Abstract
Objectives
To investigate the hypothesis that a transient episode of raised intensity phonation causes a significant increase in vocal fold inflammatory messenger RNA (mRNA) expression in-vivo.
Study Design
Prospective animal study.
Setting
Laboratory.
Subjects and Methods
Ten New Zealand white breeder rabbits received 30 minutes of experimentally induced modal or raised intensity phonation, followed by a 30 minute recovery period. A separate group of five rabbits served as sham controls. Real-time PCR was performed to investigate the mRNA expression of Interleukin-1beta (IL-1β), Transforming Growth Factor beta-1 (TGFβ-1), and Cyclooxygenase-2 (COX-2). Separate one-way analysis of variance (ANOVA) tests were used to investigate differences in gene expression across groups, with an appropriate alpha correction of .016 to control for type I error. Significant main effects were further examined using Fisher’s Least Significant Difference.
Results
ANOVA revealed that there were differences for IL-1β, TGF-β1, and COX-2 between sham-control, modal phonation, and raised intensity phonation (p < .0001). Pairwise comparisons revealed that the expression of IL-1β, COX-2, and TGF-β1 increased significantly during raised intensity phonation, compared to modal phonation and sham-control (p < .0001).
Conclusions
Results provided support for the hypothesis that a transient episode of raised intensity phonation causes a significant increase in vocal fold inflammatory mRNA expression. Future studies will investigate the signal transduction pathways and mechanisms regulating the vocal fold inflammatory response. The long-term goal of these studies is to advance understanding of the molecular and cellular events underlying phonation-related tissue alterations.
Introduction
It has been suggested that phonotraumatic voice use contributes to dysphonia and vocal pathology.1–3 Despite the clinical ramifications and importance of phonation as it relates to the development of vocal fold lesions, in-vivo studies on the cellular alterations resulting from phonation are largely unavailable. In particular, there remains a lack of empirical data on the duration of voice use that is safe and/or unsafe, the effects of physiologic and phonotraumatic phonation on the initiation of inflammation, and the cycles of tissue injury and repair that are unique to the vocal folds.
What we do know based on currently available data, is that prolonged phonation results in structural damage to the vocal fold epithelium and basement membrane, with associated alterations in the biochemical pathway involved in the maintenance of vocal fold tissue.4–6 Thus, the vibration exposure of the vocal folds during phonation appears to play an important role in phonation-related tissue alterations. What remains unknown, however, is: 1) the effects of physiologic and phonotraumatic phonation on the initiation of the inflammatory cascade, 2) the time course of tissue recovery, and 3) the molecular and cellular events leading to the development of vocal fold pathology. As inflammation plays an important role in the release of chemotactic cues for orchestration of tissue remodeling, it is likely an important component of phonation related injury. Thus, there is a critical need for information on the effects of acute phonotrauma on inflammation and tissue recovery.
Our laboratory has recently characterized physiologic and raised intensity phonation in an evoked rabbit phonation model.7 Based on an animal model described in our previous work,6,8 subsequent experimentation led to the finding that an increase in the rate of transglottal airflow, resulted in a change in glottal configuration and an increase in mean phonation intensity. 7 The objective of the present study was to investigate the hypothesis that a transient episode of raised intensity phonation causes a significant increase in vocal fold inflammatory messenger RNA (mRNA) expression in-vivo.
Methods
Animals
Fifteen New Zealand White breeder rabbits weighing 3 to 5 kg were used in this study. Five animals were assigned to one of three groups and received experimentally induced phonation as described previously. 6–8 Five animals received modal phonation, five received raised intensity phonation, and five underwent sham surgery without phonation to serve as a control group. Each animal underwent a 30 minute time dose of modal phonation, raised intensity phonation, or sham surgery, followed by a 30 minute recovery period. Induction of anesthesia was achieved with ketamine 35mg/kg, xylazine 5mg/kg, and acepromazine 0.75 mg/kg intramuscular sedation. Subsequent intramuscular injections of ketamine (17.5 mg/kg) and acepromazine (0.375 mg/kg) were provided as needed to maintain a surgical plane of anesthesia.
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center.
