailer et al., 2006) and in electron microscopy (Hu et al., 2001a; Kaufmann et al., 2009). In the present study, both antibodies were used for SDS-FRL as well as post-embedding immunogold cytochemistry and yielded identical results. Specificity of immunolabeling was confirmed on tissue samples from BKCa channel null mice, kindly provided from Peter Ruth (Institute of Pharmacy, University of Tuebingen, Germany).
Anti-mSlo maxi-K+ channel
A monoclonal anti-BKCa channel antibody was obtained from the UC Davis/NIH NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Neurobiology, Physiology and Behavior, College of Biological Sciences, University of California, Davis, CA 95616, USA (Cat.No. 75-022). The antibody was produced against a fusion protein corresponding to amino acids 690–1196 of mouse Slo1 (clone L6/60) and recognized a single band of approximately 125 kDa in immunoblot analysis of rat hippocampal membrane preparations. In the present study, the antibody was applied successfully in post-embedding immunogold labeling yielding same results as anti-BKα (913–926) and anti-BKα (1118–1132). Specificity of immunolabeling was tested and confirmed on tissue samples from BKCa channel null mice.
Animals and tissue preparation
Immunochemical studies were performed on samples from adult male Sprague–Dawley rats (250–300 g; Department Laboratory Animals and Genetics, Medical University, Vienna, Austria), adult male C57Bl/6 mice (10–12 weeks; Medical University, Vienna, Austria) and adult male BKCa channel null mice (10–12 weeks; Sausbier et al., 2004). All experimental protocols were approved by the Animal Experimentation Ethics Board, in compliance with both, the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS no. 123) and the European Communities Council Directive of November 24, 1986 (86/609/EEC). The authors further attest that all efforts were made to minimize the number of animals used and their suffering. Animals were deeply anesthetized by intraperitoneal injection of thiopental (12 mg/100 g body weight) and perfused transcardially with phosphate buffered saline (PBS; 25 mM, 0.9% NaCl, pH 7.4) followed by chilled fixative (buffer conditions are given below for the different techniques used). After fixation, brains were removed from the skull immediately, washed in phosphate buffer (PB; 0.1 M, pH 7.4) and stored in 0.1 M PB containing 0.05% sodium azide at 4 °C.
SDS-digested freeze-fracture replica labeling (SDS-FRL)
SDS-FRL was performed with some modifications to the original method (Fujimoto, 1995; Masugi-Tokita et al., 2007). The experimental animals (n=15: five Sprague–Dawley rats, five C57Bl/6 mice, and five BKCa channel null mice) were perfusion fixed with PB (0.1 M, pH 7.4) containing 1% formaldehyde and 15% of a saturated solution of picric acid. The brains were removed from the skull immediately and forebrains were cut into 140 μm coronal sections with a Vibroslicer (VT1000S; Leica Microsystems, Vienna Austria). Samples were dissected from respective brain areas and cryoprotected with 30% glycerol in 0.1 M PB overnight at 4 °C. Samples were then frozen by use of a high-pressure freezing machine (HPM 010; Bal–Tec, Balzers, Liechtenstein) and fractured by a double replica method in a freeze-etching device (BAF 060; Bal–Tec). Fractured faces were replicated by evaporation of carbon (rotating) by means of an electron beam gun positioned at a 90° angle to a thickness of 5 nm and shadowed unidirectionally with platinum-carbon at a 60° angle (thickness 2 nm). Finally, a 15 nm thick layer of carbon was applied from a 90° angle (rotating). Tissue was solubilized in a solution containing 2.5% SDS and 20% sucrose made up in 15 mM Tris buffer, pH 8.3, on a shaking platform for 18 h at 80 °C. Replicas were kept in the same solution at room temperature until they were processed further.
On day of immunolabeling, replicas were washed in Tris-buffered saline (TBS; 50 mM, 0.9% NaCl, pH 7.4) containing 0.05% BSA and incubated in a blocking solution containing 5% BSA in 50 mM TBS for 1 h at room temperature (RT). Subsequently, the replicas were incubated in the primary antibody diluted in the same solution as described above, overnight at 7 °C. Dilution of antibodies used was: anti-BKα (913–926) 1.8 μg/ml; anti-BKα (1118–1132) 2 μg/ml. After several washes in TBS, the replicas were reacted with gold-conjugated goat anti-rabbit secondary antibodies made up in 50 mM TBS (1:30) containing 5% BSA overnight at 7 °C. They were then washed in double-distilled water, mounted on formvar-coated 100-line copper grids and analyzed in a Philips CM120 TEM equipped with a Morada CCD camera (Soft Imaging Systems, Münster, Germany). Whole images were level adjusted, sharpened, and cropped in Photoshop (Adobe) without changing any specific features within. The specificity of the immunolabeling was controlled and confirmed applying the respective pre-immune serum, applying the immune serum preadsorbed with the synthetic immunogenic peptides (concentration 10 μM), and applying the immune serum on samples from BKCa channel null mice, respectively.
Sampling and analysis of SDS-FRL data
Five to seven replicas were used for quantification of BKCa channel immunolabeling per respective brain area, which were: somatosensory cortex, field CA3 of hippocampus, DG, and basolateral nucleus of the amygdala (BL). Within these areas, aspects of principal cells were selected at random and electron micrographs were taken at a magnification of ×43,000–×125,000. The magnification was verified by use of a calibration grid. Clusters of more than nine intramembrane particles (IMP) labeled with more than three immunogold particles were considered BKCa immunoreactive clusters. The outline of clusters was demarcated by hand, and immunogold particles within 20 nm from the cluster edge were included in the analysis since particles can be as distant from the respective epitope (Matsubara et al., 1996). Quantification was performed by means of AnalySIS (Soft Imaging Systems), and data were expressed as mean±SD. Statistical analysis was carried out in Prism (GraphPad Software, Inc.). Group comparisons were performed by 1way ANOVA followed by post hoc Bonferroni's multiple comparison tests (α=0.05).
For estimating the total number of immunoparticles labeling BKCa channels per DG granule cell soma, as well as the ratio of BKCa channels in the scattered and the clustered pool of channels, the surface of an average granule cell soma was calculated considering it as a spheroid (prolate ellipsoid) with a height=18 μm and width=10 μm; these values were based on specifications from Amaral and Witter (1995).
