Abstract
Proper visual function of the vertebrate retina requires the maintenance of the integrity of the retinal outer nuclear layer (ONL), which is often affected in many blinding human retinal diseases.While the structural integrity of the ONL has long been considered to be maintained primarily through the outer limiting membrane (OLM), we have little knowledge on the development and maintenance of the OLM itself. Here, by analyzing the adhering properties of photoreceptors in zebrafish N-cad and nok mutants, we demonstrated for the first time that the nok gene is essential for the establishment and/or maintenance of the OLM. In addition, our results imply the possibility that Nok, Crumbs, and their associated proteins may constitute a type of photoreceptor-photoreceptor junctional complex that has not been described before. Thus, our study provides novel insights into the mechanisms by which the integrity of the ONL is maintained in the vertebrate retina.
Keywords: retinal patterning, nagie oko (nok), outer limiting membrane, outer nuclear layer, zebrafish, photoreceptor
Introduction
In the mature vertebrate retina, Muller glial cells and six major types of neurons are segregated into three cellular layers via mechanisms that are largely not understood (Rodieck, 1973; Dowling, 1987). It has been proposed that specific cell-cell adhesions define and stabilize the cellular pattern formation in tissues during development. For example, Roger W. Sperry proposed a “Chemoaffinity Theory” that suggested that cell-cell adhesions are mediated through a large set of molecules via specific complementary adhesion (Sperry, 1963). On the other hand, Gerald Edelman proposed a “Modulation theory” that suggested that a smaller number of cell-cell adhesional molecule genes are responsible for the complex pattern of cell-cell adhesion by modulating the activities and specificities of their products (Edelman, 1983). While current progress suggests that the combination of the two theories reflects reality better, much more work at molecular levels is needed to elucidate the roles of cell-cell and cell-ECM (extracellular matrix) adhesion in tissue pattern formation.
In the past several years, a number of cell surface adhesion molecules have been found to be important for the structural development of the retina. These adhesion molecules can be generally characterized into three groups: cell-matrix adhesion molecules, pan-retina cell-cell adhesion molecules, and specific cell-cell adhesion molecules. Members of the cell-matrix adhesion molecules group function primarily in adhering retinal cells to the ECM, e.g. ECM components WIF-1 (Hunter et al., 2004) and laminins (Colognato and Yurchenco, 2000; Libby et al., 1999), and transmembrane proteins Collagen XVII (Claudepierre et al., 2005) and α6 integrin (Georges-labouesse et al., 1998). Members of the pan-retina cell-cell adhesion molecules group are expressed in many cell types in the retina to mediate non-specific retinal cell adhesion. Examples of this group include the neural cell adhesion molecule (N- CAM) (Daniloff et al., 1986), L1 (Chung et al., 2004), P84 and its interacting partner Integrin Associated Protein (IAP) (Mi et al., 2000), N-cadherin (Malicki et al., 2003), Neuroligin (von Kriegstein and Schmitz, 2003), N-Syndecan (Inatani et al., 2002), and LAMP, an isoform of IgLON (Lodge et al., 2000). Members of the specific cell-cell adhesional molecules group display regional expression patterns in the retina and are likely involved in regional adhesion functions that are important for specific retinal layers. For example, Neuron-glial cell adhesion molecule (Ng-CAM) is expressed mainly in the optic fiber layer of the retina, and is presumably important for the adhesion of ganglion cell axons with Muller glial cells (Daniloff et al., 1986); Sidekick1 and Sidekick2 are expressed in different types of retinal cells and responsible for specific synaptic formation in the retina (Yamagata et al., 2002). Despite this progress, a clear picture of the mechanisms by which adhesion molecules function in retinal pattern formation is still lacking.
In the vertebrate retina, photoreceptors have two basic classes: rods and cones, and the latter can be further divided into several subtypes, e.g. four subtypes in zebrafish (Branchek and Bremiller 1984). Photoreceptors are packed into a single layer of cells (the outer nuclear layer, ONL) with specific geometric arrangements (Raymond, 1995). How the ONL maintains its integrity has been attracting increasing attention lately because a number of blinding human retinal diseases, such as Retinitis Pigmentosa and Leber Congenital Amaurosis, directly affect the stability of this retinal layer (Rivolta et al., 2002; Perrault et al., 1999). The packing of photoreceptors into a single cellular layer requires precise coordination of photoreceptor adhesion. Among the possible subcellular structures that are responsible for the assembly of the ONL, the outer limiting membrane (OLM) has attracted much attention because of its easy identification under EM and light microscopes (Dowling 1970; Raviola 1977).
The OLM is composed of specialized cell-cell adhesion complexes between photoreceptors and Muller cells as well as among the processes of Muller cells. The OLM is a distinct type of cell-cell junctional complex due to its unique molecular composition: The OLM contains N-cadherin and α- and β-catenin, proteins typically found in adherens junctions (Matsunaga et al., 1988; Paffenholz et al., 1999. The OLM also contains ZO-1 and symplekin, which are typically found in tight junctions (Tserentsoodol et al., 1998; Saitou et al., 1997; Paffenholz et al., 1999). However, the OLM does not display immunoactivity for occludin and claudins, which are the transmembrane proteins of tight junctions (Williams and Rizzolo 1997; Paffenholz et al., 1999). The OLM also contains desmosomal protein plakophilin 2 (Paffenholz et al., 1999). Interestingly, the OLM lacks plakoglobin, a protein found in all other adhering junctions (Paffenholz et al. 1999). Undoubtedly, the OLM contributes to the stabilization of the ONL. However, it is unclear whether the OLM is the primary structure responsible for the integrity of the ONL. Furthermore, it is unclear how the OLM is established during development.
In this study we present findings that demonstrate that the zebrafish nagie oko (nok) gene plays an important role in the integrity of the ONL by regulating the development of the OLM and by participating in a potential novel type of adhesion complex between photoreceptors in the mature retina. Such function of the nok gene has not been discovered before. The nok gene, zebrafish homolog of fly stardust and mouse pals1 (Hong et al., 2001; Kamberov et al., 2000), was initially identified for its function in retinal epithelial polarity and cellular pattern formation during early retinal development in zebrafish (Wei and Malicki, 2002). Our observation of Nok’s continued expression in the junctional region between the inner segments of photoreceptors in fully developed retina promoted us to perform experiments to determine the function of Nok in photoreceptor adhesion. The results of our study have provided novel insights into the maintenance of the integrity of the ONL.
Materials and Methods
Zebrafish strains and cares
Zebrafish embryos were raised at 28.5 °C in E3 egg water until desired developmental stages. AB wildtype, nokm227, and N-cadm117 mutant embryos were used for histological analysis. N-cadm117 was previously known as the glass onion (glo) mutation. ROP::GFP fish, a transgenic fish line that expresses GFP under the control of the rod opsin promoter (ROP) (Hamaoka et al., 2002), were crossed with nokm227 to obtain double carriers. The siblings of the double carriers were crossed together to generate ROP::GFP positive embryos that are homozygous for the nok mutant gene. The ROP::GFP transgenic fish line was incrossed to obtain wild type embryos that express GFP in rods. The resulting embryos were observed under a confocal microscope live to determine the mobility of GFP positive cells in either wildtype or mutant retinas.
