Abstract
Resistance to thyroid hormone (RTH) is an autosomal dominant hereditary disorder that is difficult to diagnose because of its rarity and variable clinical features. The magnitude of RTH is caused by mutations in the thyroid hormone receptor beta (TRβ) gene. We recently treated a 38-yr-old woman with RTH who had incidental papillary thyroid carcinoma. She presented with goiter and displayed elevated thyroid hormone levels with an unsuppressed TSH. She was determined to harbor a missense mutation of M310T in exon 9 of the TRβ gene, and diagnosed with generalized RTH. This mutation has not yet been reported in Korea. RTH is very rare and easily overlooked, but should be considered in patients who present with goiter and elevated thyroid hormone levels with an unsuppressed TSH. The association between thyroid cancer and RTH needs further study.
Keywords: Thyroid Hormone Resistance Syndrom, Thyroid Hormone Receptor, Thyroid Hormone Receptors beta, Mutation, Thyroid Neoplasms
INTRODUCTION
Resistance to thyroid hormone (RTH) is a rare an autosomal dominant hereditary disorder (1). In the majority of cases, RTH is caused by mutations in the thyroid hormone receptor beta (TRβ) gene. The clinical presentation is variable, but common features include goiter, an absence of the classic thyrotoxic symptoms and metabolic consequences of elevated thyroid hormone levels, and a normal or exaggerated thyroid stimulating hormone (TSH) response to thyrotropin-releasing hormone (TRH). Until now over 1,000 cases have been identified around the world, and nine cases have been reported in Korea (2-9).
We recently encountered a case of RTH with incidental micropapillary thyroid carcinoma. The association between RTH and thyroid cancer has not yet been clarified, but there is evidence to suggest that RTH may play a contributory role.
Here, we present a case of RTH with thyroid cancer along with the mutation analysis for the TRβ gene.
CASE REPORT
A 38-yr-old Korean woman visited a private clinic for the evaluation of anterior neck swelling. The initial laboratory examination revealed an elevated levels of serum thyroid hormone without accompanying TSH suppression (free T4 2.67 ng/dL [range, 0.64-1.72] and TSH 1.82 µIU/mL [range, 0.4-4.5]). She had no signs or symptoms of typical thyrotoxicosis except diffuse goiter and mild palpitations. However, due to findings of elevated serum thyroid hormone levels and goiter, she was diagnosed with Graves' disease, and prophylthiouracil (PTU) was prescribed. After three months of PTU (100 mg three times daily), she complained of generalized weakness, a weight gain of about 3 kg, and generalized edema. Consequently, she was referred to our hospital.
When first seen at our hospital on December 9, 2008, her height and weight were 162 cm and 49 kg, respectively, with a blood pressure of 97/68 mmHg and a pulse rate of 96 beats/min. Her thyroid gland was symmetrically enlarged, and exophthalmos and myxedematous skin lesions were absent. Past medical history was significant for goiter first detected at age 13. At that time, the physician recommended regular checks for thyroid function because of elevated levels of thyroid hormones, but she was lost to follow-up. Her family history included one sister who had a diffuse goiter and a thyroid nodule.
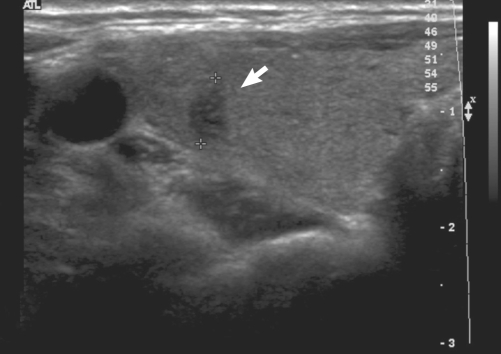
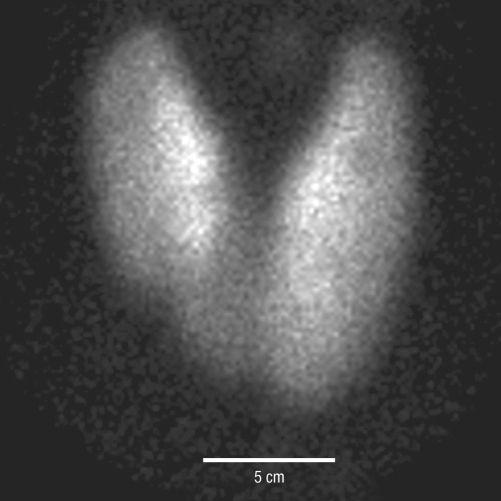
The laboratory examination revealed an elevated level of TSH despite an elevated levels of thyroid hormone [total T3 181 ng/dL (range, 76-190), free T4 2.50 ng/dL and TSH 10.23 µIU/mL]. Thyroid autoantibodies against thyroperoxidase, thyroglobulin, and TSH-receptor were all negative. Thyroid ultrasonography (USG) revealed several micro-nodules in both lobes. USG-guided fine-needle aspiration was performed for the nodule which showed suspicious features of malignancy in the right thyroid lobe (Fig. 1), and the cytologic diagnosis was papillary thyroid carcinoma. A 99m technetium thyroid scan showed diffuse enlargement of the thyroid gland with increased uptake (10.1%) (Fig. 2). PTU was discontinued due to TSH elevation and the occurrence of hypothyroid symptoms. After PTU was stopped for one month, hypothyroid symptoms disappeared and thyroid function tests (TFTs) revealed elevated thyroid hormone levels [free T4 2.20 ng/dL and total T3 141 ng/dL] and a nearly normal level of TSH (5.60 µIU/mL).