Surgical Procedure
The neck was shaved and prepped for surgery from the level of the submentum down to the chest. With the animal in the supine position, a vertical midline incision extending from the hyoid bone to the sternal notch was used to expose the larynx and trachea. The trachea was then transected just proximal to the sternum, and sutures were placed to suspend the lower portion of the trachea to the sternal fascia which secured the airway. A 3.5 cuffed endotracheal tube (RUSCh, Kernen, Germany) was inserted into the upper portion of the bisected trachea and positioned to rest approximately two cm below the glottal aperture. The cuff of this endotracheal tube was inflated to seal off the trachea and deliver airflow through the glottis. Custom, stainless steel, hooked electrodes were prepared prior to each experiment. One electrode was inserted mediolaterally into the belly of each cricothyroid muscle in a direction perpendicular to the muscle fibers (approximately at a 45 degree angle off midline) to serve as cathodes for electrical stimulation. Two additional electrodes were then inserted into the cricothyroid membrane to serve as anodes for electrical stimulation. One electrode was placed into the membrane on each side at the intersection of a longitudinal line one millimeter lateral to midline and a transverse line one millimeter inferior to the thyroid cartilage. A Gilmont Instruments flowmeter (GF-8522-1700. Barrington, IL) and Conch Therm III humidifier (Hudson, RCI, Temecula, CA) were used to deliver compressed humidified air heated to 37 degrees Celsius to the glottis.
A Grass S-88 stimulator (SA Instrumentation, Encinitas, CA) and constant current isolation unit (Grass Telefactor, model PSIU6; West Warwick, RI) were used to provide electrical stimulation to the laryngeal apparatus. The total train duration was 10 seconds (3 seconds on; 7 seconds off). Animals assigned to the sham control group received identical surgery as those in the experimental group. However, in the sham group, airflow was kept in the off position during the 30 minute experimental period, while electrical stimulation was provided to just approximate the vocal folds. Based on methods described in our earlier work, transglottal airflow was delivered at 85 cm3/sec while the stimulation current was increased in 0.2 mA steps until arriving at the phonation threshold. Modal phonation was produced using stimulation currents of 0.2 – 0.6 mA above the phonation threshold on average. This represented the current level necessary for producing sustained audible phonation. For raised intensity phonation, transglottal airflow rate was increased to 144 cm3/sec. The increase in phonation intensity was a within rabbit variable. In an earlier proof of concept study, we found that the increase in transglottal airflow rate used in the current study, produced phonations that were an average 6 dB louder than modal.7 Therefore, in the current study, raised intensity was defined as a minimum 6 dB (within rabbit) increase in the intensity of phonation and this increase in intensity from modal was maintained between 5–10 dB throughout the 30 minute stimulation period. Table 1 displays mean phonation intensity and standard deviations for rabbits in the modal phonation and raised intensity phonation groups during the 30 minute stimulation period. Table 2 displays mean phonation frequency and standard deviations for rabbits in the modal phonation and raised intensity phonation groups during the 30 minute stimulation period.
Table 1.
Mean phonation intensity
| Rabbit # | Mean Intensity (dB) | Standard Deviation (Hz) | Group |
|---|---|---|---|
| 8142 | 56.90 | 1.27 | Modal |
| 8145 | 60.18 | 0.73 | Modal |
| 8146 | 55.38 | 0.98 | Modal |
| 8152 | 55.85 | 0.98 | Modal |
| 8157 | 61.52 | 1.06 | Modal |
| 8148 | 52.49 | 0.74 | Raised Intensity |
| 8149 | 65.92 | 0.43 | Raised Intensity |
| 8150 | 60.04 | 1.52 | Raised Intensity |
| 8151 | 63.93 | 1.22 | Raised Intensity |
| 8156 | 59.04 | 1.69 | Raised Intensity |
Table 2.
