Abstract
Traditionally, epilepsy has been considered to be a disorder of neuronal dysfunction. Based on this dogma, drug development efforts have largely focused on neurocentric model systems to screen for compounds that affect the function of neurons. Unfortunately, about 30% of all patients with epilepsy – or more than 20 million worldwide – are refractory to classical neurocentric pharmacotherapy. The failure of neurocentric pharmacotherapy in epilepsy requires radical rethinking and the search for novel therapeutic targets. Research from recent years suggests that epilepsy is a disorder of astrocyte dysfunction. Astrocytes are key regulators of the brain’s own anticonvulsant adenosine. Thus, any dysfunction in astrocyte metabolism will drastically affect the brain’s ability to control excitability via adenosinergic neuromodulation. This review will focus on the astrocyte-based enzyme adenosine kinase (ADK) as the key regulator of synaptic adenosine. Astrogliosis – a pathological hallmark of the epileptic brain – leads to overexpression of the adenosine-removing enzyme ADK and therefore to adenosine deficiency. Evidence from transgenic animals demonstrates that overexpression of ADK per se is sufficient to trigger seizures. Consequently, pharmacological inhibition of ADK is very effective in suppressing seizures that are refractory to classical antiepileptic drugs. The recent definition of ADK as rational target to predict and to prevent seizures in epilepsy has prompted the development of focal adenosine augmentation therapies (AATs) that have been designed to selectively reconstitute adenosinergic signalling within an area of astrogliosis-based adenosine-dysfunction. This therapeutic challenge has experimentally been met with polymeric or stem cell based brain implants to afford the focal delivery of adenosine.
Keywords: epilepsy, astrogliosis, epileptogenesis, adenosine, adenosine kinase, cell therapy, gene therapy
INTRODUCTION
Most clinically used antiepileptic drugs (AEDs) have been designed to affect neuronal function by directly affecting neuronal ion channels, presynaptic release mechanisms, or neuronal receptor function [1, 2]. Many of these therapeutic drug effects are based on solidly studied mechanisms. For example, the gabapentinoids gabapentin and pregabalin exert their anticonvulsant activity by binding to α2δ subunits of voltage-gated calcium channels [3, 4], whereas mutations in those subunits lead to spontaneous seizures in mice [5, 6]. Despite solid characterization of AEDs and the addition of new compounds to the clinical armamentarium, it is estimated that more than 30% of all patients with epilepsy remain refractory to treatment with neurocentric therapeutic strategies or suffer from intolerable side effects [2]. Therefore, alternative treatment strategies have been explored (and shown to be efficient) that range from electrical stimulation [7, 8] to the ketogenic diet [9–12]. Recent findings suggest that these alternative treatments are at least in part based on mechanisms related to adenosine [12, 13]. Moreover, astrocytes, the key regulators of synaptic adenosine, appear to be crucially involved in seizure regulation. This review will first discuss the role of astrocyte dysfunction in general and then focus on adenosine and its regulation within the context of epilepsy, whereas other components of purinergic signalling will be covered in different reviews of this issue.
AN ASTROCYTIC BASIS OF EPILEPSY
Astrogliosis – the pathological proliferation and hypertrophy of astrocytes – is a pathological hallmark of the epileptic brain and alterations in distinct astrocyte membrane channels, receptors and transporters have all been associated with the epileptic state [14]. The concept of the tripartite synapse in which astrocytic processes ensheath neuronal synapses and contribute to the regulation of synaptic transmission [15] is an excellent explanation how astrocytes can influence and modify neuronal function and, consequently, dysfunctional gliotransmission plays an important role in the pathophysiology of epilepsy [16]. An astrocytic basis of epilepsy has first been proposed by Nedergaard and colleagues based on the demonstration that paroxysmal depolarization shifts that are involved in the initiation of seizures can be triggered by the release of glutamate from extrasynaptic sources or by photolysis of caged Ca2+ in astrocytes [17]. In these studies three AEDs – valproate, gabapentin, and phenytoin – decreased the ability of astrocytes to transmit Ca2+ signalling. It was further demonstrated that astrocytic glutamate was released via SNARE-dependent exocytosis of glutamate-containing vesicles [18]. Together, these studies suggested that astrocytic glutamate release may play an epileptogenic role in the initiation of epileptic seizures under pathological conditions. In line with these findings, gene expression changes in astrocytes in temporal lobe epilepsy were suggested to contribute to an increased release of glutamate by astrocytes [19]. Therefore, astrogliosis in epilepsy might be a likely contributing factor to the glutamate overflow that is a characteristic of the epileptic brain [20]. However, the notion that astrocyte-derived glutamate contributes to epileptiform activity has recently been challenged based on pharmacological studies performed in hippocampal slice models of epileptiform activity [21]. In these studies, TTX blocked ictal- and interictal-like epileptiform activity without affecting slow inward currents that depend on astrocytic glutamate release. In contrast, NMDA receptor antagonists blocked the astrocyte dependent slow inward currents, but did not prevent the generation of epileptiform events. Thus, based on this study, the non-synaptic glutamate release from astrocytes appears not to be necessary for the generation of epileptiform activity in vitro.
In addition to the direct contribution of astrocytes to calcium and glutamate signalling, astrocytes have the capability to affect several epileptogenic and ictogenic mechanisms more indirectly. Thus, specific inflammatory pathways are chronically activated during epileptogenesis and astrocytes have been shown to sustain those inflammatory processes by activating the interleukin 1 beta system [22]. Astrocytes of the epileptic brain are also characterized by structural reorganization and a loss of astrocytic domain organization, morphological alterations that are thought to contribute to the structural basis for recurrent excitation of the epileptic brain [23]. Astroglial gap junctions provide an activity-dependent intercellular pathway for the delivery of energetic metabolites from blood vessels to distal neurons and thereby play critical roles in coupling neuronal function to the metabolic state of an organism [24]. In particular, astrocytes play important roles in ATP-signalling [16] and astrocytic connexin 43 hemichannels may contribute significantly to extrasynaptic ATP efflux [25]. It was recently hypothesized that ATP released from astrocytes in response to neuronal activity is a source of surround inhibition to adjacent neurons, a mechanism that could prevent seizure propagation [16]. The identification of non-neuronal and non-chemical synaptic signalling pathways described here offers new and promising targets for the therapy of epilepsy [26].
