Abstract
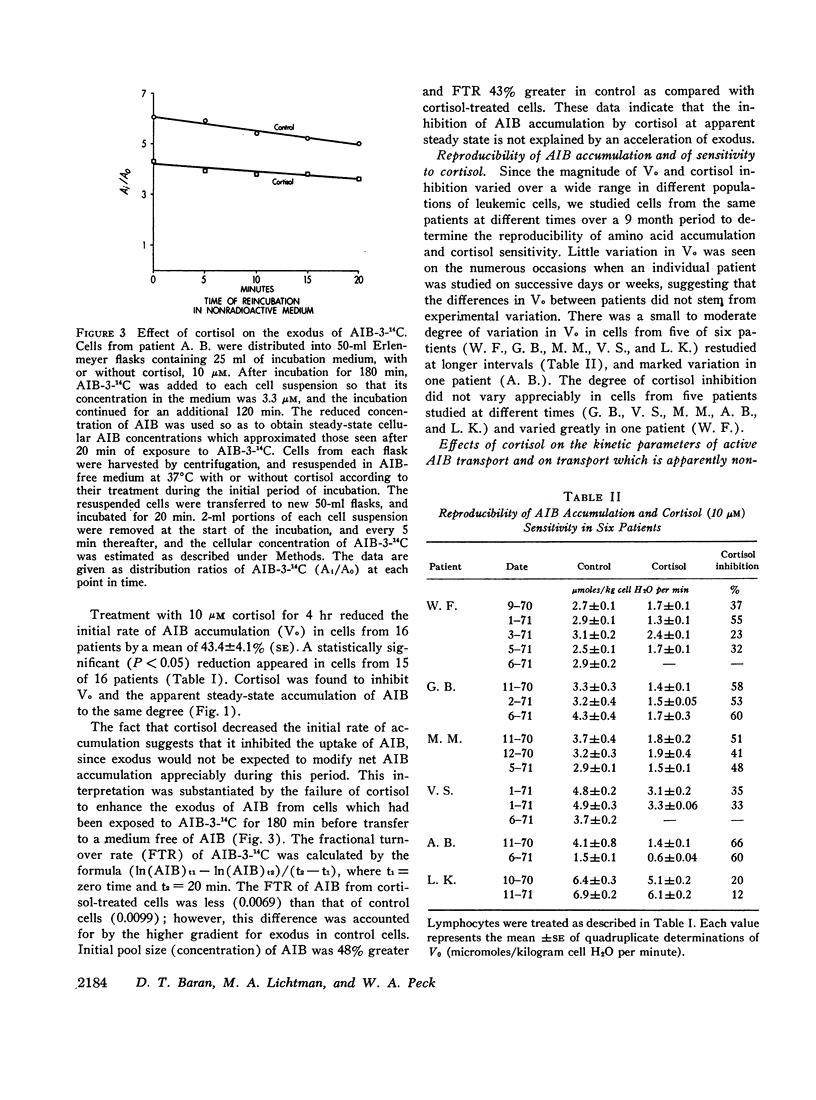
We have studied the transport of alpha-aminoisobutyric acid (AIB)-3-14C and its response to cortisol and cycloheximide in vitro in blood lymphocytes from untreated patients with chronic lymphocytic leukemia. The accumulation of AIB-3-14C increased in a linear fashion for 60 min, and reached an apparent steady state in 120 min. The initial rate of AIB accumulation (Vo) varied from 1.1 to 10.2 μmoles/kg cell H2O per min in cells from 16 different patients; however, Vo was reproducible in cells from five of six patients which were studied repeatedly over 1-9 months, and correlated positively with the lymphocyte count (r = 0.51, P = < 0.01).