Immunohistochemistry
Embryos were fixed with 4% paraformaldehyde in 1 x PBS at room temperature for 2 hours or over night at 4 °C. For each experiment, ten fixed embryos (twenty retinas) of the desired mutant and wildtype background were infiltrated with 40% sucrose in 1x PBS and embedded in cryosection medium (Tissue-Tek, Sakura Finetek USA) and cryosectioned at 30 μm thickness. The sections were washed with PBS to remove embedding medium and blocked with blocking buffer (2% BSA, 0.5% Triton X-100, in 1 x PBS) for 40 min at room temperature. The sections were incubated with desired combination of primary antibodies in blocking buffer at 4 °C for over night under the following conditions: mouse anti-ZO-1, Zymed, 1:400; rabbit anti-crb1, gift from Dr. Jan Wijnholds, 1:150; rabbit anti-crb2, gift from Dr. Pen Rashbass, 1:2,000; zpr1 for green/red double cones, ZFIN, 1:150; anti-carbonic anhydrase for Muller glia, gift from Dr. Paul Linser, 1:100; and rabbit anti-Nok, 1:400). After four washes (1 x PBS containing 0.5% Triton X-100) at room temperature for 10 min each, sections, except for those stained with Crb1, were incubated with Cy3-conjugated goat anti-mouse IgG, Cy5-conjugated goat anti-rabbit IgG secondary antibodies, and Alexa Flour 488-conjugated phalloidin. For Crb1 staining, sections after primary antibody incubation were washed and incubated with biotin-conjugated anti-rabbit antibody, followed by incubation with Alexa Flour 488-conjugated phalloidin, cy3-conjugated avidin, and cy5-conjugated anti-mouse antibodies. Sections were then washed and sealed in mounting medium (Vector) under coverslips. Immunostained sections were observed and photographed using a BioRadMRC1024 laser scanning confocal microscope.
Live imaging of photoreceptor movement
50 hpf wildtype and nok mutant ROP::GFP transgenic embryos were mounted in 1% low melting agarose on a FluoroDish (World Precision Instrument). The embedded embryos were then immersed in E3 egg water to keep them alive. The positions of GFP expressing rods were recorded under a confocal microscope hourly. To register the orientation of confocal images obtained at different time points, TetraSpeck fluorescent beads (Molecular Probes) were included in the agarose bedding. Because the size of the retina increases slightly over a period of one hour, cells that do not show apparent movement relative to the reference beads were chosen arbitrarily as nonmoving cells during a particular given period of time. Confocal images obtained at the beginning and the end of one-hour periods were pseudocolored in green and red, respectively. The two sets of images were then merged in Photoshop with the identified non-moving cells superimposed. The distances of cell movements were measured with the Metomorph software. ROP::GFP cells were cataloged into seven groups according to the distances they traveled (Figure 6).
Figure 6. Loss of the Nok function leads to increased mobility of photoreceptors.
(A) The movement of wildtype ROP (rod opsin promoter)::GFP cells in wildtype retinas was observed live under a confocal microscope between 50 hpf and 51 hpf. The images of GFP-positive cells at the start time are shown in green and those taken one hour later in red. The images of the two time points are merged, which reveals that a majority of wildtype ROP::GFP cells are spatially stationary in the retina, even though some photoreceptors show tilting at certain angles (arrows). Apical side of the retina is oriented on top.
(B) The majority of ROP::GFP cells in nok mutant retinas between 50 hpf and 51 hpf move significantly during a period of one hour.
(C) The mobility of the GFP-positive cells in wild type, mutant, or mosaic retinas are surveyed and cells are cataloged into seven groups based on their mobility. The survey shows that over 70% of mutant ROP::GFP cells move significantly in the mutant retina, in the range of 2–6 um/hr; whereas over 70% of wildtype ROP::GFP cells fall into the category of 0–1 um/hr. Furthermore, wildtype ROP::GFP cells in nok mutant retinas show increased mobility compared to those in wildtype retinas but decreased mobility compared to those in the pure mutant retina. The number of cells surveyed is indicated in parentheses.
Scale bars indicate 10 μm. Numbers of cells examined are indicated in parentheses.
Blastomer transplantation experiment
Blastomeres from wildtype ROP::GFP transgenic embryos were transplanted to nokm227 mutation embryos at 3 hpf as described previously (Wei and Malicki 2002). The movements of GFP-positive cells were then determined using the method as described above.
Isolation of rods via flow cytometry and Western Blot analysis
We took advantage of the GFP expression in ROP::GFP fish to isolate rods. Fish were first anesthetized with 1.6 mg/ml Tricaine and their eyes were enucleated with forceps. To dissociate retinal cells, the enucleated eyes were torn into small pieces and incubated with TrypLE (Invitrogen, according to manufacture’s suggested concentration) at room temperature for one hour. After the incubation, large tissue debris was removed with a Cell-Strainer (BD Biosciences). GFP-positive rods were isolated by passing the cell suspensions through a BD FACSAria Cell Sorter twice. The isolated rods (with purity over 93%) were extracted with a lysis buffer (1 x PBS, 1% Triton X-100, and protease inhibitor cocktail (Roche)). The rod lysates were analyzed for the expression of Nok by the Western blot assay. Protein extracts from whole eyes were used as a positive control.
Results
Nok localizes to the cell membrane region of the inner segments of photoreceptors
We have previously observed the existence of Nok signal in the ONL immediately after the initial retinal neurogenesis (Wei and Malicki, 2002). However, it is unclear whether the observed Nok signal in the ONL is transient, because it may be derived from residual Nok expressed before and during initial neurogenesis. To determine if Nok continues to play an important role in retinal function after the layered retinal patterning is established, we analyzed Nok’s expression at later developmental stages. At 6 dpf (day postfertilization), Nok localizes to narrow cell-cell junctional regions where photoreceptors juxtapose tightly with each other (Fig. 1A–D). Nok’s staining is apical to the OLM and basal to the ellipsoids (Fig. 1A–D), indicating that Nok localized to the inner segments. When viewed tangentially, it becomes more evident that Nok is membrane associated, displaying a strikingly similar pattern to that of the OLM (Fig. 1E and F). In the adult retina at 12 mpf (month postfertilization), Nok maintains its localization in the inner segments (Fig. 1G–J); furthermore, Nok staining extends distally at the junctional interfaces between the members of the green/red double cones (Fig 1G–J, arrowheads). This extended Nok distribution is consistent with a previous report that the double cones in zebrafish bear the most elongated inner segments (Raymond et al., 1993). Thus, Nok is highly enriched in the cell-cell junctional regions in the inner segments throughout life.
Figure 1. Nok localizes to the cell membrane regions in the inner segments of photoreceptors.
(A–D) Nok staining (C, arrowheads, red) localizes to cell-cell junctional regions in the inner segments at 6 dpf. Green/red double cones (G/R) are visualized by zpr1 antibody (blue). The OLM (A, arrowheads) is positioned basal to the Nok staining. The regions where Nok localizes are also enriched with actin (arrows, A). Asterisks indicate the localization of ellipsoids, which are the most apical regions in the inner segments and are enriched with mitochondria but negative for zpr1 staining. A merged image of A–C is shown in D. G/R is the abbreviation for green/red double cones.