ASTROCYTES AND ADENOSINE
Astrocytes are key regulators of the brain’s endogenous anticonvulsant adenosine and an astrocyte-based adenosine cycle has been proposed [27, 28]. Astrocytes are a major source of ATP that can be released into the synaptic cleft via hemichannels [25] or via regulated synaptic release [29]. Synaptic ATP is rapidly cleaved by a series of ectonucleotidases into adenosine [30, 31]. In pioneering experiments by Newman [32] it was demonstrated in rat retina that activation of glial cells reduced the firing rate of neurons that displayed spontaneous spike activity. This effect could be blocked by an adenosine A1R antagonist or by inhibitors of ATP cleavage [32]; these findings demonstrated that activated glial cells can inhibit neurons in the retina by the release of ATP, which is converted to adenosine by ecto-enzymes and subsequently activates neuronal adenosine receptors. In an elegant series of experiments Haydon and co-workers demonstrated that astrocyte-derived ATP regulates synaptic strength and plasticity [29]. In these experiments the authors used inducible transgenic mice that expressed a dominant-negative SNARE domain selectively in astrocytes to block the release of transmitters (including ATP) from these glial cells. It was demonstrated that astrocytes tonically suppressed synaptic transmission by releasing adenosine triphosphate as a precursor of adenosine, an effect that was abolished in the mutant mice. These results and related studies, which demonstrated regulated ATP release from astrocytes through lysosome exocytosis [33], indicate that astrocytes – via regulating the release of a precursor of adenosine (i.e. ATP) – play important roles in the regulation and coordination of synaptic strength, plasticity, and synaptic networks [29, 34]. In line with these findings, the inhibition of gliotransmission in dominant-negative SNARE transgenic mice attenuated the accumulation of sleep pressure, assessed by measuring the slow wave activity of the EEG during NREM sleep, and prevented cognitive deficits associated with sleep loss [35]. Findings from this study indicate that astrocytes modulate the accumulation of sleep pressure and its cognitive consequences through a pathway involving A1 receptors [35]. Subsequent studies using adenosine microelectrode biosensors demonstrated directly in neurochemical online measurements that ATP is a precursor of synaptic adenosine [36, 37]. Using similar approaches, it was further demonstrated that astrocytic adenosine kinase (ADK) regulates basal synaptic adenosine levels and seizure activity but not activity-dependant adenosine release in the hippocampus [38].
Astrocytes contain two types of equilibrative nucleoside transporters [39], which facilitate the rapid equilibration of synaptic and intra-astrocytic levels of adenosine [27, 28]. In contrast to neurotransmitters that all have their respective energy-driven transporter-mediated re-uptake system to terminate synaptic activity of the neurotransmitter, an equivalent system lacks for adenosine. Instead, the intracellular astrocyte-based [40] enzyme ADK appears to fulfill the role of a metabolic re-uptake system for adenosine. Therefore, astrocytic ADK (see subsequent chapters for details) plays a key role for the regulation of synaptic levels of adenosine [41]. The critical contribution of astrocytes for the regulation of synaptic adenosine was further corroborated in neuron / astrocyte coculture systems, in which NMDA-evoked neuronal adenosine release was subject to metabolism by added astrocytes [42].
ADENOSINE KINASE AND ADENOSINE
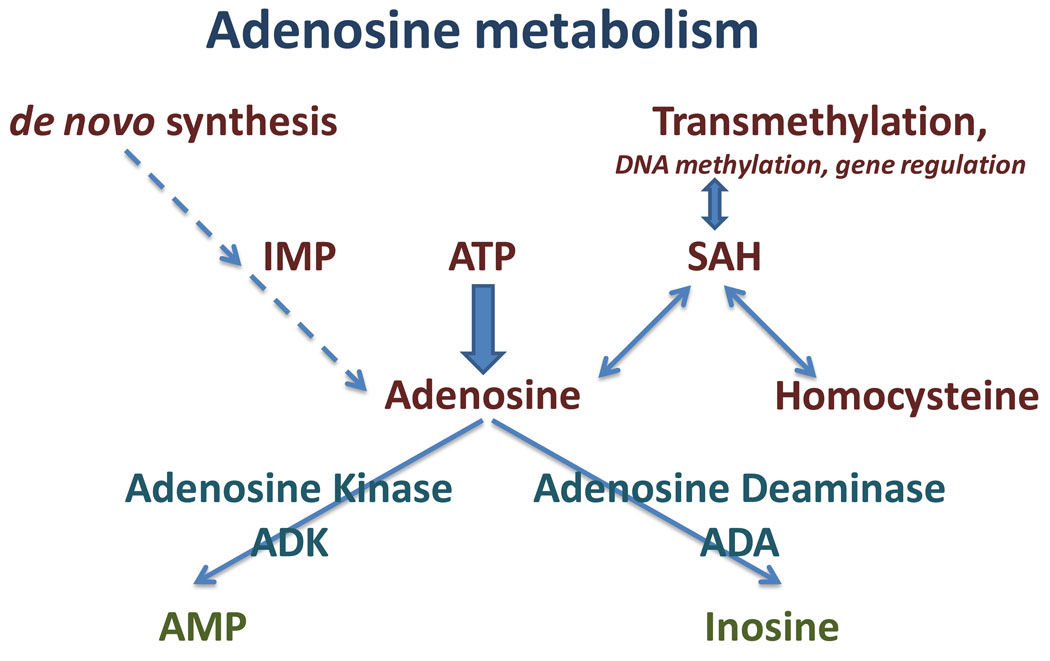
Adenosine levels can be reduced by adenosine deaminase (ADA, forming inosine), which shows highest expression levels in tongue, cells lining the intestinal tract, and thymus, whereas its expression in brain is rather low and limited to specific nuclei [43–45]. Adenosine kinase (ADK, E.C. 2.7.1.20) is responsible for the enzymatic phosphorylation of adenosine into AMP [46] (Fig. 1). In a comparative study performed in brain slices it was demonstrated that inhibition of ADK, but not of ADA, led to increases in ambient adenosine resulting in presynaptic inhibition [47]. It was realized more than 30 years ago that mammalian cells deficient in ADK are capable of excreting accumulating purines including adenosine [48]. ADK belongs to the ribokinase family of proteins, has an early evolutionary origin and is highly conserved between species [49], with only a few ADK variants from microorganisms being structurally different [50]. Based on its low KM for adenosine (0.15 µM) and a highly active substrate cycle between adenosine and AMP, ADK is considered the key enzyme for the regulation of ambient adenosine and minor changes in ADK activity can rapidly translate into major changes in ambient adenosine [51, 52]. Therefore, a genetic knockout or knockdown of ADK in cultured cells is a very effective strategy to induce cellular adenosine release [53], a strategy that has therapeutic value [54–56]. ADK appears to be regulated almost exclusively at the protein level. Studies with Leishmania ADK demonstrated that the enzyme is inactivated by aggregation, a conformation that is stabilized by ADP, whereas the active monomer is stabilized by a cyclophilin [57]. In contrast, protein phosphorylation was excluded as a potential mechanism for ADK-regulation [58].