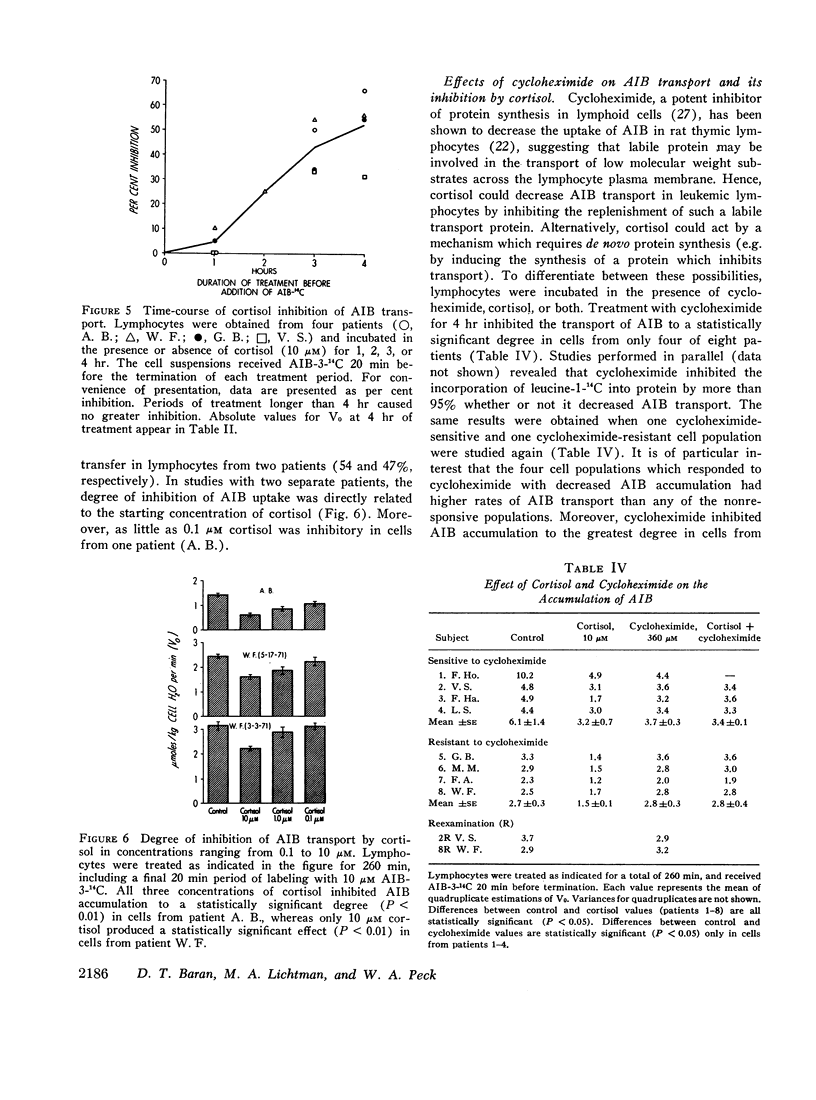
Virtually total inhibition of protein synthesis with cycloheximide was found to decrease the accumulation of AIB in cells from four patients which had high rates of AIB transport, but had no effect on transport in cells from four patients which accumulated AIB more slowly. These results indicate that active transport depends, in part, upon the presence of labile protein with a turnover rate which varies among different cell populations.
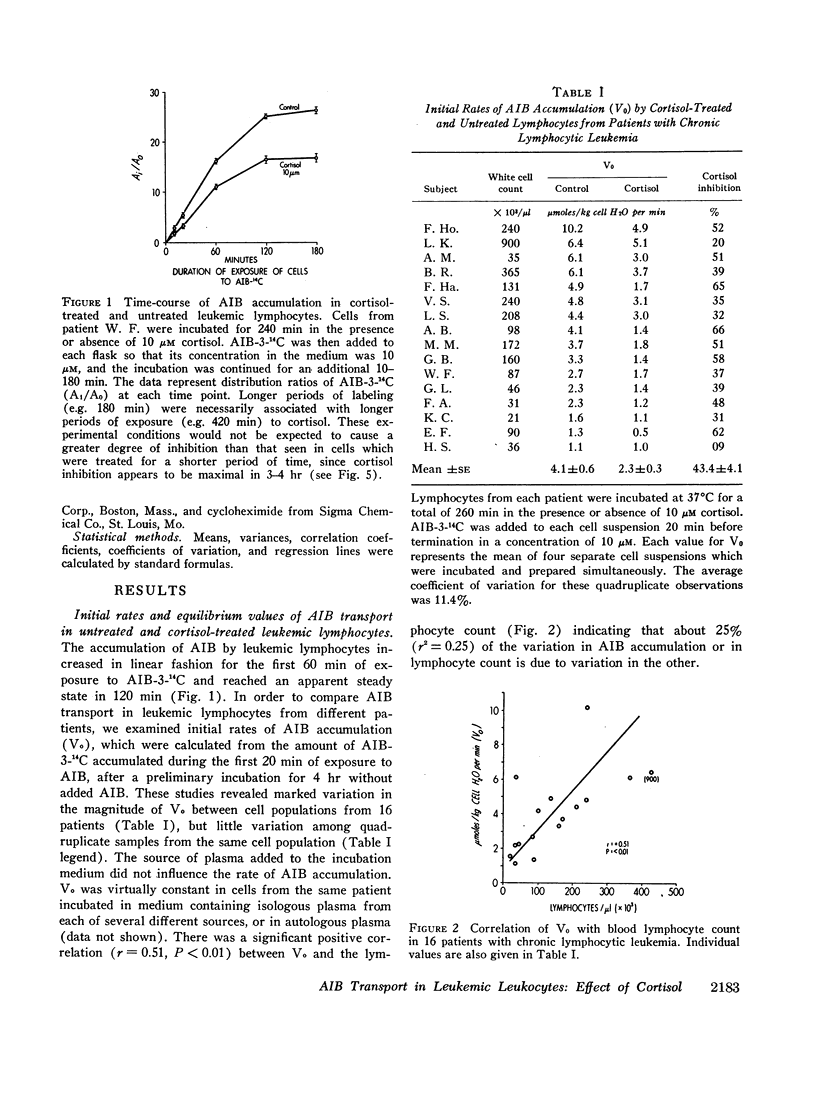
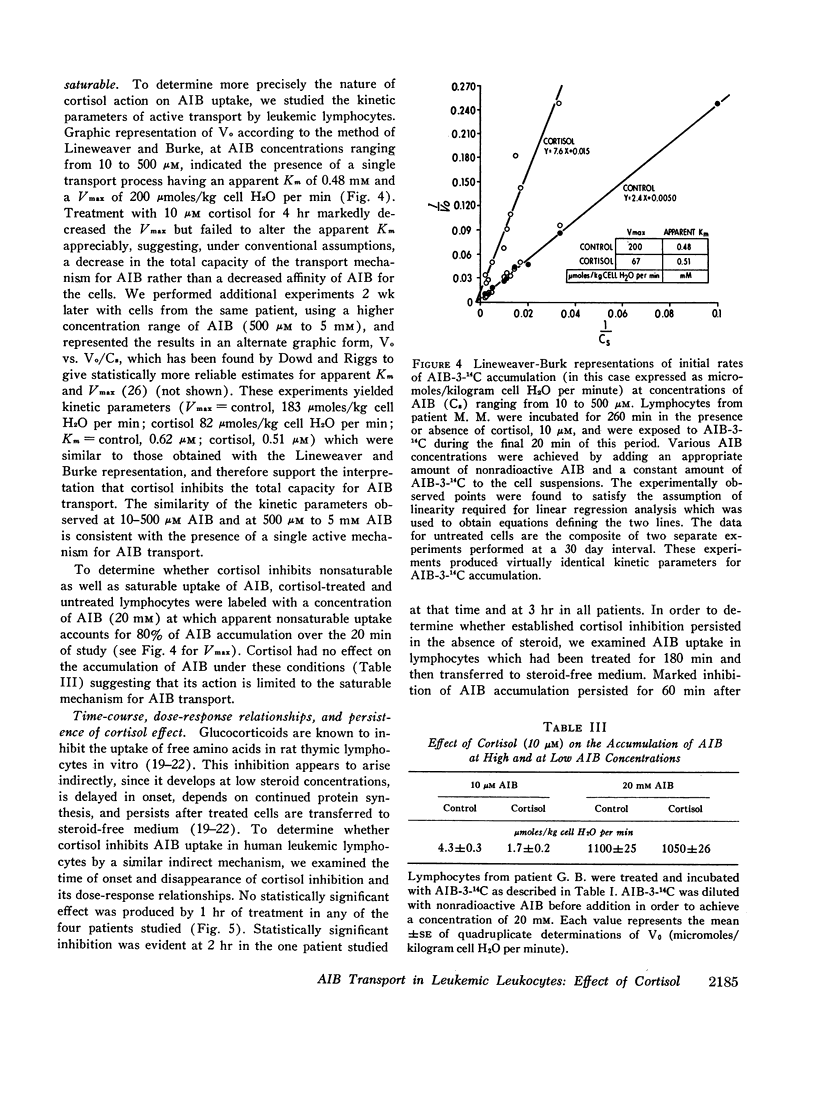
Treatment with 10 μM cortisol for 240 min in vitro reduced the initial rate of AIB-3-14C accumulation (Vo) by 43.4±4.1% (SE) (range, 9-66%) in cells from 16 patients. The degree of inhibition did not vary appreciably over a 9 month period in four of five patients. The effect of cortisol was proportional to its starting concentration, and developed at low concentrations (0.1-1.0 μM). Cortisol appears to decrease AIB accumulation by inhibiting active uptake, since it neither enhanced the exodus of AIB, nor inhibited apparently nonsaturable transport. Inhibition was noncompetitive in type, suggesting that cortisol decreases the total capacity of the active transport mechanism.
