Abstract
We undertook this study to elucidate whether baseline peritoneal membrane transport characteristics are associated with high mortality in incident automated peritoneal dialysis (APD) patients. This retrospective study includes 117 patients who started APD at Yonsei University Health System from 1996 to 2008 and had a PET within 3 months of APD initiation. High transporters were significantly older and had a higher incidence of cardiovascular disease. Patient survival for years 1, 3, and 5 were 85%, 64%, and 35% for high transporter and 94%, 81%, and 68% for non-high transporter group (P<0.01). Multivariate analysis revealed that age, diabetes, cardiovascular disease, serum albumin level, and residual renal function were independently associated with high mortality in APD patients. In contrast, high transport status was not a significant predictor for mortality in this population when the other covariates were included. Even though high transport was significantly associated with mortality in the univariate analysis, its role seemed to be influenced by other comorbid conditions. These findings suggest that the proper management of these comorbid conditions, as well as appropriate ultrafiltration by use of APD and/or icodextrin, must be considered as protective strategies to improve survival in peritoneal dialysis patients with high transport.
Keywords: Automated Peritoneal Dialysis, High Transport, Peritoneal Equilibration Test, Mortality
INTRODUCTION
Patients with end-stage renal disease (ESRD), including those who are on peritoneal dialysis (PD), are at much higher risk for mortality than the general population. Several factors such as age, comorbidities including diabetes and cardiovascular disease (CVD), malnutrition/hypoalbuminemia, reduced residual renal function, and inflammation have been associated with high mortality in PD patients (1-7). Recently, peritoneal membrane transport status has attracted considerable attention as another contributor to decreased survival in these patients.
High transport status, as defined by the increased ratio of creatinine in the dialysate to plasma after a 4-hr dwell (D/P cr4) from a peritoneal equilibration test (PET) (8), has been associated with several parameters that in themselves are well-known risk factors for mortality in PD patients. Diabetes, CVD, hypoalbuminemia, poor nutritional status, and inflammation, as well as inappropriate ultrafiltration and large peritoneal protein loss have been shown to be more prevalent in high transporters compared to other peritoneal transporters. These comorbidities may also play a role in the higher rate of adverse outcomes observed in high transporters (3, 9-13). However, most studies, investigatng the adverse effect of high transport status on outcomes in PD patients, have been performed in the patients who were mainly on continuous ambulatory PD (CAPD), or in largely prevalent patient population. The association between high peritoneal transport and the long-term survival among the incident patients treated with automated PD (APD) has not been fully explored. We, therefore, undertook this study to evaluate the impact of peritoneal membrane transport characteristics, as measured by a PET, on mortality in incident patients who were on APD.
MATERIALS AND METHODS
We retrospectively analyzed a total of 117 patients who started APD at Yonsei University Health System from January 1996 to December 2008 and had a PET within 3 months of APD initiation. This study was approved by the institutional review board (IRB number 4-2009-0680). All subjects were given a standard PET using 2.27% glucose PD fluid and were classified into one of the four peritoneal transport types according to the value for D/P cr4 defined by Twardowski et al. (8): high ≥0.81, high average 0.65 to 0.80, low average 0.50 to 0.64, and low <0.50.
Demographic and clinical data were collected based on retrospective review of patient records. The data included age, gender, body mass index (BMI, weight [kg]/height [m2]), duration of PD, cause of ESRD, comorbidities, use of icodextrin, and the patient outcome. The following laboratory data obtained at the time of PD catheter insertion (within first week after starting PD) were considered as baseline: hemoglobin, blood urea nitrogen (BUN), serum creatinine, calcium, phosphorus, total cholesterol, uric acid, and albumin concentrations, Kt/V urea, percentage of lean body mass (%LBM), normalized protein catabolic rate (nPCR), and residual glomerular filtration rate (GFR). Cardiovascular disease was defined as a history of coronary, cerebrovascular, or peripheral artery disease. Coronary disease was defined as a previous history of angioplasty, coronary artery bypass graft, myocardial infarction, or angina. Cerebrovascular disease was defined as a previous transient ischemic attack, stroke, or carotid endarterectomy, and peripheral vascular disease as a history of claudication, ischemic limb loss, and/or ulceration or a peripheral revascularization procedure. Technique failure was defined as transfer to hemodialysis due to peritonitis, ultrafiltration failure, inadequate dialysis, exit and tunnel infection, and mechanical problems.