Fig. 1.
Major routes of adenosine metabolism
In adult brain, ADK is largely expressed in astrocytes [40], and astrocytic ADK was shown to regulate the basal synaptic levels of adenosine [38]. Under conditions of acute challenge to the brain, e.g. after focal ischemia or during seizures, ADK is rapidly downregulated, likely as a physiological response to raise the protective adenosine-tone [59]. Physiological alterations of ADK have direct impact on brain function. Thus, transgenic overexpression of ADK in brain was shown to be associated with reduced adenosine and seizures [60], with increased susceptibility to stroke [61], with learning impairment and altered susceptibility to psychomimetic drugs [62], and with changes in myelination [63]. Due to its wide implications as a regulator of brain function, the pharmaceutical development of ADK inhibitors has gained much interest as potential anticonvulsant and antinociceptive agents [64, 65].
ADENOSINE KINASE IS OVEREXPRESSED IN EPILEPSY
As indicated above, astrogliosis is a pathological hallmark of the epileptic brain. Research from our laboratory has shown that astrogliosis in animal models of epilepsy is associated with increased levels of the astrocyte-based enzyme ADK [28, 66, 67]. Likewise, deficient adenosine signalling was found in the hippocampus of kindled rats [68]. The intrahippocampal injection of kainic acid (KA) in mice triggers hippocampal sclerosis and chronic recurrent seizure activity that closely mimics human mesial temporal lobe epilepsy [69–73]. During a time span of 4 weeks affected mice develop profound astrogliosis throughout the injected hippocampus that is associated with overexpression of ADK including an increase of 177% of enzyme activity compared to control hippocampus resulting in a global reduction of the adenosine-based inhibitory tone [74]. Seizures were suppressed by a low dose of the ADK-inhibitor 5-iodotubercidine (ITU) [74]. These findings suggested for the first time that astrogliosis via increasing ADK expression might contribute to the generation of seizures. However, in this complex model of epilepsy it was not possible to segregate the effects of other potential contributors to seizure generation (e.g. mossy fiber sprouting, granule cell dispersion, loss of interneurons) from astrogliosis.
To study selectively the effect of astrogliosis and ADK on seizure generation we generated a mouse model of CA3-selective epileptogenesis that develops focal astrogliosis in the absence of other confounding factors such as mossy fiber sprouting or granule cell dispersion [75]. In this model, the unilateral intraamygdaloid injection of KA leads to acute CA3-selective neuronal cell loss that constitutes a trigger for subsequent astrogliosis [75]. Three weeks after KA-injection profound CA3-selective astrogliosis had developed with colocalized overexpression of ADK. Electrographic seizures could be recorded from the astrogliotic CA3, but not from any other brain region [75]. Strikingly, during epileptogenesis seizures coincided temporally with the development of astrogliosis and overexpressed ADK [41]. Seizures in this model could be suppressed by the ADK-inhibitor ITU or by the A1R agonist CCPA. Together, these findings suggest that astrogliosis (with overexpression of ADK) is sufficient to trigger seizures in the absence of any other epileptogenetic histopathological alteration. Based on these findings, pharmacological inhibition of ADK should be highly effective in preventing seizures. Indeed, ADK inhibitors are very effective anticonvulsant agents that have the potential to amplify endogenous adenosine-signalling thereby minimizing side effects [64, 76–78]. Most importantly, the inhibition of ADK was shown to be effective in a model of pharmacoresistant epilepsy [74].
ADK expression levels have not yet been evaluated in human epileptic brain. However, microdialysis samples indicate lower adenosine baseline levels in epileptic compared to control human hippocampus [79]. Human epileptic brain is also characterized by changes in A1R expression and downregulation [80] as well as upregulation [81] of A1Rs have been reported.