Mean fundamental frequency
| Rabbit # | Mean Fundamental Frequency (Hz) | Standard Deviation (Hz) | Group |
|---|---|---|---|
| 8142 | 1099.67 | 24.19 | Modal |
| 8145 | 1202.90 | 14.28 | Modal |
| 8146 | 1002.64 | 12.91 | Modal |
| 8152 | 1137.72 | 25.24 | Modal |
| 8157 | 1104.47 | 12.29 | Modal |
| 8148 | 784.44 | 29.37 | Raised Intensity |
| 8149 | 1294.34 | 15.98 | Raised Intensity |
| 8150 | 1657.62 | 9.90 | Raised Intensity |
| 8151 | 1294.84 | 20.88 | Raised Intensity |
| 8156 | 821.51 | 26.19 | Raised Intensity |
Data Collection
A 5° 2.7mm rigid endoscope (Karl Storz Endoscopy, Endoscopy–America, Inc. CA) and Telecam-C camera (Karl Storz Endoscopy, Endoscopy–America, Inc. CA) were used to obtain video-documentation of vocal fold positioning and glottal closure during phonation. Acoustic signals were recorded using a Shure SM48 unidirectional dynamic microphone (Shure Incorporated, Niles, IL.), placed 10 cm from the opening of the laryngoscope and digitized using the Computerized Speech Lab (CSL ™ Model 4500, KayPENTAX, Lincoln Park, NJ). Three to five, 0.5–1.0 second samples were selected from the most stable portion of the acoustic waveform, and the CSL main program was used to extract mean phonation intensity and fundamental frequency. Immediately following the 30 minute phonation and recovery period, all animals were euthanized and the larynges were harvested. The lamina propria of each animal’s vocal folds was then dissected and removed with the aid of microscopic visualization. Dissection was limited to the mucosal layers of the vocal fold above the thyroarytenoid and did not include muscle. Harvested tissue was subsequently frozen and stored for later analysis in a sub−80° freezer.
Real-Time PCR
A Mixer Mill MM 301 (Retsch Inc. Pittsburgh, PA, USA) was used to homogenize vocal fold specimens. Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and treated with ribonuclease-free deoxyribonuclease I (QIAGEN, Valencia, CA, USA) to minimize contamination from genomic DNA. The quantity of total RNA was determined using the A260/A280 ratio and electrophoresis was used to evaluate the quality based on the appearance of the 18S and 28S ribosomal RNA bands. Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) using the manufacturers recommended reaction protocol. Reactions were performed with a Biometra TGradient Thermocycler (LABREPCO, Horsham, PA, USA) using the following parameters: 25°C for 10 min, 37°C for 120 min, 85°C for 5 sec, and 4°C for 5 min.
Rabbit-specific primers for interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2), transforming growth factor β-1 (TGFβ-1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were synthesized by Integrated DNA Technologies (Coralville, IA, USA) based on previously published sequences.9–12 Specific primer sequences are displayed in Table 3. All primers generated a single PCR band of the expected size. DNA sequencing was used to verify the PCR products. Real-time PCR was performed using the iQ™SyBR Green Supermix Kit (BioRad, CA, USA) in a 25 µL volume reaction mixture composed of 500 nM primer1, 500 nM primer 2, 12.5 µL iQ SyBR Green Supermix, 1 µL of template complementary DNA, and 9 µL of ribonuclease-free water. The following protocol was used for real-time PCR: 1 cycle at 95°C for 3 min, followed by 45 cycles at 95°C for 20 seconds, 58.7°C for 20 seconds, 72°C for 40 seconds, and 1 cycle at 95°C for 2 min, 55°C for 2 min, and then 1 cycle at 55°C to 95°C in 0.2°C increments to make a melting curve. An iCycler iQ™ Optical System (Software version 2.0; Bio-Rad, Hercules, CA, USA) was used to detect the PCR products. PCR products were separated by electrophoresis in 1 % agarose gels containing 0.5µg/mL ethidium bromide for verification of PCR products according to fragment size.
Table 3.
Primer Sequences
| GAPDH | Forward: 5_-TCG GCA TTG TGG AGG GGC TC-3_ |
| Reverse: 5_-TCC CGT TCA GCT CGG GGA TG-3_ | |
| IL-1β | Forward: 5_-GAA TCT GAA CCA ACA AGT GG-3_ |
| Reverse: 5_-ATG TAC CAG TTG GGG AAC T-3_ | |
| COX-2 | Forward: 5_-CAA ACT GCT CCT GAA ACC CAC TC-3_ |
| Reverse: 5_-GCT ATT GAC GAT GTT CCA GAC TCC-3_ | |
| TGF-β1 | Forward: 5_-CGG CAG CTG TAC ATT GAC TT -3_ |
| Reverse: 5_-AGC GCA CGA TCA TGT TGG AC -3_ | |
GAPDH - glyceraldehyde-3-phosphate dehydrogenase; IL– 1β - interleukin-1β; COX-2 - cyclooxygenase-2; TGF-β1 - transforming growth factor beta-1.
Standard curves were used to determine the relative ratio of gene expression. Target gene ratios from sham-control, modal phonation, and raised intensity phonation groups were normalized using expression ratios of the internal control gene (GAPDH). Bilateral vocal fold specimens were averaged for each animal before doing the group analysis with five animals per group.