Fig. 1.
The ultrasonography of thyroid showed 0.6 cm hypoechoic nodule in the right lobe with taller-than-wide appearance, which was suspicious for malignancy.
Fig. 2.
The 99mTechnetium thyroid scan showed diffuse enlargement of both lobes of the thyroid with homogenously increased uptake (10.1%).
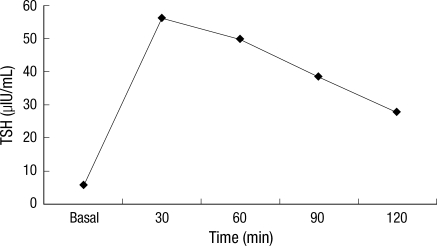
The differential diagnosis at this point was between RTH and a TSH-secreting pituitary adenoma based on the TFTs findings. TSH-secreting pituitary adenoma was ruled out because the patient had no clinical features of thyrotoxicosis, a negative MRI of the pituitary gland, a normal level of the α-subunit of TSH (0.36 mIU/mL, range, 0-0.9), and an exaggerated response of TSH to a thyrotropin-releasing hormone (TRH) stimulation test (Fig. 3).
Fig. 3.
Exaggerated response of TSH to TRH stimulation was observed in the patient with resistance to thyroid hormone.
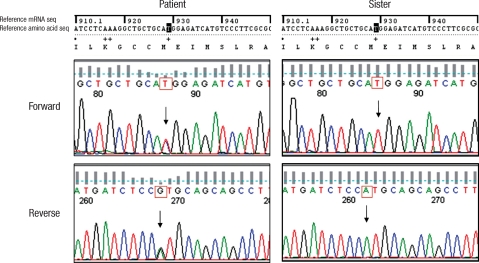
Accordingly, genomic DNA was isolated from the peripheral blood leukocytes of the patient with a G-DEX™ Ilb Genomic DNA Extraction Kit (Intron Biotechnology, Seongnam, Korea). PCR amplification was conducted on seven coding exons (from exon 4 to 10) of the TRβ gene. The sequences were analyzed using the computational software ABI 3730 XL (Applied Biosystems, Foster City, USA). A point mutation was detected in exon 9 of the TRβ gene that caused the substitution of threonine (ACG) for methionine (ATG) at codon 310 (M310T). This mutation has not yet been reported in Korea. The patient's sister had normal TFTs and no mutation by DNA sequencing analysis (Fig. 4).
Fig. 4.
The DNA sequence analysis of the TRβ gene showed a substitution of threonine (ACG) for methionine (ATG) at codon 310 (M310T) of exon 9.
Ultimately, the patient was diagnosed with RTH and papillary thyroid carcinoma. She underwent a total thyroidectomy and the pathologic diagnosis was two micropapillary thyroid carcinomas in both lobes (0.4 cm in the right and 0.2 cm in the left lobe). After surgery, she received levothyroxine (L-T4) at 150 µg/day). After three months of L-T4 therapy, her TSH level was still elevated (TSH 45.1 µIU/mL) and she complained about symptoms of hypothyroidism; therefore, we increased the dose of L-T4 to 200 µg/day. After two months, her TSH level decreased to 15.5 µIU/mL, and most of her hypothyroid symptoms disappeared. Ultimately the dose of L-T4 was increased to 250 µg/day titrated to achived a TSH level comparable to the preoperative value.