(E and F) Tangential imaging of the inner segments reveals that Nok (red) is associated with the cell membrane of photoreceptors. Nok’s expression pattern resembles that of the OLM (green), which is immediately basal to Nok.
(G–J) Nok localizes in the inner segments of the adult retina at 12 mpf. Counter staining for G/R reveals that Nok localizes to the junctional interface between photoreceptors in the inner segments. The arrowheads indicate that the Nok staining is in the junctional regions between the pairing members of G/R, which extend into the distal region of the ONL. Asterisks indicate the localization of ellipsoids. Merged image of G–I is shown in J.
Sections, except for E and F, are oriented with apical side of the retina on top. Scale bars indicate 10 μm.
As cell-cell junctional complexes, the OLM is important for the integrity of the ONL. The resemblance between Nok’s cell-cell junctional localization and that of the OLM raises a tempting possibility that Nok may also participate in maintaining the integrity of the ONL in the mature retina by mediating photoreceptor-photoreceptor (P-P) adhesion in the ONL.
Establishment of photoreceptor rosettes without the participation of Muller cells in N-cad
It is, however, technically challenging to determine if and to what extent Nok participates in the adhesion of photoreceptors in the ONL. This is due to the potential functional redundancy between the adhesion mediated by the OLM and any possible adhesion mediated by Nok.
To solve this problem, we decided to analyze N-cad mutants in which the N-cadherin gene is mutated (Masai et al., 2003; Erdmann et al., 2003; and Malicki et al., 2003). N-cadherin (N-cad), a transmembrane homophilic adhesion molecule, is expressed in the OLM and is likely responsible for the cell-cell adhesion in the OLM (Matsunaga et al., 1988). We reason that the loss of N-cad function will likely impair the potential adhesion function of the OLM and therefore allow us to analyze Nok’s adhesion function. Because the OLM contains the cell-cell junctional complexes between Muller cells and photoreceptors as well as among the processes of Muller cells (Rodieck, 1973; Dowling, 1987), we set out to study the spatial relationship between the Muller cells and the photoreceptors in N-cad mutant retinas to determine if the adhesion between Muller cells and photoreceptors is impaired by the loss of N-cad function.
In wildtype zebrafish retinas, some of the Muller cell processes extend into the OLM as expected (Fig. 2A–D), confirming the EM observations that the Muller cell processes participate in the formation of the OLM (Rodieck, 1973; Dowling, 1987). In N-cad mutant retinas, while the loss of N-cad function disrupts the formation of the ONL, photoreceptors still adhere to each other and form spherical structures called rosettes, which contain both cones and rods (Pujic and Malicki, 2001). In the rosettes, the apical ends and the residual inner segments of photoreceptors face towards the interior of the rosettes (Erdmann et al., 2003), where actin is enriched (Fig 2. H, arrow). The symmetrical organization of photoreceptors in the rosettes argues against the possibility that the formation of rosettes is a result of random aggregation of photoreceptors. Rather, it indicates the existence of an adhesion force among photoreceptors in the interior regions of the rosettes, analogous to holding a dozen balloons at the base of strings Immunohistochemistry demonstrates that no processes of Muller cells are detectable in the apical junctional regions between photoreceptors in rosettes (Fig. 2E–H, arrow), suggesting that N-cad is directly or indirectly required for the formation of cell-cell junction between Muller and photoreceptor cells in the OLM. More importantly, the formation of rosettes under no participation from Muller cells demonstrates the existence of direct adhesion between photoreceptors.
Figure 2. Photoreceptors form rosettes in N-cad mutant retinas without the participation of Muller cells.
(A–D) Muller glial cells extend processes basally into the inner limiting membrane (arrowhead) and apically into the OLM (arrow) in wildtype embryo retinas at 4.5 dpf. The Muller glial processes in the OLM (B, arrows, red) colocalize with actin staining (C, green, arrows) in the OLM (D, arrows). A merged image of B–C and the image of G/R (blue) is shown in D. The apical side of the neural retina is on top.
(E–H) Loss of N-cad function leads to disruption of retinal layers in N-cad mutant retinas at 4.5 dpf. However, the photoreceptor cells adhere to each other and form rosettes (E, bracket). The rosettes are visualized by staining for G/R (blue). Dashed lines indicate presumable photoreceptor cells other than G/R in a rosette (E). Strong actin staining (green) is found at the apical ends of the photoreceptors in the interior of rosettes (H, arrow). Processes of Muller glial cells (red) are not detectable between photoreceptors in the interior of the rosettes (F and H). A merged image of E–G is shown in H.
Scale bars indicate 10 μm.
These findings intrigue us to question the nature of the adhesion force that enables photoreceptors to form rosettes. Could it be possible that Nok is responsible for the adhesion between photoreceptors in the rosettes?
Nok is essential for photoreceptor adhesion
If Nok does play a role in the adhesion of photoreceptors in the rosettes, we expect that Nok localizes to the interior regions in the rosettes. We thus performed immunohistological analysis to confirm this. As expected, Nok localizes exclusively in the interior regions of photoreceptor rosettes (Fig. 3A–D). Because the apical end of photoreceptors faces inward in the rosettes (Erdmann et al., 2003), Nok’s interior location in the rosettes is topologically equivalent to its inner segment localization in the normal ONL. Thus, Nok’s location in the rosettes is consistent with the hypothesis that Nok participates in the adhesion between photoreceptors.
Figure 3. Nok is required for photoreceptor adhesion.
(A–D) In the N-cad mutant retina at 4.5 dpf, Nok (C, red, arrows) is only found in the interior regions of photoreceptor rosettes, where actin is also enriched. Blue indicates G/R. Dashed lines in D indicate the regions presumably occupied by other types of photoreceptors. A merged image of A–C is shown in D.
(E–H) In the nok mutant retina at 4.5 dpf, photoreceptors do not form rosettes but rather scatter randomly at 5 dpf. G/R are visualized in blue. Nok staining (red) in retinal cells is diffuse. Some Nok positive cells are zpr1-positive as well, indicating that these are G/R (arrows); other Nok-positive cells are zpr1-negative (arrowheads), indicating that they are other types of photoreceptor cells. H is the merged image of E–G.
(I). Western Blot analysis of protein extract of about one fifth of an adult eye (lane 1) and the extract of 190, 000 purified rods (lane 2) demonstrates that rods express Nok. Molecular weight markers are indicated on the right. The arrow indicates the position of full length Nok protein. Two weaker bands are noticeable in lane 2 between 50 and 37 kDa markers; they likely derive from the degradation of Nok during the process of rod isolation.
Scale bar indicates 10 μm.
The interior localization of Nok in the rosettes also allows us conclude that Nok in the ONL in the wildtype retina is expressed by photoreceptors. This conclusion was not obtainable from our previous study (Wei and Malicki, 2002), because Muller cells extend processes to the regions of OLM in the wildtype retina, making it impossible to rule out the possibility that Muller cells may contribute to the Nok signal in the ONL (Fig. 2A). Since Muller cells do not participate in the formation of rosettes (Fig. 2), the exclusive expression of Nok in the interior of the rosettes demonstrate that photoreceptors but not Muller cells express Nok in the mature neural retina. Supporting this, our Western blot analysis of isolated rod photoreceptors reveals positive expression of Nok in rods (Fig. 3I). These results, together with the fact that Nok signal is observed at the distal regions of the inner segments of the green/red double cones (Fig. 1G–H) where no Muller cell processes have been found under EM study (Fig 3 and 8 of Mariani, 1986), allow us to conclude that green cones and/or red cones and rods express Nok. We yet to determine if blue and UV cones also express Nok.
