Abstract
This study was performed in order to assess whether acute stress can increase mast cell and enterochromaffin (EC) cell numbers, and proteinase-activated receptor-2 (PAR2) expression in the rat colon. In addition, we aimed to investigate the involvement of corticotrophin-releasing factor in these stress-related alterations. Eighteen adult rats were divided into 3 experimental groups: 1) a saline-pretreated non-stressed group, 2) a saline-pretreated stressed group, and 3) an astressin-pretreated stressed group. The numbers of mast cells, EC cells, and PAR2-positive cells were counted in 6 high power fields. In proximal colonic segments, mast cell numbers of stressed rats tended to be higher than those of non-stressed rats, and their PAR2-positive cell numbers were significantly higher than those of non-stressed rats. In distal colonic segments, mast cell numbers and PAR2-positive cell numbers of stressed rats were significantly higher than those of non-stressed rats. Mast cell and PAR2-positive cell numbers of astressin-pretreated stressed rats were significantly lower than those of saline-pretreated stressed rats. EC cell numbers did not differ among the three experimental groups. Acute stress in rats increases mast cell numbers and mucosal PAR2 expression in the colon. These stress-related alterations seem to be mediated by release of corticotrophin-releasing factor.
Keywords: Corticotropin-releasing Factor; Enterochromaffin Cells; Irritable Bowel Syndrome; Mast cells; Receptor, PAR-2; Stress
INTRODUCTION
Irritable bowel syndrome (IBS) is known to be related to stress, which is likely to induce corticotrophin-releasing factor (CRF) release (1). CRF family peptides and/or receptors may activate immune cells, inducing inflammation via an increase in intestinal permeability or degranulated mast cells (2). Acute stress, including water avoidance and immobilization stress, alters intestinal motility and increases fecal pellet output in animal studies. These effects of stress are suggested to be mediated by release of CRF (3-5). Furthermore, motility alterations induced by acute stress are reported to be blocked by administration of the CRF antagonist (3, 6). Stress is implicated in certain neuroinflammatory disorders, such as neurogenic pruritus and interstitial cystitis (7). These two diseases are associated with mast cell activation. Colonic responses to immobilization stress such as mucin and prostaglandin E2 release are reported to be related to mast cell degranulation (8).
Immune activation and mucosal inflammation alter gut motility, leading to gut dysfunction. Evidence has shown that previous infection and persistent low-grade inflammation may result in gut dysfunction, generating IBS symptoms (9, 10). Increased intraepithelial lymphocytes and lamina propria lymphocytes, together with elevated numbers of enterochromaffin (EC) cells, are observed in patients with post-infectious IBS (11, 12). The immune activation is also found in patients with IBS and no history of previous gastrointestinal (GI) infection. Increased numbers of mast cells have been demonstrated in a subset of IBS patients without history of previous GI infection as well as postinfectious IBS patients (12-14). However, the mechanism underlying increased mast cells observed in IBS patients without history of previous GI infection remains unclear.
We hypothesized that CRF released by stress may increase the numbers of mast cells in the colon and enhance protease-activated receptor 2 (PAR2) expression in the colon. Thus, in the present study, we aimed to evaluate whether acute restraint stress alters the numbers of mast cells and EC cells and PAR2 expression in the rat colon. This study also aimed to investigate the involvement of CRF in these stress-related alterations.
MATERIALS AND METHODS
Animals
Eighteen adult male Wistar rats (250-300 g), aged 14-20 weeks, were used for this study. All rats were acclimated for 7 days before experimentation and allowed free access to food and water. Rats were kept on a 12-hr light:dark cycle and isolated from environmental stressors (e.g., noise) as much as possible. The animals were housed in pairs in cages and kept in a temperature-controlled room (21±1℃). All protocols were approved by the Institutional Animal Care and Use Committee at Ajou University School of Medicine (AMC-69).