Postembedding immunogold labeling
The experimental animals (n=15: five Sprague–Dawley rats, five C57Bl/6 mice, and five BKCa channel null mice) were perfusion fixed with PB (0.1 M, pH 7.4) containing 4% formaldehyde and 0.05% glutaraldehyde. The brains were taken out and respective tissue blocks were dissected, freeze-substituted and embedded at low temperature in Lowicryl HM20 resin (Takumi et al., 1999). Ultrathin sections (70–80 nm) were cut on a Leica ultramicrotome (Ultracut S; Leica, Vienna, Austria), mounted on formvar-coated nickel mesh grids, and processed for immunogold cytochemistry (Matsubara et al., 1996). Sections were etched with 1% H2O2 in TBS for 20 min at RT, followed by incubation in 50 mM glycine in TBS containing 0.1% Triton X-100 (TBS-T; pH 7.4) for 10 min at RT. After application of 2% BSA, 2% normal goat serum and 0.2% milk powder in TBS-T for 20 min to block non-specific binding sites, sections were incubated with primary antibodies diluted in TBS-T containing 2% BSA overnight at 7 °C. Concentration of antibodies used were: affinity purified anti-BKα (913–926) 1.8 μg/ml; anti-BKα (1118–1132) 2.4 μg/ml; anti-mSlo maxi-K+ channel 2.2 μg/ml. After rinsing in TBS-T (four times 10 min each), gold-conjugated goat anti-rabbit or goat anti-mouse antibodies, respectively, were applied (diluted 1:30 in TBS-T containing 2% BSA and 0.05% polyethylene glycol; for 90 min at RT). The sections were then rinsed in double-distilled water and air-dried. The sections were stained with Uranyl Acetate and Lead Citrate and examined in a TECNAI G2 as well as a Philips CM120 TEM, both equipped with a Morada CCD camera. Whole images were level adjusted, sharpened, and cropped in Photoshop (Adobe) without changing any specific features within. Specificity of immunolabeling was controlled and confirmed by applying the respective pre-immune serum, applying antibodies preadsorbed with excess immunogenic peptides (concentration 10 μM), and applying antibodies on sections from samples of BKCa channel null mice.
Sampling and analysis of postembedding immunogold data
Five samples were taken at random from the DG granule cell layer of the rat dorsal hippocampus (approximate anteroposterior level −3.4 mm; Paxinos and Watson, 1998), and four sections were analyzed per sample. Electron micrographs were taken at a magnification of ×43,000. The magnification was verified by use of a calibration grid, nonspecific immunolabeling was determined in preadsorption experiments. To establish the subcellular distribution profile of immunogold particles, the distance of particles perpendicular to the inner leaflet of the somatic plasma membrane was analyzed within a range of 25 nm toward the extracellular side (indicated with negative values) and 200 nm toward the cell interior (n=500). Quantification was performed by means of AnalySIS (Soft Imaging Systems), and data were expressed as mean±SD. For the comparison of the BKCa channel distributions in areas of the plasma membrane with and without underlying SSC, the subcellular particle distribution was established for each of the areas perpendicular to the inner leaflet of the plasma membrane within a range of 25 nm toward the extracellular side (indicated with negative values) and 80 nm toward the cell interior (n=200 particles each).
Results
Two pools of somatic BKCa channels in principal neurons of the central nervous system: clustered and scattered channels
The somatic localization and spatial distribution of BKCa channels were studied by means of SDS-FRL in a subset of central principal neurons, namely in hippocampal pyramidal cells, DG granule cells, neocortical layer 5 pyramidal cells and principal cells of the central as well as basolateral nuclei of the amygdala. The SDS-FRL technique allows localization and density analysis of integral membrane proteins beyond limitations of thin-section electron microscopy. Freeze-fracturing and replication of membrane leaflets, with subsequent solubilization of unfractured tissue constituents facilitate the view of large areas of the plasma membrane which are also accessible to antibody binding without diffusion restrictions.
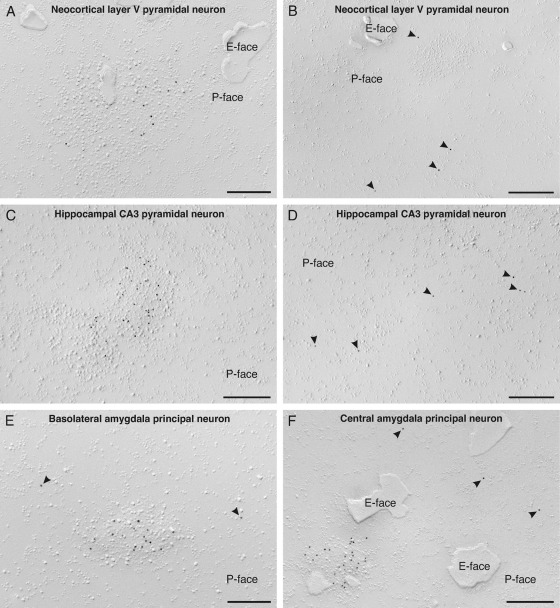
We found that BKCa channels form two distinct pools in the somatic plasma membrane of all cell types analyzed, although at different densities. All cell types showed both scattered channels and clusters of channels. Such a dual distribution pattern was seen specifically in layer 5 pyramidal neurons of the somatosensory cortex (SSCx; Fig. 1A, B), hippocampal CA3 pyramidal neurons (Fig. 1C, D), principal neurons of the basolateral (BL; Fig. 1E) as well as central amygdaloid nuclei (Fig. 1F), and hippocampal DG granule cells (Fig. 2A–H). These findings indicate that a separation into two distinct pools of channels, which we found for the first time in cerebellar PC (Kaufmann et al., 2009), is likely to represent a common organizational pattern for BKCa channels in the soma of central principal neurons.
Fig. 1.
Localization of BKCa channels in the somatic plasma membrane of central principal neurons revealed with SDS-digested freeze-fracture replica labeling in rat brain. (A) A high-power photograph of an aspect of the somatic plasma membrane of a layer V pyramidal neuron of the somato-sensory cortex reveals a cluster of immunolabeled BKCa channels associated with a cluster of intramembrane particles (IMPs). Antibodies conjugated to 10 nm gold particles and directed against epitopes at an intracellular C-terminal domain of the α subunit (anti-BKα, residues 913–926) were applied, thus labeling is detected in the P-face of the plasma membrane. The E-face is free of any immunolabeling. (B) In the same pyramidal neuron, scattered BKCa channels are detected in the P-face of the somatic plasma membrane indicated with an arrowhead. (C) Clustering of BKCa channels, associated with clusters of IMPs, is also observed in the somatic plasma membrane P-face of a hippocampal CA3 pyramidal neuron. The density of immunolabeling is higher as in the neocortical pyramidal neuron, shown in (A). (D) Scattered immunoparticles (arrowheads) are observed in the P-face of the CA3 pyramidal neuron too. (E) Clustered and scattered BKCa channels (the latter are indicated with an arrowhead) were also found in the plasma membrane P-face of a principal neuron of the basolateral amygdaloid nucleus. (F) The same distribution pattern of BKCa channels with clustered and scattered channels (arrowheads) is observed in the plasma membrane P-face of a principal neuron of the central amygdaloid nucleus. The E-face is free of any immunolabeling. Scale bar=200 nm in (A, E); 300 nm in (B); 275 nm in (C, F); 250 nm in (D).
