Abstract
Controlling low-density lipoprotein cholesterol is one of the major focuses of cardiovascular care. However, the twin global pandemics of obesity and diabetes are promoting an increased prevalence of associated cardiometabolic risk factors. These factors include mixed dyslipidemia, which is prevalent among several important subgroups of the overall population. Cardiovascular risk increases as women reach and extend beyond menopause, partly reflective of dyslipidemia. In addition, women with polycystic ovary syndrome display a cluster of risk factors reminiscent of the metabolic syndrome. Certain ethnic groups are also at increased risk of type 2 diabetes or the metabolic syndrome. Dyslipidemia contributes significantly to overall cardiovascular risk in the elderly, and the frequency of children and adolescents presenting with type 2 diabetes or metabolic syndrome is increasing worldwide. Physicians should be aware of the possibility of mixed dyslipidemia in patients at elevated cardiometabolic risk. However, while combination therapy may successfully correct the associated dyslipidemia, it remains to be established whether the addition of a second agent improves coronary risk beyond statin monotherapy.
Keywords: mixed dyslipidemia, HDL-cholesterol, triglycerides, metabolic syndrome, cardiometabolic risk, special populations
Introduction
Cardiovascular disease remains the greatest source of premature mortality in the developed world. Although cardiovascular event rates have been falling in recent decades, at least partly due to our improved management of cardiovascular disease, further optimization of our approach to cardiovascular care will be needed in order to make further inroads into the remaining burden of cardiovascular disease. The structured environment of clinical trials tends to promote focus on the management of single cardiometabolic parameters. In routine community-based clinical practice however, patients often present with a complex mixture of cardiovascular, metabolic and other conditions that present a clinical challenge to the healthcare practitioner. In addition, different populations of patients may differ both in their presentation, in terms of their principal risk factors, and in their response to treatment.
The purpose of this review is to summarize current knowledge on the therapeutic challenge posed by mixed dyslipidemia (hypercholesterolemia occurring in concert with hypertriglyceridemia and low HDL-C), with special reference to the clinically important subpopulations of patients, based on obesity status, gender, ethnicity, age and the presence of the metabolic syndrome.
Mixed dyslipidemia, where hypercholesterolemia presents in concert with other clinically important disturbances of the lipid profile, provides an example of a complex condition that can be a therapeutic challenge. The development of mixed dyslipidemia strongly impacts the overall risk of an adverse cardiovascular outcome, but this condition is often sub-optimally managed.
Obesity as a driving force for cardiovascular risk
Increasing prevalence of obesity
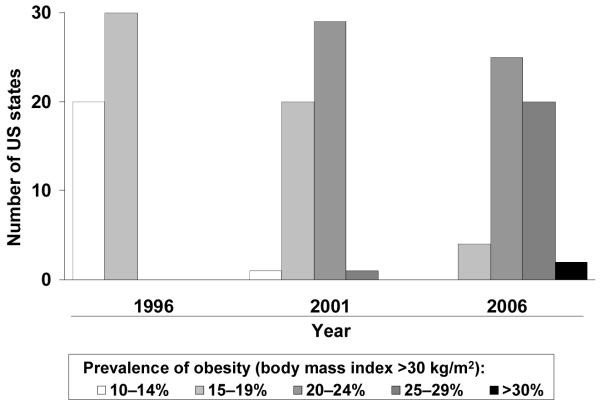
The prevalence of obesity continues to increase. As recently as 1996, 37 US States had a prevalence of obesity (defined as body mass index [BMI] ≥30 kg/m2) of 14% or less (Fig. 1a).1 The most recent available data, for 2006, indicate that no State has a prevalence of obesity below 20%, and that more than one quarter of citizens are obese in almost half of the States. Analyses from the National Health and Nutrition Examination Survey (NHANES) confirm the marked increases in generalized adiposity that have occurred during the last decade alone. The prevalence of overweight or obesity (BMI ≥25 kg/m2) increased from 56% to 65% between the 1988–1994 and 1999–2002 NHANES cohorts.2
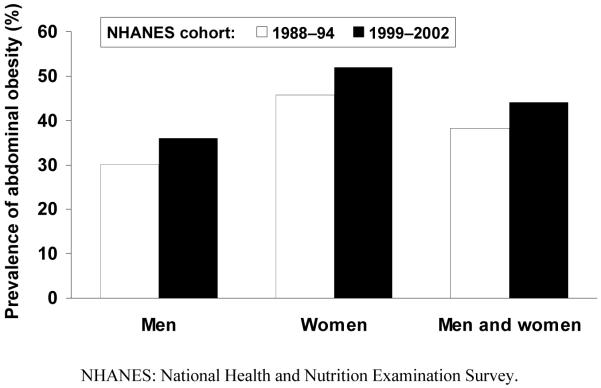
Fig. 1. Increase in the prevalence of obesity and abdominal obesity in the USA in a single decade.
