Abstract
Objective
We tested the efficacy of a cognitive-behavioral intervention in reducing environmental tobacco smoke exposure (ETSE) and improving pregnancy outcomes among African-American women.
Methods
We recruited 1,044 women to a randomized controlled trial during 2001-2004 in Washington, DC. Data on 691 women with self-reported ETSE were analyzed. A subset of 520 ETSE women and salivary cotinine levels (SCLs) <20 ng/ml was also analyzed. Individually tailored counseling sessions adapted from evidence-based interventions for ETSE and other risks, were delivered to the intervention group. The usual care group received routine prenatal care as determined by their provider. Logistic regression models were used to predict ETSE before delivery and adverse pregnancy outcomes.
Results
Women in the intervention were less likely to self-report ETSE before delivery when controlling for other covariates (OR=0.50, 95%CI=0.35-0.71). Medicaid recipients were more likely to have ETSE (OR=1.97, 95%CI=1.31-2.96). With advancing maternal age, the likelihood of ETSE was less (OR=0.96, 95%CI=0.93-0.99). For women in the intervention the rates of very low birth weight (VLBW) and very preterm birth (VPTB) were significantly improved (OR=0.11, 95%CI=0.01-0.86; OR=0.22, 95%CI=0.07-0.68, respectively). For women with SCL <20 ng/ml, maternal age was not significant. Intimate partner violence at baseline significantly increased the chances of VLBW and VPTB (OR=3.75, 95%CI=1.02-13.81; OR=2.71, 95%CI=1.11-6.62, respectively). These results were true for mothers who reported ETSE overall and for those with SCL <20 ng/ml.
Conclusions
This is the first randomized clinical trial demonstrating efficacy of a cognitive-behavioral intervention targeting ETSE in pregnancy. We significantly reduced ETSE as well as VPTB and VLBW, leading causes of neonatal mortality and morbidity in minority populations. This intervention may reduce health disparities seen in reproductive outcomes.
Keywords: behavioral intervention, environmental tobacco smoke, pregnant women, African-American, birth weight, preterm birth
Introduction and Background
Disparities in reproductive outcomes represent a significant public health challenge. Characteristics including biological, behavioral, psychosocial and ecological may contribute to racial/ethnic disparities.1-6 Studies have shown the impact of such factors on adverse pregnancy outcomes, including fetal loss, intrauterine growth restriction, preterm birth, birth defects and infant mortality in African-American women.7,8 Environmental toxicants, such as pesticides and environmental tobacco smoke exposure (ETSE) are hazards that burden socioeconomically challenged communities disproportionately.9 The long term effects of environmental toxicant exposure in utero are exacerbated by circumstances of financial deprivation by those who live in disadvantaged neighborhoods.10 The current literature has emphasized the significance of such hazards but very few studies have attempted to test the efficacy of interventions that aim to reduce their impact on pregnancy outcomes.11,12
The prevalence of active smoking among African-American pregnant women is lower than among pregnant Caucasian women (10.6% versus 18.1%).13 ETSE during pregnancy, however, may be more frequent as a result of high rates of smoking among African-American men (26.1%) and non-pregnant African-American women (18.5%). These rates are higher than or equivalent to those reported for Caucasian individuals (23.5% for men and 18.8% for women).14 African-American women may be more likely to live in overcrowded households and unable to exert effective control over their living and work environments.15,16 Regulations have significantly decreased workplace exposure, but such restrictions do not exist inside the home. The need for evidence-based individualized strategies to help decrease risks of home ETSE during pregnancy is urgent.
In this study, we explored rates of ETSE during pregnancy as self-reported and biologically confirmed in a high risk population of African-Americans in Washington, DC. We tested the efficacy of a cognitive-behavioral intervention, delivered in this randomized controlled trial (RCT), comparing women who were randomly assigned to the intervention group with those received usual care in their ability to avoid ETSE during pregnancy. Comparisons are also made with regard to reproductive outcomes.