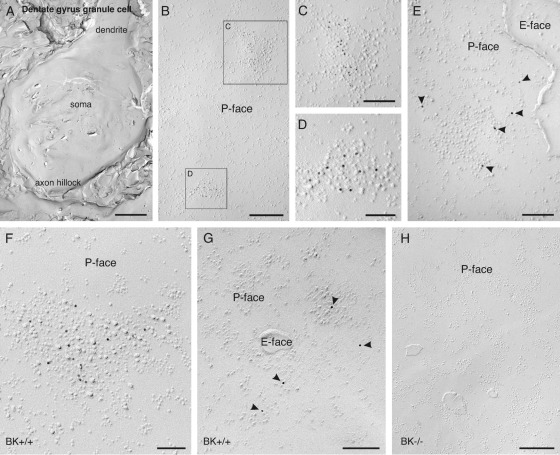
Fig. 2.
Clustered and scattered BKCa channels in the somatic plasma membrane of DG granule cells detected with SDS-digested freeze-fracture replica labeling in rat (A–E) and mouse brain (F–H). (A) A low-power photograph shows a large aspect of a granule cell soma, including the very proximal portion of the principal dendrite and the axon hillock. (B) A high-power photograph of an aspect of the granule cell somatic plasma membrane shows the distribution of BKCa channels in the P-face of the somatic plasma membrane. Antibodies conjugated to 10 nm gold particles and directed against epitopes at the intracellular C-terminal end of the α subunit were applied. (C, D) Clusters of immunoparticles labeling BKCa channels in the plasma membrane are associated with clusters of IMPs (aspects of the plasma membrane at higher magnification, indicated by a boxed area in B). (E) Some immunoparticles labeling BKCa channels are scattered in the plasma membrane P-face (indicated with an arrowhead). The E-face of the plasma membrane is free of any immunolabeling. (F, G) The same distribution pattern of BKCa channels is observed in the plasma membrane of DG granule cell somata in mouse (C57Bl/6; BK+/+), with a clustered (F) and a scattered (G) pool of channels in the plasma membrane P-face. (H) Antibody specificity is controlled and confirmed in replicas of BKCa channel null mice (BK−/−). Scale bar=2.75 μm in (A); 350 nm in (B, D, H); 175 nm in (C); 150 nm in (E, G); 100 nm in (F).
Different densities of clustered and scattered BKCa channels
Detailed quantitative analysis of BKCa channel densities was performed in DG granule cells, CA3 pyramidal neurons, layer 5 pyramidal neurons of the SSCx, and principal neurons of the BL (Table 1). With respect to the abundance of clustered channels, large differences were observed in immunoparticle densities within clusters ranging from 102±14 particles per μm2 membrane in BL principal neurons to 175±24 particles per μm2 membrane in CA3 pyramidal neurons (total number of clusters analyzed in each cell type=40). The average number of particles per cluster was similar in DG granule cells, SSCx pyramidal neurons and BL principal neurons with about eight particles per cluster. Compared to these cell types, the average number of particles per cluster was significantly higher in CA3 pyramidal neurons (P<0.001) with about 12 particles per cluster. The density of clusters per area of somatic plasma membrane was similar in all four cell types with about one cluster per 13 μm2 membrane (total membrane area analyzed in each cell type=600 μm2). Significant difference in cluster density was only observed for DG granule cells compared to CA3 pyramidal neurons (5.8±1.8 versus 9.2±3.7 clusters per 100 μm2 membrane, respectively; P<0.01). Also the average size of clusters (≈0.07 μm2) was similar in the four cell types, and no significant difference in cluster size was observed.
Table 1.
Density of clustered and scattered immunoparticles labeling BKCa channels in the soma of dentate gyrus granule cells (GC), hippocampal CA3 pyramidal neurons (CA3), layer V pyramidal neurons of the somatosensory cortex (SSCx) and principal neurons of the basolateral amygdala (BL) compared to cerebellar Purkinje cells (PC¥; values for PC were published by Kaufmann et al., 2009)
| GC | CA3 | SSCx | BL | PC¥ | |
|---|---|---|---|---|---|
| Average size of a cluster (in μm2; n=40) |
|
|
|
|
0.151±0.036 |
| Particle density within a cluster (in particles per μm2; n=40) |
|
|
|
|
341±31 |
| Number of particles per cluster (in particles per cluster; n=40) |
|
|
|
|
46.8±4.9 |
| Density of clusters (in clusters per 100 μm2; area=600 μm2) |
|
9.2±3.7 | 7.5±2.1 | 8.1±3.0 | 9.5±2.2 |
| Density of scattered particles (in particles per μm2; area=250 μm2) |
|
|
|
|
5.68±0.43 |
| Background levels (in particles per μm2; area=250 μm2) | 0.26±0.03 | n.d. | n.d. | n.d. | 0.32±0.18 |
n.d., not determined.
Group comparisons were performed by 1 way ANOVA followed by post hoc Bonferroni's (α=0.05): †, GC versus CA3; ‡, GC versus SSCx; #, GC versus BL; *, GC versus PC; ‡, CA3 versus SSCx; #, CA3 versus BL; *, CA3 versus PC; #, SSCx versus BL; *, SSCx versus PC; *, BL versus PC; P<0.01, two symbols; P<0.001, three symbols.
For the second pool of immunoparticles labeling BKCa channels scattered in the somatic plasma membrane (Fig. 2E, G), the density was considerably lower compared to that within clusters, and differed largely in the cell types analyzed (Table 1). In DG granule cells and CA3 pyramidal neurons, about one immunoparticle was detected per 1.0 μm2 of plasma membrane, whereas in SSCx pyramidal neurons and BL principal neurons, a significantly minor density was seen with about 1 particle per 1.4 μm2 plasma membrane (P<0.001; total membrane area analyzed in each cell type=250 μm2; background levels established in BKCa channel null mice=0.26±0.03 particles per μm2 membrane).
We then compared the density of somatic BKCa channels in the four cell types to that established for cerebellar PC (Kaufmann et al., 2009). With respect to the clustered pool, the average size of clusters, particle density within clusters, and average number of particles per cluster were all significantly larger in PC (P<0.001). Noteworthy, the density of clusters per unit membrane area was instead comparable between most cell types, with the only exception of DG granule cells, which showed a considerably lower density of clusters compared to PC and CA3 pyramidal neurons (Table 1). With respect to the scattered pool, the density of immunoparticles was significantly higher in PC compared to all other cell types (ratio of particle density in PC to other cell types ≈6:1; Table 1).