Cortisol inhibited AIB transport indirectly by a process which involved de novo protein synthesis, since inhibition (a) appeared only after 60 min of treatment, (b) was present in treated cells which were subsequently incubated for 60 min in cortisol-free medium, and (c) failed to develop during simultaneous blockade of protein synthesis with cycloheximide, even when cycloheximide itself did not decrease AIB transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKEDO H., CHRISTENSEN H. N. Nature of insulin action on amino acid uptake by the isolated diaphragm. J Biol Chem. 1962 Jan;237:118–122. [PubMed] [Google Scholar]
- Adamson L. F., Langeluttig S. G., Anast C. S. Inhibition by puromycin of amino acid transport by embryonic chick bone. Biochim Biophys Acta. 1966 Feb 28;115(2):355–360. doi: 10.1016/0304-4165(66)90435-1. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Cline M. J. Prediction of in vivo cytotoxicity of chemotherapeutic agents by their effect on malignant leukocytes in vitro. Blood. 1967 Aug;30(2):176–188. [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Elsas L. J., 2nd, MacDonell R. C., Jr, Rosenberg L. E. Influence of age on insulin stimulation of amino acid uptake in rat diaphragm. J Biol Chem. 1971 Nov;246(21):6452–6459. [PubMed] [Google Scholar]
- Elsas L. J., Albrecht I., Rosenberg L. E. Insulin stimulation of amino acid uptake in rat diaphragm. Relationship to protein sythesis. J Biol Chem. 1968 Apr 25;243(8):1846–1853. [PubMed] [Google Scholar]
- Elsas L. J., Rosenberg L. E. Inhibition of amino Acid transport in rat kidney cortex by puromycin. Proc Natl Acad Sci U S A. 1967 Feb;57(2):371–378. doi: 10.1073/pnas.57.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. O., Pardee A. B. Transport of amino acids by confluent and nonconfluent 3T3 and polyoma virus-transformed 3T3 cells growing on glass cover slips. J Biol Chem. 1969 May 25;244(10):2675–2681. [PubMed] [Google Scholar]
- Kattwinkel J., Munck A. Activities in vitro of glucocorticoids and related steroids on glucose uptake by rat thymus cell suspensions. Endocrinology. 1966 Aug;79(2):387–390. doi: 10.1210/endo-79-2-387. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Korner A. Effect of cycloheximide on protein and ribonucleic acid synthesis in cultured human lymphocytes. Biochem J. 1966 Sep;100(3):815–822. doi: 10.1042/bj1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick A. F., Milholland R. J., Rosen F. Stereospecific glucocorticoid binding to subcellular fractions of the sensitive and resistant lymphosarcoma P1798. Nat New Biol. 1971 Aug;232(33):216–218. doi: 10.1038/newbio232216a0. [DOI] [PubMed] [Google Scholar]
- Kostyo J. L., Redmond A. F. Role of protein synthesis in the inhibitory action of adrenal steroid hormones on amino acid transport by muscle. Endocrinology. 1966 Sep;79(3):531–540. doi: 10.1210/endo-79-3-531. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesch M., Gorlin R., Sonnenblick E. H. Myocardial amino acid transport in the isolated rabbit right ventricular papillary muscle. General characteristics and effects of passive stretch. Circ Res. 1970 Sep;27(3):445–460. doi: 10.1161/01.res.27.3.445. [DOI] [PubMed] [Google Scholar]
- MORITA Y., MUNCK A. EFFECT OF GLUCOCORTICOIDS IN VIVO AND IN VITRO ON NET GLUCOSE UPTAKE AND AMINO ACID INCORPORATION BY RAT-THYMUS CELLS. Biochim Biophys Acta. 1964 Oct 9;93:150–157. doi: 10.1016/0304-4165(64)90269-7. [DOI] [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Evidence for induction by cortisol in vitro of a protein inhibitor of transport and phosphorylation processes in rat thymocytes. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1269–1273. doi: 10.1073/pnas.68.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makman M. H., Dvorkin B., White A. Influence of cortisol on the utilization of precursors of nucleic acids and protein by lymphoid cells in vitro. J Biol Chem. 1968 Apr 10;243(7):1485–1497. [PubMed] [Google Scholar]