Methods
The National Institutes of Health-DC Initiative to Reduce Infant Mortality in Minority Populations is a congressionally mandated research project. As part of this project, we recruited women to a multicenter RCT at six clinic sites in Washington, DC between July 2001 and October 2003, to evaluate the efficacy of an integrated behavioral intervention delivered during prenatal care (PNC) in reducing risk during pregnancy and improving pregnancy outcomes. Women were followed until July 2004. Details of the design of the study and the findings with respect to the primary objectives are presented elsewhere.12,17-19 This study was reviewed and approved by the institutional review boards of the participating institutions.
Study Population
As described elsewhere,17 women were eligible for the study when they self-identified as being an ethnic minority, were ≥18 years, ≤28 weeks pregnant, were a DC resident, spoke English and reported at least one of the following risk factors: cigarette smoking, ETSE, depression or intimate partner violence (IPV). A total of 1,070 women were eligible, interviewed and consented for random assignment into the intervention group or the usual-care group. Block randomization was site- and risk-specific. Risks considered in randomization were smoking, depression, and IPV. The field staff were blinded with respect to the block size. Eight women (6 intervention and 2 usual care) were identified as suicidal during intervention or data collection and were referred immediately to mental health care and excluded from additional participation. Of those who remained, 1,044 were African-American and still pregnant at the time of the baseline interview.
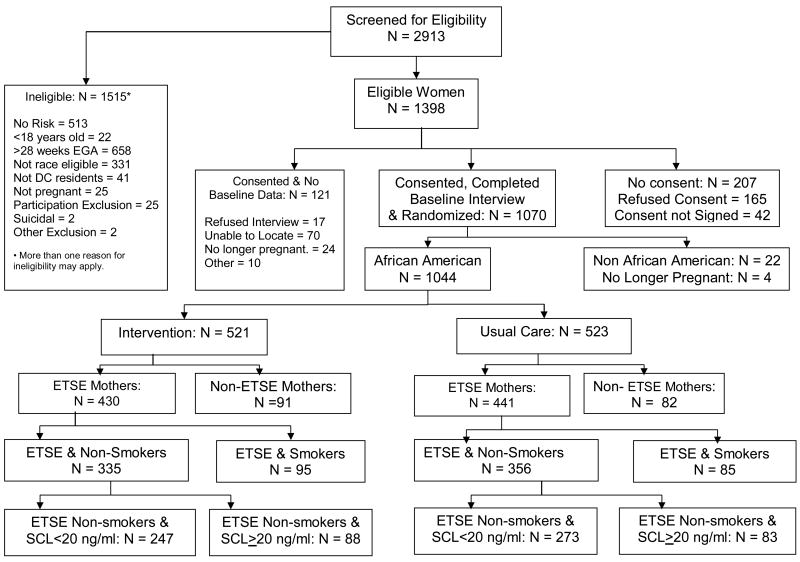
The analyses reported in this article will be confined to 691 pregnant African-American women who self-reported ETSE during the initial screening and did not self report actively smoking during pregnancy (intervention group =335, usual care group =356) (Figure 1). ETSE risk was identified when a mother reported exposure to cigarette smoking in her home, same room or a car with a smoker during a typical week. A more refined subset of 520 women with ETSE and salivary cotinine levels (SCLs) <20 ng/ml at baseline was analyzed separately (intervention group =247, usual care group =273). An SCL of 20 ng/ml was chosen because this level was associated with zero median daily cigarettes smoked.20 Cutoff levels have been used to differentiate smokers from non-smokers with ranges between 10 and 20 ng/ml.15,21-23 A detailed description of instruments used to assess other risks is available.18 Changes in ETSE and other psycho-behavioral risks were assessed during telephone interviews conducted in the 2nd and 3rd trimesters (22-26 and 34-38 weeks of gestation, respectively). Interviewers were blinded to the group assignment of participants. Medical risks during pregnancy and pregnancy outcome data on mothers and infants were abstracted from clinic and hospital medical records.
Figure 1. Profile of Project DC-HOPE Randomized Controlled Trial.