To estimate the ratio of scattered to clustered BKCa channels in the soma of a central principal model neuron, we calculated the total number of immunoparticles labeling BKCa channels for both pools in DG granule cells, since this cell type exhibits cell bodies of relatively homogeneous dimensions with a smooth surface membrane (Laatsch and Cowan, 1966; Amaral and Witter, 1995). In this cell type, we detected about one cluster of immunoparticles per 17 μm2 of somatic plasma membrane (5.8±1.8 clusters per 100 μm2 membrane). Considering the total surface area of an average granule cell soma as 648 μm2 (see Experimental Procedures), this yields approximately 38 clusters per soma. Since 8.5 particles were detected per average cluster, this results in about 323 particles per DG granule cell soma in the clustered pool. The density of scattered particles was 0.98±0.21 particles per μm2 somatic plasma membrane. This yields about 635 particles per granule cell soma in the scattered pool, that is almost twice as many particles as in the clustered pool. Thus, presuming a similar labeling efficacy for both pools of channels, about two-thirds of BKCa channels in an average principal cell soma would belong to the scattered pool and about one-third to the clustered pool.
Clustered BKCa channels are associated with plasma membrane domains overlying SSC
Since we observed that clustered BKCa channels were always associated with SSC in cerebellar PC using thin section electron microscopy (Kaufmann et al., 2009), we thus analyzed (i) the cyto-architecture beneath domains of the plasma membrane enriched in BKCa channels in DG granule cells, and studied (ii) possible localization of BKCa channels to intracellular organelles. We have chosen DG granule cells for this analysis since SSC are distinct and well described in this cell type (Laatsch and Cowan, 1966; Kolde and Themann, 1982). We applied post-embedding immunogold labeling on freeze-substituted and Lowicryl embedded ultra-thin sections (van Lookeren Campagne et al., 1991). This method holds the advantage of high spatial resolution in the subcellular dimension, although at cost of labeling sensitivity.
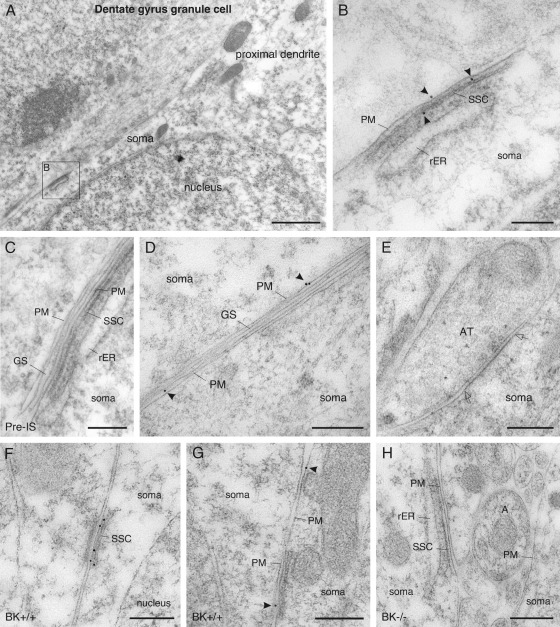
Consistent with our findings in SDS-FRL, also post-embedding immunogold labeling revealed two pools of BKCa channels in the plasma membrane of DG granule cells: one pool of scattered channels and a second pool of clustered channels in the somatic plasma membrane (Fig. 3A–H). Clustering of BKCa channels was exclusively observed in areas of the plasma membrane overlying SSC (Fig. 3B, F). In DG granule cells, SSC are usually formed by a 0.5–1.0 μm long outer cistern of the endoplasmic reticulum (ER), free of ribosomes. These cisterns run in parallel to the inner leaflet of the plasma membrane at a regular distance of about 9–13 nm and usually widen at their lateral edges. One (Fig. 3B) or two inner cisterns of rough ER can be closely apposed at a distance of 30–40 nm from the superficial cistern and from each other (Le Beux, 1972; Kolde and Themann, 1982).
Fig. 3.
Localization of BKCa channels in dentate gyrus granule cell somata of rat (A–E) and mouse brain (F–H) revealed by post-embedding immunogold labeling. Antibodies conjugated to 10 nm gold particles were applied on Lowicryl embedded ultra-thin sections. (A) A low-power micrograph shows an aspect of a granule cell soma and the very proximal portion of the raising principal dendrite. (B) Immunoparticles labeling BKCa channels are clustered in areas of the somatic plasma membrane with underlying subsurface cisterns (boxed area in A; immunoparticles are indicated with an arrowhead). (C) Specificity of immunolabeling in rat tissue is tested and confirmed by applying the respective pre-immune serum. (D) Outside areas of the plasma membrane with underlying subsurface cisterns, immunoparticles are scattered in the extrasynaptic plasma membrane with low abundance (arrowheads). (E) The postsynaptic membrane specialization of symmetric, presumably inhibitory synapses, is free of BKCa channel immunolabeling (margins of the postsynaptic membrane specialization are indicated with open arrows). (F) As in the rat, clustering of BKCa channels in areas of the plasma membrane with underlying subsurface cisterns is seen in the granule cell soma of mouse (C57Bl/6; BK+/+). (G) Outside these areas, scattered immunoparticles are localized in the extrasynaptic plasma membrane with low abundance (arrowheads). (H) Specificity of immunolabeling is tested and confirmed in sections of BKCa channel null mice (BK−/−). A, axonal profile; AT, axon terminal; GS, glial sheath; PM, plasma membrane; Pre-IS, pre-immune serum; rER, rough endoplasmic reticulum; SSC, subsurface cistern. Scale bar=1.5 μm in (A); 200 nm in (B, D, G); 250 nm in (C, E, H); 500 nm in (F).
Outside these clusters, scattered immunoparticles labeling BKCa channels were regularly observed in the extrasynaptic plasma membrane (Fig. 3D, G). Postsynaptic membrane specializations of axo-somatic synapses (symmetric, Gray's type II synapses; Laatsch and Cowan, 1966) were free of BKCa channel immunolabeling (Fig. 3E). Specificity of immunolabeling was tested and confirmed in sections of BKCa channel null mice (Fig. 3H). For establishing the abundance of scattered channels, the linear density of immunoparticles was established along the somatic plasma membrane. Portions of the somatic plasma membrane were randomly selected (aside from areas overlying SSC) and all particles within a range of ±20 nm to the inner leaflet of the plasma membrane were sampled. Post-embedding immunogold labeling techniques are based on indirect immunodetection of antigens and hence, the localization of immunoparticles does not directly represent the localization of the epitope. Specifically, gold particles may be as far as 20 nm from its epitope because of the size of the interposed immunoglobulins, the Fab' fragments, and the size of the gold particles themselves (Matsubara et al., 1996). This analysis revealed a density of 0.35±0.08 particles per μm extrasynaptic plasma membrane (total length analyzed=60 μm). The density of particles within postsynaptic specializations of axo-somatic synapses was 0.19±0.05 particles per μm membrane (number of synapses analyzed=40). The densities in post-synaptic specializations are close to background levels established in samples of BKCa channel null mice (0.15±0.04 particles per μm membrane; total length analyzed=80 μm), indicating a lack of BKCa channel localization in postsynaptic membrane specializations of the soma.