All values are expressed as mean±standard deviation (SD) or percentages. Statistical analyses were performed using SPSS for Windows ver 13.0 (SPSS, Inc., Chicago, IL, USA). Data were analyzed using Student's t-test, chi-square test, or Fisher exact test for comparisons. Patient and technique survival was assessed using life table methods and comparisons were made with Kaplan-Meier analysis and log-rank tests. Patients were censored at the point of transplantation or if lost to follow-up. To determine risk factors for mortality in APD patients, multivariate Cox regression was performed, in which we included all of the significant covariates from the univariate analysis. A P value less than 0.05 was considered statistically significant.
RESULTS
Demographic characteristics and clinical data
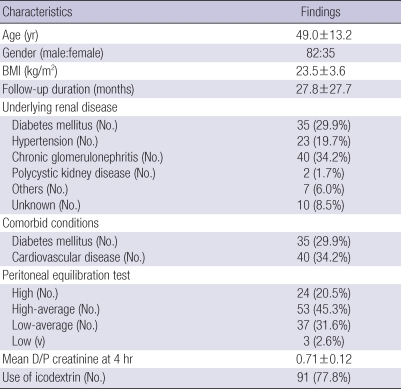
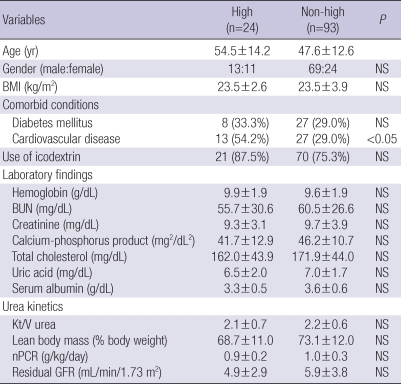
Table 1 details baseline characteristics of 117 incident APD patients. The mean age of patients was 49.0 yr (range 20 to 86 yr), 70.1% were males, and patients were on PD for a mean period of 27.8 months (range 3.5 to 155.2 months). Chronic glomerulonephritis was the most common cause of ESRD (34.2%), followed by diabetes (29.9%) and hypertension (19.7%). Among the 117 patients, 24 patients (20.5%) were high transporters, and 91 patients (77.8%) used icodextrin. The baseline mean hemoglobin was 9.6±1.9 g/dL, and serum albumin was 3.5±0.6 g/dL. The baseline Kt/V urea and residual GFR were 2.2±0.6 and 2.9±3.7 mL/min/1.73 m2, respectively. When the patients were divided into two groups based on a PET, high transporters (High group) were significantly older (P<0.05) and had a higher incidence of CVD (P<0.05) compared to non-high transporters (Non-high group) (Table 2).
Table 1.
Demographic and clinical characteristics of the study subjects (n=117)
Data are expressed as mean±standard deviation.
BMI, body mass index; D/P, ratio of dialysate to plasma.
Table 2.
Baseline characteristics in high and non-high transport groups
Data are expressed as mean±standard deviation or number of patients (percent).
BMI, body mass index; BUN, blood urea nitrogen; Kt/V, fractional urea clearance; nPCR, normalized protein catabolic rate; GFR, glomerular filfration rate, NS, not significant.
Patient outcomes
During the period of study, 66 patients (56.4%) were living on APD, and 20 patients (17.1%) died. Infection (50.0%) was the most common cause of death, with four patients dying of peritonitis, followed by CVD (40.0%), upper gastrointestinal bleeding (8.3%), and unknown (8.3%). There was a tendency for higher incidence of infection in High group (75% vs. 33.3%) and CVD in Non-high group (50% vs. 25%), but this difference was not statistically significant. Transfer to hemodialysis occurred in 21 patients (17.9%), mainly due to peritonitis (57.1%). Other reasons of technique failure were ultrafiltration failure (23.8%), inadequate dialysis (14.3%), and noncompliance (4.8%). There was no difference between either transport groups for the causes of technique failure. Another 9 patient (7.7%) underwent kidney transplantation, and only 1 patient (0.9%) moved to another center.