Integrated Behavioral Intervention and Measures
The intervention arm of this RCT was designed to be delivered during PNC and was based on the behavioral change literature, specific to the designated psycho-behavioral risks.18 The ETSE intervention focused on introducing strategies within the home to eliminate or minimize exposure of the expectant mother to ETS. Such strategies stressed, through role play and skills practice, the importance of building negotiation skills with partners and other household members who smoked. Mothers were informed about the potential risks of ETS to the mother herself and her unborn fetus, as well as risks to the newborn. Household smoking bans along with designated safe smoking areas outside the home were encouraged during pregnancy and postpartum. The intervention was delivered by a team of pregnancy advisors who were masters' trained psychologists or social workers. The pregnancy advisors were trained on assessing the stage of change of the individual mother and tailoring the intervention to her stage of change.
Mothers who were identified by the Hopkins Scale as being depressed received a cognitive behavioral therapy intervention that addressed negative cognition and relationships that contribute to depressive symptoms. Strategies for mood management and increased positive social interaction were the primary focus. Mothers who identified as experiencing IPV by use of the Conflict Tactics Scale also received a brochure-based intervention that focused on options for developing a personal safety plan and lists of community resources that were provided to all intervention women. The brochure also provided information about the types of abuse (e.g., emotional, sexual and physical) and the cycle of violence (e.g., escalation, IPV, honeymoon period). The intervention focused on danger assessment to identify risks for harm and preventive options women may consider.
Outcome measures included self-reported ETSE before delivery and infant's birth weight and gestational age. Self-reported ETSE was defined as whether a woman was exposed (yes/no) during the interview before delivery. Infant's birth weight and gestational age were abstracted from the medical records. Low birth weight (LBW) was defined as infants weighting <2,500 grams, while very low birth weight (VLBW) was defined as infants <1,500 grams. Gestational age was prioritized in the following order: ultrasound, exam and menstrual history. Preterm birth (PTB) was defined as gestational age at delivery <37 weeks, while very preterm birth (VPTB) was defined as gestational age at delivery <34 weeks.
Cotinine level, the major proximate metabolite of nicotine, was used as a biomarker of tobacco exposure,24 and was determined using saliva samples. Cotinine level was then determined using gas chromatography–mass spectrometry with lower detection limits of 10 ng/ml.
Statistical Analysis
To preserve randomization, participant data were analyzed using an intent-to-treat approach. Bivariate analyses were conducted to compare the baseline characteristics and pregnancy outcomes in the intervention group versus the usual-care group and to compare groups by self-reported ETSE before delivery and by infant birth weight (LBW/no LBW, VLBW/no VLBW) and preterm status (PTB/no PTB, VPTB/no VPTB). Logistic regression was used to model LBW, VLBW, PTB, VPTB and ETSE before delivery, controlling for relevant covariates, including care group and other psycho-behavioral risks. Initial models to predict birth outcomes (as previously defined) and ETSE before delivery included predictors with a significance level of <0.20 in the bivariate analyses. Reduced models were then developed retaining care group and covariates with significance <0.05. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were produced. Models were constructed for mothers self-reporting ETSE at the initial screening and did not report active smoking at baseline. Additional analyses were conducted for a subset of the mothers who had ETSE and whose cotinine level at baseline was <20 ng/ml.
Results
Of the 691 mothers who self-reported ETSE and denied actively smoking, approximately one quarter (27.3%) had SCLs of ≥20 ng/ml at baseline. When all mothers with an SCL of ≥20 ng/ml were excluded, a more refined subgroup of 520 mothers with ETSE were identified. The baseline characteristics of the 691 women who were randomly assigned to the intervention group and the usual care group are shown in Table 1. There seemed to be no differences between the two groups in sociodemographic, psychosocial or behavioral characteristics. Results of intervention group and usual care group comparisons of the baseline characteristics for women with ETSE and an SCL of <20 ng/ml also showed no differences between the two groups (data not shown.)
Table 1.