a) Generalized obesity (high body mass index)
b) Abdominal obesity (high waist circumference)
Abdominal obesity
Observational data suggest that abdominal obesity has an adverse effect on cardiovascular health that is additional to and independent of generalized obesity signified by elevated BMI.3 These data brought researchers and clinicians to observe the waist circumference of patients more closely. Clinics and researchers are now using the US criteria of waist circumference (>102 cm in men or >88 cm in women) to identify abdominal obesity. Certain populations, such as people of South Asian4 or Hispanic5 descent, appear particularly susceptible to the adverse cardiometabolic influence of abdominal obesity. Further data from NHANES confirmed a sharp increase in the prevalence of abdominal obesity during a single decade, diagnosed using US criteria of waist circumference (Fig. 1b).6,7
Obesity, metabolic syndrome and type 2 diabetes
An increasing tendency towards sedentary lifestyles and excessive consumption of high-energy diets is fuelling the rise in the prevalence of obesity in developed and developing nations alike.8 These obesity trends are also driving an increased prevalence of the metabolic syndrome (commonly diagnosed9 on the basis of 3 or more of high waist circumference [>102 cm in men or >88 cm in women], low HDL-C [<40 mg/dL in men or <50 mg/dL in women], high blood pressure [≥130/85 mmHg], high triglycerides [≥150 mg/dL] or increased fasting plasma glucose [≥100 mg/dL]) and type 2 diabetes, which themselves markedly increase the risk of an adverse cardiovascular outcome.4,9 A recent analysis has suggested that the presence of the metabolic syndrome accounts for approximately one-sixth of the burden of cardiovascular disease, and between about one-quarter and one-half of the burden of type 2 diabetes.10 The onset of type 2 diabetes increases the risk of cardiovascular mortality by approximately 2–4-fold compared with a non-diabetic subject.11 Moreover, the risk of mortality is highest when both type 2 diabetes and the metabolic syndrome coexist. This increased risk was shown by a study conducted in Finland during which the presence of the metabolic syndrome increased the risk of coronary heart disease by 2.2-fold in patients with diabetes and by 1.7-fold in non-diabetic subjects.12
The atherogenic dyslipidemia phenotype
Low HDL-C, elevated non–HDL-C and hypertriglyceridemia contribute to elevated cardiovascular risk in addition to elevated LDL-C.4,7,13,14 The adverse metabolic trends apparent throughout the world are promoting the appearance of a characteristic presentation of mixed dyslipidemia featuring low high-density lipoprotein cholesterol (HDL-C), elevated triglycerides, elevated ApoB (but not necessarily elevated low-density lipoprotein cholesterol [LDL-C]) and elevated levels of small, dense LDL3,. Consistent with an increased concentration of atherogenic particles, this phenotype is strongly associated with increased cardiovascular risk. For example, an analysis from 2072 men free of ischemic heart disease at baseline in the Québec Cardiovascular Study showed that an increasing proportion of LDL-C contained within small, dense LDL (highest vs. lowest tertile) was associated with an increase in the risk of developing ischemic heart disease of more than 2-fold during 7 years of follow-up.15
Managing cardiometabolic in clinically important patient populations
Women
The nature of cardiovascular risk in women
Cardiovascular disease is the primary cause of death in women, as in men. Although premenopausal women are at lower risk of cardiovascular events than men, the risk heightens by about 3-fold after the menopause.16,17 This elevated risk reflects in large part the decline in circulating estrogen concentrations and associated changes in lipid metabolism. These include a reduction in HDL-C, increased triglycerides and ApoB-containing lipoproteins and potentially adverse changes in lipid sub-profiles.16,18
Otherwise, women demonstrate the same cardiometabolic risk factors as men, despite some differences in individual contributions to overall cardiometabolic risk. Specifically, women generally demonstrate a higher prevalence of abdominal obesity (see Fig. 1b for example) which is largely responsible for the greater observed prevalence of the metabolic syndrome6 and higher rates of type 2 diabetes in women.17 Women also have higher average circulating levels of C-reactive protein (CRP), a marker of chronic, low-grade systemic inflammation that is believed to contribute to atherogenesis. This relationship is independent of gender differences in adiposity.19 Observational and prospective studies have demonstrated that women with dyslipidemia and/or coronary disease are less likely than men to receive lipid-modifying therapy20, 21
Women with polycystic ovary syndrome (PCOS) represent a subgroup at highest risk for infertility in western populations, with an estimated prevalence between 5 and 10% of women of childbearing age.22 Women with PCOS typically display insulin resistance and a cluster of cardiometabolic risk factors consistent with the metabolic syndrome. These factors include abdominal obesity, hypertension, hyperinsulinemia, low HDL-C and hypertriglyceridemia, in addition to generalized obesity and hypercholesterolemia. Women with PCOS appear to be at elevated risk of adverse cardiovascular outcomes relative to women without this condition,22 consistent with their adverse metabolic profile.