OVEREXPRESSION OF ADENOSINE KINASE TRIGGERS SEIZURES
The studies described above show a close association of astrogliosis, overexpression of ADK, and the expression of spontaneous recurrent seizures. To address the question, whether astrogliosis or overexpression of ADK per se are responsible for seizure expression, an ADK transgene was ubiquitously overexpressed in brain (Adk-tg mice) [75]. Transgenic overexpression of ADK was found to be sufficient to trigger spontaneous recurrent electrographic hippocampal seizures at a rate of about 4 seizures per hour, with each seizure lasting about 20 seconds [41]. Most importantly, those seizures were recorded in the absence of any other epileptogenic events such as neuronal cell loss, astrogliosis, mossy fiber sprouting, or granule cell dispersion [41, 75]. To provide a further molecular dissection of astrogliosis from ADK expression levels, Adk-tg mice were subjected to 20 minutes of intraamygdaloid KA-induced status epilepticus (SE). This treatment resulted in a degree of acute seizure severity and corresponding acute neuronal cell loss that was comparable to data from wild-type mice subjected to 30 minutes of SE [82]. The acute KA-induced injury (i.e. trigger for subsequent epileptogenesis) led to an ablation of ADK-expressing CA3 neurons. During a time span of three weeks Adk-tg mice subjected to 20 minutes of SE developed ipsilateral CA3-selective astrogliosis, however without associated overexpression of ADK, whereas the contralateral hippocampus was still characterized by overexpression of transgenic ADK. EEG recordings performed three weeks after KA-injection revealed recurrent electrographic seizures in the ADK-overexpressing contralateral CA3 (in the absence of astrogliosis), but not a single seizure could be detected in the ipsilateral injured CA3 that was characterized by astrogliosis in the absence of overexpressed ADK [82]. These findings further highlight that overexpression of ADK as such rather than astrogliosis is sufficient to trigger seizures. If this assumption is true, then a genetic reduction of ADK should render the brain resistant to seizure development. To test this possibility, fb-Adk-def mice were generated that are characterized by reduced levels of ADK in forebrain amounting to approximately 60% of total expression levels in matched wild-type mice. These animals were completely resistant to acute KA-induced SE and injury; following intraamygdaloid KA injection, injurious seizures were never observed and not a single TUNEL-positive cell was found 24h after KA-injection [75]. However, when KA was paired with the A1R antagonist DPCPX wild-type like seizure activity was restored and the level of the acute CA3-restrictive injury closely matched wild-type controls. Co-injection of KA with DPCPX permitted us to recreate a wild type-like trigger for subsequent epileptogenesis in fb-Adk-def mice, however, subsequently those animals were under the control of reduced forebrain ADK [75]. Strikingly, three weeks after KA-injection, these animals did not show overt signs of astrogliosis, ADK levels continued to be reduced and no seizures were detected [75]. These findings suggest that a genetic reduction of ADK renders the brain resistant to the development of spontaneous seizures. Therefore, ADK constitutes a valid therapeutic target for the prediction and prevention of seizures in epilepsy. Importantly, ADK regulates basal synaptic adenosine levels and seizure activity, however without affecting activity dependent adenosine-release in hippocampus [38]. Together the studies described above constitute a direct molecular explanation why pharmacological inhibition of ADK is such a powerful tool for seizure suppression [64, 65, 78, 83–85]: because endogenous adenosine levels rise during times of stress [86] (e.g. seizures, lack of oxygen), agents (e.g. the ADK inhibitor ABT-702 [87–89]) that amplify this site- and event-specific surge of adenosine could provide antiseizure activity similar to that of adenosine receptor agonists [64, 77]. Therefore, pharmacological inhibition of ADK is considered to be an efficient tool for the inhibition of epileptic seizures [74, 77] and chronic pain [90]; these successes were associated with an improved therapeutic window compared to A1R agonists [91]. However, systemic application of ADK inhibitors might not be a therapeutic option for epilepsy due to interference with methionine metabolism in liver [92, 93] and the risk of brain hemorrhage [90, 94].
THE ADENOSINE KINASE HYPOTHESIS OF EPILEPSY
The findings described above form the basis for the ADK hypothesis of seizure generation that has been reviewed previously [28, 66]. Briefly, any type of injury or stress to the brain (e.g. traumatic brain injury, seizures, or stroke) triggers an acute surge of micromolar levels of adenosine that go far beyond normal levels that are in the 20 to 300 nM range (see review from Bertil Fredholm in this issue). The acute surge in adenosine is likely a combined consequence of increased ATP degradation and decreased adenosine clearance [59, 74]. These high levels of adenosine can constitute a trigger for several downstream effects that all combine to trigger subsequent epileptogenesis. Likely pathways involved are: (i) Changes in adenosine receptor expression levels. Most importantly, a decrease in A1R expression and an increase in A2AR expression on astrocytes influence astrocyte proliferation and may contribute to the development of astrogliosis [95–97]. (ii) Adenosine is an important modulator of the brain immune system [98]. It is well known that inflammatory responses, microglial activation, and changes in the blood brain barrier play early roles during epileptogenesis [22, 99–101]. Thus, an acute surge in adenosine might trigger several immuno-modulatory systems that may contribute to trigger subsequent astrogliosis.
Astrogliosis is a pathological hallmark of several neurological conditions. Studies from our lab have demonstrated that upregulation of ADK is always a consequence of, but not a cause for, astrogliosis [41, 75, 82]. Astrogliosis, together with upregulation of ADK has not only been observed in animal models of epilepsy, but also in animal models of stroke and Alzheimer’s disease (Boison, unpublished observations). Therefore it is fair to assume that astrogliotic upregulation of ADK might be a common pathophysiological pathway that might explain for instance the increased incidence of seizures in patients with Alzheimer’s disease [102–104] or following stroke [105]. Thus, according to the ADK hypothesis of epileptogenesis, an acute surge of adenosine following any type of brain insult might trigger astrogliosis, which in turn leads to overexpression of ADK and a resulting adenosine deficiency, which – as pointed out above – is sufficient to trigger seizures.
It is important to note that the ADK-associated seizures are frequent, but electrographic and subclinical in nature. ADK-associated seizures in the CA3 model of epileptogenesis can only be recorded with intracranial electrodes and these seizures do not spread, likely because the adenosine system in the vicinity of the epileptogenic focus is still intact [41, 75]. Interestingly, injection of these animals with a non-convulsive dose of the A1R antagonist DPCPX can turn those electrographic seizures into clinical grade convulsive seizures (Boison, unpublished observations). It is tempting to speculate that ADK-dependent focal electrographic seizures are (i) fairly common, but commonly not diagnosed with surface EEG recording electrodes; and (ii) that these seizures constitute an early event in epileptogenesis requiring secondary events or a secondary “hit”, leading to network rewiring or failure of other endogenous antiepileptogenic systems to turn those (undiagnosed?) pre-existing “silent” seizures into clinical seizures. In conclusion, dysfunction of the adenosine system might constitute a very early event in the epileptogenic cascade and targeting the adenosine system is therefore an effective means to prevent seizures in epilepsy, but possibly also to prevent epileptogenesis as such.