Data Analysis
Three, one-way repeated measures analysis of variance (ANOVA) tests were used to investigate differences in IL-1β, TGFβ-1, and COX-2 gene expression across groups. Alpha was adjusted to .016 to control for type I error. Significant main effects were further examined using Fisher’s Least Significant Difference. For post hoc testing, a significance level of P < .016 was used to control for multiple pairwise comparisons. All analyses were performed with the use of two-tailed P values. Data were analyzed using SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL).
Results
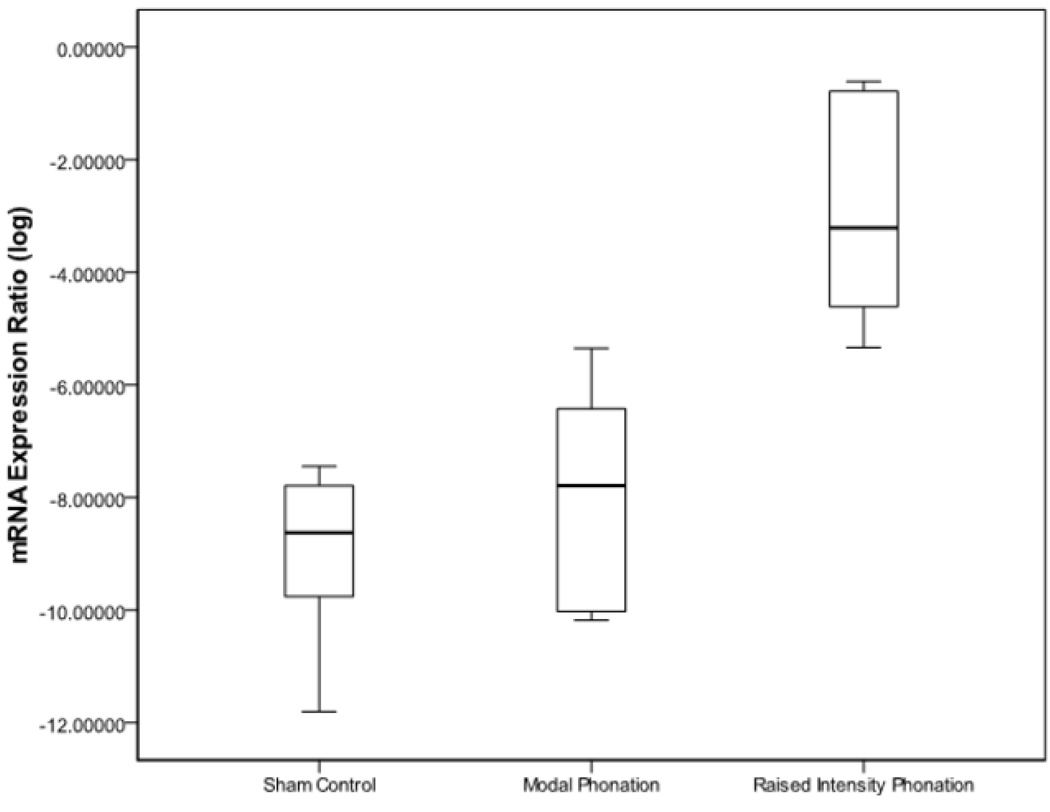
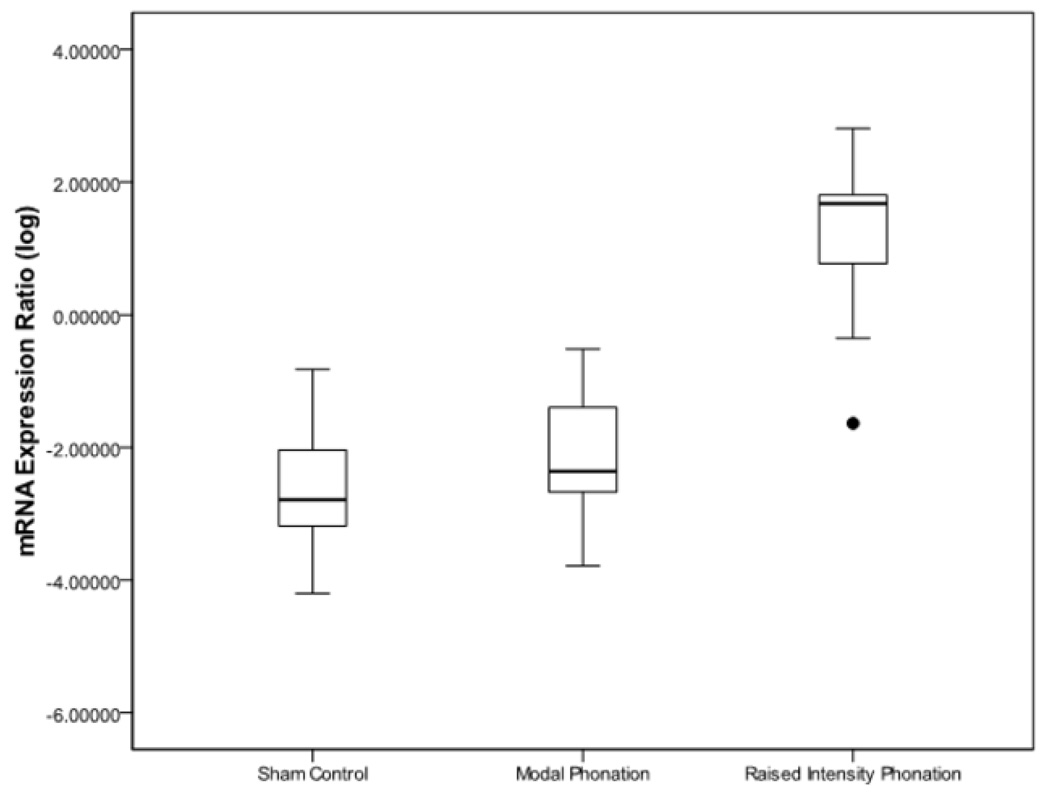