Managing cardiovascular risk in women
Recent management guidelines from the American Heart Association have addressed the management of cardiovascular risk in women.23 These guidelines acknowledge that most of the cardiovascular risk burden in women can be managed with appropriate interventions and stress the importance of careful estimation of overall cardiovascular risk.24 With regard to risk assessment, the importance of lifetime cardiovascular risk is emphasized, rather than the short-term prognosis suggested by 10-year algorithms and based on the Framingham risk score (FRS). For example, women with a low 10-year risk score may have other CHD risk biomarkers not reflected in the FRS. Thus, whereas a woman with a 10-year FRS >20% is at indisputably high risk of an adverse cardiovascular outcome, and should be managed aggressively; women with lower risk FRS may be provided a false sense of reassurance that they are at truly reduced risk of CHD. To this end, the Reynolds Risk Score has been recently suggested as a useful tool to more closely approximate global CHD risk in women 25
In general, lifestyle changes remain the cornerstone of therapy in low risk men and women with mixed dyslipidemia. They include interventions aimed at weight loss through diet and exercise,26 followed by weight maintenance that employs low saturated fat intake.27 Pharmacotherapy is reserved when blood pressure, lipids and glucose levels remain sufficiently elevated based upon national guidelines. Physicians should be aware of barriers to the achievement of good cardiovascular care in women. They include confusion arising from mixed messages from the media and from the medical profession, regarding cardiovascular health, and a tendency among women to underestimate their cardiovascular risk and to lack understanding of how to improve their cardiovascular prognosis.
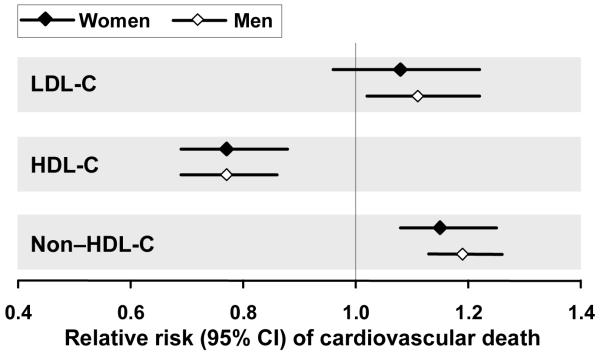
Undoubtedly, lipid-modifying interventions will have a substantial impact on reducing disease burden in women as clinical experience dictates that women derive similar benefit as men from such interventions.24 Moreover, HDL-C and non-HDL-C were shown to be at least as strong a predictor of cardiovascular death as LDL-C in women, as these parameters significantly influence cardiovascular outcome in women, while an increase in LDL-C did not (Fig. 2).28 Diagnosing and managing mixed dyslipidemia intensively is therefore important in women.
Fig. 2. Influence of lipid parameters on the risk of cardiovascular death in women and men.Error! Bookmark not defined.28.
A population of 4462 men and women were followed for an average of 19 years. The risks of cardiovascular death shown were associated with increases in lipid parameters of 0.78 mmol/L (30 mg/dL) for LDL-C and non-HDL-C and of 0.26 mmol/L (10 mg/dL) for HDL-C.