To test the hypothesis that Nok is required for photoreceptor adhesion, we set out to determine if the loss of Nok function results in the disruption of photoreceptor adhesion by investigating the existence of photoreceptor rosettes in nok mutant retinas. The examination was conducted under three criteria: rosette appearance of groups of photoreceptors; accumulation of actin in the interior regions of the potential rosettes; and localization of rosette interior marker ZO-1 within the potential rosettes (see next section for details of the biological significance of ZO-1 expression). Our results demonstrate that photoreceptors do not form rosettes in nok mutant retinas. Instead, they scatter randomly in the retina. In addition, mutant Nok protein distributes diffusely within photoreceptors (Fig 3. E–H). Thus, we conclude that Nok plays an essential role in photoreceptor adhesion, hence the integrity of the ONL.
The observation of Nok’s essentiality in photoreceptor adhesion is intriguing because while Nok associates to the cell membrane, it also localizes within photoreceptors. Thus Nok’s function of photoreceptor adhesion must be carried out via mechanisms that involve other molecules including but not limited to transmembrane proteins. We thus carried out the following experiments to investigate these mechanisms.
Nok is required for the establishment and/or maintenance of the OLM
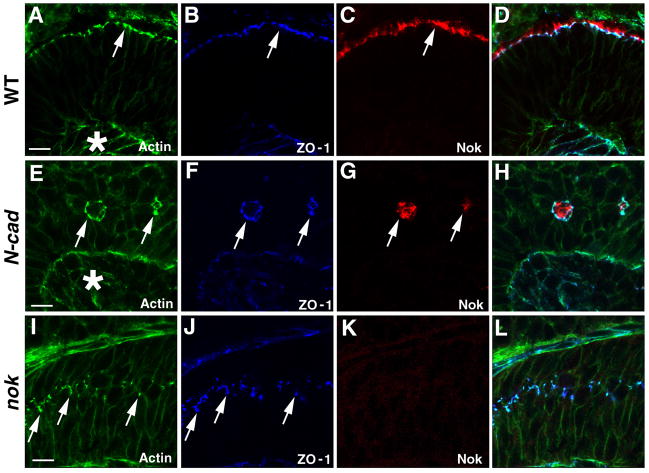
The OLM has long been thought to be the primary structure responsible for the adhesion between Muller cells and photoreceptors and consequently the integrity of the ONL (Dowling, 1970; Raviola, 1977). It is thus tempting to first determine if Nok is functionally related to the OLM. To investigate the OLM, we first determined if ZO-1, a typical tight junction protein in the epithelia (Gonzalez-Mariscal et al., 2003), localizes exclusively to the OLM in the zebrafish retina as in other vertebrate species (Paffenholz et al., 1999; Saitou et al., 1997; Keon et al., 1996; Williams and Rizzolo, 1997). As shown in Figure 4. A–D, ZO-1 localizes to the OLM and is basal to the Nok staining (Fig 4. A–D). Thus ZO-1 serves as an excellent marker for the OLM in the zebrafish retina.
Figure 4. Nok is required for the establishment and/or maintenance of the OLM and OLM-like structures.
(A–D) In wildtype retinas at 4.5 dpf, ZO-1 (blue) localizes to the OLM, basal to the Nok staining (red). The OLM is indicated by actin staining (A, arrow). A merged image of A–C is shown in D. Apical side of the retina is oriented on top.
(E–H) ZO-1 staining (blue) reveals the existence and location of the OLM-like structures in the interior regions of the rosettes in N-cad mutant retinas at 4.5 dpf (white arrows). The inset shows the magnification of a portion of the interior region, which is indicated by a white arrow in H. The Nok staining (red) inside the rosettes is concentrated more apically (black arrow) than that of ZO-1 (black arrowhead) (H, inset). A merged image of E–G is shown in H.
(I–L) ZO-1 forms rudimentary assemblies in nok mutant retinas at 4.5 dpf. Unlike Nok, subcellular accumulation of ZO-1 in photoreceptors is not affected by the loss of Nok function, as evidenced by the punctate ZO-1 staining in nok mutant retinas (J, red, arrows). These ZO-1 positive foci are also enriched with actin, as in the wildtype OLM (I and K, arrows), and they localize to membrane regions of the retinal cells that are presumably photoreceptors (L, arrows, asterisks). K is the merged image of I and J. L shows a magnified region of K.
Scale bars indicate 10 μm.
Since no intact ONL is developed in N-cad mutant retinas, we expect that there will be no intact OLM developed in N-cad retinas as well. This, however, does not rule out the possibility that OLM components assemble into structures similar to the OLM (designated OLM-like structures hereafter). These potential OLM-like structures may mediate photoreceptor adhesion. To investigate this possibility, we next performed immunohistological analysis to determine the distribution of OLM marker ZO-1 in the rosettes. Because Muller cells participate in development of the normal OLM in wildtype retinas and no Muller cells are found participating the formation of the rosettes, we expect no OLM-like structure to be found in photoreceptor rosettes. To our surprise, we found that ZO-1 accumulates in ring-like structures in the interior of rosettes (Fig 4. E–H). In addition, ZO-1’s spatial relationship with Nok in the rosettes is the same as in wildtype retinas, with ZO-1 localizing basal relative to Nok (Fig. 4H). The localization of ZO-1 in the rosettes implies that OLM-like structures are indeed established in the rosettes, despite the fact that Muller cells do not participate in rosette formation (Fig. 2E–H). These OLM-like structures might be formed directly between photoreceptors via transmembrane proteins other than N-cad. Consequently the OLM-like structures contribute to the adhesion of photoreceptors. Thus, even though the overall cellular pattern formation in the retina is disrupted in N-cad, photoreceptors are still capable to adhere to each other.
The random distribution of photoreceptors in nok mutant retinas implies the complete loss of adhesion between photoreceptors (Fig. 3E–H). It is thus intriguing to determine whether the loss of Nok function directly results in the dissociation of the OLM-like structures. We thus analyzed the distribution of ZO-1 in nok mutant retinas at 5 dpf. As shown in Fig. 4I–L, ZO-1 is concentrated at numerous bright punctate sites. These ZO-1 foci are enriched with actin and are closely associated with the cell membrane as well (Fig. 4L), suggesting that the molecular components of the OLM are still capable of forming certain rudimentary structures within individual photoreceptor cells. However, these ZO-1 containing punctate structures are not able to assemble into OLM-like structures as they do in N-cad mutant retinas, despite the fact that the expression of all molecular components of the OLM, such as N-cad, are likely not affected in nok mutant retinas. Thus, Nok is required for the establishment and/or maintenance of the OLM in wildtype retinas and the OLM-like structures in N-cad mutant.