- Makman M. H., Nakagawa S., Dvorkin B., White A. Inhibitory effects of cortisol and antibiotics on substrate entry and ribonucleic acid synthesis in rat thymocytes in vitro. J Biol Chem. 1970 May 25;245(10):2556–2563. [PubMed] [Google Scholar]
- Makman M. H., Nakagawa S., White A. Studies of the mode of action of adrenal steroids on lymphocytes. Recent Prog Horm Res. 1967;23:195–227. doi: 10.1016/b978-1-4831-9826-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher K. M., Young D. A., Munck A. Evidence for irreversible, actinomycin D-sensitive, and temperature-sensitive steps following binding of cortisol to glucocorticoid receptors and preceding effects on glucose metabolism in rat thymus cells. J Biol Chem. 1971 Feb 10;246(3):654–659. [PubMed] [Google Scholar]
- Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971 Winter;14(2):265–269. doi: 10.1353/pbm.1971.0002. [DOI] [PubMed] [Google Scholar]
- Munck A. Metabolic site and time course of cortisol action on glucose uptake, lactic acid output, and glucose 6-phosphate levels of rat thymus cells in vitro. J Biol Chem. 1968 Mar 10;243(5):1039–1042. [PubMed] [Google Scholar]
- Ning M., Reiser S., Christiansen P. A. Variation in intestinal transport of L-valine in relation to age. Proc Soc Exp Biol Med. 1968 Dec;129(3):799–803. doi: 10.3181/00379727-129-33428. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Fina J. R., Milholland J., Rosen F. Inhibition of glucose uptake in lymphosarcoma P1798 by cortisol and its relationship to the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1970 Apr 25;245(8):2074–2080. [PubMed] [Google Scholar]
- Rosen J. M., Milholland R. J., Rosen F. A comparison of the effect of glucocorticoids on glucose uptake and hexokinase activity in lymphosarcoma P1798. Biochim Biophys Acta. 1970 Dec 1;219(2):447–454. doi: 10.1016/0005-2736(70)90222-1. [DOI] [PubMed] [Google Scholar]
- SCHREK R. PREDNISOLONE SENSITIVITY AND CYTOLOGY OF VIABLE LYMPHOCYTES AS TESTS FOR CHRONIC LYMPHOCYTIC LEUKEMIA. J Natl Cancer Inst. 1964 Nov;33:837–847. doi: 10.1093/jnci/33.5.837. [DOI] [PubMed] [Google Scholar]
- States B., Segal S. Developmental aspects of cystine transport in rat intestinal segments. Biochim Biophys Acta. 1968 Sep 17;163(2):154–162. doi: 10.1016/0005-2736(68)90093-x. [DOI] [PubMed] [Google Scholar]
- Stuart J. J., Ingram M. The effect of cortisol on viability and glucose uptake in rat thymocytes in vitro. Proc Soc Exp Biol Med. 1971 Apr;136(4):1146–1150. doi: 10.3181/00379727-136-35448. [DOI] [PubMed] [Google Scholar]
- Tews J. K., Harper A. E. Amino acid transport in rat-liver slices during development. Biochim Biophys Acta. 1969;183(3):635–637. doi: 10.1016/0005-2736(69)90176-x. [DOI] [PubMed] [Google Scholar]
- Webber W. A. A comparison of the effux rates of AIB from kidney cortex slices of mature and newborn rats. Can J Physiol Pharmacol. 1968 Sep;46(5):765–769. doi: 10.1139/y68-119. [DOI] [PubMed] [Google Scholar]
- Webber W. A., Cairns J. A. A comparison of the amino acid concentrating ability of the kidney cortex of newborn and mature rats. Can J Physiol Pharmacol. 1968 Mar;46(2):165–169. doi: 10.1139/y68-027. [DOI] [PubMed] [Google Scholar]
- Werthamer S., Amaral L. The response of leukemic lymphocytes to cortisol: a suggested role of transcortin. Blood. 1971 Apr;37(4):463–472. [PubMed] [Google Scholar]
- Young D. A. Glucocorticoid action on rat thymus cells. II. Interrelationships between ribonucleic acid and protein metabolism and between cortisol and substrate effects on these metabolic parameters in vitro. J Biol Chem. 1970 May 25;245(10):2747–2752. [PubMed] [Google Scholar]
- Young D. A. Glucocorticoid action on rat thymus cells. Interrelationships between carbohydrate, protein, and adenine nucleotide metabolism and cortisol effects on these functions in vitro. J Biol Chem. 1969 Apr 25;244(8):2210–2217. [PubMed] [Google Scholar]