Baseline Characteristics for Mothers Reporting ETSE and no Active Smoking in Pregnancy by Care group (N=691)
| Characteristic | Intervention (n=335) |
Usual Care (n=356) |
|---|---|---|
| Maternal Age (mean±SD) | 23.4±4.8 | 24.1±5.1 |
| Education: < High School | 26.3% | 28.4% |
| Employed During Pregnancy | 36.1% | 39.9% |
| Marital Status: Married/Living with partner | 25.7% | 23.9% |
| Medicaid | 79.6% | 76.3% |
| Early PNC Initiation | 61.1% | 57.6% |
| Previous Preterm Delivery | 12.2% | 9.7% |
| Previous Multiple Birth | 0.0% | 1.5% |
| Alcohol Use during Pregnancy | 16.2% | 18.5% |
| Illicit Drug Use during Pregnancy | 10.2% | 7.3% |
| Depression | 40.0% | 39.9% |
| IPV | 31.0% | 29.8% |
ETSE: Environmental Tobacco Smoke Exposure
PNC: Prenatal Care
IPV: Intimate Partner Violence
Analyses for mothers who self-reported ETSE and no active smoking (n=691)
At the follow-up interview before delivery, rates for ETSE in the intervention group were significantly lower than in the usual care group. The rate of IPV was also significantly lower in the intervention group. No similar effect was seen on rates of depression (Table 2).
Table 2.
Behavioral Characteristics Before Delivery According to Care Group for Mothers Who Had ETSE and Did Not Report Active Smoking (A) and Those with an SCL of < 20 ng/ml (B)
| Behavioral Risks Before Delivery | |||
|---|---|---|---|
| A. All ETSE Mothers Excluding Smokers | |||
| Intervention (n=335) |
Usual Care (n=356) |
p-value | |
| ETSE | 53.9 | 68.2 | <0.001 |
| Depression | 32.5 | 36.5 | 0.31 |
| IPV | 4.7 | 9.9 | 0.02 |
| B. All ETSE Mothers Non-Smokers with SCL <20 ng/ml | |||
|
Intervention (n=247) |
Usual Care (n=273) |
p-value | |
| ETSE | 53.4 | 66.2 | 0.01 |
| Depression | 31.1 | 34.1 | 0.51 |
| IPV | 5.7 | 7.4 | 0.48 |
ETSE: Environmental Tobacco Smoke Exposure
IPV: Intimate Partner Violence
SCL: Salivary Cotinine Level
Logistic regression models for the 691 predicting ETSE before delivery controlled for the covariates with a significance level <0.20 in the bivariate analysis. These covariates were: care group, maternal age, marital status, Medicaid and depression. Reduced logistic models were constructed retaining care group and those covariates with significance <0.05 (Table 3). Women who were randomly assigned to the intervention were less likely to self-report ETSE before delivery controlling for other covariates (OR=0.50, 95%CI=0.35-0.71). In addition, being a Medicaid recipient increased significantly the likelihood of ETSE before delivery. In this group of 691 mothers, as age got older, the risk for ETSE before delivery decreased significantly.
Table 3.
Reduced Logistic Regression Models for Predicting ETSE Before Delivery
| Characteristic | Odds Ratio (95% CI) |
|---|---|
| A. All ETSE Mothers Excluding Smokers (n=691)† | |
| Care Group | 0.50 (0.35-0.71) |
| Maternal Age | 0.96 (0.93-0.99) |
| Medicaid | 1.97 (1.31-2.96) |
| B. All ETSE Mothers Non-Smokers with SCL < 20 ng/ml (n=520)† | |
| Care Group | 0.57 (0.38-0.84) |
| Medicaid | 1.74 (1.11-2.72) |
CI: Confidence interval.
ETSE: Environmental Tobacco Smoke Exposure
SCL: Salivary Cotinine Level
Actual numbers used in the analysis are smaller because of missing follow-up data.
In Table 4, reproductive outcomes were compared by care group. Infant outcome results were available on 82% of live births for birth weight and 85% for gestational age. The intervention group had a significantly lower incidence of VLBW and VPTB.
Table 4.
