Abstract
AIM: To assess the clinical outcomes of pre-, pro- and synbiotics therapy in patients with acute pancreatitis.
METHODS: The databases including Medline, Embase, the Cochrane Library, Web of Science and Chinese Biomedicine Database were searched for all relevant randomized controlled trials that studied the effects of pre-, pro- or synbiotics in patients with acute pancreatitis. Main outcome measures were postoperative infections, pancreatic infections, multiple organ failure (MOF), systemic inflammatory response syndrome (SIRS), length of hospital stay, antibiotic therapy and mortality.
RESULTS: Seven randomized studies with 559 acute pancreatic patients were included. Pre-, pro- or synbiotics treatment showed no influence on the incidence of postoperative infections [odds ratios (OR) 0.30, 95% confidence interval (CI): 0.09-1.02, P = 0.05], pancreatic infection (OR 0.50, 95% CI: 0.12-2.17, P = 0.36), MOF (OR 0.88, 95% CI: 0.35-2.21, P = 0.79) and SIRS (OR 0.78, 95% CI: 0.20-2.98, P = 0.71). There were also no significant differences in the length of antibiotic therapy (OR 0.75, 95% CI: 0.50-1.14, P = 0.18) and the mortality (OR 0.75, 95% CI: 0.25-2.24, P = 0.61). However, Pre-, pro- or synbiotics treatment was associated with a reduced length of hospital stay (OR -3.87, 95% CI: -6.20 to -1.54, P = 0.001). When stratifying for the severity of acute pancreatitis, the main results were similar.
CONCLUSION: Pre-, pro- or synbiotics treatment shows no significant influence on patients with acute pancreatitis. There is a lack of evidence to support the use of probiotics/synbiotics in this area.
Keywords: Probiotics, Synbiotics, Prebiotics, Nutrition support, Acute pancreatitis
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory process in mild to severe forms with a high mortality rate, frequently associated with necrosis of the gland[1]. The infected pancreatic necrosis is a principal late complication and a major cause of morbidity and mortality in patients with severe acute pancreatitis. In view of the deleterious effects of infected necrotizing pancreatitis, prophylactic antibiotics have been widely used. However, some randomized controlled trials (RCTs) and meta-analysis have not demonstrated significant benefits of prophylactic antibiotics on patients with necrotizing AP[2-4]. It is also not recommended for the routine use in sterile pancreatic necrosis[5].
A new anti-infectious strategy is needed. The administration of probiotics/synbiotics modulating the intestinal microbiota may be a valuable treatment option. Lilly and Stillwell first defined the term “probiotics” as ingestible microorganisms that benefited the host by improving intestinal microbial balance[6]. Previous studies showed the clinical benefits of probiotics (including Lactobacilli and Bifidobacteria), such as inhibiting proliferation of harmful bacteria, protecting the intestinal barrier, preventing gut bacterial translocation to blood and distant sites, and modulating the immune function[7,8], all of which contributed to the reduction of the incidence of nosocomial infections related to intestinal microbial imbalance. Prebiotics are also beneficial to enhancing the effects of enteral nutrition and probiotics[9-11]. For example, as one of the most important prebiotics, fibers can selectively stimulate growth or activity of certain colonic bacteria[12]. In addition, fibers are broken down by the probiotic bacteria to produce a whole series of nutrients including short-chain fatty acids which can stimulate mucosal cell growth, reduce translocation, and enhance the intestinal immune function in colon. Combined use of probiotics and prebiotics, which are called synbiotics, have been shown to enhance immunomodulating ability, balance gut microbiota, inhibit bacterial translocation and reduce the incidence of nosocomial infections in clinically surgical patients.
Evidences showed that the use of pro- or synbiotics might reduce postoperative infections after abdominal surgery[13-15]. Several RCTs have also been performed demonstrating a therapeutic and preventive effect of pre-, pro- or synbiotics treatment in patients with acute pancreatitis[16-18]. However, these studies were small in size, and have been underpowered. Recently, a multi-center RCTs reported some unexpected results contradicting the previous studies[19]. This trial was controversial for some shortcomings. Overall, the magnitude of the therapeutic effect remains unknown. We therefore performed this meta-analysis to assess the potential effects of pre-, pro- or synbiotics treatment in patients with acute pancreatitis by reviewing the current literature, and synthesizing the available data.
MATERIALS AND METHODS
Literature search strategy
A systematic review of the literature was performed to identify all RCTs assessing the effects of pre-, pro- or synbiotics treatment in acute pancreatitis. Two authors independently searched the database including Medline (1966 to March 2010), Embase (1980 to March 2010), Web of Science, Chinese Biomedicine Database (1979 to March 2010) and the Cochrane Library (2010, issue 1) with no language restriction using the following terms: “(prebiotic* OR probiotic* OR synbiotic* OR lactobacillus OR Bifidobacterium OR Lactobacilli) AND (acute pancreatitis)”. We identified relevant studies initially by title, abstract, and finally by full text. The reference lists of all selected RCTs and previous systematic reviews were also searched by hand. If duplicate article was published by the same author using the same case series, the data from the most recent manuscript publications was included.