FOCAL ADENOSINE AUGMENTATION THERAPIES (AATs) FOR THE TREATMENT OF EPILEPSY
The paragraphs above suggest that augmentation of the adenosine system is an effective strategy for the prevention of seizures in epilepsy. Based on this neurochemical rationale adenosine augmentation therapies (AATs) have been assessed for therapeutic effectiveness [106–108]. While pharmacological AAT approaches are very effective in preventing seizures (see above), in general those approaches are hampered by significant peripheral and central side effects [106]. To circumvent those side effects focal AATs have been developed with the premise that focal reconstitution of adenosine signalling restricted to a local region of adenosine dysfunction (i.e. the epileptogenic astrogliotic “scar”) would restore normal adenosine signalling rather than leading to excessive amounts of adenosine. These strategies are considered to be safe since adenosine is an endogenous anticonvulsant and subject to normal metabolic clearance. Therefore any excessive or detrimental adenosine levels are unlikely to be reached in focal AAT approaches. A proof of principle for this therapeutic concept was first established by implanting adenosine-loaded ethylene vinyl acetate copolymers releasing 20 to 50 ng adenosine per day into the brain ventricles of kindled rats [109]. These polymeric implants provided robust reduction of stage 5 seizures for at least 7 days [109]. To prolong the duration of adenosine release from polymeric brain implants, hamster fibroblasts engineered to release adenosine based on genetic disruption of their Adk gene were encapsulated and thus immuno-isolated into semipermeable polyethersulfone-based hollow fibers and transplanted into the brain ventricles of fully kindled rats. These implants released about 40 ng adenosine per hour and provided almost complete seizure suppression for a duration of 12 days, which corresponded to the life expectancy of the encapsulated cells [110, 111]. The use of encapsulated adenosine releasing myoblasts lead to an extension of the therapeutic time window to up to 8 weeks [112]. In those studies, it was documented that prolonged implant-derived adenosine-release did not lead to desensitization of adenosine receptors, nor to the development of sedative side effects [112]. The effectiveness of focal adenosine augmentation for seizure suppression was further validated by an independent laboratory that used focal adenosine injections to prevent seizures in rats [113].
In order to provide more refined seizure control, mouse embryonic stem cells were engineered in our laboratory to release therapeutically effective doses of adenosine based on a bi-allelic genetic disruption of the endogenous Adk gene [114, 115]. These cells were subjected to a defined in vitro differentiation protocol to generate adenosine releasing ES-cell derived neural progenitor cells that can be transplanted directly into brain without the risk of tumor formation. Direct implants of these cells into the infrahippocampal fissure of immunosuppressed rats formed dense implants within the infrahippocampal fissure and some of the cells migrated into the ipsilateral CA1 region and differentiated into NeuN-positive neurons [56]. Most importantly, these implants effectively suppressed kindling epileptogenesis. The same type of graft prevented the development of epilepsy in the mouse model of CA3-selective epileptogenesis [75].
Embryonic stem cells are ethically controversial and cannot be used as autologous grafts in patients. In order to develop a cell-based system for the focal delivery of adenosine that would be compatible with future clinical applications, human mesenchymal stem cells were engineered to release adenosine using a lentivirus expressing a micro RNA directed against Adk. This strategy led to efficient knockdown of ADK and triggered the release of adenosine from these cells [54]. Subsequently, it was shown that infrahippocampal implants of these cells in mice attenuated both acute seizures and acute injury [54], and ameliorated the consequences of KA-induced epileptogenesis [116]. The natural biopolymer silk constitutes an excellent substrate to promote the release of adenosine from stem cells [53]. Thus, silk-based polymers seeded with mesenchymal stem cells derived from a patient and engineered to release adenosine might comprise a viable system for human epilepsy therapy.
To assess the therapeutic potential of silk-based adenosine release, silk based brain implants were engineered to release defined doses of adenosine with a stable release kinetic over several days [117]. Infrahippocampal implants of these constructs provided dose-dependent suppression of kindling epileptogenesis in rats [117]. Silk-based adenosine delivery at a dose of 1000 ng per day was shown to prevent fully kindled seizures, but most importantly novel and powerful antiepileptogenic effects of focal adenosine delivery were demonstrated [118].
CONCLUSIONS AND OUTLOOK
Compelling evidence suggests that a focal dysfunction of adenosine signalling – caused by astrogliosis-induced overexpression of ADK – is sufficient to trigger focal electrographic seizures, possibly a very early event in the epileptogenic cascade that finally leads to the expression of spontaneous recurrent, clinical seizures. Due to the central role of this pathway, focal AAT is highly effective in preventing seizures and likely a viable strategy to prevent epilepsy from developing. Thus, the modulation of adenosinergic signalling is a powerful strategy to interfere with epilepsy on different levels of its genesis. Is this approach ripe for translation into clinical practice?
Human mesial temporal lobe epilepsy is an ideal medical condition for the safe and step-wise introduction of AATs into clinical practice. Patients foreseen for surgical removal of the epileptogenic hippocampus are normally subjected to invasive diagnostics prior to their resection surgery. It would be highly feasible to combine pre-surgical diagnostic evaluation that includes intracranial EEG-recording electrodes with focal intrahippocampal infusion of adenosine. This procedure is feasible since adenosine is already approved by the US Food and Drug Administration (FDA) for intravenous infusion to prevent supraventricular tachycardia. Furthermore, experience is available from phase I clinical trials in which adenosine was infused into the intrathecal space in an attempt to provide a novel strategy for pain control [119–121]. These examples demonstrate the safety of adenosine delivery to a human patient. Furthermore, adenosine is an endogenous metabolite and subjected to rapid clearance. Therefore it is highly unlikely that toxic levels of adenosine can be reached. Transient intrafocal adenosine infusion into a patient would allow the following assessments: (i) demonstration that adenosine is effective in preventing epileptiform discharges in a patient with pharmacoresistant epilepsy; (ii) dose escalation studies can be used to identify the most effective dose range; (iii) finally, the epileptogenic hippocampus would be resected and subjected to detailed histopathological analysis. This procedure is considered to be ethically acceptable, since a patient would not be deprived of current standard of care. In a next step, silk-based adenosine releasing polymers could transiently be implanted into the epileptogenic hippocampus (e.g. for several days) prior to its surgical resection. Thus step-wise procedures are possible to implement focal AAT into clinical practice.
Despite this clinical promise there are also critical questions that need to be addressed. Preliminary assessment indicates that focal AATs are not associated with overt side effects [112]. To date however, potential central side effects of focal AATs have not been evaluated systematically, since a stable dose of adenosine over several days or weeks would be necessary for those studies. Future studies are needed to address in detail potential CNS-side effects of focal AAT including psychomotor and cognitive function. Of note is the observation that overexpression of ADK and adenosine deficiency triggers severe cognitive impairment in mice [62]; therefore AATs might eventually be beneficial in ameliorating cognitive impairment, which is a characteristic comorbidity of epilepsy. In addition to the therapeutic potential for epilepsy, AATs might also be useful for the amelioration of adenosine-dependent symptoms in conditions as diverse as chronic pain, Alzheimer’s disease, and schizophrenia [27, 122].
ACKNOWLEDGMENT
The work of the author is supported by grants R01NS058780, R01NS061844, R01MH083973, R21NS057475-01, and R21NS057538-01 from the National Institutes of Health (NIH).