BKCa channels are mainly integrated in the plasma membrane
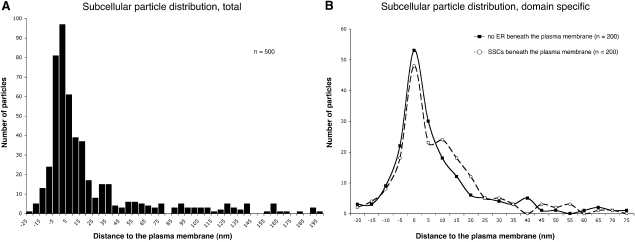
In order to analyze possible localization of BKCa channels to intracellular organelles in addition to the plasma membrane, we established a vertical frequency profile of immunogold particles along an axis perpendicular to the somatic plasma membrane. The inner leaflet of the plasma membrane was used as reference line, and the vertical distribution of gold particles was analyzed within a range of 25 nm toward the extracellular side and 200 nm toward the cell interior. Portions of DG granule cell somata were randomly selected, and all gold particles within the given range were analyzed and binned in 5 nm intervals. This plot revealed a peak corresponding with the plasma membrane, indicating that the majority of the immunosignal originated from plasma membrane labeling (Fig. 4A). Of all particles analyzed (n=500), 71.4% were localized within a range of ±20 nm from the inner leaflet of the plasma membrane. However, the distribution profile was not exactly Gaussian, but appeared slightly skewed toward the cell interior. This inward skew seems too large to being fully explained by the localization of the epitope in the cytoplasmic domain of the alpha subunit. It indicates that small subpopulations of BKCa channels may be localized to intracellular organelles, in addition to the main population of channels in the plasma membrane.
Fig. 4.
Subcellular distribution profile of gold particles labeling BKCa channels [anti-BKα (913–926); 10 nm gold] assessed along an axis perpendicular to the inner leaflet of the somatic plasma membrane of rat dentate gyrus granule cells. (A) The distribution profile (n=500, binning=5 nm) was analyzed within a range of 25 nm toward the extracellular side (indicated with negative values) and 200 nm toward the cell interior. Zero is defined as the center of the inner membrane leaflet. The distribution profile is peaking over the plasma membrane with the mode slightly skewed toward the cell interior. (B) The subcellular particle distribution was reanalyzed and compared in areas of the somatic plasma membrane with underlying subsurface membrane cisterns and in areas of the plasma membrane without visible endoplasmic reticulum (ER) lying beneath it. Particles within a range of 20 nm toward the extracellular side (indicated with negative values) and 75 nm toward the intracellular side were included in this analysis. Zero is defined as the center of the inner membrane leaflet. The particle distribution in areas of the plasma membrane without visible ER beneath it (solid line; n=200 particles, binning=5 nm) shows a Gaussian-shaped distribution profile peaking over the plasma membrane. In areas of the plasma membrane with underlying subsurface cisterns, the particle distribution (dashed line; n=200, binning=5 nm) displays a prominent peak at the level of the plasma membrane and a small shoulder at an interval of about 10–25 nm toward the cytoplasmic side.
To establish whether a BKCa channel subpopulation could be localized to junctional ER components like SSC, we re-analyzed the vertical frequency profile of immunogold particles with respect to (i) areas of the plasma membrane with SSC lying beneath it and (ii) areas of the plasma membrane outside SSC without any visible ER beneath it. Gold particles were sampled within a range of 20 nm toward the extracellular side and 75 nm toward the cell interior (n=200 particles, each) along an axis perpendicular to the inner leaflet of the plasma membrane used as reference line. In areas of the plasma membrane outside SSC, the particle distribution appeared approximately Gaussian with the peak coinciding with the plasma membrane. This re-affirms that BKCa channels are primarily localized in the plasma membrane (Fig. 4B). Conversely, in areas of the plasma membrane with underlying SSC, the distribution profile showed a peak coinciding with the plasma membrane and a small shoulder at a distance of about 10–20 nm toward the cell interior. This may indicate a small subpopulation of BKCa channels being localized in SSC, in addition to the main population of channels in the plasma membrane (Fig. 4B).
Discussion
Two pools of somatic BKCa channels in principal neurons of the central nervous system
Our ultra-structural investigations revealed two pools of BKCa channels in the somata of central principal neurons–one pool consisting of clustered and another of scattered channels in the extrasynaptic plasma membrane. This characteristic distribution pattern of somatic BKCa channels was observed in all types of neurons examined, specifically in cortical layer 5 pyramidal neurons, hippocampal CA3 pyramidal neurons, principal neurons of the central as well as basolateral amygdaloid nuclei, and DG granule cells. Recently, we reported a similar non-homogeneous distribution for BKCa channels in the soma of cerebellar PC (Kaufmann et al., 2009). Thus, the formation of a clustered and a scattered pool of BKCa channels seem to represent a common organizational principle for somatic BKCa channels in principal neurons of different parts of the mammalian brain. The two pools of channels are likely independent from each other, although switching between both pools may play a role as it was shown for neurotransmitter receptors migrating between synaptic and extrasynaptic membranes (Triller and Choquet, 2005).
Different densities of clustered and scattered BKCa channels in different cell types
Despite the common organizational principle in somatic BKCa channels with a clustered and a scattered pool, we found striking differences between different cell types regarding the densities of BKCa channels, and such differences were found for both channel pools. Noteworthy, cerebellar PC seem to constitute a class of their own with respect to BKCa channel densities (Kaufmann et al., 2009). Their massive BKCa channel clusters stand out against those of all the other cell types, both in terms of size and channel density within a cluster. In PC, the number of immunoparticles labeling BKCa channels per cluster is on average 3.8–6.3 times than that of the other cell types. The difference in density of scattered immunoparticles is even more evident, and 5.5–8.5 times higher in PC than in any of the other cells.