Patient survival
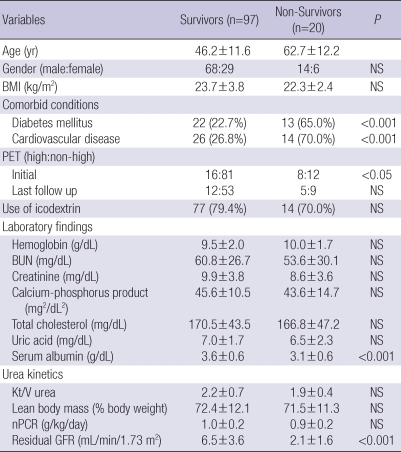
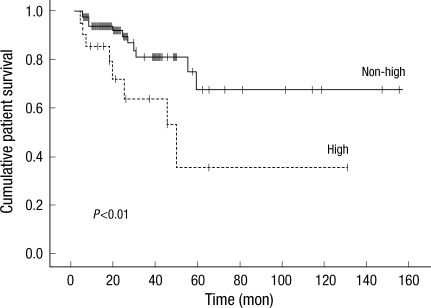
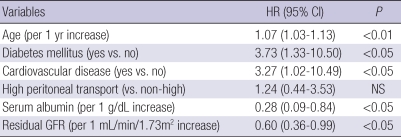
Table 3 presents the differences between survivors (n=97) and non-survivors (n=20). In non-survival group, diabetes (P<0.001), CVD (P<0.001), and high peritoneal membrane transport (P<0.05) were more prevalent, and serum albumin level (P<0.001) and residual GFR (P<0.001) at baseline were significantly lower than those observed in the survivor group. Besides, non-survivors were significantly older than survivors (P<0.001). Fig. 1 shows patient survival for both peritoneal membrane transport groups. Patient survival for years 1, 3, and 5 were 85%, 64%, and 35% for High group and 94%, 81%, and 68% for Non-high group (P<0.01). As shown in Table 4, multivariate Cox regression revealed that age (P<0.01), diabetes (P<0.05), CVD (P<0.05), serum albumin level (P<0.05), and residual GFR (P<0.05) were independently associated with high mortality in patients treated with APD. However, high transport status was not a significant predictor for mortality in this population when the other covariates were included.
Table 3.
Comparisons between survivors and non-survivors
Data are expressed as mean±standard deviation or number of patients (percent).
BMI, body mass index; PET, peritoneal equilibration test; nPCR, normalized protein catabolic rate; NS, not significant.
Fig. 1.
Kaplan-Meier survival plots for patient survival according to peritoneal transport type. The survival rate was significantly higher for the patients who were in the nonhigh transport group (P<0.01).
Table 4.
Multivariate Cox proportional hazard model for patient mortality
HR, hazard ratio; CI, confidence interval; NS, not significant.
Technique survival
Estimated mean pure (death-censored) and combined (patient and death-censored) technique survival were 90.5±23.1 and 49.8±13.1 months in High group, and 100.5±11.9 and 79.1±10.4 months in Non-high group, respectively. Cumulative combined technique survival at the end of 1, 3, and 5 yr were 76%, 57%, and 16% for High group, and 83%, 66%, and 30% for Non-high group. There were no significant differences in the risk of either technique failure between patients in two transport groups.
DISCUSSION
It is well established that PD patients have different peritoneal membrane transport properties. These differences can best be classified and determined by the use of a PET, which helps to characterize the relationships among dwell time, solute transport, glucose absorption, drain volume, and net solute clearance (14). Patients who have a greater rate of membrane solute transport are classified as high (or fast) transporters, and will tend to have enhanced clearance of small solutes, such as urea and creatinine, early in short dwells. However, these patients will have larger peritoneal loss of protein, will be more likely to fluid overload as a result of rapid reabsorption of glucose from the dialysate and subsequent ultrafiltration dysfunction, and will have greater systemic exposure to glucose (15-19). In addition, high transporters have been associated with poor nutritional status, more prevalent comorbid diseases, and chronic inflammation (3, 9-13).
Many conflicting results have been reported on the relationship between high peritoneal transport and mortality in PD patients. The single-center Stroke PD study (18, 19) and the multicenter CANUSA study (2) found that high transport was associated with worse patient and technique survival independent of other important risk factors, such as age, comorbidities, and residual renal function. A recent meta-analysis of 20 observational studies (20) also demonstrated that a higher peritoneal membrane solute transport rate was associated with a higher mortality risk and a trend to higher technique failure. However, these findings were not confirmed by several other studies including ADEMEX trial which showed that high transport does not have any influence on patient survival in PD patients (21-23). Furthermore, some investigations have found an association on univariate analysis only, or with morbidity but not mortality (24-26). The reasons for these conflicting observations are not clear, but most studies investigating outcomes in PD patients have been performed in patients who were mainly on CAPD, or included a greater portion of prevalent patients. Even though there has been a study, with small number of patients, reporting that APD patients with high peritoneal transport had a lower probability of patient survival, there is some evidence that APD mitigates the adverse effects of high transport (27). Most recently, analysis from the ANZDATA registry, by far the largest study published to date, has confirmed that peritoneal transport type was only a significant predictor of mortality in patients on CAPD, not in those on APD (28). In another recent single-center observation cohort study (29), high transport status predicted a higher risk of death in patients who started PD between 1990 and 1997, but not in patients who started PD between 1998 and 2005. During the latter vintage, patients were more likely to be treated with APD or icodextrin. Similarly, in the EAPOS study (30) of patients receiving APD, transport status had no bearing on increased risk of death at one year. Taken together, it is likely that an increased peritoneal transport rate appears to be less important as a predictor of mortality in APD patients when compared to CAPD patients.