REFERENCES
- 1.White HS, Smith MD, Wilcox KS. Mechanisms of action of antiepileptic drugs. Int Rev Neurobiol. 2007;81:85–110. doi: 10.1016/S0074-7742(06)81006-8. [DOI] [PubMed] [Google Scholar]
- 2.Vajda FJE. Pharmacotherapy of epilepsy: New armamentarium, new issues. Journal of Clinical Neuroscience. 2007;14:813–823. doi: 10.1016/j.jocn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Rogawski MA, Bazil CW. New molecular targets for Antiepileptic drugs: alpha 2 delta, SV2A, and K(v)7/KCNQ/M potassium channels. Current Neurology and Neuroscience Reports. 2008;8:345–352. doi: 10.1007/s11910-008-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Brill J, Klocke R, Paul D, Boison D, Gouder N, Klugbauer N, Hofmann F, Becker C-M, Becker K. Entla: A novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem. 2004;279:7322–7330. doi: 10.1074/jbc.M308778200. [DOI] [PubMed] [Google Scholar]
- 6.Donato R, Page KM, Koch D, Nieto-Rostro M, Foucault I, Davies A, Wilkinson T, Rees M, Edwards FA, Dolphin AC. The ducky(2J) mutation in Cacna2d2 results in reduced spontaneous Purkinje cell activity and altered gene expression. Journal of Neuroscience. 2006;26:12576–12586. doi: 10.1523/JNEUROSCI.3080-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonck K, De Herdt V, Boon P. Vagal nerve stimulation--a 15-year survey of an established treatment modality in epilepsy surgery. Adv Tech Stand Neurosurg. 2009;34:111–146. doi: 10.1007/978-3-211-78741-0_5. [DOI] [PubMed] [Google Scholar]
- 8.Boon P, Raedt R, de Herdt V, Wyckhuys T, Vonck K. Electrical stimulation for the treatment of epilepsy. Neurotherapeutics. 2009;6:218–227. doi: 10.1016/j.nurt.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Gulati S, Kalra V, Agarwala A, Kabra M. Seizure control and biochemical profile on the ketogenic diet in young children with refractory epilepsy--Indian experience. Seizure. 2009;18:446–449. doi: 10.1016/j.seizure.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic Diets: An Update for Child Neurologists. J Child Neurol. 2009 doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- 11.Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6:406–414. doi: 10.1016/j.nurt.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends in Neurosciences. 2008;31:273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, Bakos R, Nedergaard M. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 14.Binder DK, Steinhauser C. Functional changes in astroglial cells in epilepsy. Glia. 2006;54:358–368. doi: 10.1002/glia.20394. [DOI] [PubMed] [Google Scholar]
- 15.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Kumaria A, Tolias C, Burnstock G. ATP signalling in epilepsy. Purinergic Signalling. 2008;4:339–346. doi: 10.1007/s11302-008-9115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian GF, Azmi H, Takano T, Xu QW, Peng WG, Lin J, Oberheim N, Lou NH, Wang XH, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nature Medicine. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- 19.Lee TS, Mane S, Eid T, Zhao H, Lin A, Guan Z, Kim JH, Schweitzer J, King-Stevens D, Weber P, Spencer SS, Spencer DD, de Lanerolle NC. Gene expression in temporal lobe epilepsy is consistent with increased release of glutamate by astrocytes. Mol Med. 2007;13:1–13. doi: 10.2119/2006-00079.Lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia. 2008;49 Suppl 2:42–52. doi: 10.1111/j.1528-1167.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 21.Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 2006;26:9312–9322. doi: 10.1523/JNEUROSCI.2836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B, Nedergaard M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 25.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szente M. Possible exploitation of non-neuronal and non-chemical synaptic signalling pathways in epilepsy therapy. Current Signal Transduction Therapy. 2008;3:215–230. [Google Scholar]
- 27.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 30.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 32.Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 34.Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda) 2006;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- 35.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wall MJ, Atterbury A, Dale N. Control of basal extracellular adenosine concentration in rat cerebellum. Journal of Physiology-London. 2007;582:137–151. doi: 10.1113/jphysiol.2007.132050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alanko L, Porkka-Heiskanen T, Soinila S. Localization of equilibrative nucleoside transporters in the rat brain. J Chem Neuroanat. 2006;31:162–268. doi: 10.1016/j.jchemneu.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy J-M, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamzow CR, Xiong W, Parkinson FE. Adenosine Produced by Neurons Is Metabolized to Hypoxanthine by Astrocytes. Journal of Neuroscience Research. 2008;86:3447–3455. doi: 10.1002/jnr.21789. [DOI] [PubMed] [Google Scholar]
- 43.Geiger JD, Nagy JI. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci. 1986;6:2707–2714. doi: 10.1523/JNEUROSCI.06-09-02707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geiger JD, Nagy JI. Ontogenesis of adenosine deaminase activity in rat brain. J Neurochem. 1987;48:147–153. doi: 10.1111/j.1471-4159.1987.tb13139.x. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Geiger JD, Daddona PE, Nagy JI. Subcellular, regional and immunohistochemical localization of adenosine deaminase in various species. Brain Res Bull. 1987;19:473–484. doi: 10.1016/0361-9230(87)90152-3. [DOI] [PubMed] [Google Scholar]
- 46.Kornberg A, Pricer WE. Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. J. Biol. Chem. 1951;193:481–495. [PubMed] [Google Scholar]
- 47.Pak MA, Haas HL, Decking UKM, Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacol. 1994;33:1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 48.Chan T-S, Ishii K, Long C, Green H. Purine excretion by mammalian cells deficient in adenosine kinase. J. Cell. Physiol. 1973;81:315–322. doi: 10.1002/jcp.1040810304. [DOI] [PubMed] [Google Scholar]