Why do cerebellar PC have so much higher BKCa channel densities than the others? PC have a larger cell body (Amaral and Witter, 1995), are neurochemically different since being GABAergic (Paxinos, 1995), and the duration of their action potential is far briefer than in any of the other cell types examined (Hu and Storm, 2001b; Edgerton and Reinhart, 2003; Sausbier et al., 2004; Brenner et al., 2005). The most striking difference in all measured properties, however, is between cerebellar PC and DG granule cells. The large difference in channel density might be represented functionally by the considerable difference in action potential duration, or more specifically, in the different speed of spike repolarization. The action potential in PC is much briefer than in DG granule cells, the spike duration at half amplitude being only ∼0.2 ms versus ∼0.7–1.2 ms (at ∼32–34 °C), respectively (Geiger and Jonas, 2000; Hu and Storm, 2001b; Edgerton and Reinhart, 2003; Sausbier et al., 2004; Brenner et al., 2005). Despite BKCa channels may play just a minor role in spike repolarization in either of these cell types (Geiger and Jonas, 2000; Hu and Storm, 2001b; Womack and Khodakhah, 2002; Edgerton and Reinhart, 2003; Sausbier et al., 2004; Brenner et al., 2005), it can be surmised that higher channel densities are needed to generate faster spikes, because a larger current is required to charge the membrane capacitance more rapidly. Specifically, rapid spike repolarization requires high densities of repolarizing potassium channels. Since PC spikes repolarize extremely rapidly, likewise many other GABAergic neurons, they may need a large BKCa current and hence a high channel density for contributing appreciably to spike repolarization. However, spike repolarization might depend primarily on the activity of voltage-gated potassium (KV) channels.
Somatic BKCa channels seem to be needed mainly for generating a fAHP that contributes to recovery of sodium channels from inactivation during interspike intervals thus allowing high-frequency firing (Sausbier et al., 2004). In order to activate a sufficient BKCa channel current to produce an efficient fAHP, a larger number of BKCa channels is needed in a cell with brief spikes (i.e. cerebellar PC) than in a cell with more long-lasting spikes (i.e. DG granule cells), since a briefer spike will activate a smaller fraction of available channels, again assuming that other factors are equal. Although this may be a main reason for the large difference in the overall density of somatic BKCa channels, it is unlikely that all other factors are in fact equal. Obviously, the opening kinetics of the BKCa channels themselves, the nature of the specific Ca2+ source and the speed as well as efficiency in the coupling with the Ca2+ source are important factors that may differ between the two cell types. Since cerebellar PC are also substantially larger than DG granule cells, their far larger surface area and, hence, larger membrane capacitance requires a larger BKCa channel current and thus a larger total number of channels, if similar functions are to be performed. However, as long as we consider isopotential cells, these size factors cannot fully explain differences in channel densities by themselves, because both the channel number and membrane capacitance are proportional to the surface area. Nevertheless, the fact that each cerebellar PC is equipped with a massive dendritic tree, which dwarfs that of the DG granule cells, might be relevant. Thus, the dendrites, unless they have the same channel density as the soma, and the channels all activate almost simultaneously, may represent a large sink that may require a larger somatic channel density in cerebellar PC for achieving a similar impact compared to the less branched DG granule cells. In addition, there are likely to be several other, cell type-specific differences, including generation of dendritic Ca2+ spikes that can be regulated by BKCa channels (Rancz and Häusser, 2006).
Participation of BKCa channels in the formation of ‘PLasmERosomes’
As in Purkinje cells, BKCa channels were clustered in the plasma membrane at sites of SSC in DG granule cells. Such cisterns are specialized subcompartments of the ER, which contributes crucially to spatial Ca2+ buffering and signaling in neurons (Verkhratsky, 2005). Increasing evidence suggests that neuronal ER does not represent a uniform Ca2+ pool but rather a spatially heterogeneous system encompassing multiple Ca2+ subdomains (calciosomes). These specialized ER domains are supposed to unload and refill Ca2+ independently (Blaustein and Golovina, 2001), and SSC are likely to represent such kind of calciosomes in central principal neurons. Areas of the plasma membrane overlying calciosomes also form specialized microdomains that contain unique sets of membrane proteins (Moore et al., 1993; Putney and Ribeiro, 2000). These domains are proposed to function as a coordinated unit, a “PLasmERosome”, together with the underlying calciosome (Blaustein and Golovina, 2001). The present study demonstrating BKCa channel clustering in areas of the plasma membrane overlying SSC provides strong morphological evidence that BKCa channels participate in building such PLasmERosome in central principal neurons.
The presence of PLasmERosomes may be correlated with some of the distinctive physiological properties of the neuronal surface (Blaustein and Golovina, 2001). They seem to be important for regulating the membrane potential (Satin and Adams, 1987; Akita and Kuba, 2000), and may also play a role in shaping of action potentials and modulation of firing rate (Mikoshiba, 2007). They may even represent a signal initiation site through modulation of junctional ER Ca2+ content by altering the activity of local Na+ pump isoforms, local Ca2+ release events (Ca2+ sparks) or calcium induced Ca2+ release (CICR; Blaustein and Golovina, 2001).
Non-homogenous distribution of BKCa channels
Non-homogenous distribution of potassium channels in central neurons was reported previously for members of the voltage-gated potassium channel family, in particular KV2.1 channels (Lim et al., 2000), KV4.2 channels (Alonso and Widmer, 1997; Jinno et al., 2005), and KV4.3 channels (Kollo et al., 2006, 2008). Although extrasynaptic channel clusters have been proposed to participate in novel forms of intercellular communication, the functional roles of such clusters are still far from being understood. Obviously, ion channel clustering induces local changes in the electrophysiological properties of the neuronal surface membrane. Although nerve cell membranes are often assumed to be uniform with respect to electrical properties like excitability, capacitance and resistance, there is mounting evidence for spatial compartmentalization and heterogeneity in signaling within neuronal subdomains (Berridge, 2006; Dai et al., 2009).
Regarding differential spatiotemporal Ca2+ activation, the scattered and the clustered pool of BKCa channels may play different roles in the processing and shaping of electrical signals. Whereas the scattered pool of channels may be involved primarily in rapid, transient events such as spike repolarization (Storm, 1987a; Shao et al., 1999; Gu et al., 2007), the clustered pool may convey slower and more long-lasting effects (slower activating and slower decaying) that are triggered by CICR. Depolarization-dependent Ca2+ influx through voltage-gated Ca2+ (CaV) channels may induce CICR from SSC during high-frequency activity, thus activating BKCa channels in the plasma membrane (Llano et al., 1994; Akita and Kuba, 2000). Clustered BKCa channels may also be activated via spontaneous CICR and hence may represent a spike-independent signal initiation site (Bardo et al., 2006). Anyway, CICR-triggered BKCa channel activation likely happens in a rather slow manner occurring only after repolarization of a spike unlike the more direct activation of BKCa channels upon Ca2+ influx through CaV channels (Storm, 1987b; Brenner et al., 2000; Fakler and Adelman, 2008). BKCa channels are thus opened already during or shortly after a spike in many neurons (Storm, 1987a,b; Lancaster and Nicoll, 1987; Hu and Storm, 2001b; Edgerton and Reinhart, 2003; Sausbier et al., 2004). With respect to the scattered BKCa channels, we observed no ER component within less than 80 nm distance, which would be required for serving as an effective Ca2+ source. It seems most likely that scattered BKCa channels are activated within CaV-BKCa complexes. Within the six major groups of CaV channels known, L-type (CaV1.2), P/Q-type (CaV2.1) and N-type (CaV2.2) channels can all reliably activate BKCa channels within milliseconds if present at nanometer distances. A proteomic approach combining affinity purification with mass spectrometry showed that these CaV channel types indeed form macro-molecular complexes with BKCa channels (Fakler and Adelman, 2008). Hence, scattered BKCa channels could be activated during the brief depolarization phase of an action potential, thus providing a rapid K+ conductance contributing to spike repolarization and a fAHP component (Storm, 1987a,b; Shao et al., 1999; Hu and Storm, 2001b; Sausbier et al., 2004; Gu et al., 2007).