Inclusion and exclusion criteria
Only RCTs evaluating the use of pre-, pro- or synbiotics in patients with acute pancreatitis were included in this review. The trials should have at lease one of the followings as a primary outcome variable: number of infections and pancreatic infectious complications, number of multiple organ failure (MOF) and systemic inflammatory response syndrome (SIRS), surgical interventions, length of hospital stay, and mortality. Major reasons for exclusion of studies were (1) animal studies; (2) duplicate publication; and (3) no usable data reported.
Data extraction
Data was abstracted independently by two reviewers according to the following selection criteria: study design and period, population, intervention, and outcome variables listed above. Disagreement was resolved by discussion.
Methodological quality
We assessed the quality of the studies based on the random method, allocation concealment, blinding and follow-up. The methodological quality of the studies included in the meta-analysis was also scored with the Jadad scale[20], which was a 5-point quality scale defining low quality studies as having a score of < 3 and high-quality studies as having a score of ≥ 3.
Statistical analysis
The statistical analysis was performed using the free software Review Manager (Version 5.0; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2008). Dichotomous data was presented as odds ratio (OR) with 95% confidence intervals (CI). Statistical heterogeneity was measured using the χ2 test and the inconsistency index (I2). A Chi-squared P value < 0.05 was considered to indicate statistically significant heterogeneity. Fixed effects model was used when there was no heterogeneity of the results. Otherwise, the random effects model was used. Subgroup analyses stratified by the severity of acute pancreatitis were performed. Sensitivity analyses were performed only in high quality trials to avoid errors caused by poor quality studies. Visual inspection of asymmetry in funnel plots was conducted to assess the potential for publication bias.
RESULTS
Main characteristics of the studies
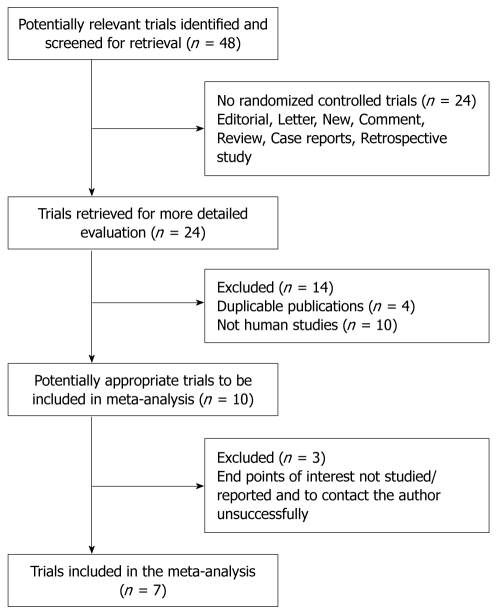
A total of 48 papers relevant to the searching words were identified through the bibliographic search. After initial eligibility screening, 41 of these papers were excluded, of which 24 were not randomized controlled studies, 10 were not conducted in humans, 3 did not report usable data, and 4 were duplicate publications[21-24]. Only 7 RCTs involving 559 patients met the inclusion criteria and were included in the meta-analysis[16-19,25-27]. The flow chart of study selection is summarized in Figure 1.
Figure 1.
Flow chart showing the study selection procedure.
The main characteristics of the included patients between two groups were well matched in all RCTs (including age and gender). Five studies compared the score of the second acute physiology and chronic health evaluation (APACHE II)[18,19,25-27], and five studies compared C-reactive protein [16-19,25]. Two of the studies tested a probiotics[16,18], one of the studies tested a prebiotics[25], while the remaining four studies tested a synbiotics (probiotics plus prebiotics)[17,19,26,27]. Five studies recruited patients with severe acute pancreatitis[17,19,25-27], and two studies recruited patients with mild, moderate and severe degrees of pancreatitis[16,18]. Patients with biliary tract diseases were excluded in one of the studies[16]. Only three studies reported adverse effects associated with administration of pre-, pro- or synbiotics, which included bowel ischemia, catheter-related sepsis, tube intolerance and reintube. The study details are summarized in Table 1. The surgical outcomes from the RCTs included in this meta-analysis are presented in Table 2.
Table 1.