To further investigate the requirement of Nok in the development of the OLM, we set out to analyze the ZO-1 and Nok staining in wildtype, nok, and N-cad mutant retinas at 35 hpf, when the majority of retinal cells are undifferentiated and photoreceptors have not been specified. Analyzing ZO-1 and Nok staining at this early developmental stage will likely provide us with information regarding the precursors of the OLM and consequently their involvement in OLM development. In wildtype, ZO-1 staining, immediately basal to Nok staining, colocalizes with adherens junction-associating actin at the apical regions of the retina (Fig. 5. A–D), suggesting that adherens junctions in the retinal neuroepithelium are likely the precursors of the OLM. In N-cad mutant retinas at 35 hpf, adherens junction-bearing retinal cells tend to assemble into structures that are similar to the photoreceptor rosettes observed in N-cad mutant retinas at 5 dpf (Fig. 5E–H). Within these cell assemblies, ZO-1 and actin localize to the interior regions and organize into ring-like structures. Furthermore, Nok localizes to the interior as well, immediately apical to the ZO-1 staining (Fig. 5H). This rosette-like organization of retinal epithelial cells is not, however, established in nok mutant retinas even though adherens junctions localize ectopically in both nok and N-cad mutant retinas (Fig. 5. E–L). This demonstrates that Nok is required for the adhesion between ZO-1-expressing cells even at early retinal developmental stages. Furthermore, the concurrence of the OLM-like structure in photoreceptor rosettes and the ZO-1 containing ring-like structure in retinal neuroepithelium in N-cad but not in nok mutant retinas strongly suggests that ZO-1 containing adherens junctions are the precursors of the OLM.
Figure 5. Nok is required for the adhesion between retinal neuroepithelial cells even before the differentiation of photoreceptor cells.
(A–D) In wildtype retinal neuroepithelium, ZO-1(blue) colocalizes to the adherens junction-associated actin bundles (green) at 35 hpf (arrows). Nok (red) localizes immediately apical to the ZO-1 staining. D is the merged image of A–C. The asterisk indicates the lens. Apical side of the retina is oriented on top.
(E–H) In N-cad mutant retinas at 35 hpf, ZO-1 (blue) and actin bundle staining (green) indicate that retinal epithelial cells adhere to each other to form a ring-like structure (arrows) at 35 hpf. Nok (red) localizes apical to the ZO-1 staining in a more interior region within the cluster of cells. H is the merged image of E–G. The asterisk indicates the lens.
(I–L) In nok mutant retinas at 35 hpf, ZO-1(blue) and adherens junction-associated actin bundles (green) localize ectopically to the interior of the retinal neuroepithelium (arrows). Mutant Nok (red) does not concentrate to the adherens junctions (K). L is the merged image of I–K.
Scale bars indicate 10 μm.
Evidence for the possibility of a Nok-mediated photoreceptor-photoreceptor (P-P) junctional complex
While the above experiments demonstrate the essential role of Nok in photoreceptor adhesion through its function in establishing/maintaining the OLM or the OLM-like structures, the far more extensive distribution of Nok in the cell-cell junctional regions of the inner segments of photoreceptors than that of the OLM (Fig 1) suggests the possibility of a Nok-mediated P-P junctional complex in the inner segments. If this is true, this junctional complex likely works along with the OLM to collectively secure the integrity of the OLN.
Considering that no OLM-like structures are developed in nok mutant retinas, the above hypothesis predicts that nok mutant photoreceptors are less adhesive than wildtype cells and are consequently more mobile. To test this prediction, we performed live imaging analysis to monitor the mobility of GFP-expressing rods in either wildtype or nok mutant retinas. Our results indicate that photoreceptors in nok mutant retinas are much more mobile than those in wildtype retinas (Fig. 6. A and B). The movement of photoreceptors in mutant retinas displays a number of interesting features: 1) cells can move closer to each other over time; 2) cells can also move away from each other; 3) the body shapes of photoreceptors undergo extensive changes due to surface extension/retraction and/or rotation of cell body; and 4) the majority of photoreceptors are mobile in nok mutant retinas (Fig. 6B, C). On the other hand, photoreceptors in wildtype retinas remain mostly stationary even though slight tilting of some photoreceptors has been observed (Fig. 6A, C). More interestingly, when transplanted in nok mutant retinas, wildtype photoreceptors are less mobile than mutant cells in nok mutant retinas (Fig 6. C, black bars vs. red bars); but more mobile than wildtype cells in wildtype retinas (Fig 6. C, black bars vs. green bars). Thus, in the case that no OLM-like structures are formed in nok mutant retinas, the reciprocal correlation between photoreceptor mobility, which reflects photoreceptor adhesiveness, and the level of the expression of functional Nok is consistent with the hypothesis that Nok mediates a novel type of P-P junctional complex. In addition, this junctional complex functions independently of the OLM or OLM-like structures.
If Nok is involved in a direct binding force important for photoreceptor adhesion, Nok, as a cytoplasmic protein, must mediate the adhesion through one or more transmembrane proteins. The Crumbs proteins are good candidates for the mediation for the following reasons: First, Crumbs, a transmembrane protein initially identified in fly that interacts with Stardust, the fly homolog of Nok, has three homologs in vertebrates: Crb1, Crb2, and Crb3 (Tepass and Knust, 1993; Hong, et al., 2001; Makarova et al., 2003; Katoh, 2004; den Hollander, 2001b). Second, Crb1, 2, and 3 have been observed to be expressed in the ONL in the mouse retina and Crb1 physically interacts with Pals1, the mouse homolog of Nok, in retinal lysates (van de Pavert et al., 2004). Third, the extracellular domains of Crumbs contain modules similar to Liminin-A G domain and EGF polypeptide modules that are known to interact with other proteins (Stenflo et al., 2000; Hohenester et al., 1999), likely rendering Crumbs the capability to adhere to other molecules. Finally, many mutations on the extracellular domain of Crb1 cause inherited retinal Dystrophies in humans (den Hollander et al., 2004). We thus set out to first determine whether Crumbs proteins colocalize with Nok in the inner segments of the zebrafish retina and therefore serve as possible transmembrane proteins essential for Nok-mediated P-P adhesional complex.
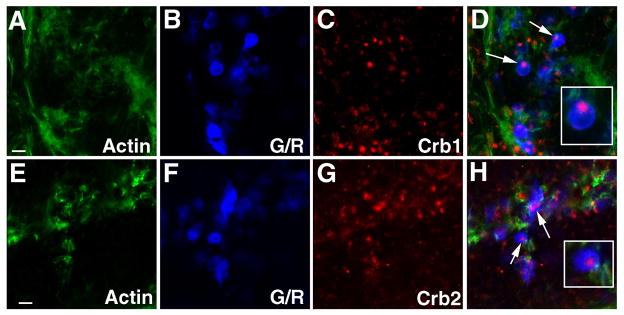
Immunohistochemistry using anti-Crb1 and anti-Crb2 antibodies shows that both proteins localize to the inner segments apical to the OLM at 6 dpf and 12 mpf and that both are enriched in the junctional regions between green and red cones (Fig. 7. A–L; data not shown). Moreover, like Nok, they are also concentrated in the interior regions of rosettes in N-cad mutant retinas (Fig. 7M–T). Therefore, the expression patterns of Crb1 and Crb2 are identical to that of Nok in the mature retina (Fig 1; Fig. 3), consistent with the hypothesis that that Crb1 and Crb2 might be the transmembrane proteins responsible for Nok-mediated P-P junctional complex.