The two pools of channels could also differ in their mediation of local and spatially directed chemical signals. Being part of macromolecular signaling complexes, BKCa channel activity can be modulated by a wide spectrum of physiologically relevant factors such as phosphorylation, oxidation, steroid hormones, and gases (NO, CO; Hou et al., 2009). Thus, the different pools of channels might be linked to different subcellular signaling pathways.
Conclusion
In conclusion, this study provides the first ultrastructural localization of BKCa channels in the somatic plasma membrane of various central principal neurons, and demonstrates a common organizational principle in the distribution of these channels. In all neuronal cell types we examined, BKCa channels form two, probably independent pools: one consisting of clustered and one of scattered channels in extrasynaptic membrane areas. These two pools differ most likely in their routes of Ca2+ activation, with clustered BKCa channels being in position for activation via Ca2+ release from internal stores, and scattered channels being activated via an ER independent mechanism. The pool of clustered BKCa channels may thus represent a key element of somatic PLasmERosomes with potent contributions to spatial signaling within central principal neurons.
Acknowledgments
This work was supported by a grant from the Innsbruck Medical University, MFI-4305 to W.A.K., the Austrian Science Fund (FWF) S102 to F.F., and by the Centre of Excellence Programme of the Norwegian Research Council (NFR/SFF) to J.F.S. The excellent technical assistance of Barbara Knoll-Kreidl and Dr. Barbara Kapelari at Innsbruck Medical University, Dept. Pharmacology (Austria), is gratefully acknowledged. We thank Dr. Michael Hess at Innsbruck Medical University, Div. Histology and Embryology, for support in high-pressure freezing, Prof. Hans-Günther Knaus at Innsbruck Medical University, Div. Molecular and Cellular Pharmacology, for providing BKCa channel antibodies and Prof. Peter Ruth at the University of Tuebingen, Inst. Pharmacy (Germany), for providing BKCa null mice. We also gratefully acknowledge Prof. Ryuichi Shigemoto and Dr. Yugo Fukazawa at the National Institute for Physiological Sciences, Div. Cerebral Structure (Japan), for continuous support and discussions concerning the SDS-digested freeze-fracture replica labeling technique.
References
- Adelman J.P., Shen K.Z., Kavanaugh M.P., Warren R.A., Wu Y.N., Lagrutta A., Bond C.T., North R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Akita T., Kuba K. Functional triads consisting of ryanodine receptors, Ca2+ channels, and Ca2+-activated K+ channels in bullfrog sympathetic neurons: Plastic modulation of action potential. J Gen Physiol. 2000;116:697–720. doi: 10.1085/jgp.116.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G., Widmer H. Clustering of KV42 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. doi: 10.1016/s0306-4522(96)00561-1. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Witter M.P. Hippocampal formation. In: Paxinos G., editor. The rat nervous system. 2nd edn. Elsevier/Academic Press Inc; San Diego, CA: 1995. pp. 443–493. [Google Scholar]
- Bardo S., Cavazzini M.G., Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. Review. [DOI] [PubMed] [Google Scholar]
- Benhassine N., Berger T. Homogeneous distribution of large-conductance calcium-dependent potassium channels on soma and apical dendrite of rat neocortical layer 5 pyramidal neurons. Eur J Neurosci. 2005;21:914–926. doi: 10.1111/j.1460-9568.2005.03934.x. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Blaustein M.P., Golovina V.A. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. Review. [DOI] [PubMed] [Google Scholar]
- Brenner R., Chen Q.H., Vilaythong A., Toney G.M., Noebels J.L., Aldrich R.W. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Brenner R., Jegla T.J., Wickenden A., Liu Y., Aldrich R.W. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- Cui J., Cox D.H., Aldrich R.W. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca2+-activated K+ channels. J Gen Physiol. 1997;109:647–673. doi: 10.1085/jgp.109.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Hall D.D., Hell J.W. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton J.R., Reinhart P.H. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber E.S., Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 2003;9:181–194. doi: 10.1177/1073858403009003011. Review. [DOI] [PubMed] [Google Scholar]
- Faber E.S., Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B., Adelman J.P. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. Review. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins: Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Geiger J.R., Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Golding N.L., Jung H.Y., Mickus T., Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M., Kaufmann W.A. Coassembly of big conductance Ca2+-activated K+ channels and L-type voltage-gated Ca2+ channels in rat brain. J Biol Chem. 2004;279:36445–36453. doi: 10.1074/jbc.M402254200. [DOI] [PubMed] [Google Scholar]
- Gu N., Vervaeke K., Storm J.F. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Heinemann S.H., Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology. 2009;24:26–35. doi: 10.1152/physiol.00032.2008. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Shao L.R., Chavoshy S., Gu N., Trieb M., Behrens R., Laake P., Pongs O., Knaus H.G., Ottersen O.P., Storm J.F. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Storm J.F. Differential BK channels contribute to action potential repolarization in granule cells of the rat dentate gyrus. Society for Neuroscience, program no 27. 2001:1002. Abstract. [Google Scholar]
- Jinno S., Jeromin A., Kosaka T. Postsynaptic and extrasynaptic localization of Kv42 channels in the mouse hippocampal region, with special reference to targeted clustering at gabaergic synapses. Neuroscience. 2005;134:483–494. doi: 10.1016/j.neuroscience.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Kaufmann W.A., Ferraguti F., Fukazawa Y., Kasugai Y., Shigemoto R., Laake P., Sexton J.A., Ruth P., Wietzorrek G., Knaus H.G., Storm J.F., Ottersen O.P. Large-conductance calcium-activated potassium channels in purkinje cell plasma membranes are clustered at sites of hypolemmal microdomains. J Comp Neurol. 2009;515:215–230. doi: 10.1002/cne.22066. [DOI] [PubMed] [Google Scholar]
- Knaus H.G., Eberhart A., Koch R.O., Munujos P., Schmalhofer W.A., Warmke J.W., Kaczorowski G.J., Garcia M.L. Characterization of tissue-expressed alpha subunits of the high conductance Ca2+-activated K+ channel. J Biol Chem. 1995;270:22434–22439. doi: 10.1074/jbc.270.38.22434. [DOI] [PubMed] [Google Scholar]