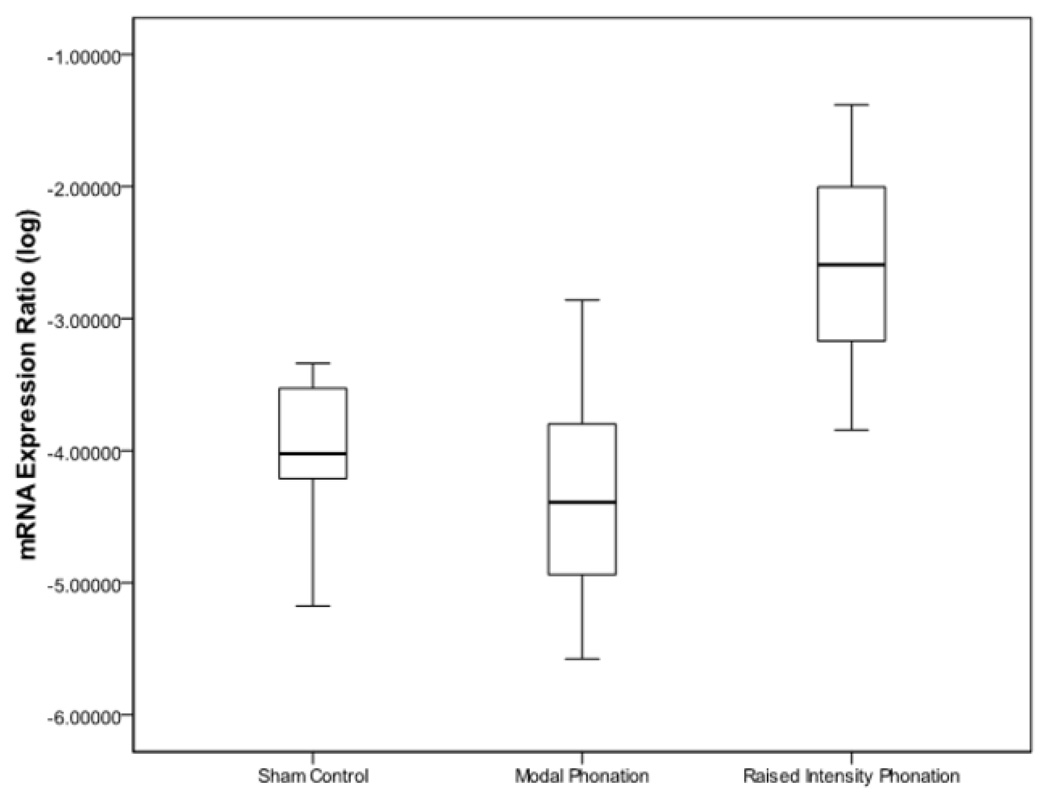
Results from ANOVA revealed that there were differences for IL-1β (p < .0001), TGF-β1 (p < .0001), and COX-2 (p < .0001) between sham-control, modal phonation, and raised intensity phonation. Pairwise comparisons revealed that the expression of IL-1β increased significantly during raised intensity phonation, compared to modal phonation and sham-control (p < .0001) (Fig 1); COX-2 increased significantly during raised intensity phonation, compared to modal phonation and sham-control (p < .0001) (Fig 2), and TGF-β1 increased significantly during raised intensity phonation, compared to modal phonation and sham-control (p < .0001) (Fig 3).