In the present study, we found that there was a significant difference, as determined by univariate analysis, in mortality between high and non-high transporters. However, high transport status was confirmed not to be a significant risk factor associated with mortality of the incident APD patients. This situation was particularly evident when adjusted for age and other comorbidities such as diabetes, CVD, serum albumin level, and residual GFR. As these are well known risk factors for mortality on PD, it is probable that the association seen in this study between high transporter and mortality is related to the presence of these comorbid conditions. Furthermore, it might be said that the strategies including optimization of the short dwell lengths using APD combined with icodextrin which will result in sustained ultrafiltration and thus prevention of reabsorption in the long dwell have been attributed to minimize the adverse effects of high transport on patient survival in these patients.
There are several limitations in the present study. It was an observational study based on retrospective data collected from a relatively small sample size. Therefore, the causality of our findings needs further confirmation. Also we did not include other relevant data representing overall nutritional status and systemic inflammation such as subjective global assessment (SGA) and other inflammatory markers to fully assess the impact of nutritional and inflammatory status. Finally, the impact of icodextrin-based products on mortality could not be evaluated in this study because most of our patients were using icodextrin.
In conclusion, the present study showed that age, diabetes, CVD, serum albumin level, and residual GFR were independent predictors for mortality in incident PD patients who were on APD. Even though high transport was significantly associated with mortality in the univariate analysis, this association was not an independent risk factor for mortality. Its role seemed to be influenced by other comorbid conditions. These findings suggest that the proper management of these comorbid conditions, as well as appropriate ultrafiltration by use of APD and/or icodextrin, must be considered as a protective strategy to improve survival in PD patients with high transport.
References
- 1.Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. J Am Soc Nephrol. 1996;7:198–207. doi: 10.1681/ASN.V72198. [DOI] [PubMed] [Google Scholar]
- 2.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J Am Soc Nephrol. 1998;9:1285–1292. doi: 10.1681/ASN.V971285. [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 4.Chung SH, Heimburger O, Stenvinkel P, Qureshi AR, Lindholm B. Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol Dial Transplant. 2003;18:590–597. doi: 10.1093/ndt/18.3.590. [DOI] [PubMed] [Google Scholar]
- 5.Yoon HB, Park HC, Lee H, Han SS, Kim S, Joo KW, Kim YS, Ahn C, Han JS, Kim S, Oh Kh. Treatment outcomes and prognostic factors for peritoneal dialysis patients based on single center experience over 18 years. Korean J Nephrol. 2009;28:19–31. [Google Scholar]
- 6.Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int Suppl. 2003;88:S3–S12. doi: 10.1046/j.1523-1755.2003.08801.x. [DOI] [PubMed] [Google Scholar]
- 7.Bargman JM, Thorpe KE, Churchill DN CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 8.Twardowski ZJ, Nolph KD, Khanna R, Prowant BF, Ryan LP, Moore HL, Nielsen MP. Peritoneal equilibration test. Perit Dial Bull. 1987;7:138–147. [Google Scholar]
- 9.Kang DH, Yoon KI, Choi KB, Lee R, Lee HY, Han DS, Cho EY, Lee JH. Relationship of peritoneal membrane transport characteristics to the nutritional status in CAPD patients. Nephrol Dial Transplant. 1999;14:1715–1722. doi: 10.1093/ndt/14.7.1715. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Heimburger O, Cheng HH, Bergstrom J, Lindholm B. Does a high peritoneal transport rate reflect a state of chronic inflammation? Perit Dial Int. 1999;19:17–22. [PubMed] [Google Scholar]