Methodological characteristics of the clinical trials included in this meta-analysis
| Trial | Patients (synbiotics/control) | Characteristics of patients (synbiotics/control) | Intervention | Control group | Length of treatment (d) |
| Oláh et al[16] (UK, 2002) | 45 (22/23) | Mean Glasgow score (2.5/2.8), mean CRP (206.5/188.7) mg/L | 109L. plantarum 299 + EN + 10 g oat fiber | EN + heat-killed L. plantarum 299 + oat fiber | 7 |
| Oláh et al[17] (UK, 2007) | 62 (33/29) | Mean Imrie score (2.9/3.1), mean CRP (216.7/191.2) mg/L | Four LAB: 1010P. pentosaceus, Leuconostoc mesenteroides, L. paracasei and L. plantarum + four bioactive fiber (Synbiotics 2000) + EN | EN + four bioactive fiber | 7 |
| Karakan et al[25] (Turkey, 2007) | 30 (15/15) | Mean APACHE II score (9.4/9.6), mean CRP (232/244) mg/L | 24 g multi-fibers including soluble fibers and insoluble fibers + EN | EN | 6-13 |
| Qin et al[18] (China, 2008) | 74 (36/38) | Mean APACHE II score (8.8/8.9), mean CRP (125/136) mg/L | 108L. plantarum + EN + PN | PN | 7 |
| Besselink et al[19] (Netherlands, 2008) | 296 (152/144) | Mean APACHE II score (8.6/8.4), mean Imrie score (3.3/3.4), mean CRP (268/270) mg/L | Six LAB: 1010L. acidophilus, L. casei, L. salivarius, L.lactis, B. bifidum, and B. lactis (Ecologic 641) + cornstarch + maltodextrins + fiber-riched EN | Cornstarch + maltodextrins + fiber-riched EN | 28 |
| Li et al[26] (China, 2007) | 25 (14/11) | APACHE II score 8-20 | Three LAB:7.2 × 107B. longum, L. bulgaricus and S. thermophilus (Golden Bifid) | Water | 7 |
| Wu et al[27] (China, 2009) | 27 (14/13) | APACHE II score 8-20 | Three strains: > 6 × 104L. lactis + L. acidophilus and S. lactis | N/A | 7 |
N/A: Not applicable; APACHE II: Score of the second acute physiology and chronic health evaluation; CRP: C-reactive protein; LAB: Lactic acid bacteria; L.: Lactobacillus; B.: Bifidobacterium; E.: Enterococcus; P.: Pediococcus; S.: Streptococcus; EN: Enteral nutrition; PN: Parenteral nutrition.
Table 2.
Surgical outcomes from randomized studies included in this meta-analysis
| Trial | No. of patients | Septic morbidity (%) | MOF (%) | SIRS (%) | Pancreatic infections (%) | Surgical interventions (%) | Hospital stay (d) | Mortality (%) |
| Oláh et al[16] | 22/23 | 5 (22.7)/ 20 (87) | 2 (9.1)/ 2 (8.7) | 11 (50)/ 6 (26.1) | 1 (4.5)/ 7 (30.4) | 1 (4.5)/ 7 (30.4) | 13.7/21.4 (median) | 1 (4.5)/2 (8.7) |
| Oláh et al[17] | 33/29 | 9 (27.3)/ 15 (51.7) | 5 (15.1)/ 9 (31) | 3 (9)/ 5 (17.2) | 4 (12.1)/ 8 (27.6) | 4 (12.1)/ 7 (24.1) | 14.9/19.7 (median) | 2 (6.1)/6 (20.7) |
| Karakan et al[25] | 15/15 | 2 (13.3)/ 2 (13.3) | 1 (6.7)/ 2 (13.3) | N/A | N/A | N/A | 10 ± 4/15 ± 6 | 2 (13.3)/4 (26.7) |
| Qin et al[18] | 36/38 | 11 (30.6)/ 29 (76.3) | 4 (11.1)/ 7 (18.4) | 6 (16.7)/ 14 (36.8) | N/A | N/A | 20.9/24.2 (median) | 0 (0)/0 (0) |
| Besselink et al[19] | 152/144 | 46 (30.3)/ 41 (28.47) | 33 (22)/ 15 (10) | N/A | 21 (14)/ 14 (10) | 28 (18)/ 14 (10) | 28.9 ± 41.5/23.5 ± 25.9 | 24 (16)/9 (6) |
| Li et al[26] | 14/11 | N/A | N/A | N/A | N/A | N/A | 42 ± 5/49 ± 6.8 | N/A |
| Wu et al[27] | 14/13 | N/A | N/A | N/A | N/A | N/A | 34 ± 6/40 ± 6 | 5 (35.7)/6 (46.2) |
N/A: Not applicable; MOF: Multi-organ failure; SIRS: Systemic inflammatory response syndrome.
Quality assessment
Four of these studies were double blind[16,17,19,25]. Allocation concealment was adequate in 3 studies[17,19,25], and unclear in 4 studies[16,18,26,27]. The Jadad score of the studies was evaluated and ranged from 1 to 5 (mean 2.9). Most of the studies were small in size (85.7% had 100 or less participants). Follow-up was only reported in two studies[18,19]. The quality assessment of the studies is presented in Table 3.
Table 3.