Figure 1.
Log-transformed mRNA expression ratios between the sham control, modal phonation, and raised intensity phonation groups for interleukin-1β (IL-1β). The error bars represent 95.0% confidence intervals of the mean.
Figure 2.
Log-transformed mRNA expression ratios between the sham control, modal phonation, and raised intensity phonation groups for cyclooxygenase-2 (COX-2). The error bars represent 95.0% confidence intervals of the mean. Outlier variable denoted by ●.
Figure 3.
Log-transformed mRNA expression ratios between the sham control, modal phonation, and raised intensity phonation groups for transforming growth factor- β1 (TGF-β1). The error bars represent 95.0% confidence intervals of the mean.
Discussion
Following trauma, the inflammatory response plays an important role in the release of chemotactic cues for orchestration of tissue remodeling. Animal studies over the last several years have allowed us to gain a much better understanding of the inflammatory cycle and process of wound healing in the vocal fold following mechanical trauma. Most relevant to the current study, Welham et al found the cytokines IL-1β, COX-2, and TNF-α, to be significantly upregulated in the rat vocal fold one hour after mechanical injury.13 With the exception of TNF-β, mRNA levels of these cytokines remained elevated for up to 72 hours after injury.14 In an attempt to better understand the mRNA expression of key extracellular matrix components during the inflammatory, proliferative, and remodeling phases of wound healing, Ohno et al reported time-dependent alterations in the mRNA expression of procollagen –I, -III, decorin, and hyaluronan synthase (HAS)-1, -2, and -3 between 1 and 56 days following mechanical injury to the rat vocal fold.15 While these studies have provided important information regarding the biochemical events involved in mechanical trauma to the vocal folds, less is known about the cycles of tissue injury and repair that are unique to phonation. Although the effects of inflammation on connective tissue healing has been the focus of research in other mobile tissues,16–20 the mechanical forces produced during phonation are unlike any other tissue found in the body. Some histological and physiological comparisons can be made between the vocal folds and other mobile tissues in the body however, it is likely that the cellular response to repeated cycles of trauma and inflammation secondary to phonation are unique to this specialized tissue.
Clinically, it is often observed that patients who use phonotraumatic vocal behaviors are at increased risk for developing vocal pathology, ranging from edema and mild dysfunction of the vocal folds, to discrete vocal fold lesions and marked voice impairment. Reportedly, management of voice is designed to alter the process of inflammation in a way that provides an optimal environment for healing following phonotrauma or phonosurgery.21 However, a more complete understanding of the cycles of inflammation and recovery, and the progression of these cycles of repair during the development of phonotraumatic lesions is needed.
The purpose of the current study was to investigate the effects of modal and raised intensity phonation on vocal fold inflammatory gene expression. Using the in-vivo rabbit phonation model described previously, we investigated the hypothesis that a transient episode of raised intensity phonation causes a significant increase in vocal fold inflammatory messenger RNA (mRNA) expression in-vivo.7 Each animal underwent a 30 minute time dose of modal phonation, raised intensity phonation, or sham surgery, followed by a 30 minute recovery period. Our aim was to more clearly define raised intensity phonation at the mRNA level and confirm its potential for use as a phonotraumatic model in future investigations. To do this, we measured gene expression of known inflammatory markers in the vocal folds following a transient episode of raised intensity phonation, compared to modal phonation and sham-control. We used experimental controls that underwent surgical preparation with electrical stimulation of the laryngeal apparatus for 30 minutes without full closure of the glottis and without transglottal airflow.