Stress protocol
The rats were handled daily for a week by the same examiner and then submitted to acute restraint stress or sham stress for 90 min. Rats were divided into 3 experimental groups with 6 rats per treatment group: 1) saline-pretreated non-stressed group (rats were pretreated with saline 0.1 mL and then placed freely in their home cage for 90 min), 2) saline-pretreated stressed group (rats were pretreated with saline 0.1 mL and then placed into the restraint tube for 90 min), 3) astressin-pretreated stressed group (rats were pretreated with the CRF antagonist astressin, 20 µg/kg in 0.1 mL and then placed into the restraint tube for 90 min). In all stress sessions, the total body of the animal from head to lower hindlimbs was tightly placed in a plexiglass cylindrical restrainer for immobilization. Restraint stress has been used in visceral hypersensitivity and intestinal permeability studies (15, 16). Partial restraint stress model, in which the animal's fore-shoulders, upper fore-limbs and thoracic trunk are restricted for 2 hr, is reported to increase plasma levels of adrenocorticotropic hormone and cortisone, which indicates activation of the hypothalamic-pituitary-adrenal axis (17). We assumed that total restraint could cause more stress than partial restraint. Thus, we applied total restraint stress for 90 min. Control sham stressed rats were placed in their home cages for 90 min without any restraint stress. Fecal pellet output is known to increase under stress conditions (18). During the experiments, feces were collected. Immediately after completing the protocol, all rats were sacrificed by stunning and posterior exsanguinations. Just after the sacrifice, blood and tissues were collected. Astressin was purchased from Sigma Chemical Company (St. Louis, MO, USA).
Measurement of plasma cortisol levels
Blood samples were collected in the central vein immediately after completing the experimental protocols and sacrificing rats. Blood in a heparinized Eppendorf tube was immediately centrifuged (10,000 rpm, 1 min at 4℃) to separate plasma, which was then frozen at -70℃ until assay. Plasma cortisol levels were quantified using a radioimmunoassay kit (Rat Corticosteroid Coat-a-Count Kit, Diagnostic Products Corp., Los Angles, CA, USA).
Histologic studies
Two segments (0.5 cm) of proximal colon, taken from 2 cm from the cecocolonic junction, and two distal segments, taken at 2 cm from the anorectal junction, were excised for histologic studies. Colonic segments were fixed in Carnoy's solution, cleared in xylene, and embedded in paraffin blocks. Paraffin-embedded specimens were sliced in 4 µm sections with a microtome, and mounted on precoated slides (Dako, Glostrup, Denmark). Following dewaxing, tissue sections were incubated with 0.5% hydrogen peroxide in methanol at 22℃ for 10 min, and washed under running tap water for 15 min. Sections were treated with 0.1% trypsin (Sigma, Pool, UK) mixed in 0.1% calcium chloride (pH 7.8) for 10 min at 37℃. Nonspecific binding was blocked by incubation in normal rabbit serum diluted to 1:5 in Tris-buffered saline (TBS; pH 7.6) for 15 min.
Toluidine blue staining was performed in order to identify mast cell population. The cytoplasm of mast cells contains granules (metachromatic) composed of heparin and histamine. Toluidine blue stains mast cells red-purple (metachromatic staining) and the background blue (orthochromatic staining) (Fig. 1A). Mast cells in the mucosa and submucosa were counted in 6 non-overlapped high-powered fields (HPF, final magnification, ×400) by a pathologist blinded for each experiment.
Fig. 1.
Staining of mast cells, EC cells, and PAR2-positive cells (magnification, ×400). Toluidine blue stain was performed in order to identify mast cells (A), and immunostaining using anti-chromogranin A antibodies for EC cells (B) and anti-PAR2 antibodies for PAR2 protein expression (C) was carried out.
The slides were incubated for 18 hr at 4℃ with anti-chromogranin A antibodies for EC cells and to anti-PAR2 antibodies for PAR2 protein expression. Immunostaining was performed as previously described using the Dako Techmate 500 Plus and Techmate reagents (Dako, Carpinteria, CA, USA). EC cell numbers (Fig. 1B) and the levels of PAR2 protein expression (Fig. 1C) were assessed 6 non-overlapped HPF by a pathologist in blind.