Quality assessment of the included randomized trials
| Trial | Generation of random | Allocation concealment | Blinding | Follow-up | Baseline similarity | Jadad score |
| Oláh et al[16] | Unclear | Unclear | Double blinded | No | Similar | 4 |
| Oláh et al[17] | Unclear | Adequate | Double blinded | No | Similar | 4 |
| Karakan et al[25] | Clear | Adequate | Double blinded | No | Similar | 3 |
| Qin et al[18] | Clear | Unclear | No blinding | Yes | Similar | 3 |
| Besselink et al[19] | Clear | Adequate | Double blinded | Yes | Similar | 5 |
| Li et al[26] | Unclear | Unclear | No blinding | No | Similar | 1 |
| Wu et al[27] | Unclear | Unclear | No blinding | No | Similar | 1 |
Infectious complications and pancreatic infection
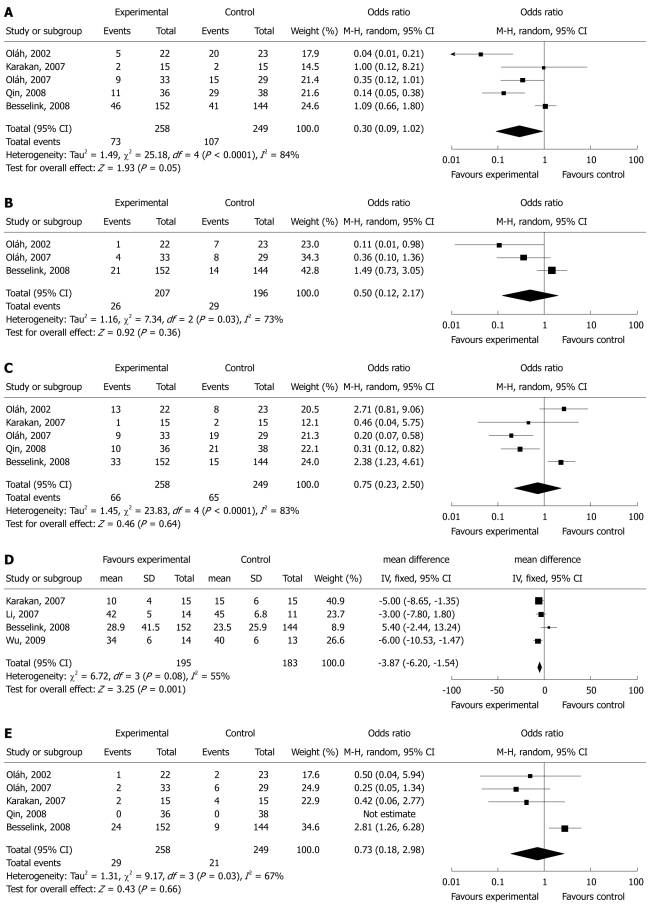
The incidence of infectious complications was reported in all 7 RCTs in the meta-analysis. The type of infections included pancreatic abscess, infected pancreatic necrosis, pneumonia, catheter-related septic complication, urinary tract infections, wound infections, and sepsis or bacteremia. Overall, there were no significant differences of incidence of total infections in pancreatic patients between the probiotics/synbiotics group and the control group (OR = 0.3, 95% CI: 0.09-1.02, P = 0.05) (Figure 2A). There was significant heterogeneity between studies (I2 = 84%, P < 0.0001).
Figure 2.
Forest plot for the effects of probiotics/synbiotics in patients with acute pancreatitis. A: Infectious morbidity; B: Pancreatic infections; C: Multiple organ failure (MOF) and systemic inflammatory response syndrome (SIRS); D: Length of hospital stay; E: Mortality.
Only three studies reported pancreatic infections[16,17,19]. Pancreatic infections included pancreatic abscess and infected pancreatic necrosis. There was no significant difference in the pancreatic infections between the probiotics/synbiotics group and the control group (OR 0.50, 95% CI: 0.12-2.17, P = 0.36) (Figure 2B). The test result for heterogeneity was also significant (I2 = 73%, P = 0.03).
MOF and SIRS
There were no significant differences in the incidence of MOF and SIRS between the probiotics/synbiotics group and the control group. The odds ratio and 95% CI: were 0.88 (0.35-2.21, P = 0.79) in MOF based on five studies[16-19,25], and 0.78 (0.20-2.98, P = 0.71) in SIRS based on three studies[16-18]. Combination analysis of MOF and SIRS also showed no significant differences between the two groups (OR 0.75, 95% CI: 0.23-2.50, P = 0.64) (Figure 2C). The significant heterogeneity was present among the studies (I2 = 83%, P < 0.0001).
Length of antibiotic therapy, and hospital stay
Four studies involved in the meta-analysis provided applicable data on length of hospital stay[19,25-27]. The length of hospital stay was significantly shorter in the probiotics/synbiotics group (OR -3.87, 95% CI: -6.20 to -1.54, P = 0.001) (Figure 2D). However, there was no significant difference in the length of antibiotic therapy (three RCTs, OR 0.75, 95% CI: 0.50-1.14, P = 0.18) between the probiotics/synbiotics group and the control group[16,18,19]. The test result for heterogeneity was not significant (P = 0.08 in hospital stay, P = 0.33 in antibiotic therapy).
Surgical intervention and mortality
Three studies reported significant difference in surgical intervention (OR 0.59, 95% CI: 0.11-3.07). Mortality was reported in six of seven RCTs[16-19,25,27]. There was no significant difference in the mortality between the probiotics/synbiotics group and the control group (OR 0.75, 95% CI: 0.25-2.24, P = 0.61) (Figure 2E). The significant heterogeneity was present between studies (P = 0.009 in surgical intervention; P = 0.04 in mortality).
Stratification, sensitivity analysis and publication bias
We analyzed the surgical outcomes stratified by the severity of acute pancreatitis. The use of pre-, pro- or synbiotics had no significant influence on the main surgical outcomes including septic morbidity, pancreatic infections, surgical intervention, mortality, MOF and SIRS in severe acute pancreatitis (Table 4).
Table 4.