Results from ANOVA revealed that there were differences for IL-1 β, TGF- β 1, and COX-2 between sham-control, modal phonation, and raised intensity phonation. Pairwise comparisons revealed that the expression of all three inflammatory markers measured in this study increased significantly during raised intensity phonation, compared to modal phonation and sham-control. IL-1β, TGF- β1 and COX-2 are well characterized inflammatory markers. The cytokine TGF- β1 is a major promoter of fibrosis and scar formation.22 If left unchecked, increased TGF- β1 expression may result in excessive cell proliferation and repair signaling to fibroblasts. In the current study, TGF- β1 mRNA levels were upregulated in the vocal fold following raised intensity phonation, compared to modal phonation and control. As TGF- β1 is an important mediator of inflammation, the modulation of TGF- β1 signaling may provide a logical target for the manipulation of vocal fold inflammatory signaling. It is generally believed that phonotrauma from prolonged, raised-intensity phonation causes inflammatory changes in the lamina propria, often progressing to dysphonia and vocal fold pathology. COX-2 and its enzymatic product prostaglandin are known mediators of inflammation and fibroblast signaling. In the present study, COX-2 mRNA levels were upregulated in the vocal fold following raised intensity phonation, compared to modal phonation and control, and similar to the COX-2 upregulation that has been reported in the vocal fold following mechanical injury. 14 IL-1 β is a prototypical pro-inflammatory cytokine that plays an important role in the regulation of the extracellular matrix during wound healing. It has been shown that IL-1 β stimulation of lung and mucosal fibroblasts results in an increase in the production of hyaluronic acid.23,24 In an earlier study, we found IL-1 β to be non-significantly increased in the rabbit vocal fold after experimentally induced phonation (2 seconds on; 3 seconds off for 180 minutes), compared to control.6 Interestingly, although accumulated voicing time in the current study was less (3 seconds on; 7 seconds off for 30 minutes), IL-1β mRNA was significantly increased after raised intensity phonation, compared to modal phonation and control. One possible explanation is that the longer recovery time (1 hour) employed in the 2008 study, a period which was twice as long as the recovery period used in the current study (30 minutes), may have facilitated the post-phonation recovery of IL-1β gene expression. Albeit, we are reluctant to make direct inferences between the two studies as the stimulation parameters, placement of electrodes, and data/specimen collection procedures in the two experiments was different.
Results of the present study provided support for the hypothesis that a transient episode of raised intensity phonation causes a significant increase in vocal fold inflammatory mRNA expression. We plan to utilize the model of acute phonotrauma used in the current study to develop an improved understanding of cycles of tissue injury and repair that are unique to phonation. The long-term goal of these studies will be to improve our understanding of the molecular and cellular events underlying phonation-related tissue alterations in order to begin testing pharmacologic interventions for the management of acute phonotrauma. It should be noted that although rabbits share similarities with humans with respect to vocal fold extracellular matrix components, interspecies variation in tissue layered structure may also influence the inherent tissue response to mechanical trauma, and should be considered when making comparisons between species.
Conclusion
An in-vivo rabbit phonation model was used to investigate the effects of modal and raised intensity phonation on the vocal fold inflammatory response. Experimental animals were subjected to either 30 minutes of modal phonation or raised intensity phonation, followed by a 30 minute recovery period. Results revealed that a transient episode of raised intensity phonation causes a significant increase in vocal fold inflammatory mRNA expression. This increase in inflammatory mediator expression persisted despite a 30 minute recovery period, while the same duration of modal phonation resulted in no change in inflammatory mRNA expression, compared to sham-control.