- 11.Chung SH, Chu WS, Lee HA, Kim YH, Lee IS, Lindholm B, Lee HB. Peritoneal transport characteristics, comorbid diseases and survival in CAPD patients. Perit Dial Int. 2000;20:541–547. [PubMed] [Google Scholar]
- 12.Margetts PJ, McMullin JP, Rabbat CG, Churchill DN. Peritoneal membrane transport and hypoalbuminemia: cause or effect? Perit Dial Int. 2000;20:14–18. [PubMed] [Google Scholar]
- 13.Pecoits-Filho R, Araujo MR, Lindholm B, Stenvinkel P, Abensur H, Romao JE, Jr, Marcondes M, De Oliveira AH, Noronha IL. Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant. 2002;17:1480–1486. doi: 10.1093/ndt/17.8.1480. [DOI] [PubMed] [Google Scholar]
- 14.Twardowski ZJ. Clinical value of standardized equilibration tests in CAPD patients. Blood Purif. 1989;7:95–108. doi: 10.1159/000169582. [DOI] [PubMed] [Google Scholar]
- 15.Heaf J. Pathogenic effects of a high peritoneal transport rate. Semin Dial. 2000;13:188–193. doi: 10.1046/j.1525-139x.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- 16.Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int. 2000;20(Suppl 4):S22–S42. [PubMed] [Google Scholar]
- 17.Chung SH, Heimburger O, Lindholm B. Poor outcomes for fast transporters on PD: the rise and fall of a clinical concern. Semin Dial. 2008;21:7–10. doi: 10.1111/j.1525-139X.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 18.Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int. 1998;54:2207–2217. doi: 10.1046/j.1523-1755.1998.00180.x. [DOI] [PubMed] [Google Scholar]
- 19.Davies SJ, Phillips L, Russell GI. Peritoneal solute transport predicts survival on CAPD independently of residual renal function. Nephrol Dial Transplant. 1998;13:962–968. doi: 10.1093/ndt/13.4.962. [DOI] [PubMed] [Google Scholar]
- 20.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol. 2006;17:2591–2598. doi: 10.1681/ASN.2006030194. [DOI] [PubMed] [Google Scholar]
- 21.Passadakis PS, Thodis ED, Panagoutsos SA, Selisiou CA, Pitta EM, Vargemezis VA. Outcome for continuous ambulatory peritoneal dialysis patients is not predicted by peritoneal permeability characteristics. Adv Perit Dial. 2000;16:2–6. [PubMed] [Google Scholar]
- 22.Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Fang W, Bargman JM, Oreopoulos DG. High peritoneal permeability is not associated with higher mortality or technique failure in patients on automated peritoneal dialysis. Perit Dial Int. 2008;28:82–92. [PubMed] [Google Scholar]
- 24.Cueto-Manzano AM, Correa-Rotter R. Is high peritoneal transport rate an independent risk factor for CAPD mortality? Kidney Int. 2000;57:314–320. doi: 10.1046/j.1523-1755.2000.00817.x. [DOI] [PubMed] [Google Scholar]
- 25.Park HC, Kang SW, Choi KH, Ha SK, Han DS, Lee HY. Clinical outcome in continuous ambulatory peritoneal dialysis patients is not influenced by high peritoneal transport status. Perit Dial Int. 2001;21(Suppl 3):S80–S85. [PubMed] [Google Scholar]
- 26.Szeto CC, Law MC, Wong TY, Leung CB, Li PK. Peritoneal transport status correlates with morbidity but not longitudinal change of nutritional status of continuous ambulatory peritoneal dialysis patients: a 2-year prospective study. Am J Kidney Dis. 2001;37:329–336. doi: 10.1053/ajkd.2001.21298. [DOI] [PubMed] [Google Scholar]
- 27.Hung KY, Lin TJ, Tsai TJ, Chen WY. Impact of peritoneal membrane transport on technique failure and patient survival in a population on automated peritoneal dialysis. ASAIO J. 1999;45:568–573. doi: 10.1097/00002480-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006;17:271–278. doi: 10.1681/ASN.2005050566. [DOI] [PubMed] [Google Scholar]
- 29.Davies SJ. Mitigating peritoneal membrane characteristics in modern peritoneal dialysis therapy. Kidney Int Suppl. 2006;103:S76–S83. doi: 10.1038/sj.ki.5001920. [DOI] [PubMed] [Google Scholar]
- 30.Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, Divino Filho JC, Vonesh E, van Bree M. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol. 2003;14:2948–2957. doi: 10.1097/01.asn.0000092146.67909.e2. [DOI] [PubMed] [Google Scholar]