Surgical outcomes stratified by severity of pancreatitis in this meta-analysis
| No. of studies | Synbiotic | Control | OR (95% CI) | P for effect size | P for heterogeneity | |
| Septic morbidity | 3 | 57/200 | 58/188 | 0.89 (0.57, 1.37) | 0.58 | 0.16 |
| Pancreatic infections | 2 | 25/185 | 22/173 | 1.06 (0.58, 1.96) | 0.84 | 0.07 |
| Surgical intervention | 2 | 32/185 | 21/173 | 1.07 (0.23, 4.92) | 0.94 | 0.04 |
| MOF and SIRS | 3 | 43/200 | 36/188 | 0.65 (0.09, 4.44) | 0.66 | 0.0005 |
| Mortality | 4 | 33/214 | 25/201 | 0.77 (0.22, 2.72) | 0.69 | 0.02 |
OR: Odds ratio; CI: Confidence interval; MOF: Multi-organ failure; SIRS: Systemic inflammatory response syndrome.
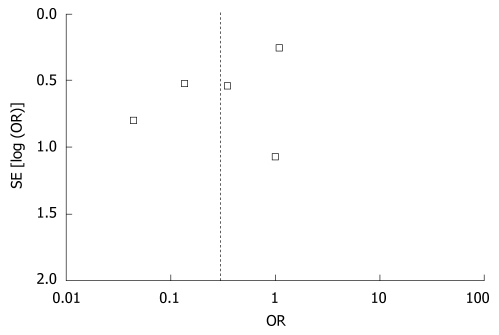
Allocation concealment and blinding were unclear in three of included studies[18,26,27]. The results of the sensitivity analysis based on the remaining four studies, after excluding trials of low quality, are similar to the previous results, indicating that the results of meta-analysis are relatively credible. Review of funnel plots is present in Figure 3, which can not rule out the potential for publication bias in all analyses.
Figure 3.
Funnel plot of publication bias.
DISCUSSION
The microbiota colonizing the gastrointestinal tract has been considered as the main causes of the pathogenesis of the nosocomial infections[28,29]. Surgical trauma, especially surgical trauma of the digestive tract, may result in physical injury and atrophy of the gastrointestinal mucosa, increased intestinal permeability, microbial imbalance, and intraluminal bacterial or bacterial products translocation cross the intestinal barrier to local lymph nodes and distant organs, and ultimately induce an increased morbidity of infectious complications and sepsis[30,31]. At present, probiotics and prebiotics have been administrated in surgical trauma[28,32-34], cancer[30,35,36], active ulcerative colitis[37], and critically ill patients[31,38,39].
Up to now, nine RCTs including 733 patients have been published reporting the effects of pre-, pro- or synbiotics treatment in patients undergoing abdominal surgery. Six studies showed a significantly positive effect of probiotics with or without prebiotics in postoperative septic complications, and the remaining three studies showed no effects. A meta-analysis based on these studies demonstrated that the use of pre-, pro- or synbiotics might reduce the incidence of postoperative infections, duration of hospital stay and length of antibiotic therapy after abdominal surgery[40]. Another meta-analysis based on eight randomized studies reported that the use of pre-, pro- or synbiotics in critically ill adult patients showed no statistically significant benefit in the length of ICU stay, hospital mortality and the incidence of nosocomial infections[41]. However, this meta-analysis did not include postoperative patients and trauma patients admitted to ICU, most of them benefited from probiotics treatment[29-31]. From our meta-analysis, we found that the use of pre-, pro- or synbiotics had no influence on the main outcomes including postoperative infections, pancreatic infections, MOF, SIRS and mortality in patients with acute pancreatitis. In the subgroup analysis and sensitivity analysis, a similar result was observed, after the stratification was conducted by the severity of acute pancreatitis and the trials of low quality were excluded.
There were controversies on the use of pre-, pro- or synbiotics in patients with acute pancreatitis among the recent studies. Three studies suggested that probiotics administration was effective in preventing complications of the experimental acute pancreatitis by decreasing bacterial translocation[42-44]. The first clinical trial was performed by Oláh et al[16] to evaluate the use of probiotics and fiber in clinical patients with acute pancreatitis. The study demonstrated that a dose of 109 of L. plantarum 299 together with oat fiber significantly reduced infected pancreatic necrosis and the number of surgical interventions. Subsequently, several studies reported similarly positive effects of probiotics with or without prebiotics[17,18,25-27]. However, the sample size in these studies was small and the conclusion from them was inconclusive. A large multi-center, randomized double-blinded controlled trial by Besselink et al[19] was designed to see whether probiotics could reduce the incidence of infectious complications in patients with severe acute pancreatitis. The trial involved 296 patients in 15 hospitals, and compared the use of a multi-species probiotics preparation with a placebo. The results showed that infectious complications occurred in 30% of the patients in the probiotics group and in 28% of the placebo group. Nine patients developed bowel ischaemia (8 died) in the probiotics group, whereas none developed this complication in the placebo group. Multiple organ failure occurred in 22% of the patients in the probiotics group and in 10% in the placebo group. In all, 16% patients in the probiotics group and 6% in the placebo group died. The results were opposite to previous evidences, and raised safety question on the use of pre-, pro- or synbiotics. Although Dutch’s study was criticized for its design, approval, and conduct, this trial should still be carefully considered for its large sample size[45]. The previous studies have demonstrated that probiotics are safe for use in healthy persons, but should be used with caution in patients with underlying immune compromise, chronic disease, or debilitation because of the risk of sepsis[46]. However, bowel ischemia has never been reported in the previous studies including acute pancreatitis, trauma, critical illness and elective abdominal surgery, and the high incidence of MOF and mortality associated with probiotics treatment were only reported in Besselink’s study. Besselink et al[19] explained that it was probably not the combination of probiotics but the administration of the combined probiotics together with the severity of the disease that was largely responsible for the effects obtained. This explanation may be one of potential reasons, but not the only one.