Acknowledgments
Research supported by NIH grant R03 DC 008400 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Paper presented at the Annual Meeting of the American Academy of Otolaryngology-Head and Neck Surgery, San Diego, CA. Oct 4-7, 2009.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1.Roy N, Merrill RM, Gray SD, et al. Voice disorders in the general population: prevalence, risk factors, and occupational impact. Laryngoscope. 2005;115(11):1988–1995. doi: 10.1097/01.mlg.0000179174.32345.41. [DOI] [PubMed] [Google Scholar]
- 2.Behrman A, Sulica L. Voice rest after microlaryngoscopy: current opinion and practice. Laryngoscope. 2003;113(12):2182–2186. doi: 10.1097/00005537-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Behlau M, Oliveira G. Vocal hygiene for the voice professional. Curr Opin Otolaryngol Head Neck Surg. 2009;17(3):149–154. doi: 10.1097/MOO.0b013e32832af105. [DOI] [PubMed] [Google Scholar]
- 4.Gray SD, Titze I, Lusk RP. Electron Microscopy of Hyperphonated Canine Vocal Cords. J of Voice. 1987;1:109–115. [Google Scholar]
- 5.Gray SD, Titze IR. Histologic investigation of hyperphonated canine vocal cords. Ann Otol Rhinol Laryngol. 1998 Jul-Aug;97:381–388. doi: 10.1177/000348948809700410. [DOI] [PubMed] [Google Scholar]
- 6.Rousseau B, Ge PJ, French LC, et al. Experimentally Induced Phonation Increases Matrix Metalloproteinase-1 Gene Expression in Normal Rabbit Vocal Fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson ER, Abdollahian D, Ohno T, et al. Characterization of raised phonation in an evoked rabbit phonation model. Laryngoscope. 2009;119(7):1439–1443. doi: 10.1002/lary.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge PJ, French LC, Ohno T, et al. Model of evoked rabbit phonation. Ann Otol Rhinol Laryngol. 2009;118(1):51–55. doi: 10.1177/000348940911800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K, Funata N, Ikuta F, et al. Neuronal apoptosis and inflammatory responses in the central nervous system of a rabbit treated with shiga toxin-2. J Neuroinflammation. 2008;5:11. doi: 10.1186/1742-2094-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majima T, Lo IK, Randle JA, et al. ACL transection influences mRNA levels for collagen type I and TNF alpha in MCL scar. J Orthop Res. 2002;20(3):520–525. doi: 10.1016/S0736-0266(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau B, Ge P, Ohno T, et al. Extracellular matrix gene expression after vocal fold injury in a rabbit model. Ann Otol Rhinol Laryngol. 2008;117(8):598–603. doi: 10.1177/000348940811700809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studer RK, Gilbertson LG, Georgescu H, et al. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008;26(7):991–998. doi: 10.1002/jor.20604. [DOI] [PubMed] [Google Scholar]
- 13.Welham N, Lim X, Tateya I, et al. Inflammatory factor profiles one hour following vocal fold injury. Ann Otol Rhinol Laryngol. 117(2):145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 14.Lim X, Tateya I, Tateya T, et al. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol. 115(12):921–929. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- 15.Ohno T, Hirano S, Rousseau B. Extracellular matrix gene expression during wound healing of the injured rat vocal fold. Otolaryngol Head Neck Surg. 2009;140:757–761. doi: 10.1016/j.otohns.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 16.Ferretti M, Srinivasan A, Deschner J, et al. Anti-Inflammatory effects of continuous passive motion on meniscal fibrocartilage. J Orthop Res. 2005;23(5):1165–1171. doi: 10.1016/j.orthres.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal S. Low magnitude of tensile strain inhibits IL-1beta dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 human periodontal ligament cells in vitro. J Dent Res. 2001;80:1416–1420. doi: 10.1177/00220345010800050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal S, Long P, Gassner R, et al. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44(3):608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gassner R, Buckley MJ, Georgescu H, et al. Cyclic tensile strain exerts anti-inflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;163:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- 20.Long P, Liu F, Piecso NP, et al. Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone. 2002;30:547–552. doi: 10.1016/s8756-3282(02)00673-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postma GN, Courey MS, Ossoff RH. The Professional Voice. In: Cummings CW, Flint PW, Harker LA, et al., editors. Otolaryngology - Head and Neck Surgery. 4th ed. Philadelphia: Mosby; 2005. pp. 2143–2146. [Google Scholar]
- 22.Lanning DA, Nwomeh BC, Montante SJ, et al. TGF β-1 alters the healing of cutaneous fetal excisional wounds. J Pediatr Surg. 1999;34:695–700. doi: 10.1016/s0022-3468(99)90358-5. [DOI] [PubMed] [Google Scholar]
- 23.Tufveeson E, Westergren-Thorsson G. Alteration of proteoglycan synthesis in human lung fibroblasts induced by interleukin-1β and tumor necrosis factor-β. J Cell Biochem. 2000;77:298–309. doi: 10.1002/(sici)1097-4644(20000501)77:2<298::aid-jcb12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Hano N, Hata K, et al. Differential regulation by IL-1 β and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using RT-PCR. J Invest Dermatol. 2004;122:631–639. doi: 10.1111/j.0022-202X.2004.22332.x. [DOI] [PubMed] [Google Scholar]