Statistical analysis
All data are expressed as means±S.E.M for each group of rats. The numbers of mast cells, EC cells, PAR2-positive cells and plasma cortisol levels obtained after each treatment were compared using a ANOVA and Student's t-test. P values <0.05 were considered statistically significant. SPSS for Windows version 11 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
RESULTS
Mast cell and EC cell numbers
In proximal colonic segments, mast cell numbers of stressed rats tended to be higher than those of non-stressed rats (12.7±3.5 vs. 4.2±1.1; P=0.06). Mast cell numbers in proximal colonic segments of astressin-pretreated stressed rats were significantly lower than those of saline-pretreated stressed rats (4.0±1.3 vs. 12.7±3.5; P=0.05) (Fig. 2A). In distal colonic segments, mast cell numbers of stressed rats were significantly higher than those of non-stressed rats (18.2±2.7 vs. 10.2±2.1; P=0.04). Mast cell numbers in distal colonic segments of astressin-pretreated stressed rats were significantly lower than those of saline-pretreated stressed rats (7.7±1.9 vs. 18.2±2.7; P=0.01) (Fig. 2B). No significant differences in EC cell numbers among the three experimental groups were observed, both in the proximal colonic segments (2.0±0.3 vs. 1.7±0.2; P=0.32) (Fig. 3A) and in the distal colonic segments (1.5±0.2 vs. 1.3±0.2; P=0.52) (Fig. 3B).
Fig. 2.
Comparison of mast cell numbers in colonic segments. In proximal colonic segments, mast cell numbers of stressed rats tended to be higher than those of non-stressed rats, and mast cell numbers of astressin-pretreated stressed rats were significantly lower than those of saline-pretreated stressed rats (*P=0.05) (A). In distal colonic segments, mast cell numbers of stressed rats were significantly higher than those of non-stressed rats (*P=0.04), and mast cell numbers of astressin-pretreated stressed rats were significantly lower than those of saline-pretreated stressed rats (†P=0.01) (B).
Fig. 3.
Comparison of EC cell numbers in colonic segments. No significant differences in EC cell numbers among the three experimental groups were observed, both in the proximal colonic segments (A) and in the distal colonic segments (B).
PAR2 expression
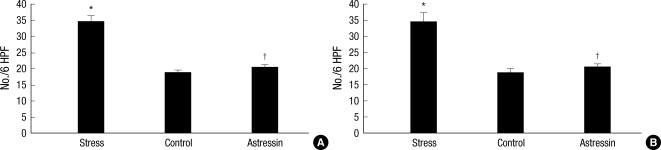
PAR2 protein expression was evaluated by immunohistochemistry using a specific monoclonal antibody. PAR2-positive cell numbers in proximal colonic segments of stressed rats were significantly higher than those of non-stressed rats (34.7±4.7 vs. 18.8±2.0; P=0.02). PAR2-positive cell numbers in proximal colonic segments of the astressin-pretreated stressed group were significantly lower than those of the saline-pretreated stressed group (20.5±2.5 vs. 34.7±4.7; P=0.02) (Fig. 4A). PAR2-positive cell numbers in distal colonic segments of stressed rats were significantly higher than those of non-stressed rats (55.5±7.0 vs. 31.5±2.9; P=0.01). PAR2-positive cell numbers in distal colonic segments of the astressin-pretreated stressed group were significantly lower than those in the saline-pretreated stressed group (33.8±2.5 vs. 55.5±7.0; P=0.02) (Fig. 4B).
Fig. 4.
Comparison of PAR2-positive cell numbers in colonic segments. In proximal colonic segments, PAR2-positive cell numbers of stressed rats were significantly higher than those of non-stressed rats (*P=0.02), and PAR2-positive cell numbers of the astressin-pretreated stressed group were significantly lower than those of the saline-pretreated stressed group (†P=0.02) (A). In distal colonic segments, PAR2-positive cell numbers of stressed rats were significantly higher than those of non-stressed rats (*P=0.01), and PAR2-positive cell numbers of the astressin-pretreated stressed group were significantly lower than those in the saline-pretreated stressed group (†P=0.02) (B).
Fecal pellet output and plasma cortisol levels
There was significantly higher pellet output in stressed rats compared to non-stressed controls (9.0±0.27 vs. 3.5±0.16; P<0.001). Pellet output was significantly reduced by pretreatment of astressin in stressed rats (4.8±0.22 vs. 9.0±0.27; P=0.001). Plasma cortisol levels from stressed rats were significantly higher than those from non-stressed rats (4.1±0.16 vs. 2.3±0.19 µg/dL; P=0.02). Astressin treatment tended to decrease plasma cortisol levels in stressed rats (2.9±0.15 vs. 4.1±0.16 µg/dL; P=0.07).