- Knaus H.G., Schwarzer C., Koch R.O., Eberhart A., Kaczorowski G.J., Glossmann H., Wunder F., Pongs O., Garcia M.L., Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde G., Themann H. Subsurface cisterns and lamellar bodies in the granule cells of the guinea-pig fascia dentata. Cell Tissue Res. 1982;223:455–461. doi: 10.1007/BF01258502. [DOI] [PubMed] [Google Scholar]
- Kollo M., Holderith N.B., Nusser Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J Neurosci. 2006;26:2684–2691. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollo M., Holderith N., Antal M., Nusser Z. Unique clustering of A-type potassium channels on different cell types of the main olfactory bulb. Eur J Neurosci. 2008;27:1686–1699. doi: 10.1111/j.1460-9568.2008.06141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatsch R.H., Cowan W.M. Electron microscopic studies of the dentate gyrus of the rat: I. Normal structure with special reference to synaptic organization. J Comp Neurol. 1966;128:359–395. doi: 10.1002/cne.901280305. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R.A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Brauchi S. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. Review. [DOI] [PubMed] [Google Scholar]
- Le Beux Y.J. Subsurface cisterns and lamellar bodies: particular forms of the endoplasmic reticulum in the neurons. Z Zellforsch Mikrosk Anat. 1972;133:327–352. doi: 10.1007/BF00307242. [DOI] [PubMed] [Google Scholar]
- Lim S.T., Antonucci D.E., Scannevin R.H., Trimmer J.S. A novel targeting signal for proximal clustering of the Kv21 K+ channel in hippocampal neurons. Neuron. 2000;25:385–397. doi: 10.1016/s0896-6273(00)80902-2. [DOI] [PubMed] [Google Scholar]
- Lingle C.J., Solaro C.R., Prakriya M., Ding J.P. Calcium-activated potassium channels in adrenal chromaffin cells. Ion Channels. 1996;4:261–301. doi: 10.1007/978-1-4899-1775-1_7. Review. [DOI] [PubMed] [Google Scholar]
- Llano I., DiPolo R., Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron. 1994;12:663–673. doi: 10.1016/0896-6273(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Masugi-Tokita M., Tarusawa E., Watanabe M., Molnár E., Fujimoto K., Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A., Laake J.H., Davanger S., Usami S., Ottersen O.P. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S., Pape H.C. Properties of a Ca2+-activated K+ conductance in acutely isolated pyramidal-like neurons from the rat basolateral amygdaloid complex. J Neurophysiol. 1997;78:1256–1262. doi: 10.1152/jn.1997.78.3.1256. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. Review. [DOI] [PubMed] [Google Scholar]
- Misonou H., Menegola M., Buchwalder L., Park E.W., Meredith A., Rhodes K.J., Aldrich R.W., Trimmer J.S. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.D., Etter E.F., Philipson K.D., Carrington W.A., Fogarty K.E., Lifshitz L.M., Fay F.S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365:657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Paxinos G. 2nd edn. Elsevier/Academic Press Inc; San Diego, CA: 1995. The rat nervous system. [Google Scholar]
- Paxinos G., Watson C. 4th edn. Elsevier/Academic Press Inc; San Diego, CA: 1998. The rat brain in stereotaxic coordinates. [Google Scholar]
- Putney J.W., Jr, Ribeiro C.M. Signaling pathways between the plasma membrane and endoplasmic reticulum calcium stores. Cell Mol Life Sci. 2000;57:1272–1286. doi: 10.1007/PL00000765. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli G., Saviane C., Mohajerani M.H., Pedarzani P., Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz E.A., Häusser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. J Neurosci. 2006;26:5428–5437. doi: 10.1523/JNEUROSCI.5284-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Garcia M.L., Kaczorowski G.J., Charlton M.P. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–653. doi: 10.1016/s0301-0082(02)00004-7. Review. [DOI] [PubMed] [Google Scholar]
- Sailer C.A., Kaufmann W.A., Kogler M., Chen L., Sausbier U., Ottersen O.P., Ruth P., Shipston M.J., Knaus H.G. Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur J Neurosci. 2006;24:442–454. doi: 10.1111/j.1460-9568.2006.04936.x. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Butler A., Ferreira G., Santi C., Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. Review. [DOI] [PubMed] [Google Scholar]
- Satin L.S., Adams P.R. Spontaneous miniature outward currents in cultured bullfrog neurons. Brain Res. 1987;401:331–339. doi: 10.1016/0006-8993(87)91417-x. [DOI] [PubMed] [Google Scholar]
- Sausbier M., Hu H., Arntz C., Feil S., Kamm S., Adelsberger H., Sausbier U., Sailer C.A., Feil R., Hofmann F., Korth M., Shipston M.J., Knaus H.G., Wolfer D.P., Pedroarena C.M., Storm J.F., Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier U., Sausbier M., Sailer C.A., Arntz C., Knaus H.G., Neuhuber W., Ruth P. Ca2+-activated K+ channels of the BK-type in the mouse brain. Histochem Cell Biol. 2006;125:725–741. doi: 10.1007/s00418-005-0124-7. [DOI] [PubMed] [Google Scholar]
- Shao L.R., Halvorsrud R., Borg-Graham L., Storm J.F. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J.F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J.F. Intracellular injection of a Ca2+ chelator inhibits spike repolarization in hippocampal neurons. Brain Res. 1987;435:387–392. doi: 10.1016/0006-8993(87)91631-3. [DOI] [PubMed] [Google Scholar]
- Storm J.F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. Review. [DOI] [PubMed] [Google Scholar]
- Takumi Y., Ramírez-León V., Laake P., Rinvik E., Ottersen O.P. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Triller A., Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. Review. [DOI] [PubMed] [Google Scholar]
- van Lookeren Campagne M., Oestreicher A.B., van der Krift T.P., Gispen W.H., Verkleij A.J. Freeze-substitution and Lowicryl HM20 embedding of fixed rat brain: suitability for immunogold ultrastructural localization of neural antigens. J Histochem Cytochem. 1991;39:1267–1279. doi: 10.1177/39.9.1833448. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. Review. [DOI] [PubMed] [Google Scholar]
- Wanner S.G., Koch R.O., Koschak A., Trieb M., Garcia M.L., Kaczorowski G.J., Knaus H.G. High-conductance calcium-activated potassium channels in rat brain: pharmacology, distribution, and subunit composition. Biochemistry. 1999;38:5392–5400. doi: 10.1021/bi983040c. [DOI] [PubMed] [Google Scholar]
- Wei A.D., Gutman G.A., Aldrich R., Chandy K.G., Grissmer S., Wulff H. International Union of Pharmacology. LII: Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. Review. [DOI] [PubMed] [Google Scholar]
- Womack M.D., Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci. 2002;16:1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]