Bivariate Comparisons of Pregnancy Outcomes by Care group
| Pregnancy Outcome | Intervention | Usual Care | p-value |
|---|---|---|---|
| All ETSE Mothers Excluding Smokers | |||
| (n=335) | (n=356) | (n=691) | |
| Miscarriage | 1.4% | 2.8% | 0.21 |
| Perinatal Deaths (20 weeks-28 days) | 1.4% | 1.9% | 0.59 |
| Non-Live Birth | 2.7% | 4.7% | 0.19 |
| Neonatal Hospitalization days (mean±SD) | 3.1±3.3 | 5.5±22.9 | 0.08 |
| LBW (<2,500 grams) | 9.5% | 13.9% | 0.11 |
| VLBW (<1,500 grams) | 0.4% | 3.1% | 0.02 |
| PTB (<37 weeks) | 11.6% | 13.5% | 0.49 |
| VPTB (<34 weeks) | 1.4% | 5.6% | 0.01 |
| ETSE Mothers Non-Smokers with Baseline SCL <20 ng/ml | |||
| (n=247) | (n=273) | (n=520) | |
| Miscarriage | 0.9% | 1.6% | 0.47 |
| Perinatal Deaths (20 weeks-28 days) | 1.3% | 1.3% | 0. 93 |
| Non-Live Birth | 2.2% | 2.9% | 0.65 |
| Neonatal Hospitalization days (mean±SD) | 3.1±2.8 | 6.0±25.6 | 0.10 |
| LBW (<2,500 grams) | 11.3% | 12.9% | 0.59 |
| VLBW (<1,500 grams) | 0.5% | 2.6% | 0.07 |
| PTB (<37 weeks) | 11.8% | 13.5% | 0.59 |
| VPTB (<34 weeks) | 0.5% | 5.5% | 0.01 |
ETSE: Environmental Tobacco Smoke Exposure
LBW: Low Birth Weight
VLBW: Very Low Birth Weight
PTB: Preterm Birth
VPTB: Very Preterm Birth
SCL: Salivary Cotinine Level
To construct the reduced logistic models, we conducted bivariate analyses comparing mothers who delivered VLBW infants with those who delivered infants who weighed ≥1,500 grams. Variables in this bivariate comparison included care group, previous preterm delivery, depression and IPV. Significant differences for mothers who delivered VLBW infants were randomization to the intervention group (10.0% vs. 48.8%, p=0.02), previous preterm delivery (40.0% vs. 10.9%, p<0.01) and IPV at baseline (60.0% vs. 29.4%, p=0.04). In the case of VPTB, variables with significant differences included randomization to the intervention group (19.1% vs. 49.5%, p<0.01), previous preterm delivery (28.6% vs. 11.1%, p=0.01) and IPV at baseline (52.4% vs. 29.3%, p=0.02). For these significant variables, when included in reduced logistic models, randomization to the intervention group significantly reduced the chances of VLBW and VPTB (Table 5). Previous preterm delivery was significantly associated with increased chances of VLBW and VPTB. IPV at baseline significantly increased the chances of VLBW and VPTB.
Table 5.
Reduced Logistic Regression Models for Predicting Very Low Birth Weight and Very Preterm Birth
| Characteristic | VLBW OR (95% CI) |
VPTB OR (95% CI) |
|---|---|---|
| All ETSE Mothers Excluding Smokers (n=691)† | ||
| Care Group | 0.11 (0.01-0.86) | 0.22 (0.07-0.68) |
| Previous Preterm Delivery | 5.43 (1.44-20.55) | 3.16 (1.15-8.67) |
| IPV at Baseline | 3.75 (1.02-13.81) | 2.71 (1.11-6.62) |
| ETSE Mothers Non-smokers with Baseline SCL<20 ng/ml (n=520)† | ||
| Care Group | 0.18 (0.02-1.49) | 0.07 (0.01-0.57) |
| IPV at Baseline | --- | 2.97 (1.00-8.79) |
VLBW: Very Low Birth Weight
VPTB: Very Preterm Birth
CI: Confidence interval.
ETSE: Environmental Tobacco Smoke Exposure
IPV: Intimate Partner Violence
SCL: Salivary Cotinine Level
Actual numbers used in the analysis are smaller because of missing pregnancy outcomes data.
Analyses for mothers who self-reported ETSE and no active smoking with SCL < 20 ng/ml (n=520)
For this group of mothers, the follow-up interview before delivery also showed rates for ETSE to be significantly lower in the intervention group as compared with the usual-care group. Depression and IPV were not significant (Table 2).