DISCUSSION
Inappropriate secretion of TSH and the co-existence of elevated thyroid hormone has been noted in several circumstances, including intermittent administration with L-T4, TSH-secreting pituitary adenoma, and RTH (10). RTH is distinguished from TSH-secreting pituitary adenoma by an exaggerated response to TRH, normal levels of the TSH α subunit, and a normal pituitary gland on sella-MRI. RTH is characterized by reduced clinical and biochemical manifestations of thyroid hormone action relative to the elevated circulating thyroid hormone levels. The linkage between RTH and the TRβ gene was detected in 1988 (11). About 90% of RTH patients carry mutations in the T3-binding domain between exon 4 and exon 10 of the TRβ gene. The majority of previously described mutations were missense mutations that induced single amino acid substitutions.
Most patients of RTH are clinically euthyroid, some individuals may appear to be hypothyroid or hyperthyroid. Furthermore, the same subject can manifest signs and symptoms of hypothyroidism in one tissue, while the findings may be suggestive of thyrotoxicosis in other tissues (1). On the basis of clinical features, three different forms of RTH have been described: generalized RTH (GRTH), pituitary RTH (PRTH), and peripheral tissue RTH (PTRTH). GRTH patients, the most common form, appear eumetabolic despite elevated level of thyroid hormones maintained by the hypersecretion of TSH in response to hypothalamic TRH. PRTH patients generally display thyrotoxic. Administration of an anti-thyroid drugs may improve the symptoms. PTRTH patients manifest symptoms and signs of hypothyroidism, despite of normal serum thyroid hormone and TSH levels (12).
Treatment of RTH should be aimed at maintaining a normal metabolic status. In our case, administration of anti-thyroid drugs under the misdiagnosis of Graves' disease resulted in an increase in serum TSH and the appearance of symptoms of hypothyroidism, while thyroid hormone levels remained elevated. As expected, large amounts of L-T4 were needed in our patient after total thyroidectomy. Titration should be achieved by assessing tissue responses. Restoration of the preoperative levels of TSH is the recommended target in GRTH.
The RTH in our patient was accompanied by two small papillary thyroid cancers. The precise contribution of RTH to thyroid tumorigenesis is not fully understood, but there is evidence to suggest that they may play contributory roles. About 95% of RTH patients have diffuse goiter, and continuous stimulation of the thyroid by excess TSH may be related to the formation of thyroid nodules and the growth of thyroid cancer (13). Previous pathological examinations of the thyroid in RTH cases have revealed hyperplasia of the follicular epithelium, sometimes accompanied by papillary proliferation probably due to continuous stimulation by TSH (12). Not only the effect of thyroid, but the cases of co-existence of pituitary tumor were reported (14). TRβ mutants may also have oncogenic actions. Abnormal expression and somatic mutation at various sites of the TRβ gene have been described in human cancers of the liver, kidney, breast and colon (15-19). Puzianowska-Kuznicka et al. (16) conducted the sequencing of TRβ1 and TRα1 cDNAs. TRβ1 and TRα1 mutations were found in 93.8% and 62.5% of papillary cancer cases, respectively. But no mutations were found in healthy thyroid controls, and only 11.1% and 22.2% of thyroid adenomas had such TRβ1 or TRα1 mutations. Suzuki et al. (20) generated a mouse model (TRβPV/PV mice) of thyroid cancer that harbored a C-terminal 14 amino acid frame-shift mutation in the TRβ gene.
Despite accumulating evidence for a possible role for TR mutants in thyroid tumorigenesis, an elevated rate of thyroid cancers in RTH patients has not yet been observed. It may be that most cases of RTH in humans have heterozygous mutations of TRβ, unlike the TRβPV/PV homozygous mouse model. Other genetic alterations such as BRAF mutation, which is commonly detected the Korean patients with PTC, may also be required to initiate thyroid cancer. Unfortunately, analyses of BRAFV600E mutation or activities of PI3K could not be conducted in our case.
In conclusion, RTH is very rare and easily overlooked, but must be considered in patients who present with goiter and elevated thyroid hormone levels with unsuppressed TSH. Further studies to address the association between thyroid cancer and RTH will shed insight on the molecular and cellular mechanisms of thyroid tumorigenesis.