There are three major differences between Besselink’s study and the other studies in the meta-analysis: the patients in the former received a higher number and more strains of probiotic organisms (six strains of probiotics vs 1-4 strains of probiotics in other studies); patients received probiotics administration for a longer period in Besselink’s study (28 d vs 7 d in the other studies), and the pressor agents were administrated during probiotics feeding in some patients. Little work has been performed and no accepted recommendation was reported on the therapeutic strategy (including the optimal dose and time) of probiotics. The dose of probiotics administered was usually based on the previous studies showing clinical benefit from probiotics. Furthermore, the dose of probiotics varies considerably among different studies. In this meta-analysis, three of the studies tested a probiotics or prebiotics[16,18,25] and showed preventive effects. The remaining four studies tested different doses of synbiotics (probiotics plus prebiotics)[17,19,26,27], 3 of which showed positive results[17,26,27]. Combination of several strains of probiotics was regarded as more powerful in previous opinion. This study indicates that the inappropriate combination of probiotic strains may be harmful, while a single use of probiotics or prebiotics may be safer. Evidences suggest that intestinal blood flow at the mucosal level is generally reduced in acute pancreatitis and critically ill patients[47]. The reduced blood flow and oxygen supply in intestinal mucosa might be further compromised by the administration of enteral nutrition and probiotic bacteria[48,49]. On the other hand, feeding probiotics might cause local mucosal damage with an inflammatory response of the small bowel wall. Multispecies probiotics mixture may strongly increase the concentration of IL-10, and reduce the concentration of IL-6[50]. Experimental studies demonstrated that the increasing IL-10 is able to induce intestinal damage during intestinal ischemia/reperfusion[51], whereas IL-6 is able to protect enterocytes[52]. One may speculate that a long duration of probiotics treatment and a high number of bacterial organisms may lead to probiotics overload in the small bowel, which aggravate the disorder of inflammatory response and reduction of intestinal blood flow and oxygen supply at the phase of acute stage of severe acute pancreatitis, and ultimately lead to bowel ischemia.
The unexpected deleterious effects may refer to more biologic actions of probiotics. It is clear that different strains of probiotics can have different effects. Moreover, their effects may vary in healthy people and patients, in different disease states, and among different age groups. Thus, clinical effects of one probiotics strain in one population cannot be automatically generalized to other strains or to other populations[46]. Although the individual strains used in this study are credible on the basis of their capacity to inhibit growth of pathogenic bacteria and to modulate immune responses, the benefits, disadvantages and interaction of different probiotics strains and their mechanisms of action in special illness is still unclear, and the combination of several strains should be thoroughly evaluated for safety and clinical benefits.
Limitations of study
This meta-analysis has several limitations. The included studies were significantly heterogeneous, so the results should be interpreted with caution. Since these studies include diverse patient groups with diverse severity of disease, the effects of pre- pro- or synbiotics may differ among them. The type and the concentration of the probiotics vary considerably in these reviewed studies, which may explain some of the differences in results between studies. In addition, some strains are effective and some may be harmful, and the interaction of several stains of probiotics as a mixture may lead to the deleterious effects in some special illness. Most of these studies are small in scale and two of them are of low quality, which may influence the analysis. There were also differences in the study design, the population and study team among these studies. Finally, meta-analysis remains retrospective that is subject to the methodological deficiencies of the studies, it is therefore possible that studies with different results may be unpublished, which leads to publication bias.
In conclusion, there are a few recommendations can be offered and further studies are required. In view of the questions raised in safety and efficacy, use of pre- pro- or synbiotics should be cautious in critically ill patients, especially in patients with severe acute pancreatitis. Unless new and strong evidence is presented, probiotics use can no longer be considered risk-free. However, we can not draw a conclusion that the use of pre- pro- or synbiotics is dangerous, and it is too early and inappropriate to deny the beneficial effects of all of pre- pro- or synbiotics in critically ill patients and severe acute pancreatitis. After all, a single large trial is not sufficient to draw a definitive conclusion. Well-designed randomized controlled trials are needed to further explore the mechanistic issues and probiotic interactions, and assess the effect and safety of pre-, pro- or synbiotics.
COMMENTS
Background
The administration of pre-, pro- or synbiotics has been shown to enhance immunomodulating ability, modulate the intestinal microbiota, inhibit bacterial translocation and reduce the incidence of nosocomial infections in clinically surgical patients, and may be a new anti-infectious strategy for patients with acute pancreatitis.