Logistic regression models for the 520 mothers predicting ETSE before delivery controlled for the covariates with significance level <0.20 in the bivariate analysis. These covariates were care group, maternal age, marital status, educational level, Medicaid and illicit drug use during pregnancy. Reduced logistic models were constructed retaining care group and covariates with significance <0.05 (Table 3). Randomization to the intervention group significantly reduced ETSE before delivery (OR=0.57, 95%CI=0.38-0.84). In addition, being a Medicaid recipient increased significantly the likelihood of ETSE before delivery.
Reproductive outcomes were compared by care group. Infant outcome results on live births are available on 86% for birth weight and 88% for gestational age. Results for the group of 520 showed only VPTB to be significantly different between the two groups (Table 4).
To construct the reduced logistical models, we conducted bivariate analyses comparing mothers who delivered VLBW infants with those who delivered infants who weighed ≥1,500 grams. Variables in this bivariate comparison included care group, previous preterm delivery, depression and IPV. Significant differences for mothers who delivered VLBW infants were randomization to the intervention group (10.0% vs. 48.8%, p=0.02), previous preterm delivery (40.0% vs. 10.9%, p<0.01) and IPV at baseline (60.0% vs. 29.4%, p=0.04). In the case of VPTB, variables with significant differences included randomization to the intervention group (19.1% vs. 49.5%, p<0.01), previous preterm delivery (28.6% vs. 11.1%, p=0.01) and IPV at baseline (52.4% vs. 29.3%, p=0.02).
For this subgroup of mothers, the only significant differences for mothers who delivered VPTB compared to those who delivered infants born at ≤34 weeks' gestation were randomization to the intervention group (OR=0.07, 95%CI=0.01-0.57) and IPV at baseline (OR=2.97, 95%CI=1.00-8.79) (Table 5).
Discussion
The built environment and toxicant exposure within it have been shown to represent a disproportionately high health risk to populations who live in poverty.9 Our study shows ETSE to be a commonly encountered problem by pregnant African-American women who may themselves smoke during pregnancy or non-smokers who live or work with smokers. Of the pregnant African-American women eligible to participate in our study, three times as many acknowledged ETSE as compared with women who reported smoking. The lower rates of smoking in pregnancy in African-American women should not be misinterpreted as an overall low risk of exposure to tobacco smoke.
Our intervention was successful in significantly reducing reported ETSE during pregnancy. The only other factor associated with reduced exposure was maternal age. Medicaid (a marker for poverty) was associated with increased exposure. The only other intervention study that attempted to reduce ETSE in pregnancy and of which we are aware was unsuccessful in reaching its goal.11 The author's results agreed with our own in that older mothers seemed to have lower ETSE, although the differences in that study did not reach significance.
ETSE during pregnancy is proved to be strongly associated with poor reproductive outcomes, including elevated rates of LBW. ETSE lowers the infants' adjusted mean birth weight by 33 to 36 grams, with a significant dose response relationship.25,26 Factors such as advancing maternal age have been shown to increase the risk for LBW among smokers.27 It is unclear what role such modifying factors may play in mothers exposed to ETS. Our results do not show an association of VLBW and VPTB with advancing maternal age, but other risks such as IPV were associated with increased rates of VLBW and/or VPTB. The history of a previous preterm delivery remains consistently the strongest predictor of VLBW and VPTB among non-smoking mothers with ETSE.
There seems to be disagreement in the literature regarding the effect of ETSE on gestational age. Two recent publications showed no association between ETSE and prematurity25,26; one of them,25 a large meta-analysis, concluded that there was no effect of ETSE on gestational age. Another study did not show a significant association between ETSE and PTB but found association with the rates of VPTB (adjusted OR=2.4, 95%CI=1.0-5.3).27 Our study shows similar results. Our cognitive-behavioral integrated intervention that addressed various risk factors including IPV reduced the rates for VPTB after adjusting for confounders in both groups that were included in these analyses. The intervention showed a significant reduction in VPTB.