References
- 1.Refetoff S. The Thyroid. 9th ed. Philadelphila: Lippincott Williams & Wilkins; 2005. pp. 1109–1129. [Google Scholar]
- 2.Yun YS, Hong SK, Ahn CW, Nam JH, Park SW, Cha BS, Song YD, Lee EJ, Lim SK, Kim KR, Lee HC, Huh KB. Mutations in thyroid hormone receptor-beta with patients with generalized resistance and pituitary resistance to thyroid hormone. J Korean Soc Endocrinol. 2000;15:113–120. [Google Scholar]
- 3.Hwang JK, Kim KW, Kim TY, Kim SW, Park YJ, Park DJ, Kim SY, Lee HK, Cho BY. A case of syndrome of resistance to thyroid hormone associated with mutation (M313T) in thyroid hormone receptor beta gene. J Korean Soc Endocrinol. 2003;18:206–213. [Google Scholar]
- 4.Seo JY, Yoon IS, Yoo JH, Ahn SY, Jeong HL, Shin CH, Yang SW. A case of thyroid hormone resistance (RTH) J Korean Soc Pediatr Endocrinol. 2005;10:100–104. [Google Scholar]
- 5.Park TJ, Kang JK, Seo KW, Kim HJ, Chung YS, Lee KW, Jeong SY, Kim HJ, Kim DJ. A case of resistance syndrome to thyroid hormone associated with mutation (G345D) in the thyroid hormone receptor beta gene. J Korean Soc Endocrinol. 2007;22:277–281. [Google Scholar]
- 6.Shin JY, Ki CS, Kim JK. Identification of a de novo mutation (H435Y) in the THRB gene in a Korean patient with resistance to thyroid hormone. Korean J Pediatr. 2007;50:576–579. [Google Scholar]
- 7.Kim JH, Park TS, Baek HS, Kim GH, Yoo HW, Park JH. A newly identified insertion mutation in the thyroid hormone receptor-beta gene in a Korean family with generalized thyroid hormone resistance. J Korean Med Sci. 2007;22:560–563. doi: 10.3346/jkms.2007.22.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DY, Kim YM, Choi HH, Lee JY, Han JP, Lee SJ, Choi MG. A case of pituitary resistance to thyroid hormone with nonfunctioning pituitary microadenoma. Korean J Med. 2008;74:94–99. [Google Scholar]
- 9.Kim JY, Choi ES, Lee JC, Lee KU, Kim YJ, Kim SJ, Lee YW. Resistance to thyroid hormone with missense mutation (V349M) in the thyroid hormone receptor beta gene. Korean J Intern Med. 2008;23:45–48. doi: 10.3904/kjim.2008.23.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho BY. Clinical thyroidology. 2nd ed. Seoul: Korea Medical Book; 2005. [Google Scholar]
- 11.Usala SJ, Bale AE, Gesundheit N, Weinberger C, Lash RW, Wondisford FE, McBride OW, Weintraub BD. Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erbA beta gene. Mol Endocrinol. 1988;2:1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- 12.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 13.Taniyama M, Ishikawa N, Momotani N, Ito K, Ban Y. Toxic multinodular goitre in a patient with generalized resistance to thyroid hormone who harbours the R429Q mutation in the thyroid hormone receptor beta gene. Clin Endocrinol (Oxf) 2001;54:121–124. doi: 10.1046/j.1365-2265.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 14.Berker D, Aydin Y, Tutuncu YA, Isik S, Delibasi T, Berker M, Guler S, Kamel N. Somatotropin adenoma and resistance to thyroid hormone. J Endocrinol Invest. 2009;32:284–286. doi: 10.1007/BF03346468. [DOI] [PubMed] [Google Scholar]
- 15.Lin KH, Shieh HY, Chen SL, Hsu HC. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol Carcinog. 1999;26:53–61. doi: 10.1002/(sici)1098-2744(199909)26:1<53::aid-mc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Puzianowska-Kuznicka M, Krystyniak A, Madej A, Cheng SY, Nauman J. Functionally impaired TR mutants are present in thyroid papillary cancer. J Clin Endocrinol Metab. 2002;87:1120–1128. doi: 10.1210/jcem.87.3.8296. [DOI] [PubMed] [Google Scholar]
- 17.Puzianowska-Kuznicka M, Nauman A, Madej A, Tanski Z, Cheng S, Nauman J. Expression of thyroid hormone receptors is disturbed in human renal clear cell carcinoma. Cancer Lett. 2000;155:145–152. doi: 10.1016/s0304-3835(00)00416-x. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Meng ZH, Chandrasekaran R, Kuo WL, Collins CC, Gray JW, Dairkee SH. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002;62:1939–1943. [PubMed] [Google Scholar]
- 19.Horkko TT, Tuppurainen K, George SM, Jernvall P, Karttunen TJ, Makinen MJ. Thyroid hormone receptor beta1 in normal colon and colorectal cancer-association with differentiation, polypoid growth type and K-ras mutations. Int J Cancer. 2006;118:1653–1659. doi: 10.1002/ijc.21556. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12:963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]