- 49.Park J, Gupta RS. Adenosine kinase and ribokinase - the RK family of proteins. Cellular and Molecular Life Sciences. 2008;65:2875–2896. doi: 10.1007/s00018-008-8123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long MC, Shaddix SC, Moukha-Chafiq O, Maddry JA, Nagy L, Parker WB. Structure-activity relationship for adenosine kinase from Mycobacterium tuberculosis II. Modifications to the ribofuranosyl moiety. Biochem Pharmacol. 2008;75:1588–1600. doi: 10.1016/j.bcp.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bontemps F, Van den Berghe G, Hers HG. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983;80:2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arch JR, Newsholme EA. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and nvertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978;174:965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uebersax L, Fedele DE, Schumacher C, Kaplan DL, Merkle HP, Boison D, Meinel L. The support of adenosine release from adenosine kinase deficient ES cells by silk substrates. Biomaterials. 2006;27:4599–4607. doi: 10.1016/j.biomaterials.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 54.Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pignataro G, Studer FE, Wilz A, Simon RP, Boison D. Neuroprotection in ischemic mouse brain induced by stem cell-derived brain implants. J Cereb Blood Flow Metab. 2007;27:919–927. doi: 10.1038/sj.jcbfm.9600422. [DOI] [PubMed] [Google Scholar]
- 56.Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- 57.Sen B, Chakraborty A, Datta R, Bhattacharyya D, Datta AK. Reversal of ADP-mediated aggregation of adenosine kinase by cyclophilin leads to its reactivation. Biochemistry. 2006;45:263–271. doi: 10.1021/bi0518489. [DOI] [PubMed] [Google Scholar]
- 58.Sahin B, Kansy JW, Nairn AC, Spychala J, Ealick SE, Fienberg AA, Greene RW, Bibb JA. Molecular characterization of recombinant mouse adenosine kinase and evaluation as a target for protein phosphorylation. Eur J Biochem. 2004;271:3547–3555. doi: 10.1111/j.1432-1033.2004.04291.x. [DOI] [PubMed] [Google Scholar]
- 59.Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- 60.Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 61.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- 62.Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci. 2007;26:3237–3252. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 63.Wu NL, Boison D. Adenosine kinase expression modulates expression of myelin proteolipid protein. The Open Neuroscience Journal. 2007;1:15–19. [Google Scholar]
- 64.McGaraughty S, Cowart M, Jarvis MF, Berman RF. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5:43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- 65.Boyer SH, Ugarkar BG, Solbach J, Kopcho J, Matelich MC, Ollis K, Gomez-Galeno JE, Mendonca R, Tsuchiya M, Nagahisa A, Nakane M, Wiesner JB, Erion MD. Adenosine kinase inhibitors. 5. Synthesis, enzyme inhibition, and analgesic activity of diaryl-erythro-furanosyltubercidin analogues. J Med Chem. 2005;48:6430–6441. doi: 10.1021/jm0503650. [DOI] [PubMed] [Google Scholar]
- 66.Boison D. Astrogliosis and adenosine kinase: a glial basis of epilepsy. Future Neurology. 2008;3:221–224. [Google Scholar]
- 67.Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- 69.Riban V, Bouilleret V, Pham-Le BT, Fritschy JM, Marescaux C, Depaulis A. Evolution of hippocampal epileptic activity during the development of hippocampal sclerosis in a mouse model of temporal lobe epilepsy. Neuroscience. 2002;112:101–111. doi: 10.1016/s0306-4522(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 70.Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience. 2000;97:47–58. doi: 10.1016/s0306-4522(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 71.Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM. Early loss of interneurons and delayed subunit-specific changes in GABA(A)-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus. 2000;10:305–324. doi: 10.1002/1098-1063(2000)10:3<305::AID-HIPO11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 72.Bouilleret V, Boyet S, Marescaux C, Nehlig A. Mapping of the progressive metabolic changes occurring during the development of hippocampal sclerosis in a model of mesial temporal lobe epilepsy. Brain Res. 2000;852:255–262. doi: 10.1016/s0006-8993(99)02092-2. [DOI] [PubMed] [Google Scholar]
- 73.Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999;89:717–729. doi: 10.1016/s0306-4522(98)00401-1. [DOI] [PubMed] [Google Scholar]
- 74.Gouder N, Scheurer L, Fritschy J-M, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGaraughty S, Cowart M, Jarvis MF. Recent developments in the discovery of novel adenosine kinase inhibitors: mechanism of action and therapeutic potential. CNS Drug Rev. 2001;7:415–432. doi: 10.1111/j.1527-3458.2001.tb00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs. 2000;9:551–564. doi: 10.1517/13543784.9.3.551. [DOI] [PubMed] [Google Scholar]
- 78.Kowaluk EA, Bhagwat SS, Jarvis MF. Adenosine kinase inhibitors. Current Pharmaceutical Design. 1998;4:403–416. [PubMed] [Google Scholar]
- 79.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 80.Glass M, Faull RL, Bullock JY, Jansen K, Mee EW, Walker EB, Synek BJ, Dragunow M. Loss of A1 adenosine receptors in human temporal lobe epilepsy. Brain Res. 1996;710:56–68. doi: 10.1016/0006-8993(95)01313-x. [DOI] [PubMed] [Google Scholar]
- 81.Angelatou F, Pagonopoulou O, Maraziotis T, Olivier A, Villemeure JG, Avoli M, Kostopoulos G. Upregulation of A1 adenosine receptors in human temporal lobe epilepsy: a quantitative autoradiographic study. Neurosci Lett. 1993;163:11–14. doi: 10.1016/0304-3940(93)90217-9. [DOI] [PubMed] [Google Scholar]
- 82.Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biology. 2009 doi: 10.1017/S1740925X09990135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ugarkar BG, DaRe JM, Kopcho JJ, Browne CE, 3rd, Schanzer JM, Wiesner JB, Erion MD. Adenosine kinase inhibitors. 1. Synthesis, enzyme inhibition, and antiseizure activity of 5-iodotubercidin analogues. J Med Chem. 2000;43:2883–2893. doi: 10.1021/jm000024g. [DOI] [PubMed] [Google Scholar]
- 84.Ugarkar BG, Castellino AJ, DaRe JM, Kopcho JJ, Wiesner JB, Schanzer JM, Erion MD. Adenosine kinase inhibitors. 2. Synthesis, enzyme inhibition, and antiseizure activity of diaryltubercidin analogues. J Med Chem. 2000;43:2894–2905. doi: 10.1021/jm0000259. [DOI] [PubMed] [Google Scholar]
- 85.Wiesner JB, Ugarkar BG, Castellino AJ, Barankiewicz J, Dumas DP, Gruber HE, Foster AC, Erion MD. Adenosine kinase inhibitors as a novel approach to anticonvulsant therapy. J Pharmacol Exp Ther. 1999;289:1669–1677. [PubMed] [Google Scholar]
- 86.Schrader J, Wahl M, Kuschinsky W, Kreutzberg GW. Increase of adenosine content in cerebral cortex of the cat during bicuculline-induced seizure. Pflugers Arch. 1980;387:245–251. doi: 10.1007/BF00580977. [DOI] [PubMed] [Google Scholar]