The effect of the intervention on the reduction of the higher spectrum of morbidity (VPTB and VLBW) is in itself intriguing. Mothers with the higher level of reproductive morbidity seemed to exhibit the highest rates of psycho-social and behavioral risk. Such mothers may have benefited significantly from the integrated approach that addressed multiple co-occurring risks simultaneously. Our results did not confirm significant changes in the intervention group except in the case of IPV reduction for mothers who reported ETS exposure and no smoking during pregnancy. Nonetheless reduction in degree of depression or IPV may not be detectable in our comparisons. Furthermore, a catalystic effect as a result of modest levels of reduction in multiple co-occurring risks may not be captured in a significant reduction in each risk alone. This is a topic that deserves additional study in larger population samples.
The main strengths of our study include the prospective, controlled trial and pregnancy outcomes as the end-points. The limitations of the study include its restriction to high risk African-American women and the lack of its generalizability to a broader population. The sample size of this study was calculated using a presumed reduction of the designated risks in the intervention group. The extent of the effect of the intervention on pregnancy outcomes was not part of our assumptions in sample size calculations. This limitation in sample size may have prevented differences across groups from reaching statistical significance, especially for more rare outcomes. Lower rates of miscarriage and longer hospital stays as well as higher rates of non-live births and low birth weight were noted in some subgroup analyses but may have not reached significance due to inadequacy of sample size. The non-disclosure of active smoking in 27% of mothers who self-reported only as ETS exposed is in agreement with other investigators who reported reluctance of pregnant women to share information about smoking during pregnancy.28 In addition, the significant results for both all mothers with ETSE and those with an SCL of <20 ng/ml are consistent. The chemical confirmation by SCL measurement is reassuring that our findings are valid even for mothers with relatively low levels of salivary cotinine. Additional studies with even lower cut-off points are needed.
Our intervention required a specialized staff of master's level trained social work and mental health professionals who may not be readily available at most PNC sites. Adding comparably trained professionals to a PNC team may present some financial challenges.
Finally the intervention did not encourage participation of other household members who could have assisted the pregnant mother in negotiating and implementing a household smoking ban. Existing evidence shows the added efficacy of household support in environmental tobacco smoke avoidance.15
Conclusions
Our study is the first, to our knowledge, to show that a cognitive-behavioral intervention that is delivered during PNC can assist African-American mothers in reducing their risk of ETSE and improving their pregnancy outcomes. A simple screening method that is used in the clinic identified a population at risk. It was evident that the intervention was associated with reduced ETSE and a varying degree of improved pregnancy outcomes. The most consistent association with ETSE reduction was a significant improvement in the rates of VPTB. This is most relevant in the potential impact on the reduction of infant mortality rates because the smallest and most premature infants are those that are at the highest risk of death and significant morbidity.13,29
Acknowledgments
This study was supported by grants no. 3U18HD030445; 3U18HD030447; 5U18HD31206; 3U18HD03919; 5U18HD036104, Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center on Minority Health and Health Disparities.
Abbreviations
- ETSE
environmental tobacco smoke exposure
- RCT
randomized controlled trial
- PNC
prenatal care
- IPV
intimate partner violence
- SCL
salivary cotinine level
- LBW
low birth weight
- VLBW
very low birth weight
- PTB
preterm birth
- VPTB
very preterm birth
- OR
odds ratio
- CI
confidence interval
- DC
District of Columbia
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- 1.Ehrenthal DB, Jurkovitz C, Hoffman M, Kroelinger C, Weintraub W. A population study of the contribution of medical comorbidity to the risk of prematurity in blacks. Am J Obstet Gynecol. 2007;197:409.e1–409.e6. doi: 10.1016/j.ajog.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Calvo J, Jackson J, Hansford C, Woodman C. Psychosocial factors and birth outcomes: African American women in case management. J Health Care Poor Underserved. 1998;9:395–419. doi: 10.1353/hpu.2010.0409. [DOI] [PubMed] [Google Scholar]