DISCUSSION
Our results of the present study showed that acute immobilization stress increased mast cell numbers and PAR2 expression in the colon and these stress effects were inhibited by the CRF antagonist astressin. Thus, these results suggest that CRF released by acute stress mediates increase in mast cell numbers and PAR2 expression. These alterations can lead to increased intestinal permeability, inducing inflammation and hypersensitivity of the intestine. These stress-induced alterations may explain low-grade inflammation observed in a subset of IBS patients. Fecal pellet output significantly increased in stressed rats, which was inhibited by the pretreatment of the CRF antagonist astressin. Accordingly, it is conceivable that CRF released by stress plays a role in the pathogenesis of IBS. In keeping with our findings, previous studies have shown that stress can enhance experimental colitis, stimulate colonic motility, and induce rectal hyperalgesia (2, 5). These effects of stress have suggested to be mediated by CRF, the main regulator of the hypothalamic-pituitary-adrenal axis (3, 6). CRF receptors are found in different immune cells including macrophages, lymphocytes and mast cells (19, 20). Thus, these immune cells are likely to be affected by the release of CRF. Similarly, it has been shown that CRF released during immobilization stress plays a role in increasing colonic transit, colonic mucin secretion and the histamine content of colonic mast cell (21).
Immune activation and mucosal inflammation alter gut motility and sensitivity. Increased lymphocytes, together with elevated numbers of mast cells and EC cells, are observed in patients with post-infectious IBS (11, 14). In our previous study, we confirmed that EC cells, mast cells and lamina propria T lymphocytes were definitely increased in patients with post-infectious IBS. In that study, we found that mast cells increased in a subset of IBS patients without history of previous GI infection (12). Accordingly, mast cells are likely to be associated with the pathogenesis of IBS, irrespective of the presence of history of previous GI infection. An increase in mast cell density is reported to be most remarkable in diarrhea-predominant IBS patients (22). Mast cells release potent mediators including tryptase and histamine. These mediators affect the enteric nerves and smooth muscle, leading to abnormal secretomotor function and visceral hypersensitivity (23, 24). However, the mechanism underlying increased mast cells observed in IBS patients without history of previous GI infection remains unclear. Since stress is known to be closely related to IBS, our results of the present study provide evidence that stress-mediated release of CRF may play a role in increasing mast cell number in IBS. In contrast, EC cell numbers are not likely to be altered by stress or CRF.
PAR2 is a G-protein coupled receptor for trypsin and mast cell tryptase. Tryptase released upon mucosal mast cell degranulation can activate PAR2 (26). The results of the present study confirmed that PAR2 expression was significantly increased by stress. This stress effect was inhibited by the CRF antagonist astressin, suggesting the role of CRF. Thus, it is plausible that CRF released during acute stress increases mast cell numbers and degranulates mast cells, which upregulates PAR2 expression in the colon. PAR2 is highly expressed in the intestine, where it is found in colonic myocytes, enterocytes, enteric neurons, terminals of mesenteric afferent nerves, and immune cells (25, 26). Activation of PAR2 modulates diverse gastrointestinal functions, such as motility, ionic exchange, paracellular permeability, sensory functions and inflammation (27). Intracolonic administration of the synthetic selective PAR2 agonist in rats increases paracellualr permeability and produces visceral hyperalgesia (28). PAR2-mediated dysfunction of colonic epithelial barrier and subsequent allodynia or hyperalgesia may play an important role in the pathogenesis of IBS. Actually, elevated colonic luminal serine protease activity is seen in diarrhea-predominant IBS patients (29, 30). In mice, colonic exposure to supernatants from diarrhea-predominant IBS patients results in allodynia and increased colonic paracellular permeability, which is dependent on PAR2 expression (30).
The model of total restraint used in the present study produces physical stress in addition to psychological stress. Restraint models have been used in studies on colonic visceral hypersensitivity and intestinal permeability (15, 16). Our results showed that total restraint in rats for 90 min increased plasma cortisol levels, suggesting activation of the hypothalamic-pituitary-adrenal axis. We speculate that a 90 min restraint session permits the release of CRF, which is able to increase mast cells and PAR2 expression in the colon. This speculation is of importance in understanding the role of stress in triggering IBS symptoms. Indeed, psychological factors have been suggested to be involved in the pathogenesis of IBS.