Research frontiers
Some randomized controlled studies (RCTs) demonstrated a therapeutic and preventive effect of pre-, pro- or synbiotics in patients with acute pancreatitis. However, a multicenter RCTs reported some unexpected results. These studies were controversial for their shortcomings. Overall, the magnitude of the therapeutic effect remains unknown. This meta-analysis was performed to assess the potential effect and safety of pre-, pro- or synbiotics in patients with acute pancreatitis.
Innovations and breakthroughs
Currently, there is still a lack of evidence to support the use of pre-, pro- or synbiotics in patients with acute pancreatitis.
Applications
Pre-, pro- or synbiotics treatment shows no statistically significant benefit in the main outcome of patients with acute pancreatitis. In view of the questions in safety and efficacy, use of pre- pro- or synbiotics should be cautious in critically ill patients, especially in patients with severe acute pancreatitis. Further studies are needed to explore mechanistic issues and probiotic interactions in critical illnesses.
Terminology
Probiotics, is defined as ingestible microorganisms that benefit the host by improving intestinal microbial balance.
Peer review
In this meta-analysis, the use of pre- pro- and synbiotics was compared to placebo in patients with acute pancreatitis. In general, I find the study interesting and clinically relevant. However, the manuscript needs to undergo major revision.
Footnotes
Supported by The Grant for the Research Projects of Sichuan Province, China. No. 07FG002-032
Peer reviewers: Kristin Verbeke, PhD, Professor, Laboratory Digestion and Absorption, University Hospital Leuven, E462, Herestraat 49, B-3000 Leuven, Belgium; Mr. Morten Hylander Møller, Anaesthesiology and Intensive Care Medicine, Copenhagen University Hospital Herlev, Skolevej 14B, Holte, 2840, Denmark
S- Editor Wang YR L- Editor Ma JY E- Editor Ma WH
References
- 1.Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF, et al. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674–683. doi: 10.1097/01.sla.0000250414.09255.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997–1004. doi: 10.1053/j.gastro.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 4.Mazaki T, Ishii Y, Takayama T. Meta-analysis of prophylactic antibiotic use in acute necrotizing pancreatitis. Br J Surg. 2006;93:674–684. doi: 10.1002/bjs.5389. [DOI] [PubMed] [Google Scholar]
- 5.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 6.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 7.Macfarlane GT, Cummings JH. Probiotics, infection and immunity. Curr Opin Infect Dis. 2002;15:501–506. doi: 10.1097/00001432-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 9.Tuohy KM, Rouzaud GC, Brück WM, Gibson GR. Modulation of the human gut microflora towards improved health using prebiotics--assessment of efficacy. Curr Pharm Des. 2005;11:75–90. doi: 10.2174/1381612053382331. [DOI] [PubMed] [Google Scholar]
- 10.Bengmark S. Immunonutrition: role of biosurfactants, fiber, and probiotic bacteria. Nutrition. 1998;14:585–594. doi: 10.1016/s0899-9007(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 11.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 12.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura T, Tsuchiya Y, Nashimoto A, Yabusaki H, Takii Y, Nakagawa S, Sato N, Kanbayashi C, Tanaka O. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology. 2007;54:661–663. [PubMed] [Google Scholar]
- 14.Rayes N, Seehofer D, Theruvath T, Mogl M, Langrehr JM, Nüssler NC, Bengmark S, Neuhaus P. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg. 2007;246:36–41. doi: 10.1097/01.sla.0000259442.78947.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy BS, Macfie J, Gatt M, Larsen CN, Jensen SS, Leser TD. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br J Surg. 2007;94:546–554. doi: 10.1002/bjs.5705. [DOI] [PubMed] [Google Scholar]
- 16.Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 17.Oláh A, Belágyi T, Pótó L, Romics L Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology. 2007;54:590–594. [PubMed] [Google Scholar]
- 18.Qin HL, Zheng JJ, Tong DN, Chen WX, Fan XB, Hang XM, Jiang YQ. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr. 2008;62:923–930. doi: 10.1038/sj.ejcn.1602792. [DOI] [PubMed] [Google Scholar]
- 19.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Kecskés G, Belágyi T, Oláh A. [Early jejunal nutrition with combined pre- and probiotics in acute pancreatitis--prospective, randomized, double-blind investigations] Magy Seb. 2003;56:3–8. [PubMed] [Google Scholar]
- 22.Oláh A, Belágyi T, Issekutz A, Olgyai G. [Combination of early nasojejunal feeding with modern synbiotic therapy in the treatment of severe acute pancreatitis (prospective, randomized, double-blind study)] Magy Seb. 2005;58:173–178. [PubMed] [Google Scholar]
- 23.Besselink MG, Timmerman HM, Buskens E, Nieuwenhuijs VB, Akkermans LM, Gooszen HG. Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949] BMC Surg. 2004;4:12. doi: 10.1186/1471-2482-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. [Probiotic prophylaxis in patients with predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial] Ned Tijdschr Geneeskd. 2008;152:685–696. [PubMed] [Google Scholar]