- 3.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(suppl2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 4.Orr ST, James FA, Blackmoore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm birth among African-American women in Baltimore Maryland. Am J Epidemiol. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 5.Murphy CC, Schei B, Myhr TL, Du Mont J. Abuse: A risk factor for low birthweight? A systematic review and meta-analysis. CMAJ. 2001;164:1567–1572. [PMC free article] [PubMed] [Google Scholar]
- 6.deFur PL, Evans GW, Cohen Hubal EA, et al. Vulnerability as a function of individual and group resources in cumulative risk assessment. Environ Health Perspect. 2007;115:817–824. doi: 10.1289/ehp.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sims M, Sims TL, Bruce MA. Urban poverty and infant mortality rate disparities. J Nat Med Assoc. 2007;99:349–356. [PMC free article] [PubMed] [Google Scholar]
- 8.Wigle DT, Arbuckle TE, Turner MC, et al. Epidemiological evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 9.Perera FP, Rauh V, Tsai WY, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects to exposure to environmental tobacco smoke and material hardship among inner city children. Neurotoxicol Teratol. 2004;26:373–385. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pletsch PK. Reduction of primary and secondary smoke exposure for low income black pregnant women. Nurs Clin N Am. 2002;37:315–329. doi: 10.1016/s0029-6465(01)00011-1. [DOI] [PubMed] [Google Scholar]
- 12.El-Mohandes AE, Kiely M, Joseph JG, et al. An intervention to improve postpartum outcomes in African American mothers: A randomized clinical trial. Obstet Gynecol. 2008;112:611–620. doi: 10.1097/AOG.0b013e3181834b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin JA, Hamilton BE, Sutton PD, et al. National vital statistics reports. 6. Vol. 56. Hyattsville, MD: National Center fro Health Statistics; 2007. Births: final data for 2005. [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. Health, United States, 2007 with chart book on trends in the health of Americans. Hyattsville, MD: 2008. [PubMed] [Google Scholar]
- 15.Blake SM, Murray KD, El-Khorazaty MN, et al. Environmental tobacco smoke avoidance among pregnant African American non-smokers. Am J Prev Med. 2009;36:225–234. doi: 10.1016/j.amepre.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra DP, Nguyen RH. Environmental tobacco smoke and low birth weight a hazard in the workplace? Environ Health Perspect. 1999;107(Suppl 6):897–904. doi: 10.1289/ehp.99107s6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Khorazaty MN, Johnson AA, Kiely M, et al. Recruitment and retention of low-income minority women in a behavioral intervention to reduce smoking, depression, and intimate partner violence during pregnancy. BMC Public Health. 2007;7:233. doi: 10.1186/1471-2458-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz KS, Blake SM, Milligan RA, et al. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy and Childbirth. 2008;8:22. doi: 10.1186/1471-2393-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph JG, El-Mohandes AAE, Kiely M, et al. Results of a randomized clinical trial in high-risk pregnant African American women to reduce psychosocial and behavioral risk factors in pregnancy. Am J Public Health. 2009;99(6):1053–1061. doi: 10.2105/AJPH.2007.131425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Mohandes AE, Kiely M, Gantz MG, Blake SM, El-Khorazaty MN. Prediction of birth weight by cotinine levels during pregnancy in a population of Black smokers. Pediatrics. 2009;124(4):1053–1061. doi: 10.1542/peds.2008-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegaard HK, Kjærgaardm H, Møller LF, Wachmann H, Ottesen B. The effect of environmental tobacco smoke during pregnancy on birth weight. Acta Obstet Gyncol. 2006;85:675–681. doi: 10.1080/00016340600607032. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis MJ, Tunstall-Pedeo H, Feyerbend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from non-smokers. Am J Public Health. 1987;7:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction. 2008;103:1553–1561. doi: 10.1111/j.1360-0443.2008.02297.x. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107S:349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93:F351–361. doi: 10.1136/adc.2007.133553. [DOI] [PubMed] [Google Scholar]
- 26.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7:81. doi: 10.1186/1471-2458-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11:427–433. doi: 10.1097/00001648-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Webb DA, Boyd NR, Messina D, Windsor RA. The discrepancy between self-reported smoking status and urine cotinine levels among women enrolled in prenatal care at four publicly funded clinical sites. J Public Health Manag Pract. 2003;9:322–325. doi: 10.1097/00124784-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008;51:360–370. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]