Ethnicity
The tendency of certain ethnic groups, such as South Asians and Hispanic patients to present with abdominal obesity and cardiometabolic risk factors associated with insulin resistance and the metabolic syndrome, including the atherogenic dyslipidemia phenotype, has been described above. Moreover, some ethnic groups (e.g. South Asians) are at higher risk of cardiovascular disease at a lower level of BMI.29 The diagnostic criteria for the metabolic syndrome proposed by the International Diabetes Federation recognize this phenomenon and provide ethnic-specific cut-off values for waist circumference to diagnose abdominal obesity.30
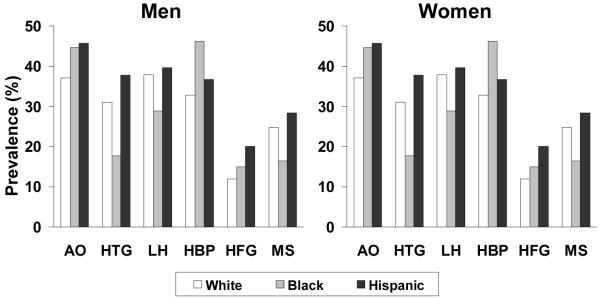
Differences in anthropometry also exist between black and white subjects, particularly with regard to women who tend to be more abdominally obese (Fig. 3).31-33 Black subjects are also more likely to be insulin resistant and have higher blood pressure (at similar BMI) than white subjects, and consequently bear a heavier burden of hypertension (see Fig. 3) and type 2 diabetes (and associated nephropathy) at a younger age. Although triglycerides tend to be lower, and HDL-C, higher, in black vs. white subjects, black subjects are more likely than white subjects to display an LDL subclass profile featuring a shift towards smaller, more atherogenic particles.34 A higher prevalence of heart failure among the black population has also been linked to the development of left ventricular hypertrophy in association with the metabolic syndrome.32
Fig. 3. Prevalence of components of the metabolic syndrome according to ethnicity in the 1988–94 cohort of the US National Health and Nutrition Examination Survey.Error! Bookmark not defined.33.
AO: abdominal obesity (waist circumference >102 cm in men or >88 cm in women); HTG: hypertriglyceridemia (>1.69 mmol/L [150 mg/dL]); LH: low HDL-C (<1.04 mmol/L [40 mg/dL] in men and <1.29 mmol/L [50 mg/dL] in women); HBP: high blood pressure (≥130/85 mmHg); HFG: high fasting glucose (≥6.1 mmol/L [110 mg/dL]); MS: National Cholesterol Education Program/Adult Treatment Panel III metabolic syndrome (three of more individual components as described above).
In view of the 40% higher cardiovascular death rates in black vs. white subjects in the USA.Error! Bookmark not defined.31 guidelines for the management of cardiovascular risk in African-Americans have recently been provided by an expert consensus panel.Error! Bookmark not defined.31 These guidelines reproduce the lipid, blood pressure and physical activity goals from general cardiovascular management guidelines, but also note that Framingham scores were derived from a largely white population. As such, there is the potential concern that FRS may underestimate higher cardiovascular risk in African-Americans. However, when applied to several cohorts that included a sizeable proportion of African-Americans, the FRS prediction was validated. 35 Nevertheless, other barriers persist, including socioeconomic issues that may limit the access of members of ethnic minorities to adequate healthcare.36 In addition, dyslipidemic white patients are more likely to receive lipid-modifying therapy than their black, Hispanic or Asian counterparts.20
Age
The prevalence of metabolic syndrome, diabetes and dyslipidemia increases with age, and contributes to increased cardiovascular risk.37, 38 A decline in mitochondrial function in older individuals also contributes to the development of insulin resistance.39 Older subjects derive benefit from lipid-modifying therapy, although the delivery of care is likely to be complicated by comorbidity and polypharmacy in this population. Nevertheless, the older patient with mixed dyslipidemia should be actively considered for lipid-modifying therapy.40
An increasing frequency of children and adolescents presenting with the metabolic syndrome or type 2 diabetes represents another consequence of the increasing prevalence of sedentary habits and obesity.41,42 Presentation with these conditions at a young age implies a long duration of exposure of the cardiovascular system to cardiometabolic risk factors, with a consequent increase in the lifetime risk of cardiovascular disease.43,44 Hypercholesterolemia, male gender, smoking, hypertension, diabetes, and low HDL-C contribute to overall risk in this population and should be managed aggressively. Current guidelines for the management of cardiovascular risk in pediatric subjects recommend lifestyle intervention for subjects at low overall risk, followed by pharmacotherapy as required for subjects with at least two cardiometabolic comorbidities.Error! Bookmark not defined.44 In particular, the risk factors associated with the metabolic syndrome in adults are known to also cluster in children with obesity or type 2 diabetes mellitus, and should be addressed individually.