Figure 7. Nok colocalizes with Crb1 and Crb2.
(A–D) Crb1 (red) localizes to the cell membrane regions in the inner segments of photoreceptors in the adult retina at 12 mpf. The arrowheads point out the Crb1 staining in the cell membrane regions between the members of G/R (blue). Green staining indicates the localization of actin. D is the merged image of A–C.
(E–H) Crb1 (red) localizes apically to the OLM, which is visualized by ZO1 staining (blue) and actin staining (I, green, arrowheads) in the adult retina. H is the merged image of E–G.
(I–L) Crb2 (red) localizes to the cell membrane regions in the inner segments of photoreceptors in the adult retina at 12 mpf. The arrowheads point out the Crb2 staining in the cell-cell junctional regions between the members of G/R (blue). Green staining indicates the localization of actin and the OLM is indicated with arrowheads (M). L is the merged image of I–K.
(M–P) Crb1 (red, arrows) localizes to the interior regions of the photoreceptor rosettes in N-cad mutant retinas at 4.5 dpf. G/R are visualized in blue and actin is shown in green. P is the merged image of M–O.
(Q–T) Crb2 (red, arrow) localizes to the interior regions of the photoreceptor rosettes in N-cad mutant retinas at 4.5 dpf. G/R are visualized in blue and actin is shown in green. T is the merged image of Q–S.
Sections A–L are oriented with apical side of the retina on the top. Scale bars indicate 10 μm.
To address how Nok is functionally related to Crumbs, we analyzed the subcellular distribution of Crumbs in nok mutant retinas. It turns out that the majority of Crb1 and Crb2 localize internally in photoreceptors in nok mutant retinas (Fig. 8A–H). Thus, Nok is required for the proper plasma membrane localization of Crumbs.
Figure 8. Nok is required for the proper localization of Crumbs proteins in retinal photoreceptors.
(A–D) Crb1 (C, red) localizes primarily internally in photoreceptors of nok mutant retinas at 4.5 dpf. The arrows indicate the subcellular enrichment of Crb1. The inset in D shows a magnified photoreceptor. G/R are visualized in blue and actin is shown in green. D is the merged image of A–C.
(E–H) Crb2 (red) localizes primarily internally in photoreceptors of nok mutant retinas at 4.5 dpf. The arrows indicate the subcellular enrichment of Crb2. The inset in H shows a magnified photoreceptor. G/R are visualized in blue and actin is shown in green. H is the merged image of E–G.
The apical end of the retinas in panels A–L is oriented on top. Scale bars indicate 10 μm.
Taken together, the above data are consistent with our current working hypothesis of Nok’s function in P-P junctional complex: Nok targets Crumbs proteins to the proper plasma membrane of the inner segments of photoreceptors; the extracellular domains of Crumbs likely mediate physical adhesion either through homophilic binding to Crumbs on the neighboring photoreceptors or through heterophilic binding to other molecules, such as the extracellular matrix components and/or different photoreceptor surface molecules.
Discussion
By analyzing the adhesional properties of photoreceptors in nok and N-cad mutants, our present study reveals that Nok plays an indispensable role in photoreceptor adhesion. Nok’s function in photoreceptor adhesion has not been reported before and our findings provide critical insights into the molecular mechanisms by which the integrity of the ONL is maintained in the mature vertebrate retina. As discussed below, Nok’s such function is likely achieved through two mechanisms: establishing/maintaining the OLM, and mediating a novel type of P-P junctional complex (Fig. 9).
Figure 9. A model for the mechanisms by which Nok functions in photoreceptor adhesion.
(A) A schematic drawing of Nok’s position in the inner segment and its spatial relationship with the OLM.
(B) The two mechanisms by which Nok participates in maintaining the integrity of the ONL: through establishing/maintaining the OLM, and likely through establishing a potential novel type of cell-cell adhesion complex between photoreceptors. The former mechanism likely involves the participation of Muller cells in wildtype retinas; whereas the latter may concern only photoreceptors and likely involves Crumbs proteins.
Nok’s essential role in the adhesion between photoreceptors
Our current and previous (Wei and Malicki, 2002) studies reveal that Nok is expressed at a broad spectrum of developmental stages in the retina and carries distinct functions at specific stages. At retinal epithelial stages, Nok is required for the maintenance of retinal neuroepithelial polarity and the integrity of the RPE (Wei and Malicki, 2002). During initial retinal neurogenesis, Nok plays an essential role in retinal cellular pattern formation (Wei and Malicki, 2002). Here we demonstrated that Nok is continuously expressed in photoreceptors in the adult retina (Fig. 1) and plays an essential role in photoreceptor adhesion.
Nok’s function in photoreceptor adhesion is demonstrated by several lines of evidence and reasoning. First, the formation of photoreceptor rosettes and the establishment of the intact ONL occur in the presence of functional Nok (Fig. 1 and 2). Second, the loss of Nok function leads to a complete loss of photoreceptor adhesion as evidenced by the lack of photoreceptor rosettes in nok mutants (Fig. 3). Third, photoreceptor mobility, which reflects the lack of adhesiveness, displays reciprocal correlation with the expression level of functional Nok (Fig. 6). Finally, the lack of photoreceptor adhesion caused by nok mutations is not a secondary consequence derived from the defects of early retinal developmental events, such as retinal lamination, RPE integrity, and the morphogenesis of Muller cell processes. This is because both N-cad and nok mutant retinas display these defects and yet rosettes form in N-cad but not in nok. Taken together, our results unequivocally demonstrate for the first time that Nok plays an indispensable role in photoreceptor adhesion. This adds another dimension to the diversity of Nok’s biological functions during retinal development.
Nok mediates the establishment and/or maintenance of the OLM
Our finding that Nok is required for the formation of the OLM-like structures demonstrates that Nok plays an essential role in the development of the OLM (Fig 4.). Nok thus contributes to the stability of the ONL indirectly via the OLM. We also provided evidence suggesting that the OLM is derived from the adherens junction and that Nok plays an important role during the transition (Fig. 5). As a unique type of cell-cell junctional complex, the OLM has a distinct molecular makeup (Introduction). To better understand the development of the OLM and how it functions for the structural integrity of the ONL, it is necessary to study the dynamics of its molecular components during development. For example, it is necessary to determine if all OLM components are expressed in the adherens junction at early developmental stages. Such a study will help us determine at the molecular levels how Nok functions during OLM development.
Do Nok and Crb mediate P-P junctional complexes as another mechanism underlying the integrity of the ONL?
Besides Nok’s function in the establishment and/or maintenance of the OLM, our present study also suggests that Nok contributes to the integrity of the ONL via a second mechanism: Nok determines the proper cell plasma membrane localization of Crumbs, whose extracellular domains likely mediate the physical adhesion among photoreceptors. This hypothesis is supported by two facts: First, Nok, Crb1, and Crb2 display identical expression patterns along the extensive cell membrane regions in the inner segments, where photoreceptor cell membranes juxtapose tightly as revealed by EM studies (Mariani, 1986). Second, the proper targeting of Crumbs proteins requires functional Nok (Fig. 7 and 8). This hypothesis is consistent with the fact that the extracellular domains of Crb1 and Crb2 contain protein-protein interaction domains. It is also consistent with the retinal phenotypes in human patients with mutations on the extracellular domain of the Crb1 (for detailed references see results section).