- 25.Karakan T, Ergun M, Dogan I, Cindoruk M, Unal S. Comparison of early enteral nutrition in severe acute pancreatitis with prebiotic fiber supplementation versus standard enteral solution: a prospective randomized double-blind study. World J Gastroenterol. 2007;13:2733–2737. doi: 10.3748/wjg.v13.i19.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YM. Adjuvant therapy for probiotics in patients with severe acute pancreatitis: An analysis of 14 cases. Shijie Huaren Xiaohua Zazhi. 2007;15:302–304. [Google Scholar]
- 27.Wu XG, Zhang QC. Adjuvant therapy for probiotics in patients with severe acute pancreatitis with hepatic lesion: an analysis of 27 cases. Clin Med. 2009;29:51–52. [Google Scholar]
- 28.Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond) 2004;106:287–292. doi: 10.1042/CS20030251. [DOI] [PubMed] [Google Scholar]
- 29.Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, Bengmark S, Neuhaus P. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant. 2005;5:125–130. doi: 10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, Asahara T, Nomoto K, Tanaka R, Nimura Y. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104–113. doi: 10.1007/s00423-004-0536-1. [DOI] [PubMed] [Google Scholar]
- 31.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30:1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 32.Rayes N, Hansen S, Seehofer D, Müller AR, Serke S, Bengmark S, Neuhaus P. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18:609–615. doi: 10.1016/s0899-9007(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 33.McNaught CE, Woodcock NP, MacFie J, Mitchell CJ. A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients. Gut. 2002;51:827–831. doi: 10.1136/gut.51.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson AD, McNaught CE, Jain PK, MacFie J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut. 2004;53:241–245. doi: 10.1136/gut.2003.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–496. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 36.Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, Nomoto K, Nimura Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706–714. doi: 10.1097/01.sla.0000219039.20924.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31:119–126. doi: 10.1177/0148607107031002119. [DOI] [PubMed] [Google Scholar]
- 39.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, Fedorak R, Madsen K. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 40.Pitsouni E, Alexiou V, Saridakis V, Peppas G, Falagas ME. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2009;65:561–570. doi: 10.1007/s00228-009-0642-7. [DOI] [PubMed] [Google Scholar]
- 41.Watkinson PJ, Barber VS, Dark P, Young JD. The use of pre- pro- and synbiotics in adult intensive care unit patients: systematic review. Clin Nutr. 2007;26:182–192. doi: 10.1016/j.clnu.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Akyol S, Mas MR, Comert B, Ateskan U, Yasar M, Aydogan H, Deveci S, Akay C, Mas N, Yener N, et al. The effect of antibiotic and probiotic combination therapy on secondary pancreatic infections and oxidative stress parameters in experimental acute necrotizing pancreatitis. Pancreas. 2003;26:363–367. doi: 10.1097/00006676-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Muftuoglu MA, Isikgor S, Tosun S, Saglam A. Effects of probiotics on the severity of experimental acute pancreatitis. Eur J Clin Nutr. 2006;60:464–468. doi: 10.1038/sj.ejcn.1602338. [DOI] [PubMed] [Google Scholar]
- 44.van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, Smidt H, Visser MR, Rijkers GT, Gooszen HG, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Sheldon T. Dutch probiotics study is criticised for its “design, approval, and conduct”. BMJ. 2010;340:c77. doi: 10.1136/bmj.c77. [DOI] [PubMed] [Google Scholar]
- 46.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256–1264; quiz 1446-1467. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 47.Andersson R, Wang X, Ihse I. The influence of abdominal sepsis on acute pancreatitis in rats: a study on mortality, permeability, arterial pressure, and intestinal blood flow. Pancreas. 1995;11:365–373. doi: 10.1097/00006676-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 48.McClave SA, Chang WK. Feeding the hypotensive patient: does enteral feeding precipitate or protect against ischemic bowel? Nutr Clin Pract. 2003;18:279–284. doi: 10.1177/0115426503018004279. [DOI] [PubMed] [Google Scholar]
- 49.Melis M, Fichera A, Ferguson MK. Bowel necrosis associated with early jejunal tube feeding: A complication of postoperative enteral nutrition. Arch Surg. 2006;141:701–704. doi: 10.1001/archsurg.141.7.701. [DOI] [PubMed] [Google Scholar]
- 50.Timmerman HM, Niers LE, Ridwan BU, Koning CJ, Mulder L, Akkermans LM, Rombouts FM, Rijkers GT. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr. 2007;26:450–459. doi: 10.1016/j.clnu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Nüssler NC, Müller AR, Weidenbach H, Vergopoulos A, Platz KP, Volk HD, Neuhaus P, Nussler AK. IL-10 increases tissue injury after selective intestinal ischemia/reperfusion. Ann Surg. 2003;238:49–58. doi: 10.1097/01.sla.0000074962.26074.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rollwagen FM, Madhavan S, Singh A, Li YY, Wolcott K, Maheshwari R. IL-6 protects enterocytes from hypoxia-induced apoptosis by induction of bcl-2 mRNA and reduction of fas mRNA. Biochem Biophys Res Commun. 2006;347:1094–1098. doi: 10.1016/j.bbrc.2006.07.016. [DOI] [PubMed] [Google Scholar]