Conclusions
The data summarized above highlight the importance of mixed dyslipidemia within the overall context cardiovascular care, particularly in the setting of obesity, insulin resistance or type 2 diabetes mellitus. Lifestyle intervention is the primary focus of lipid-modifying therapy according to current guidelines, with prescription of statins for patients unable to achieve their LDL-C goal in this way.7 However, this strategy does relatively little to address the disturbances of HDL-C or triglycerides in the patient with mixed dyslipidemia. These are seemingly important issues to resolve because of the residual risk imposed by low HDL-C45 or elevated TG,46 despite statin therapy.
Moving forward, the results of ongoing clinical outcome trials are expected to greatly assist in resolving these issues. They include the use of niacin in evaluating patients with the metabolic syndrome and vascular disease, (ie, AIM-HIGH: http://clinicaltrials.gov/ct/show/NCT00120289 and HPS-THRIVE II: http://www.ctsu.ox.ac.uk/pressreleases/2006-05-31/hps2-thrive-press-release), fenofibrate in diabetics (ie, ACCORD: http://www.accordtrial.org/web/public/index.cfm) in the wake of the microvascular benefits recently observed47 and dalcetrapib for acute coronary syndromes (Dal-OUTCOMES: http://www.clinicaltrials.gov/ct2/show/NCT00658515?term=nc20971). Only after these data become available will we be able to write the next chapter for optimizing the management of mixed dyslipidemia.
Acknowledgments
Supported by NIH grant HL-61639, a VA Merit Award and an American Heart Association GIA. The author acknowledges Michael Gwilt PhD for editorial assistance.
References
- 1.Centers for Disease Control and Prevention [accessed July 2008];U.S. Obesity Trends 1985–2006. Updated 2007. Available at http://www.cdc.gov/nccdphp/dnpa/obesity/trend/maps.
- 2.National Center for Health Statistics [accessed July 2008];Prevalence of Overweight and Obesity Among Adults: United States, 1999-2002. Updated 2007. Available at http://www.cdc.gov/nchs/products/pubs/pubd/hestats/obese/obse99.htm.
- 3.Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 4.Wild SH, Byrne CD. Risk factors for diabetes and coronary heart disease. BMJ. 2006;333:1009–1011. doi: 10.1136/bmj.39024.568738.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichley KB, Mueller WH, Hanis CL, et al. Centralized obesity and cardiovascular disease risk in Mexican Americans. Am J Epidemiol. 1987;125:373–386. doi: 10.1093/oxfordjournals.aje.a114544. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 7.National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;17:3143–3421. [PubMed] [Google Scholar]
- 8.Zimmet PZ, Alberti KGMM. Introduction: Globalization and the non-communicable disease epidemic. Obesity. 2006;14:1–3. doi: 10.1038/oby.2006.1. [DOI] [PubMed] [Google Scholar]
- 9. [accessed July 2008];Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Available at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.