The fact that proper targeting of Crumbs to the inner segments requires Nok is consistent with the conservation of the functional dependency and physical interaction between Crumbs and Nok homologs in fruit fly and other vertebrates. In mammalian MDCK cell line, Crumbs interacts with the PDZ domain of Nok’s mammalian homolog Pals1 via the COOH terminus of Crumbs (Roh et al., 2002). The physical interaction between Pals1 and Crb has also been demonstrated in mouse retinal lysate (van de Pavert et al., 2004). Interaction between Crumbs and Stardust, the fly homolog of Nok, is also observed in fly epithelium. Nok’s function in targeting Crumbs to the inner segment likely requires the help from other Nok-interacting proteins. Besides a PDZ domain, six additional protein-protein interaction domains have been identified in Nok and some of their targets have been identified in mammalian epithelial cell culture systems. Among these domains, the N-terminal conserved domain interacts with Par-6 (Hurd et al., 2003), L27N interacts with DLT (Roh et al., 2002), L27C interact with Lin7 (Kamberov et al., 2000). These proteins also likely interact with Nok in photoreceptors and assist in targeting Crumbs to the inner segments. The targets of the SH3 domain, protein Band 4.1 binding domain, and guanylate kinase domain are yet to be identified. With a Band 4.1 binding domain, Nok likely binds to cytoskeletal protein Band 4.1 or Band 4.1-like proteins, thus bridging the cytoskeleton with the potential Crumbs-containing P-P junctional complex. If true, Nok’s physical linkage of the cytoskeleton with Crumbs enhances the mechanical stability of photoreceptor adhesion, consistent with the stabilization function of the cytoskeleton in other types of cell-cell junctional complexes.
The biochemical nature of Nok/Crumbs-mediated juntional complex is likely complicated. First, three Crumbs proteins identified in vertebrates so far display identical subcellular localizations. Among the three identified Crumbs, Crb3 has a very small extracellular domain; whereas Crb1 and Crb2 have large extracellular domains, making them the most likely candidates responsible for the adhesion function. As we show in Figure 6, Crb1 and Crb2 have an identical expression pattern in the zebrafish retina. This identical expression pattern and the fact that the phenotypes of Crb1 knockout mice are much less severe than that of nok mutants (van de Pavert et al., 2004) suggest that Crb1 and Crb2 are functionally redundant in terms of cell adhesion. Second, Crumbs may mediate homophilic adhesion by binding to the same Crumbs protein of neighboring photoreceptors and/or mediate heterophilic adhesion by binding to the extracellular matrix or different surface molecules of neighboring photoreceptors.
To unequivocally prove the existence of this Nok/Crb-mediated novel type of P-P junctional complex, we still need to answer several important questions at biochemical and ultrastructure levels: What portion of the extracellular domain of Crumbs is directly involved in binding? What are the binding targets of Crumbs? How strong is the binding force? How does Nok target Crumbs to the inner segments during development? What are the structural characteristics of the potential adhesion complex at the ultrastructural level? These questions are the subjects of our future experiments.
Coordination between adhesion mediated by the OLM and by the Nok/Crb-mediated P-P adhesion complex
If a Nok/Crb-mediated P-P junctional complex does exist, its functional relationship with the OLM is likely complicated. Crumbs/Nok-mediated adhesion is likely more fundamental than that of the OLM, because the loss of Nok function leads to complete lost of photoreceptor adhesion, whereas the loss of function of OLM component N-cad does not. In addition, Crb/Nok may be directly involved in the integrity of adherens junctions, which likely develop into the OLM. In fully developed mature retina, while the OLM and the Crb/Nok-midated P-P adhesion are likely to be structurally independent of each other due to their different subcellular localizations, they may contribute synergistically to the integrity and stability of the ONL. Regardless, Nok plays indispensable roles in both mechanisms of photoreceptor adhesion.
Acknowledgments
The authors thank Drs. Wijnholds, Rashbass, and Linser for the anti-Crb1, anti-Crb2, and anti-Carbonic Anhydrase antibodies, respectively. We are grateful to Ms. Anne Catalano for her assistance in the revision of the manuscript. The work was supported by a NIH core grant (5P30EY008098-17, PI, Dr. Robert Hendricks) and the following funds to X.W.: University of Pittsburgh School of Medicine startup fund, Research to Prevent Blindness Career Development Award, NIH RO1EY016099, and UPMC Health System Competitive Medical Research Grant.
Abbreviations
- ONL
outer nuclear layer
- OLM
outer limiting membrane
- Nok
Nagie oko
- N-cad
N-cadherin
- P-P
photoreceptor-photoreceptor
- G/R
reen/red double cones
- ROP
rod opsin promoter
- dpf
day postfertilization
- mpf
month postfertilization
References
- Branchek T, Bremiller R. The Development of Photoreceptors in the Zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984;224:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Chung KY, Leung KM, Lin CC, Tam KC, Hao YL, Taylor JS, Chan SO. Regionally specific expression of L1 and sialylated NCAM in the retinofugal pathway of mouse embryos. J Comp Neurol. 2004;471:482–498. doi: 10.1002/cne.20047. [DOI] [PubMed] [Google Scholar]
- Claudepierre T, Manglapus MK, Marengi N, Radner S, Champliaud MF, Tasanen K, Bruckner-Tuderman L, Hunter DD, Brunken WJ. Collagen XVII and BPAG1 expression in the retina: evidence for an anchoring complex in the central nervous system. J Comp Neurol. 2005;487:190–203. doi: 10.1002/cne.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Daniloff JK, Chuong CM, Levi G, Edelman GM. Differential distribution of cell adhesion molecules during histogenesis of the chick nervous system. J Neurosci. 1986;6:739–758. doi: 10.1523/JNEUROSCI.06-03-00739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Davis J, van der Velde-Visser SD, Zonneveld MN, Pierrottet CO, Koenekoop RK, Kellner U, van den Born LI, Heckenlively JR, Hoyng CB, et al. CRB1 mutation spectrum in inherited retinal dystrophies. Hum Mutat. 2004;24:355–369. doi: 10.1002/humu.20093. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Johnson K, de Kok YJ, Klebes A, Brunner HG, Knust E, Cremers FP. CRB1 has a cytoplasmic domain that is functionally conserved between human and Drosophila. Human Molecular Genetics. 2001;10:2767–2773. doi: 10.1093/hmg/10.24.2767. [DOI] [PubMed] [Google Scholar]
- Dowling J. The Retina. Cambridge, Massachusetts: Harvard University Press; 1987. [Google Scholar]
- Dowling JE. Organization of vertebrate retinas. Invest Ophthalmol. 1970;9:655–680. [PubMed] [Google Scholar]