In conclusion, acute stress in rats increases mast cell numbers and mucosal PAR2 expression in the colon. These stress effects seem to be mediated by release of CRF. However, EC cell numbers are not likely to be affected by stress or CRF. Increased mast cells observed in a subset of IBS patients without history of previous GI infection may be attributed to stress-induced alterations mediated by CRF.
Footnotes
This study was supported by grants of Korean Society of Neurogastroenterology and Motility and the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A010383).
References
- 1.Fukudo S. Role of corticotrophin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42(Suppl 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 2.Gue M, Del Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 3.Monnikes H, Schmidt BG, Tache Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–723. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 4.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 5.Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–621. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]
- 6.Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotrophin-releasing factor. Gastroenterology. 1988;95:1510–1517. doi: 10.1016/s0016-5085(88)80070-2. [DOI] [PubMed] [Google Scholar]
- 7.Sant GR, Theoharides TC. The role of the mast cell in interstitial cystitis. Urol Clin North Am. 1994;21:41–53. [PubMed] [Google Scholar]
- 8.Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotrophin releasing factor and mast cells. Am J Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- 9.Annese V, Bassotti G, Napolitano G, Usai P, Andriulli A, Vantrappen G. Gastrointestinal motility disorders in patients with inactive Crohn's disease. Scand J Gastroenterol. 1997;32:1107–1117. doi: 10.3109/00365529709002989. [DOI] [PubMed] [Google Scholar]
- 10.Vermillion DL, Huizinga JD, Riddell RH, Collins SM. Altered small intestinal smooth muscle function in Crohn's disease. Gastroenterology. 1993;104:1692–1699. doi: 10.1016/0016-5085(93)90647-u. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 13.Park CH, Joo YE, Choi SK, Rew JS, Kim SJ, Lee MC. Activated mast cells infiltrate in close proximity to enteric nerves in diarrhea-predominant irritable bowel syndrome. J Korean Med Sci. 2003;18:204–210. doi: 10.3346/jkms.2003.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578–1583. doi: 10.1111/j.1572-0241.2003.07542.x. [DOI] [PubMed] [Google Scholar]
- 15.Gué M, Del Rio-Lacheze C, Eutamene H, Théodorou V, Fioramonti J, Buéno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–279. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 16.Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–621. doi: 10.1016/0016-5085(88)90231-4. [DOI] [PubMed] [Google Scholar]
- 17.Strausbaugh HJ, Dallman MF, Levine JD. Repeated, but not acute, stress suppresses inflammatory plasma extravasation. Proc Natl Acad Sci USA. 1999;96:14629–14634. doi: 10.1073/pnas.96.25.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barone FC, Deegan JF, Price WJ, Fowler PJ, Fondacaro JD, Ormsbee HS., 3rd Cold-restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol. 1990;258:G329–G337. doi: 10.1152/ajpgi.1990.258.3.G329. [DOI] [PubMed] [Google Scholar]
- 19.Singh VK. Stimulatory effect of corticotrophin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol. 1989;23:257–262. doi: 10.1016/0165-5728(89)90058-1. [DOI] [PubMed] [Google Scholar]
- 20.Webster EL, Tracey DE, Jutila MA, Wolfe SA, Jr, De Souza EB. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990;127:440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- 21.Eutamene H, Theodorou V, Fioramonti J, Bueno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotrophin-releasing factor release in rats. J Physiol. 2003;553:959–966. doi: 10.1113/jphysiol.2003.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Rhee PL, Kim HS, Lee JH, Kim YH, Kim JJ, Rhee JC. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71–78. doi: 10.1111/j.1440-1746.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 23.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 24.Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. doi: 10.1016/s0016-5085(97)70056-8. [DOI] [PubMed] [Google Scholar]
- 25.Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995;232:84–89. doi: 10.1111/j.1432-1033.1995.tb20784.x. [DOI] [PubMed] [Google Scholar]
- 26.Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 27.Vergnolle N. Clinical relevance of proteinase activated receptors (PARS) in the gut. Gut. 2005;54:867–874. doi: 10.1136/gut.2004.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology. 2002;122:1035–1047. doi: 10.1053/gast.2002.32387. [DOI] [PubMed] [Google Scholar]
- 29.Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM WEL Investigators. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 30.Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztoczy A, Izbeki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic luminal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]