- 87.Kowaluk EA, Mikusa J, Wismer CT, Zhu CZ, Schweitzer E, Lynch JJ, Lee CH, Jiang M, Bhagwat SS, Gomtsyan A, McKie J, Cox BF, Polakowski J, Reinhart G, Williams M, Jarvis MF. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin- 3-yl)pyrido[2,3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties. II. In vivo characterization in the rat. J Pharmacol Exp Ther. 2000;295:1165–1174. [PubMed] [Google Scholar]
- 88.Jarvis MF, Yu H, Kohlhaas K, Alexander K, Lee CH, Jiang M, Bhagwat SS, Williams M, Kowaluk EA. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther. 2000;295:1156–1164. [PubMed] [Google Scholar]
- 89.Suzuki R, Stanfa LC, Kowaluk EA, Williams M, Jarvis MF, Dickenson AH. The effect of ABT-702, a novel adenosine kinase inhibitor, on the responses of spinal neurones following carrageenan inflammation and peripheral nerve injury. Br J Pharmacol. 2001;132:1615–1623. doi: 10.1038/sj.bjp.0703972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGaraughty S, Jarvis MF. Purinergic control of neuropathic pain. Drug Development Research. 2006;67:376–388. [Google Scholar]
- 91.Jarvis MF, Mikusa J, Chu KL, Wismer CT, Honore P, Kowaluk EA, McGaraughty S. Comparison of the ability of adenosine kinase inhibitors and adenosine receptor agonists to attenuate thermal hyperalgesia and reduce motor performance in rats. Pharmacol Biochem Behav. 2002;73:573–581. doi: 10.1016/s0091-3057(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 92.Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99:6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annual Review of Nutrition. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 94.Erion MD, Wiesner JB, Rosengren S, Ugarkar BG, Boyer SH, Tsuchiya M. Therapeutic potential of adenosine kinase inhibitors as analgesic agents. Drug Dev Res. 2000;50 S14-06. [Google Scholar]
- 95.Hindley S, Herman MA, Rathbone MP. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J Neurosci Res. 1994;38:399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- 96.Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43:190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- 97.Rathbone MP, Middlemiss PJ, DeLuca B, Jovetich M. Extracellular guanosine increases astrocyte cAMP: inhibition by adenosine A2 antagonists. Neuroreport. 1991;2:661–664. doi: 10.1097/00001756-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vezzani A, Ravizza T, Balosso S, Aronica E. Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia. 2008;49 Suppl 2:24–32. doi: 10.1111/j.1528-1167.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 100.Uva L, Librizzi L, Marchi N, Noe F, Bongiovanni R, Vezzani A, Janigro D, de Curtis M. Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea-pig brain requires enhancement of blood-brain barrier permeability. Neuroscience. 2008;151:303–312. doi: 10.1016/j.neuroscience.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vezzani A, Baram TZ. New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr. 2007;7:45–50. doi: 10.1111/j.1535-7511.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 105.Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48 Suppl 2:13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 106.Boison D, Stewart K-A. Therapeutic epilepsy research: from pharmacological rationale to focal adenosine augmentation. Biochem Pharmacol. 2009 doi: 10.1016/j.bcp.2009.08.005. doi:10.1016/j.bcp.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009;85:131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009;6:278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boison D, Scheurer L, Tseng JL, Aebischer P, Mohler H. Seizure suppression in kindled rats by intraventricular grafting of an adenosine releasing synthetic polymer. Exp Neurol. 1999;160:164–174. doi: 10.1006/exnr.1999.7209. [DOI] [PubMed] [Google Scholar]
- 110.Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc. Natl. Acad. Sci. USA. 2001;98:7611–7616. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boison D, Huber A, Padrun V, Deglon N, Aebischer P, Mohler H. Seizure suppression by adenosine-releasing cells is independent of seizure frequency. Epilepsia. 2002;43:788–796. doi: 10.1046/j.1528-1157.2002.33001.x. [DOI] [PubMed] [Google Scholar]
- 112.Güttinger M, Padrun V, Pralong W, Boison D. Seizure suppression and lack of adenosine A1 receptor desensitization after focal long-term delivery of adenosine by encapsulated myoblasts. Exp Neurol. 2005;193:53–64. doi: 10.1016/j.expneurol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 113.Anschel DJ, Ortega EL, Kraus AC, Fisher RS. Focally injected adenosine prevents seizures in the rat. Exp Neurol. 2004;190:544–547. doi: 10.1016/j.expneurol.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 114.Fedele DE, Koch P, Brüstle O, Scheurer L, Simpson EM, Mohler H, Boison D. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci Lett. 2004;370:160–165. doi: 10.1016/j.neulet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 115.Güttinger M, Fedele DE, Koch P, Padrun V, Pralong W, Brüstle O, Boison D. Suppression of kindled seizures by paracrine adenosine release from stem cell derived brain implants. Epilepsia. 2005;46:1–8. doi: 10.1111/j.1528-1167.2005.61804.x. [DOI] [PubMed] [Google Scholar]
- 116.Li T, Ren G, Kaplan DL, Boison D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 2009;84:238–241. doi: 10.1016/j.eplepsyres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szybala C, Pritchard EM, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eisenach JC, Curry R, Hood DD. Dose response of intrathecal adenosine in experimental pain and allodynia. Anesthesiology. 2002;97:938–942. doi: 10.1097/00000542-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 120.Eisenach JC, Hood DD, Curry R. Preliminary efficacy assessment of intrathecal injection of an American formulation of adenosine in humans. Anesthesiology. 2002;96:29–34. doi: 10.1097/00000542-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 121.Eisenach JC, Hood DD, Curry R. Phase I safety assessment of intrathecal injection of an American formulation of adenosine in humans. Anesthesiology. 2002;96:24–28. doi: 10.1097/00000542-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 122.Boison D. Adenosine as a modulator of brain activity. Drug News Persp. 2007;20:607–611. doi: 10.1358/dnp.2007.20.10.1181353. [DOI] [PubMed] [Google Scholar]