- Edelman GM. Cell adhesion molecules. Science. 1983;219:450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Erdmann B, Kirsch FP, Rathjen FG, More MI. N-cadherin is essential for retinal lamination in the zebrafish. Developmental Dynamics. 2003;226:570–577. doi: 10.1002/dvdy.10266. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Morioka H, Nakamura H, Watanabe K. Gap junctions in the differentiated neural retinae of newly hatched chickens. J Cell Sci. 1976;22:597–606. doi: 10.1242/jcs.22.3.597. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A. Essential role of alpha 6 integrins in cortical and retinal lamination. Current Biology. 1998;8:983–986. doi: 10.1016/s0960-9822(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Takechi M, Chinen A, Nishiwaki Y, Kawamura S. Visualization of rod photoreceptor development using GFP-transgenic zebrafish. Genesis. 2002;34:215–220. doi: 10.1002/gene.10155. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Tisi D, Talts JF, Timpl R. The crystal structure of a laminin G-like module reveals the molecular basis of alpha-dystroglycan binding to laminins, perlecan, and agrin. Mol Cell. 1999;4:783–792. doi: 10.1016/s1097-2765(00)80388-3. [DOI] [PubMed] [Google Scholar]
- Hong Y, Ackerman L, Jan LY, Jan YN. Distinct roles of Bazooka and Stardust in the specification of Drosophila photoreceptor membrane architecture. Proc Natl Acad Sci U S A. 2003;100:12712–12717. doi: 10.1073/pnas.2135347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Honjo M, Oohira A, Kido N, Otori Y, Tano Y, Honda Y, Tanihara H. Spatiotemporal expression patterns of N-syndecan, a transmembrane heparan sulfate proteoglycan, in developing retina. Invest Ophthalmol Vis Sci. 2002;43:1616–1621. [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- Janssen-Bienhold U, Dermietzel R, Weiler R. Distribution of connexin43 immunoreactivity in the retinas of different vertebrates. J Comp Neurol. 1998;396:310–321. [PubMed] [Google Scholar]
- Janssen-Bienhold U, Schultz K, Gellhaus A, Schmidt P, Ammermuller J, Weiler R. Identification and localization of connexin26 within the photoreceptor-horizontal cell synaptic complex. Vis Neurosci. 2001;18:169–178. doi: 10.1017/s0952523801182015. [DOI] [PubMed] [Google Scholar]
- Kamberov E, Makarova O, Roh M, Liu A, Karnak D, Straight S, Margolis B. Molecular cloning and characterization of Pals, proteins associated with mLin-7. Journal of Biological Chemistry. 2000;275:11425–11431. doi: 10.1074/jbc.275.15.11425. [DOI] [PubMed] [Google Scholar]
- Katoh M. Identification and characterization of Crumbs homolog 2 gene at human chromosome 9q33.3. Int J Oncol. 2004;24:743–749. [PubMed] [Google Scholar]
- Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Chang BS, Mun GH, Chung YH, Kim J, Shin DH. An ultramicroscopic study on the distribution of Muller cell processes in the outer retinal layers of the zebrafish. Ann Anat. 2005;187:43–50. doi: 10.1016/j.aanat.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci. 1999;19:9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Fisher SK. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J Comp Neurol. 1988;268:281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Lodge AP, Howard MR, McNamee CJ, Moss DJ. Co-localisation, heterophilic interactions and regulated expression of IgLON family proteins in the chick nervous system. Brain Res Mol Brain Res. 2000;82:84–94. doi: 10.1016/s0169-328x(00)00184-4. [DOI] [PubMed] [Google Scholar]
- Makarova O, Roh MH, Liu CJ, Laurinec S, Margolis B. Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1) Gene. 2003;302:21–29. doi: 10.1016/s0378111902010843. [DOI] [PubMed] [Google Scholar]
- Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Developmental Biology. 2003;259:95–108. doi: 10.1016/s0012-1606(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond B Biol Sci. 1986;227:483–492. doi: 10.1098/rspb.1986.0035. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, et al. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Hatta K, Takeichi M. Role of N-Cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron. 1988;1:289–295. doi: 10.1016/0896-6273(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Mi ZP, Jiang P, Weng WL, Lindberg FP, Narayanan V, Lagenaur CF. Expression of a synapse-associated membrane protein, P84/SHPS-1, and its ligand, IAP/CD47, in mouse retina. J Comp Neurol. 2000;416:335–344. [PubMed] [Google Scholar]
- Paffenholz R, Kuhn C, Grund C, Stehr S, Franke WW. The arm-repeat protein NPRAP (neurojungin) is a constituent of the plaques of the outer limiting zone in the retina, defining a novel type of adhering junction. Exp Cell Res. 1999;250:452–464. doi: 10.1006/excr.1999.4534. [DOI] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet JM, Gerber S, Ghazi I, Leowski C, Ducroq D, Souied E, Dufier JL, Munnich A, Kaplan J. Leber congenital amaurosis. Mol Genet Metab. 1999;68:200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Developmental Biology. 2001;234:454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;25(Suppl):27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- Raymond P, Barthel L, Curran G. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. Journal of Comparative Neurology. 1995;359:537–550. doi: 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones. Neuron. 1993;10:1161–1174. doi: 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11:1219–1227. doi: 10.1093/hmg/11.10.1219. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The Vertebrate Retina Principles of Structure and Function. San Francisco, California: W. H. Freeman & Co; 1973. [Google Scholar]
- Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, Goyal M, Wiggins R, Margolis B. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J Cell Biol. 2002;157:161–172. doi: 10.1083/jcb.200109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita S. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222–231. [PubMed] [Google Scholar]
- Sperry R. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J, Stenberg Y, Muranyi A. Calcium-binding EGF-like modules in coagulation proteinases: function of the calcium ion in module interactions. Biochim Biophys Acta. 2000;1477:51–63. doi: 10.1016/s0167-4838(99)00262-9. [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E. Crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Developmental Biology. 1993;159:311–326. doi: 10.1006/dbio.1993.1243. [DOI] [PubMed] [Google Scholar]
- Tserentsoodol N, Shin BC, Suzuki T, Takata K. Colocalization of tight junction proteins, occludin and ZO-1, and glucose transporter GLUT1 in cells of the blood-ocular barrier in the mouse eye. Histochem Cell Biol. 1998;110:543–551. doi: 10.1007/s004180050316. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Kantardzhieva A, Malysheva A, Meuleman J, Versteeg I, Levelt C, Klooster J, Geiger S, Seeliger MW, Rashbass P, et al. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci. 2004;117:4169–4177. doi: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Schmitz F. The expression pattern and assembly profile of synaptic membrane proteins in ribbon synapses of the developing mouse retina. Cell Tissue Res. 2003;311:159–173. doi: 10.1007/s00441-002-0674-0. [DOI] [PubMed] [Google Scholar]
- Wei X, Cheng Y, Luo Y, Shi X, Nelson S, Hyde DR. The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination. Dev Biol. 2004;269:286–301. doi: 10.1016/j.ydbio.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nature Genetics. 2002;31:150–157. doi: 10.1038/ng883. [DOI] [PubMed] [Google Scholar]
- Williams CD, Rizzolo LJ. Remodeling of junctional complexes during the development of the outer blood-retinal barrier. Anat Rec. 1997;249:380–388. doi: 10.1002/(SICI)1097-0185(199711)249:3<380::AID-AR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]