- 10.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 11.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 13.Miller M, Seidler A, Kwiterovich PO, Pearson TA. Long-term predictors of subsequent cardiovascular events with coronary artery disease and ‘desirable’ levels of plasma total cholesterol. Circulation. 1992;86:1165–70. doi: 10.1161/01.cir.86.4.1165. [DOI] [PubMed] [Google Scholar]
- 14.Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101:1003–8. doi: 10.1016/j.amjcard.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 15.St-Pierre AC, Cantin B, Dagenais GR, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25:553–559. doi: 10.1161/01.ATV.0000154144.73236.f4. [DOI] [PubMed] [Google Scholar]
- 16.Bittner V. Perspectives on dyslipidemia and coronary heart disease in women. JACC. 2005;46:1628–1635. doi: 10.1016/j.jacc.2005.05.089. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease disadvantage. Arch Intern Med. 1995;155:57–61. [PubMed] [Google Scholar]
- 18.Matthews KA, Meilahn E, Kuller LH, et al. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 19.Lakoski SG, Cushman M, Criqui M. Gender and C-reactive protein: data from the Multithnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 20.O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med. 2004;164:1313–1318. doi: 10.1001/archinte.164.12.1313. [DOI] [PubMed] [Google Scholar]
- 21.Miller M, Byington R, Hunninghake D, Pitt B, Furberg CD, Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) Investigators Sex bias and underutilization of lipid-lowering therapy in patients with coronary artery disease at academic medical centers in the United States and Canada. Arch Intern Med. 2000;160:343–7. doi: 10.1001/archinte.160.3.343. [DOI] [PubMed] [Google Scholar]
- 22.Bailey CJ, Campbell IW, Chan JCN, et al., editors. Metformin. The Gold Standard. Wiley; Chichester: 2007. The role of metformin in polycystic ovary syndrome and infertility; pp. 223–232. [Google Scholar]
- 23.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 24.Mosca L. Management of dyslipidemia in women in the post-hormone therapy era. J Gen Inter Med. 2005;20:297–305. doi: 10.1111/j.1525-1497.2005.40239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 26.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller M, Beach V, Sorkin JD, Mangano C, Dobmeier C, Novacic D, Rhyne J, Vogel RA. Comparative Effects of 3 Popular Diets on Lipids, Endothelial Function and C-Reactive Protein during Weight Maintenance. J Am Diet Assoc. 2009;109:713–718. doi: 10.1016/j.jada.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 29.Dudeja V, Misra A, Pandey RM, Devina G, Kumar G, Vikram NK. BMI does not accurately predict overweight in Asian Indians in northern India. Br J Nutr. 2001;86:105–12. doi: 10.1079/bjn2001382. [DOI] [PubMed] [Google Scholar]
- 30.International Diabetes Federation [accessed July 2008];IDF consensus worldwide definition of the metabolic syndrome. Available at http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf.
- 31.Williams RA, Flack JM, Gavin JR, et al. Guidelines for management of high-risk African Americans with multiple cardiovascular risk factors: recommendations of an expert consensus panel. Ethn Dis. 2007;17:214–220. [PubMed] [Google Scholar]
- 32.Burchfiel CM, Skelton TN, Andrew ME, et al. Metabolic syndrome and echocardiographic left ventricular mass in blacks; the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2005;112:819–927. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 33.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 34.Kullo IJ, Jan MF, Bailey KR, et al. Ethnic differences in low-density lipoprotein particle size in hypertensive adults. J Clin Lipidol. 2007;1:218–224. doi: 10.1016/j.jacl.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 36.Fincher C, Williams JE, MacLean V, Allison JJ, Kiefe CI, Canto J. Racial disparities in coronary heart disease: a sociological view of the medical literature on physician bias. Ethn Dis. 2004;14:360–71. [PubMed] [Google Scholar]
- 37.Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. JACC. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 38.Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III) National Cholesterol Education Program (NCEP) NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kannel WB. Cardiovascular disease preventive measures for the older patient: an epidemiologic perspective. Am J Geriatr Cardiol. 2006;15:382–388. doi: 10.1111/j.1076-7460.2006.04397.x. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Ford ES, Mokdad AH, et al. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics. 2006;118:e1390–1398. doi: 10.1542/peds.2006-1062. [DOI] [PubMed] [Google Scholar]
- 42.Mattsson N, Rönnemaa T, Juonala M, et al. The prevalence of the metabolic syndrome in young adults. The Cardiovascular Risk in Young Finns study. J Intern Med. 2007;261:159–169. doi: 10.1111/j.1365-2796.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- 43.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk. An American Heart Association Scientific Statement from the Atherosclerosis, Hypertension and Obesity in the Young Committee. Circulation. 2003;107:1448–1453. doi: 10.1161/01.cir.0000060923.07573.f2. [DOI] [PubMed] [Google Scholar]
- 44.Kavey R-EW, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and the Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 45.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 46.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, PROVE IT-TIMI 22 Investigators Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 47.Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d’Emden MC, Crimet DC, O’Connell RL, Colman PG, FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]